Abstract
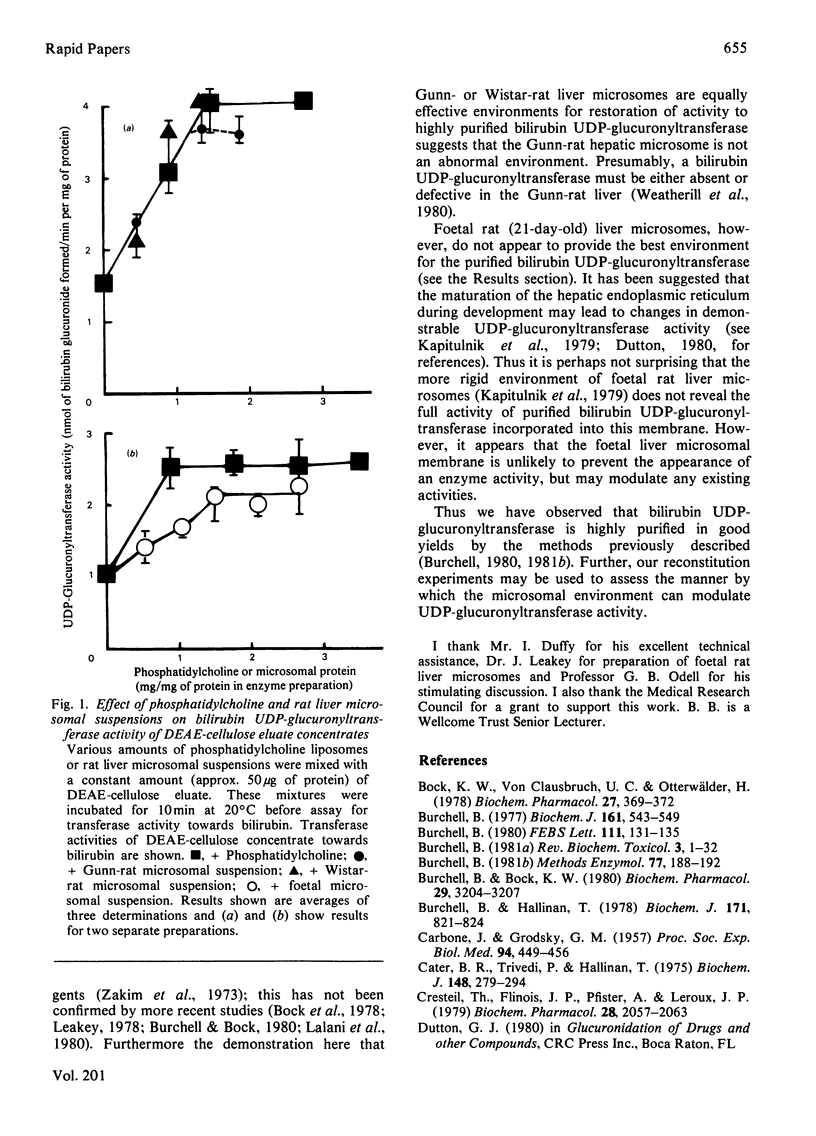
1. Reconstitution of purified bilirubin UDP-glucuronyltransferase from Wistar-rat liver into Gunn-rat liver microsomes provides a better environment than phosphatidylcholine liposomes, such that the final specific activity of the Wistar-rat liver enzyme was increased up to 85 units/mg of protein. 2. Gunn- and Wistar-rat liver microsomes were equally effective for reconstitution of the purified enzyme. 3. The transferase activity does not appear to be fully expressed in the more rigid environment of foetal Wistar-rat liver microsomes. 4. These reconstitution experiments reveal a final specific activity for the purified bilirubin UDP-glucuronyltransferase consistent with the capacity of the whole rat liver to glucuronidate bilirubin and indicate that the absence of this enzyme activity in Gunn-rat liver microsomes is not due to an abnormal microenvironment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock K. W., Clausbruch U. C., Ottenwälder H. UDP-glucuronyltransferase in perfused rat liver and in microsomes: V. Studies with Gunn rats. Biochem Pharmacol. 1978 Feb 1;27(3):369–371. doi: 10.1016/0006-2952(78)90245-9. [DOI] [PubMed] [Google Scholar]
- Burchell B., Bock K. W. Abolition of the apparent deficiency of 2-aminophenol glucuronidation in perfused Gunn rat liver by pentan-3-one. Biochem Pharmacol. 1980 Dec 1;29(23):3204–3207. doi: 10.1016/0006-2952(80)90586-9. [DOI] [PubMed] [Google Scholar]
- Burchell B., Hallinan T. Phospholipid content and activity of pure uridine diphosphate-glucuronyltransferase from rat liver. Biochem J. 1978 Jun 1;171(3):821–824. doi: 10.1042/bj1710821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell B. Isolation and purification of bilirubin UDP-glucuronyl-transferase from rat liver. FEBS Lett. 1980 Feb 25;111(1):131–135. doi: 10.1016/0014-5793(80)80777-0. [DOI] [PubMed] [Google Scholar]
- Burchell B. Studies on the purification of rat liver uridine diphosphate glucuronyltransferase. Biochem J. 1977 Mar 1;161(3):543–549. doi: 10.1042/bj1610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater B. R., Trivedi P., Hallinan T. Inhibition of glucose 6-phosphatase by pure and impure C-type phospholipases. Reactivation by phospholipid dispersions and protection by serum albumin. Biochem J. 1975 May;148(2):279–294. doi: 10.1042/bj1480279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresteil T., Flinois J. P., Pfister A., Leroux J. P. Effect of microsomal preparations and induction on cytochrome P-450-dependent monooxygenases in fetal and neonatal rat liver. Biochem Pharmacol. 1979 Jul 1;28(13):2057–2063. doi: 10.1016/0006-2952(79)90224-7. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., CARBONE J. V. The synthesis of bilirubin glucuronide by tissue homogenates. J Biol Chem. 1957 May;226(1):449–458. [PubMed] [Google Scholar]
- Kapitulnik J., Tshershedsky M., Barenholz Y. Fluidity of the rat liver microsomal membrane: increase at birth. Science. 1979 Nov 16;206(4420):843–844. doi: 10.1126/science.493984. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalani E. N., Weatherill P. J., Kennedy S. M., Burchell B. The inherited deficiency of hepatic UDP-glucuronyltransferase: structure-activity relationships of in vitro stimulators. Biochem Pharmacol. 1980 Sep 1;29(17):2367–2371. doi: 10.1016/0006-2952(80)90271-3. [DOI] [PubMed] [Google Scholar]
- Leakey J. E. An improved assay technique for uridine diphosphate glucuronosyltransferase activity towards 5-hydroxytryptamine and some properties of the enzyme. Biochem J. 1978 Dec 1;175(3):1119–1124. doi: 10.1042/bj1751119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Burchell B. The separation and purification of rat liver UDP-glucuronyltransferase activities towards testosterone and oestrone. Biochem J. 1980 Aug 1;189(2):377–380. doi: 10.1042/bj1890377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherill P. J., Kennedy S. M., Burchell B. Immunochemical comparison of UDP-glucuronyltransferase from Gunn- and Wistar-rat livers. Biochem J. 1980 Oct 1;191(1):155–163. doi: 10.1042/bj1910155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart G. J. Functional heterogeneity of UDP-glucuronosyltransferase as indicated by its differential development and inducibility by glucocorticoids. Demonstration of two groups within the enzyme's activity towards twelve substrates. Biochem J. 1978 Aug 15;174(2):485–489. doi: 10.1042/bj1740485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakim D., Goldenberg J., Vessey D. A. Regulation of microsomal enzymes by phospholipids. VI. Abnormal enzyme-lipid interactions in liver microsomes from Gunn rats. Biochim Biophys Acta. 1973 Feb 28;297(2):497–502. doi: 10.1016/0304-4165(73)90097-4. [DOI] [PubMed] [Google Scholar]


