Abstract
The external urethral sphincter (EUS), composed of skeletal muscle, along with a smooth muscle-lined internal urethral sphincter (IUS), have crucial roles in maintaining continence during bladder filling and facilitating urine flow during voiding. Disruption of this complex activity has profound consequences on normal lower urinary tract function during the micturition cycle. However, relatively little is known about the normal and pathological functions of these particular muscle types, how activity can be manipulated and regulated and why, for example, loss of EUS function and sarcopenia is associated with ageing. Here we discuss the unique physiological, biochemical and metabolic properties of striated and smooth muscle components of the urethral sphincter, which have distinct roles in maintaining continence during bladder filling. Relevant in vivo models for investigation of pathophysiological mechanisms, and for pre-clinical evaluation of therapeutic approaches are reviewed. Electromyography and Urethral Pressure Profile recordings are pivotal to understanding the function and dysfunction of the EUS and for clinical evaluation of e.g. urinary retention. Pre-clinical and clinical studies have revealed that age- or disease-related tissue remodelling that lead to filling/voiding disorders may be mitigated with emerging therapeutic approaches.
Keywords: External urethral sphincter, Striated muscle, Smooth muscle, Sarcopenia, Ageing, Urinary retention
1. Introduction
The urethral sphincter comprising the External Urethral Sphincter (EUS) and the Internal Urethral Sphincter (IUS) has received comparatively little research attention compared with the urinary bladder. Typically regarded as the external skeletal muscle layers (EUS) that surround the internal smooth muscle layers (IUS), both muscle components are important in maintaining continence during bladder filling, enabling a low-resistance conduit to urine flow during emptying. Consideration of the EUS, the main focus of this article, will therefore be in tandem with the IUS, as their combined physiological properties deliver urethral function. This article is based on the authors’ contributions to a Roundtable Discussion at the 2023 International Continence Society conference (Toronto). Here, we aim to summarise knowledge of EUS physiology alongside key elements of the IUS, highlight relevant in vivo models, review pathophysiology within clinical context and identify potential strategies and therapeutic targets to normalise EUS function.
2. Muscular tissues of the urethra
2.1. Layers of the urethra wall
The urethral wall comprises layers of skeletal muscle, smooth muscle, lamina propria (containing connective tissue and the microvasculature) and urothelium, that vary in relative proportions along its length from the bladder neck to the external environment. Moreover, there are both species and sex variations. The innermost urothelium is surrounded by the lamina propria and in the female pig urethra, this is surrounded by circular smooth muscle in the distal 75%, by longitudinal smooth muscle in the proximal 75%, and in the distal third, circular skeletal muscle is surrounded by longitudinal skeletal muscle [1]. In contrast, the rabbit urethra has better developed longitudinal muscle (skeletal and smooth) in the proximal third, and greater circular muscle in the mid-third, as shown with trichrome histology [2]. EUS skeletal muscle will be discussed in more detail in Section 3.
2.2. Urethral smooth muscle and tone
Urethral smooth muscle in circular or longitudinal orientations has some different physiological properties, notably a lower shortening velocity in circular vs. longitudinal smooth muscle [3]. This is consistent with the reported dominant contribution of urethral circular smooth muscle to the development and maintenance of tone and ultimately continence [4]. Longitudinal smooth muscle may be more dominant during emptying where its comparatively faster activity shortens the urethra, facilitating urine flow [3]. The interesting property of urethral smooth muscle preparations to develop tone after an initial stretch as demonstrated in in vitro myography or tension recordings is common across mouse, rabbit, human and pig. Urethral smooth muscle exhibits spontaneous electrical activity as observed in microelectrode recordings of spikes, slow waves and spontaneous transient depolarisations (STDs) [5], [6]. This activity underpins tonic smooth muscle contraction, and some of the contributing ion channel mechanisms are discussed below. While there is agreement that contraction of EUS skeletal muscles maintains urinary continence, it is increasingly recognised that the smooth muscle layers also have a significant contribution. In fact, urethral pressure may almost entirely reflect smooth muscle activity at rest and during bladder filling, particularly in females, with the EUS skeletal muscle becoming active during physical stress e.g. coughing or sneezing to provide additional closure during short periods of increased abdominal pressure [7]. Urethral smooth muscle is also under neural control where tetrodotoxin-sensitive neurogenic-stimulation in tension recordings evoke -adrenoceptor-mediated contractions and nitric oxide-mediated relaxations. The latter is an active mechanism underpinning opening of the urethra during micturition, reducing resistance to urine flow.
Table 1.
Skeletal muscle fibre-type distribution in human and animal external urethral sphincter. See text for explanations of fibre types.
2.3. Calcium-activated chloride channels in urethral smooth muscle
Foundational work by Callahan and Creed [5] and Hashitani et al. [6] demonstrated spontaneous or intrinsic electrical activity in urethra smooth muscle, with the latter paper providing compelling evidence that a calcium-activated chloride conductance was a key mechanism. Later, patch-clamp electrophysiology confirmed that calcium-activated chloride currents (CACC) were present in sheep urethral smooth muscle cells [16] and in novel rabbit urethral interstitial cells (UIC) [17]. Pharmacological inhibitors confirmed that CACC contributed to electrical signalling, urethral tone and neurogenic contractions [17]. The hypothesis that urethral preparations with CACC within UIC directly signal to neighbouring smooth muscle cells to contract and maintain tone is attractive with evidence from live-tissue preparations of urethra showing that Ca2+-signalling activity was sometimes correlated between UIC and neighbouring smooth muscle cells [18]. Furthermore, extensive work has elucidated the signal transduction pathways in UIC, their modulation by neurotransmitters and the origins of the oscillatory Ca2+-signals [17]. Interestingly, sex differences in urethral tone of mouse and human is reportedly associated with expression of the CACC protein, ANO1 [19], where higher levels of tone in female tissues were correlated with increased ANO1 protein expression. Given reports of the presence of UIC in human urethra [17], further research is needed to ascertain whether CACC/ANO1 in urethral smooth muscle, UIC, or both are determinants of urethral tone and importantly, does this provide therapeutic opportunity. Recent evidence supporting expression of the mechanosensitive channel, PIEZO1 in mouse urethra [20] urothelium, smooth muscle and skeletal muscle, has led to a proposed function in sensing wall tension during urine flow. Again, further work is needed in animal models and human tissue to realise the potential of this promising area.
3. Metabolic, electrophysiological and contractile properties of EUS skeletal muscle
3.1. Muscle fibre types and EUS muscle function
Skeletal muscle fibres of the EUS show a typical sarcomeric structure that in human tissue is associated with abundant mitochondria suggestive of an oxidative metabolic phenotype [8]. However, compared to musculoskeletal striated fibres, they are unusual in that they do not connect to rigid supports, so that there is less active shortening on stimulation. Moreover, a lack of muscle spindles in the muscle mass [10] implies that tension is not modified through spinal reflexes. Overall, these observations are consistent with a muscle that maintains variations in tone, consistent with a function of varying urethral fluid resistance.
This is corroborated by the fact that Type-I muscle fibres are the majority type in human EUS. These fibres, in comparison to Type-II fibres, are more fatigue-resistant under continuous, tetanic stimulation; have a smaller fibre diameter and richer capillary network and an oxidative, rather than glycolytic metabolism. This contrasts with EUS muscle from laboratory animals, that have a preponderance of Type-II fibres (Table 1). It is important to stress that there are several subtypes of Type-II fibres that range from typical glycolytic, fast, fatiguable fibres (Type-IIB) through to those (Type-IIX and Type-IIA) that have a phenotype and metabolism intermediate with Type-I fibres. However, the difference between human and animal EUS fibre type is striking and should be considered when extrapolating data from animal studies to human conditions. Various reasons may be proposed for this difference in Type-I/Type-II abundance, including the fact that most mammals, but not humans, also use urine voiding for territorial marking where rapid and intermittent control of urethral fluid resistance is necessary.
3.2. Contractile properties of EUS skeletal muscle
Stimulation of EUS in vitro or ex vivo preparations at 20 Hz or greater generates maintained tetanic contractions, after an initial partial fade, consistent with its ability to maintain a sphincter function [21], [22]. However, comparison with external anal sphincter (EAS) skeletal muscle from rats and humans showed that, despite similarities of Type-I/Type-II fibre type ratio and fibre diameter, EUS compared to EAS muscle, was far superior in maintaining a tetanic contraction [15]. The difference lay in greater succinate dehydrogenase labelling in EUS muscle, indicating greater oxidative metabolism. Interestingly, EUS activity differs between humans and rodents, particularly, the presence of coordinated contractions in small animals during micturition. These might facilitate bladder emptying and in male animals, may explain urination-territory marking behaviours whereas in humans, urethral contraction occurs during filling, facilitating continence. Although skeletal muscle contraction is not under inotropic control to the extent present in smooth muscle or myocardium, EUS contraction strength may be modulated by external factors that may exert physiological modulatory control; two examples here will suffice. The Zucker Fatty rat is an animal model of obesity that displays urine leakage when slight pressure is applied to the suprapubic area, in contrast to Zucker Lean rats. Isolated EUS preparations showed that tetanic contractions from Zucker Fatty rats show less fatigue-resistance, as well as smaller caffeine-evoked contractures (caffeine is used to release intracellular Ca2+-stores) [23]. Thus, failure of the EUS sphincter may in part be due to reduced capacity of Ca2+-stores required for contraction.
The nitric oxide (NO•) pathway exerts little action on EUS twitch contractions but does reduce the fatiguable component of the tetanic response [22]. Moreover, neuronal NO• synthase (nNOS) activity is associated with motor end-plates on EUS muscle fibres suggesting a possibility to modulate neuromuscular transmission [24].
3.3. Electrophysiological properties of EUS muscle
At a cellular level, these are similar to those in other skeletal muscles. Electrical stimulation of motor nerves to EUS muscle elicits a brief (1–2 ms) action potential that is blocked by the pre-synaptic agent, tubocurarine [21]. The depolarising phase in human and pig myotube preparations is supported by a Na current with similar voltage-dependent kinetics to those in other skeletal muscles, supplemented by inward Ca2+ currents with kinetics consistent with L-type and T-type channels, [25] that may have a role to maintain intracellular Ca2+-stores. The ionic basis of repolarisation is less well characterised and the physiological relevance of other ion channels, such as Piezo channels [20], requires further characterisation.
One pathological feature of EUS electrophysiology, considered further in more detail below (Section 5), is enhanced EUS electromyographic (EMG) activity in some patients, and associated with urinary retention [26]. The authors proposed this may result from interaction of the bioelectric fields between muscle fibres, so that an action potential conducting in one fibre may directly stimulate an adjacent one, independent of neuromuscular transmission – i.e. ephaptic transmission. This phenomenon had previously been demonstrated in nerve bundles [27] and a proposed explanation was that action potential propagation in one nerve fibre changed the excitability of an adjacent resting fibre. A detailed biophysical evaluation [28] concluded that two key properties of such a multi-fibre system would enhance ephaptic transmission: the electrical resistivity of the extracellular medium and fibre cross-sectional area. Less important factors were the separation between adjacent fibres and the electrical properties of the inactive fibre. These conclusions were consistent with experiments [27] whereby ephaptic transmission was enhanced when carried out in solutions of ‘decreased salinity’, i.e. extracellular electrical resistivity was increased. Pathologically, fibrosis is associated with such changes to extracellular resistivity. A testable hypothesis is that EUS tissue from patients showing abnormal EMG activity has increased fibrotic deposition, and if so, suggests antifibrotic therapies may offer a solution to manage such aberrant EMG activity.
4. Animal models of in vivo EUS function
4.1. Aged mice
Benign prostatic enlargement (BPE) is a highly prevalent health issue exhibited by 50% of men by age 50, and 75% by age 80 which may be associated with benign prostatic obstruction (BPO). BPE has been shown to adversely affect EUS function [29], [30] potentially due to physical impairment caused by the expansion of prostatic fibrosis. The US Federal Drug Administration (FDA) approved the use of phosphodiesterase-5 inhibitor, tadalafil, and its recent (2021) combination with finasteride (i.e., Entadfi) as a single pill for BPE/BPO [31]. However, since some patients develop refractoriness to tadalafil, the role of nitric oxide (NO•)-cGMP signalling in the pathophysiology of BPE/BPO requires thorough investigation. The ‘receptor’ for NO• is soluble guanylate cyclase (sGC) that catalyses the conversion of GTP to cGMP. The responsiveness of sGC is dependent upon cytochrome B5 reductase-3 (CYB5R3) that maintains the sGC heme in the NO• sensitive reduced state (Fe2+) [32]. However, CYB5R3 may be inhibited due to oxidative stress associated with BPE and sGC becomes unresponsive to NO•. Cinaciguat, a heme mimetic, can stimulate cGMP production when sGC is oxidised and the heme displaced [33], [34]. It has recently been reported [33] that aged (24 months) mice without hormonal manipulation recapitulated the BPO phenotype of high bladder pressure/low urine flow compared to low pressure/high flow of adult mice (9 months), therefore recapitulating human urethral/bladder symptoms in older men. Furthermore, daily oral treatment with cinaciguat (10 mg/kg/2 weeks) reversed BPO in these older mice and this could be through a urethral and/or prostatic mechanism. While mice lack a prostatic capsule that results in BPE compressing the urethra in men, the mouse prostatic EUS serves a similar function with growth of prostatic tissue within the area surrounded by the EUS compressing the urethra making the aged mouse a suitable model for studying BPO [33].
4.2. Spinal cord injured (SCI) mouse
Nearly 300,000 Americans are currently affected by SCI with approximately 18,000 new cases annually, according to the National SCI Statistics Center. SCI can cause serious impairment of motor and lower urinary tract function, with development of life-threatening comorbidities and decrease in the quality of life. SCI is associated with detrusor sphincter dyssynergia (DSD) (potentially involving the proximal urethra) which can cause significant urinary retention as well as increase the risk of urinary tract infections and damage to the kidneys. The therapeutic potential of the P75 neurotrophin receptor modulator, LM11A-31, that counters activation of cell death pathways in the early stages of SCI was recently examined. LM11A-31 improved recovery from contusion-induced SCI at thoracic level T8–T9, neurogenic detrusor overactivity (NDO) and DSD [35]. The sparing of neural connections in the spinal cord due to early treatment with LM11A-31 was demonstrated using 3D high resolution magnetic resonance imaging and improvement in DSD/NDO using EUS electromyograms and bladder cystometrograms, respectively.
4.3. Mouse model of radiation-induced cystitis
One of the initial responses following irradiation of the bladder is inflammation caused by urothelial apoptosis and urine infiltration [36]. Concurrently, there is damage to the vascular endothelium leading to ischemia. These processes cause increased collagen deposition, and decreased bladder force generation and compliance. Radiation scatter can also affect neighbouring structures including the EUS and urethra, which then experience similar ischemic and fibrotic responses [37]. Recent studies demonstrated that treatment with human relaxin 2 (hRLX2) reversed fibrosis through enhancement of extracellular matrix (ECM) degradation and suppression of collagen synthesis. It also enhanced force generation by increasing L-type Ca2+ channel CaV expression in muscle, and improved tissue perfusion through vasodilation. Furthermore, hRLX2 is anti-inflammatory, inhibiting further damage to the bladder wall. The hRLX2 hormone can bind to relaxin receptors 1 and 2 (RXFP1/2) located on immune cells, myofibroblasts and muscle cells. Thus, activation of RXFP1/2 by hRLX2 can suppress pro-inflammatory and pro-fibrotic processes, enhance ECM degradation, increase force generation in muscle [38] and reverse fibrosis [38].
5. Evaluating urethral sphincter functions in clinical practice
Neuromuscular electrical activity in the urethral sphincter can be evaluated by electromyography (EMG), and urethral functions through urodynamic pressure flow studies or specialist urethral pressure profilometry (UPP). The EUS is the more accessible component of the female urogenital sphincter complex [39] and electrical signals can be recorded using either surface EMG electrodes that are placed on the perineum or intramuscular needle electrodes inserted into the muscle. Surface EMG electrodes record from different muscles of the pelvic floor and therefore concentric needle EMG is preferred for specifically evaluating the EUS. Periurethral insertion of the needle is superior to the transvaginal approach [40] and a local anaesthetic is applied over the site of insertion and infiltrated periurethrally [41].
The EUS is tonically active in health, and profuse EMG activity can be recorded from this muscle during lower urinary tract storage phase. Electrical activity ceases during voiding, and this can be demonstrated in kinesiological EMG recordings. Needle electrodes are rarely used for this purpose given technical difficulties of retaining the needle in position during the urodynamic testing, and the inhibitory effects that the needle may have on voiding. Concomitant videofluoroscopy provides information about urethral function and is therefore currently preferred over EMG when evaluating the bladder outlet during urodynamic testing.
5.1. Urethral sphincter EMG in chronic idiopathic urinary retention
Abnormal EMG activity was first demonstrated in women with chronic idiopathic urinary retention by Clare Fowler and colleagues in the 1980s [26]. In Fowler’s syndrome, women typically present with urinary retention >1 litre; however, they lack the sense of urinary urgency or bladder pain generally associated with such large volumes. When performing intermittent self-catheterisation, patients often report an abnormal sensation as if the catheter is being “gripped”. An association with polycystic ovaries was reported in the original description of the condition. The EMG abnormalities consist of spontaneously occurring complex repetitive discharges — bizarre repetitively-firing complex discharges of variable frequency that have a characteristic sound over the loudspeaker of the EMG machine, similar to a helicopter sound. Several of the complex repetitive discharges have a prominent decelerating component, called decelerating bursts, having a myotonia-like quality (pseudomyotonia) with a sound quality similar to underwater recording of whales [26], [41]. Urethral sphincter volume and UPP have been demonstrated to be abnormal [42], and the findings from these tests suggest a primary disorder of urethral sphincter relaxation. Findings in UPP and EMG testing often correlate [42], and a pulsatile UPP trace is associated with decelerating bursts in the EMG [43].
The underlying pathophysiology responsible for Fowler’s syndrome remains unclear. Complex repetitive discharges are a non-specific finding of nerve injury and suggest an increase in muscle fibre grouping from reinnervation. Low jitter between individual discharges suggests ephaptic transmission between muscle fibres [44]. Possible mechanisms include cryptic intramuscular nerve fibre injury or an unstable muscle membrane due to a hormonally mediated channelopathy. Complex repetitive discharges have also been recorded from the EAS, suggesting wider pelvic floor dysfunction in some women [45].
Interestingly, complex repetitive discharges and decelerating bursts have been recorded from the EUS of women not reporting urinary symptoms, more prevalent in the mid-luteal phase of the menstrual cycle [46], [47]. This raises a question whether these EMG findings could in fact be present in the healthy female EUS. These findings are not reported for other skeletal muscles, and it could be speculated that the electrical activity could be contributing to the urethral continence mechanism. Most women with Fowler’s syndrome report a triggering event for going into urinary retention [48], and whether an excess level of complex repetitive discharges with decelerating bursts could predispose to developing voiding dysfunction in susceptible women needs to be evaluated further. The presence of an abnormal urethral sphincter EMG has been shown to prognosticate outcome following sacral neuromodulation [49]; moreover, urinary retention improves when botulinum toxin is injected into the EUS of women with Fowler’s syndrome with either abnormal EMG findings or elevated UPP [50].
5.2. Urethral sphincter EMG in other disorders
In women reporting stress urinary incontinence (SUI), changes of denervation have been reported in the EUS. However, these changes are unable to prognosticate surgical outcomes and therefore the practical value of urethral sphincter EMG in this setting remains unclear. Injury to the innervation of the EUS at the level of the sacral spinal cord, sacral roots or the pudendal nerve can result in neurogenic changes which can be identified in EMG. Urethral sphincter EMG is however, rarely used in clinical practice for this purpose because of the small size of the muscle; larger, more accessible muscles of the pelvic floor such as the EAS are preferred. Furthermore, assessing reinnervation by motor unit potential analysis and denervation by spontaneous activity (fibrillation potentials, positive sharp waves, fasciculations) is technically challenging due to the tonic nature of EMG activity.
6. Age-related changes and potential therapies
6.1. Age-related changes in EUS muscle
SUI results from a combination of intrinsic sphincter deficiency and urethral hypermobility due to malfunctioning of the urethra supporting structure. The prevalence of SUI increases with age, peaking in middle age and then stabilises or even decreases in old age presumably due to changes in hormonal environment or reduction in physical activity. Considering that volume and strength of skeletal muscle generally start to decline in one’s thirties and become noticeable in one’s fifties, age-related changes in the EUS appear to play a critical role in the pathogenesis of SUI. Since age-related deterioration of skeletal muscle, known as sarcopenia, occurs preferentially in fast (type II) muscle fibres, it is of interest to know whether age-related changes in human EUS, comprising predominantly of slow (type I) muscle fibres can be considered as a manifestation of sarcopenia.
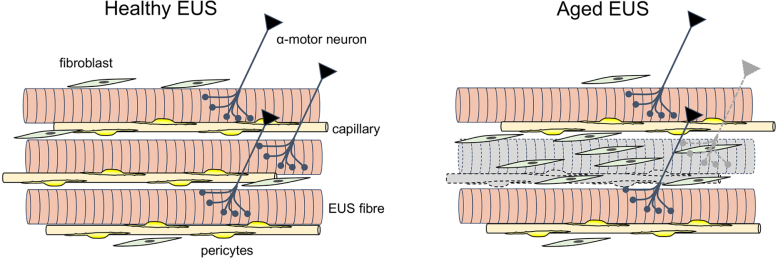
Histological examination of the female urethra using cadavers, or at autopsy, showed a decline in number and density of EUS and blood vessels, as well as an increase in connective tissue with age [51]. Histological analysis of female EUS biopsy specimens also demonstrated that SUI patients are charactered by reduced EUS fibres associated with increased connective tissue compared to continent subjects [52]. In addition, EMG revealed denervation of the EUS of SUI patients as evidenced by increased fibrillation potentials and fewer motor units than continent subjects. Thus, age-related EUS dysfunction may also be largely due to loss of motoneurons, as in the case of sarcopenia (Fig. 1).
Fig. 1.
Age-related changes in EUS. In aged EUS, the number and density of muscle fibres are reduced largely due to the loss of -motor neurons. Rarefaction of pericyte-wrapped capillaries also results in muscle fibre degeneration. Fibroblasts proliferate to replace degenerated muscle fibres with fibrotic tissue.
In clinical settings, sonographic studies demonstrated an age-related reduction in the cross-sectional area of the urethra, where EUS thickness is correlated with age or grade of SUI [53]. Importantly, magnetic resonance imaging revealed that 12-week pelvic floor muscle (PFM) training results in hypertrophy of EUS in aged SUI patients [54], indicating that age-related EUS dysfunction may be at least partly reversible.
To advance our understanding of pathogenesis of age-related EUS dysfunction, establishing animal models that can be reasonably translated to humans are required. Aged rats (>24 months) display EUS atrophy associated with fibrosis [55], or mitochondrial degeneration, and are thus characterised as displaying sarcopenia. Our preliminary studies using aged-mice also demonstrated several characteristics of sarcopenia [56] (Fig. 1), including denervation, fragmented nicotinic acetylcholine receptors, capillary rarefaction and proliferation of cells expressing platelet-derived growth factor, a marker for fibroblasts. It should be noted that, unlike slow fibres dominant in human EUS, rodent EUS is predominantly comprised of fast muscle fibres.
6.2. Therapeutic strategies and options
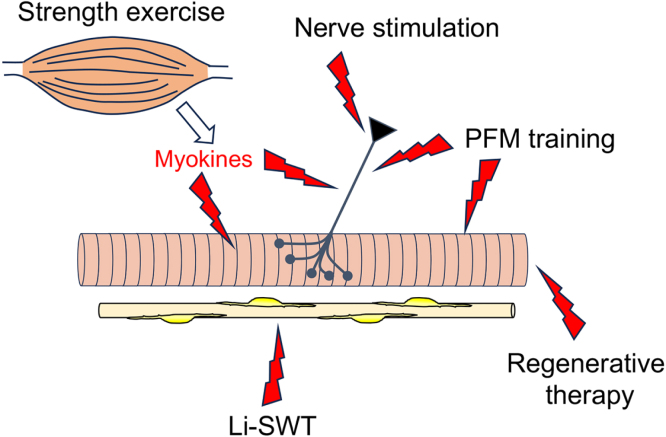
Skeletal muscle contractions exclusively arise from signal transmission from motor neurons at neuromuscular junctions. In addition, maintenance of skeletal muscle function critically relies on its motor neuron innervation as evidenced by muscle atrophy seen in sarcopenia, and functional recovery occurring upon reinnervation. Therefore, therapeutic strategies for age-related mass and/or strength loss of EUS should primarily aim to activate the motor neuron innervation of the EUS, while the EUS itself and blood vessels also have promising therapeutic potentials (Fig. 2).
Fig. 2.
Therapeutic strategies and targets for SUI. Pelvic floor muscle (PFM) training strengthens EUS fibres primarily by stimulating -motor neuron-mediated EUS contractions. Pudendal nerve stimulation also induces nerve-evoked EUS contractions. Strength exercise could trigger the release of myokines that have neurotrophic and/or anabolic actions. Low-intensity shock wave therapy (LiSWT) may activate muscle reaeration by increasing the blood supply. Regenerative therapy needs to target not only muscle but also the innervating motor neurons and vasculature.
PFM training is the optimal physical therapy that effectively improves SUI [54]. However, PFM training does not offer a complete, long-term cure in about half of SUI patients, and thus its effectiveness should be enhanced. Combination of PFM training with whole-body strength exercise could enhance PFM training-induced EUS hypertrophy by anabolic actions of myokines that are released from contracting skeletal muscles [57]. Although strength exercise is often considered inappropriate for elderly people, low-intensity resistance exercise with slow movement can be safely applied and effective for gaining muscle strength and volume [58]. Application of slow movement protocols to PFM training may also enhance its effectiveness. Importantly, preventive PFM training would be more effective than aiming to restore reduced EUS mass and/or strength.
Regenerative therapy of age-related EUS dysfunction has considerable therapeutic potential. However, because of the critical dependency of skeletal muscle function on its innervation, as well as its vascularisation to meet a high energy demand, strategies enhancing the regeneration of these structures is vital for a successful, long-term outcome. Electrical pudendal nerve stimulation that upregulates brain derived neurotrophic factors [59] to facilitate nerve regeneration and muscle reinnervation is more effective than biofeedback-assisted PFM training for the treatment of SUI [60]. Low-intensity extracorporeal shock wave therapy (Li-SWT) has been shown to improve erectile dysfunction by vasodilatory and angiogenetic actions. Li-SWT induces EUS regeneration and angiogenesis in animal model [61] and has also been shown to relieve SUI symptoms [62].
7. Summary
Although the fundamental aspects of excitation–contraction coupling of the EUS skeletal musculature have been characterised, much remains to be done to determine how these may change in pathological conditions or during natural ageing. A number of animal models are useful for mechanistic research and pre-clinical evaluation of interventions. In clinical practice, the EUS can be assessed using urodynamic testing and electromyography, which are useful to phenotype chronic idiopathic urinary retention in young women. Furthermore, as both smooth and skeletal muscle elements contribute to tone and continence, there are myriad promising therapeutic targets to be exploited.
Acknowledgements
The present study was in part supported by Grant-in-Aid for Scientific Research (C) (No. 20K09564, 23K08767) from Japan Society for Promotion of Sciences (JSPS) to HH. JNP is supported in part by funding from the United Kingdom’s Department of Health NIHR University College London Hospitals Biomedical Research Centres funding scheme. KDM has received support from the Medical Research Council, UK (MR/M012425/1). AK and CHF are supported by grant NIH R01 DK098361.
Ethics approval
The authors declare that this study did not involve Humans or Animals.
References
- 1.Brading A.F., Teramoto N., Dass N., McCoy R. Morphological and physiological characteristics of urethral circular and longitudinal smooth muscle. Scand. J. Urol. Nephrol. Suppl. 2001:12–18. doi: 10.1080/003655901750174818. discussion 106-125. [DOI] [PubMed] [Google Scholar]
- 2.Lyons A.D., Gardiner T.A., McCloskey K.D. Kit-positive interstitial cells in the rabbit urethra: Structural relationships with nerves and smooth muscle. BJU Int. 2007;99:687–694. doi: 10.1111/j.1464-410X.2006.06617.x. [DOI] [PubMed] [Google Scholar]
- 3.Arner A., Mattiasson A., Radzizewski P., Uvelius B. Shortening velocity is different in longitudinal and circular muscle layers of the rabbit urethra. Urol. Res. 1998;26:423–426. doi: 10.1007/s002400050080. [DOI] [PubMed] [Google Scholar]
- 4.Bridgewater M., MacNeil H.F., Brading A.F. Regulation of tone in pig urethral smooth muscle. J. Urol. 1993;150:223–228. doi: 10.1016/s0022-5347(17)35451-4. [DOI] [PubMed] [Google Scholar]
- 5.Callahan S.M., Creed K.E. Electrical and mechanical activity of the isolated lower urinary tract of the guinea-pig. Br. J. Pharmacol. 1981;74:353–358. doi: 10.1111/j.1476-5381.1981.tb09978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashitani H., Van Helden D.F., Suzuki H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br. J. Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venema P.L., Kramer G., Koeveringe GA.van., Heesakkers J. The maximal urethral pressure at rest and during normal bladder filling is only determined by the activity of the urethral smooth musculature in the female. J. Clin. Med. 2023;12 doi: 10.3390/jcm12072575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosling J.A., Dixon J.S., Critchley H.O., Thompson S.A. A comparative study of the human external sphincter and periurethral levator ani muscles. Br. J. Urol. 1981;53:35–41. doi: 10.1111/j.1464-410x.1981.tb03125.x. [DOI] [PubMed] [Google Scholar]
- 9.Chen X., Creed K.E. Histochemical and contractile properties of striated muscles of urethra and levator ani of dogs and sheep. Neurourol. Urodyn. 2004;23:702–708. doi: 10.1002/nau.20053. [DOI] [PubMed] [Google Scholar]
- 10.Schroder H.D., Reske-Nielsen E. Fiber types in the striated urethral and anal sphincters. Acta Neuropathol. 1983;60:278–282. doi: 10.1007/BF00691877. [DOI] [PubMed] [Google Scholar]
- 11.Tokunaka S., Okamura K., Fujii H., Yachiku S. The proportions of fiber types in human external urethral sphincter: Electrophoretic analysis of myosin. Urol. Res. 1990;18:341–344. doi: 10.1007/BF00300784. [DOI] [PubMed] [Google Scholar]
- 12.Tokunaka S., Murakami U., Okamura K., Miyata M., Yachiku S. The fiber type of the rabbits’ striated external urethral sphincter: Electrophoretic analysis of myosin. J. Urol. 1986;135:427–430. doi: 10.1016/s0022-5347(17)45658-8. [DOI] [PubMed] [Google Scholar]
- 13.Sumino Y., Sato F., Kumamoto T., Mimata H. Striated muscle fiber compositions of human male urethral rhabdosphincter and levator ani. J. Urol. 2006;175:1417–1421. doi: 10.1016/S0022-5347(05)00697-X. [DOI] [PubMed] [Google Scholar]
- 14.Whitmore I., Gosling J.A., Gilpin S.A. A comparison between the physiological and histochemical characterisation of urethral striated muscle in the guinea pig. Pflugers Arch. 1984;400:40–43. doi: 10.1007/BF00670534. [DOI] [PubMed] [Google Scholar]
- 15.Buffini M., O’Halloran K.D., O’Herlihy C., O’Connell R., Jones J.F. Comparison of the contractile properties, oxidative capacities and fibre type profiles of the voluntary sphincters of continence in the rat. J. Anat. 2010;217:187–195. doi: 10.1111/j.1469-7580.2010.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotton K.D., Hollywood M.A., McHale N.G., Thornbury K.D. Ca2+ current and Ca(2+)-activated chloride current in isolated smooth muscle cells of the sheep urethra. J. Physiol. 1997;505(Pt 1):121–131. doi: 10.1111/j.1469-7793.1997.121bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drumm B.T., Thornbury K.D., Hollywood M.A., Sergeant G.P. Role of Ano1 Ca(2+)-activated Cl(-) channels in generating urethral tone. Am. J. Physiol. Renal. Physiol. 2021;320:F525–F536. doi: 10.1152/ajprenal.00520.2020. [DOI] [PubMed] [Google Scholar]
- 18.Hashitani H., Suzuki H. Properties of spontaneous Ca2+ transients recorded from interstitial cells of Cajal-like cells of the rabbit urethra in situ. J. Physiol. 2007;583:505–519. doi: 10.1113/jphysiol.2007.136697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D., Meng W., Shu L., Liu S., Gu Y., Wang X., Feng M. ANO1 in urethral SMCs contributes to sex differences in urethral spontaneous tone. Am. J. Physiol. Renal Physiol. 2020;319:F394–F402. doi: 10.1152/ajprenal.00174.2020. [DOI] [PubMed] [Google Scholar]
- 20.Dalghi M.G., Clayton D.R., Ruiz W.G., Al-Bataineh M.M., Satlin L.M., Kleyman T.R., et al. Expression and distribution of PIEZO1 in the mouse urinary tract. Am. J. Physiol. Renal Physiol. 2019;317:F303–F321. doi: 10.1152/ajprenal.00214.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van der Werf B.A., Hidaka T., Creed K.E. Continence and some properties of the urethral striated muscle of male greyhounds. BJU Int. 2000;85:341–349. doi: 10.1046/j.1464-410x.2000.00421.x. [DOI] [PubMed] [Google Scholar]
- 22.Walters R.D., McMurray G., Brading A.F. Pudendal nerve stimulation of a preparation of isolated guinea-pig urethra. BJU Int. 2006;98:1302–1309. doi: 10.1111/j.1464-410X.2006.06498.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y.C., Lin G., Wang G., Reed-Maldonado A., Lu Z., Wang L., et al. Impaired contractility of the circular striated urethral sphincter muscle may contribute to stress urinary incontinence in female zucker fatty rats. Neurourol. Urodyn. 2017;36:1503–1510. doi: 10.1002/nau.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Pascual A., Costa G., Labadia A., Jimenez E., Triguero D., Rodriguez-Veiga E., Gonzalez-Soriano J. Partial nicotinic receptor blockade unmasks a modulatory role of nitric oxide on urethral striated neuromuscular transmission. Nitric Oxide. 2005;13:98–110. doi: 10.1016/j.niox.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Berjukow S., Margreiter E., Marksteiner R., Strasser H., Bartsch G., Hering S. Membrane properties of single muscle cells of the rhabdosphincter of the male urethra. Prostate. 2004;58:238–247. doi: 10.1002/pros.10334. [DOI] [PubMed] [Google Scholar]
- 26.Fowler C.J., Christmas T.J., Chapple C.R., Parkhouse H.F., Kirby R.S., Jacobs H.S. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: A new syndrome? BMJ. 1988;297:1436–1438. doi: 10.1136/bmj.297.6661.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz B., Schmitt O.H. Electric interaction between two adjacent nerve fibres. J. Physiol. 1940;97:471–488. doi: 10.1113/jphysiol.1940.sp003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark J.W., Plonsey R. A mathematical study of nerve fiber interaction. Biophys. J. 1970;10:937–957. doi: 10.1016/S0006-3495(70)86344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda K., Nagashima K., Murayama N., Yamanishi T., Tojo M., Shimazaki J. Change of external urethral sphincter function in prostatic patients. Urol. Int. 1991;47(Suppl 1):43–47. doi: 10.1159/000282248. [DOI] [PubMed] [Google Scholar]
- 30.Ishigooka M., Hayami S., Hashimoto T., Suzuki Y., Katoh T., Nakada T. Relative and total volume of histological components in benign prostatic hyperplasia: Relationships between histological components and clinical findings. Prostate. 1996;29:77–82. doi: 10.1002/(SICI)1097-0045(199608)29:2<77::AID-PROS2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31.Villapalos-Garcia G., Zubiaur P., Marian-Revilla C., Soria-Chacartegui P., Navares-Gomez M., Mejia-Abril G., et al. Food administration and not genetic variants causes pharmacokinetic variability of tadalafil and finasteride. J. Pers. Med. 2023;13 doi: 10.3390/jpm13111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahaman M.M., Nguyen A.T., Miller M.P., Hahn S.A., Sparacino-Watkins C., Jobbagy S., et al. Cytochrome b5 reductase 3 modulates soluble guanylate cyclase redox state and cGMP signaling. Circ. Res. 2017;121:137–148. doi: 10.1161/CIRCRESAHA.117.310705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabbarova I.V., Ikeda Y., Kozlowski M.G., Tyagi P., Birder L.A., Chakrabarty B., et al. Benign prostatic hyperplasia/obstruction ameliorated using a soluble guanylate cyclase activator. J. Pathol. 2022;256:442–454. doi: 10.1002/path.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanai A.J., Andersson K.E., Birder L.A., Fry C.H. Soluble guanylate cyclase activators to treat benign prostatic hyperplasia and associated LUTS. Continence (Amst) 2023;6 doi: 10.1016/j.cont.2023.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda Y., Zabbarova I., Tyagi P., Hitchens T.K., Wolf-Johnston A., Wipf P., Kanai A. Targeting neurotrophin and nitric oxide signaling to treat spinal cord injury and associated neurogenic bladder overactivity. Continence (Amst) 2022;1 doi: 10.1016/j.cont.2022.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zwaans B.M.M., Carabulea A.L., Bartolone S.N., Ward E.P., Chancellor M.B., Lamb L.E. Voiding defects in acute radiation cystitis driven by urothelial barrier defect through loss of E-cadherin, ZO-1 and Uroplakin III. Sci. Rep. 2021;11:19277. doi: 10.1038/s41598-021-98303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doiron R.C., Witten J., Rourke K.F. The scope, presentation, and management of genitourinary complications in patients presenting with high-grade urethral complications after radiotherapy for prostate cancer. Can. Urol. Assoc. J. 2021;15:E6–E10. doi: 10.5489/cuaj.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikeda Y., Zabbarova I.V., Birder L.A., Wipf P., Getchell S.E., Tyagi P., et al. Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice. Neurourol. Urodyn. 2018;37:2441–2451. doi: 10.1002/nau.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oelrich T.M. The striated urogenital sphincter muscle in the female. Anat. Rec. 1983;205:223–232. doi: 10.1002/ar.1092050213. [DOI] [PubMed] [Google Scholar]
- 40.Olsen A.L., Benson J.T., McClellan E. Urethral sphincter needle electromyography in women: Comparison of periurethral and transvaginal approaches. Neurourol. Urodyn. 1998;17:531–535. doi: 10.1002/(sici)1520-6777(1998)17:5<531::aid-nau9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 41.Panicker J.N., M, Fowler C.J. Fowler’s syndrome: A primary disorder of urethral sphincter relaxation. Obstet. Gynaecol. 2018;20(2) [Google Scholar]
- 42.Wiseman O.J., Swinn M.J., Brady C.M., Fowler C.J. Maximum urethral closure pressure and sphincter volume in women with urinary retention. J. Urol. 2002;167:1348–1351. doi: 10.1016/s0022-5347(05)65297-4. discussion 1351-1342. [DOI] [PubMed] [Google Scholar]
- 43.Sihra N., Malde S., Panicker J., Kightley R., Solomon E., Hamid R., et al. Does the appearance of the urethral pressure profile trace correlate with the sphincter EMG findings in women with voiding dysfunction? Neurourol. Urodyn. 2018;37:751–757. doi: 10.1002/nau.23341. [DOI] [PubMed] [Google Scholar]
- 44.Trontelj J., Stalberg E. Bizarre repetitive discharges recorded with single fibre EMG. J. Neurol. Neurosurg. Psychiatry. 1983;46:310–316. doi: 10.1136/jnnp.46.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb R.J., Fawcett P.R., Neal D.E. Electromyographic abnormalities in the urethral and anal sphincters of women with idiopathic retention of urine. Br. J. Urol. 1992;70:22–25. doi: 10.1111/j.1464-410x.1992.tb15657.x. [DOI] [PubMed] [Google Scholar]
- 46.Ramm O., Mueller E.R., Brubaker L., Lowenstein L., Kenton K. Complex repetitive discharges–A feature of the urethral continence mechanism or a pathological finding? J. Urol. 2012;187:2140–2143. doi: 10.1016/j.juro.2012.01.118. [DOI] [PubMed] [Google Scholar]
- 47.Tawadros C., Burnett K., Derbyshire L.F., Tawadros T., Clarke N.W., Betts C.D. External urethral sphincter electromyography in asymptomatic women and the influence of the menstrual cycle. BJU Int. 2015;116:423–431. doi: 10.1111/bju.13042. [DOI] [PubMed] [Google Scholar]
- 48.Swinn M.J., Wiseman O.J., Lowe E., Fowler C.J. The cause and natural history of isolated urinary retention in young women. J. Urol. 2002;167:151–156. [PubMed] [Google Scholar]
- 49.De Ridder D., Ost D., Bruyninckx F. The presence of Fowler’s syndrome predicts successful long-term outcome of sacral nerve stimulation in women with urinary retention. Eur. Urol. 2007;51:229–233. doi: 10.1016/j.eururo.2006.06.031. discussion 233-224. [DOI] [PubMed] [Google Scholar]
- 50.Wright SL.A.P., Satish M.R., Malladi P., Pakzad M., Simeoni S., Panicker J.N. Delivery of urethral sphincter botulinum toxin injections for treating urinary retention during the COVID19 pandemic. Continence Rep. 2023 [Google Scholar]
- 51.Carlile A., Davies I., Rigby A., Brocklehurst J.C. Age changes in the human female urethra: A morphometric study. J. Urol. 1988;139:532–535. doi: 10.1016/s0022-5347(17)42512-2. [DOI] [PubMed] [Google Scholar]
- 52.Hale D.S., Benson J.T., Brubaker L., Heidkamp M.C., Russell B. Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am. J. Obstet. Gynecol. 1999;180:342–348. doi: 10.1016/s0002-9378(99)70211-5. [DOI] [PubMed] [Google Scholar]
- 53.Klauser A., Frauscher F., Strasser H., Helweg G., Kolle D., Strohmeyer D., et al. Age-related rhabdosphincter function in female urinary stress incontinence: Assessment of intraurethral sonography. J. Ultrasound Med. 2004;23:631–637. doi: 10.7863/jum.2004.23.5.631. quiz 638-639. [DOI] [PubMed] [Google Scholar]
- 54.Madill S.J., Pontbriand-Drolet S., Tang A., Dumoulin C. Changes in urethral sphincter size following rehabilitation in older women with stress urinary incontinence. Int. Urogynecol. J. 2015;26:277–283. doi: 10.1007/s00192-014-2507-6. [DOI] [PubMed] [Google Scholar]
- 55.Oshiro T., Kimura R., Izumi K., Ashikari A., Saito S., Miyazato M. Changes in urethral smooth muscle and external urethral sphincter function with age in rats. Physiol. Rep. 2021;8 doi: 10.14814/phy2.14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson L., Degens H., Li M., Salviati L., Lee Y.I., Thompson W., et al. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cornish S.M., Bugera E.M., Duhamel T.A., Peeler J.D., Anderson J.E. A focused review of myokines as a potential contributor to muscle hypertrophy from resistance-based exercise. Eur. J. Appl. Physiol. 2020;120:941–959. doi: 10.1007/s00421-020-04337-1. [DOI] [PubMed] [Google Scholar]
- 58.Tanimoto M., Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J. Appl. Physiol. (1985) 2006;100:1150–1157. doi: 10.1152/japplphysiol.00741.2005. [DOI] [PubMed] [Google Scholar]
- 59.Balog B.M., Deng K., Askew T., Kuang M., Hanzlicek B., Damaser M.S. Brain derived neurotrophic factor mediates accelerated recovery of regenerative electrical stimulation in an animal model of stress urinary incontinence. Exp. Neurol. 2021;343 doi: 10.1016/j.expneurol.2021.113781. [DOI] [PubMed] [Google Scholar]
- 60.Wang S., Lv J., Feng X., Wang G., Lv T. Efficacy of electrical pudendal nerve stimulation in treating female stress incontinence. Urology. 2016;91:64–69. doi: 10.1016/j.urology.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 61.Wu A.K., Zhang X., Wang J., Ning H., Zaid U., Villalta J.D., et al. Treatment of stress urinary incontinence with low-intensity extracorporeal shock wave therapy in a vaginal balloon dilation induced rat model. Transl. Androl. Urol. 2018;7:S7–S16. doi: 10.21037/tau.2017.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin K.L., Chueh K.S., Lu J.H., Chuang S.M., Wu B.N., Lee Y.C., et al. Low intensity extracorporeal shock wave therapy as a novel treatment for stress urinary incontinence: A randomized-controlled clinical study. Medicina (Kaunas) 2021;57 doi: 10.3390/medicina57090947. [DOI] [PMC free article] [PubMed] [Google Scholar]