Abstract
The level of phosphorus must be carefully monitored for proper and effective utilization of coal and coal ash. The phosphorus content needs to be assessed to optimize combustion efficiency and maintenance costs of power plants, ensure quality, and minimize the environmental impact of coal and coal ash. The detection of low levels of phosphorus in coal and coal ash is a significant challenge due to its complex chemical composition and low concentration levels. Effective monitoring requires accurate and sensitive equipment for the detection of phosphorus in coal and coal ash. X-ray fluorescence (XRF) is a commonly used analytical technique for the determination of phosphorus content in coal and coal ash samples but proves challenging due to their comparatively weak fluorescence intensity. Fourier Transform Infrared spectroscopy (FTIR) emerges as a promising alternative that is simple, rapid, and cost-effective. However, research in this area has been limited. Until now, only a limited number of research studies have outlined the estimation of major elements in coal, predominantly relying on FTIR spectroscopy. In this article, we explore the potential of FTIR spectroscopy combined with machine learning models (piecewise linear regression—PLR, partial least square regression—PLSR, random forest—RF, and support vector regression—SVR) for quantifying the phosphorus content in coal and coal ash. For model development, the methodology employs the mid-infrared absorption peak intensity levels of phosphorus-specific functional groups and anionic groups of phosphate minerals at various working concentration ranges of coal and coal ash. This paper proposes a multi-model estimation (using PLR, PLSR, and RF) approach based on FTIR spectral data to detect and rapidly estimate low levels of phosphorus in coal and its ash (R of 0.836, RMSE of 0.735 ppm, RMSE (%) of 34.801, MBE of − 0.077 ppm, MBE (%) of 5.499, and MAE of 0.528 ppm in coal samples and R of 0.803, RMSE of 0.676 ppm, RMSE (%) of 38.050, MBE of − 0.118 ppm, MBE (%) of 4.501, and MAE of 0.474 ppm in coal ash samples). Our findings suggest that FTIR combined with the multi-model approach combining PLR, PLSR, and RF regression models is a reliable tool for rapid and near-real-time measurement of phosphorus in coal and coal ash and can be suitably modified to model phosphorus content in other natural samples such as soil, shale, etc.
Keywords: Phosphorus, Minor element, Coal, Coal ash, FTIR, Spectroscopy
Subject terms: Environmental sciences, Environmental impact
Introduction
Coal is one of the most abundant energy sources worldwide, providing reasonable, reliable, and constant power available on demand to meet energy consumption and industrial needs. Coal originates from diverse plants, accumulation of skeletal fragments, and organic elements within peat deposits1,2. Over time, coal undergoes extended geological and microbiological processes, incorporating multi-elemental impurities1. These multi-elemental impurities in coal greatly influence the environmental, economic, and combustion liability3. The elemental composition of coal is an important factor governing the liability of coal to spontaneous combustion, which is one of the most dangerous mining hazards4–6. They also affect the quality and pose deleterious effects during coal utilization. In recent years, there has been a growing focus on the determination of elements in coal and coal ash due to their significant role in the local and global energy mix. Phosphorus found in coal plays a crucial role in metallurgical processing, as it significantly impacts the quality of the resulting products. The lack of commercially viable alternative energy technologies that can significantly replace fossil fuels makes coal vital to meeting the global energy demand. Coal generates approximately 40% of the world’s power and heat, despite growing decarbonization efforts over the past 40 years7. Hence, there is a critical demand for the advancement of efficient, economical, and swift techniques to determine the elemental content in coal and coal ash samples. Analyzing the elemental impurities within coal will offer direction for refining coal purification methods, thereby enhancing the coal quality. Furthermore, improved knowledge and quantification of the elemental impurities in coal and coal ash will play an important role in their effective utilization, as the elemental makeup of the input coal significantly impacts the final quality of the products and residues.
Significance of phosphorus content in coal and coal ash
Phosphorus (P), with atomic number 15, is a crucial element for living cells, which, during organic decay in the peat bed, is released from the plant structure and re-precipitated elsewhere. Phosphorus-bearing mineral groups such as apatite, monazite, xenotime, and crandallite are ubiquitous in coal8,9. Although most of the phosphorus in coal is thought to be in the form of inorganic mineral components, researchers have identified the possibility of organic phosphorus in coal9. The Clarke value of phosphorus is 200 ± 30 ppm for brown coal and 250 ± 10 ppm for hard coal10–14. The amount of P in coal varies depending on the location and geological period. Coal from the Cretaceous era found in Western Canada and coal from the Permian era found in India and Australia have higher levels of P compared to coal from the Carboniferous era found in the United States and Europe15.
Though present in minor amounts, the presence of phosphorus in coal has garnered considerable attention due to its detrimental effect on the marketing and use of coking coals. Phosphorus is crucial for enhancing the strength of the metal, but excess can render the final metal product brittle16. The presence of phosphorus in the form of an oxide impurity results in the formation of intergranular segregation during the process of continuous casting. This results in a decrease in the ductility and toughness of the steel, which, in turn, results in an increased risk of breakage during processing, fabrication, and use17. In addition to this, phosphorus can cause deposits to form in the superheater of boilers and can harm catalysts in the process of liquefaction11. This reduces the heat transfer rate, increases pressure drop, reduces the overall combustion efficiency, and increases the maintenance cost of the system. Thus, phosphorus impurities in coal will uncover their grade and value. Phosphorus is also significant in the long-term leaching of coal waste products2,18,19. Hence, it is important to monitor and control the quality of coal during various processing stages, such as washing, blending, and pulverization, to estimate the likely interactions they may have in different coal utilization processes. This ensures that coal meets all regulatory specifications and can help optimize the performance of coal-fired power plants and other industrial processes that depend on coal as a fuel source. Accurate quantification of phosphorus content can help power plant operators adjust combustion parameters to minimize these effects. Hence, the rapid determination of phosphorus in coal is of primary interest, especially for low phosphorus steel or ferro alloy-making processes where the input material, like coke, may contribute up to 36% compared to 21% from iron ore and 43% of phosphorus from sinter20–22.
Massive amounts of coal ash produced from coal combustion can release airborne phosphorus compounds, contributing to PM10 particulate matter. Concerns over long-term phosphorus supply in nature and its environmental impact have arisen. Coal fly ash, rich in essential nutrients, including phosphorus, holds the potential for enhancing plant growth and amending acidic soils. Analyzing the elemental composition of coal ash is crucial due to its dual role as a health hazard and industrial resource23–27. Hence, there is a vital requirement for a fast and dependable method to measure and monitor phosphorus levels in coal and coal ash, necessitating the use of cost-effective and expeditious multi-element analytical techniques.
Phosphorus detection methods in coal and coal ash
Numerous analytical techniques are available for the elemental determination of coal and coal ash. Techniques like traditional wet analysis methods (gravimetric and volumetric) for measuring coal phosphorus are labour-intensive and destructive. While other advanced techniques like inductively coupled plasma optical emission spectroscopy (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), Atomic fluorescence spectrometry (AFS), and atomic absorption spectroscopy (AAS) offer efficiency, they involve complex wet digestion sample preparation and high operational costs. Sample preparation requires the use of inorganic acids (HClO4, HF, H2SO4, H3BO3), which can pose safety risks and negatively impact spectrometric techniques due to matrix effects and spectral interferences28,29. Moreover, wet digestion is prone to systematic errors, including contamination and analyte losses, exacerbated by operating at low temperatures and atmospheric pressure, leading to poor elemental extraction recoveries30. High carbon concentrations in coal can also cause spectral and non-spectral interferences in inductively coupled plasma-based detection techniques. Excessive acid and residual carbon content alter solution properties, affecting the aerosol formation and analyte transportation to the plasma, resulting in signal reduction and high background, affecting detection limits31,32. AFS is vulnerable to specific elements and is impacted by fluorescence and matrix effects, and it may not completely mitigate spectral interference33.
Non-dispersion detection methods like X-ray diffraction (XRD), X-ray Fluorescence (XRF), and X-ray photoelectron spectroscopy (XPS) are non-destructive alternatives, with XRF being common for phosphorus measurement and requiring minimal sample preparation. This is advantageous, especially for coal samples, which are difficult to dissolve due to their complex organic matrices. Above all, direct analysis with non-destructive techniques is particularly useful over destructive methods for determining volatile elements32. Matrix interferences resulting from major elements comprising the majority of the sample matrix and the need for careful standard utilization reduce the sensitivity for minor and trace elements, leading to high detection limits and poor precision in quantitative analysis. Fractionation and spectrometric interference issues can also significantly disrupt the accuracy and precision of this analysis approach32,34,35. Overall, these methods have trade-offs in terms of cost, sensitivity, and practicality for handling bulk sample numbers. For the rapid assessment of phosphorus in coal and its ash, there is a strong preference for an approach that reduces sample processing time and analytical duration, facilitates high sample throughput, and ensures acceptable accuracy.
Until now, a limited number of research studies have outlined the estimation of elements like sulfur and carbon, predominantly relying on FTIR spectroscopy and machine learning techniques36–43. Quantifying phosphorus in coal and coal ash using FTIR spectroscopy is challenging due to the element’s extremely low concentration and weak signal. Mid-infrared (MIR) spectra of coal and coal ash contain absorbance peaks due to the functional and anionic groups of minerals related to phosphorus in coal and coal ash. The objective of this current study was to assess the effectiveness of using FTIR spectroscopy in conjunction with multivariate regression techniques for the measurement of phosphorus in coal and its ash, compared to traditional analytical methods. Samples prepared in different concentration ranges, making a total of 96 FTIR spectra each for coal and its ash, were utilized to establish the model. The standard XRF method determined the reference phosphorus concentration in each sample. After establishing a model, it becomes feasible to predict the concentrations of phosphorus in an unknown sample by analyzing its spectrum in near-real time.
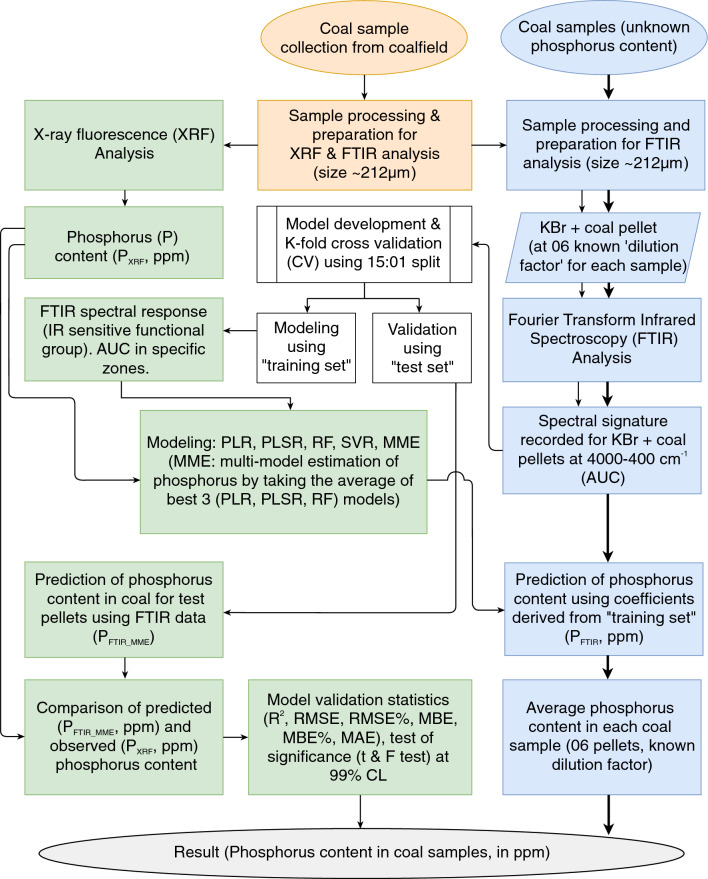
The present method provides the advantage of detecting low levels of phosphorus in coal, which is particularly crucial for the steel-making industry and environmental regulations. Unlike other methods, it requires no hazardous chemicals, much less sample size, sample preparation, and measurement time. The proposed method using FTIR stands out as a simpler, cost-effective, and rapidly working alternative to measure the phosphorus content in coal and coal ash. The approach, methods, and comparative validation analysis conducted in the study are summarized in Fig. 1.
Figure 1.
Schematic illustration of the step-by-step procedure used for model development, validation, and estimation of phosphorus content in coal used. The same procedure was repeated for the analysis of phosphorus in coal ash. (AUC, Area under the curve; PLR, piecewise linear regression; PLSR, partial least square regression; RF, random forest, SVR, support vector regression; MME, multi-model estimation).
Results
Elemental composition of coal and coal ash
The elemental composition of coal and its ash is determined at various stages of the coal mining process, from exploration and mining to transportation and combustion. This information is utilized to assess the quality and suitability of the coal (for applications such as power generation, steel production, and cement manufacturing) and its ash (for applications such as fertilizers, the construction industry, and contaminant removal). In this study, WDXRF spectroscopy is employed to quantitatively analyze the phosphorus content in coal and coal ash along with sodium, magnesium, aluminium, silicon, potassium, calcium, titanium, manganese, and iron. The phosphorus content in coal and coal ash samples is reported as P2O5 mass percent, which is converted to phosphorus mass percent using the conversion factor specific to phosphorus. All the concentrations expressed in mass percent were later converted to parts per million (ppm). The phosphorus content analyzed in coal ranges from 215.59 ppm in sample J_01 to 569.96 ppm in sample J_14 while that in coal ash ranges from 135.29 ppm in sample J_01 to 466.97 ppm in sample J_16. A comprehensive report of the WDXRF analysis conducted on coal and coal ash samples is provided in Tables 1 and 2 respectively.
Table 1.
XRF spectroscopy analysis report providing the elemental composition of coal.
| XRF Spectroscopy (mass %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Na2O | MgO | Al2O3 | SiO2 | P2O5 | P (ppm) | K2O | CaO | TiO2 | MnO | Fe2O3 | Total |
| J_01 | 0.418 | 1.428 | 24.880 | 43.123 | 0.049 | 215.591 | 0.828 | 7.240 | 2.549 | 0.241 | 19.243 | 100.00 |
| J_02 | 0.000 | 0.297 | 21.800 | 40.473 | 0.071 | 308.549 | 0.439 | 1.952 | 2.108 | 0.144 | 32.717 | 100.00 |
| J_03 | 0.190 | 1.226 | 23.819 | 47.707 | 0.114 | 497.520 | 0.576 | 10.495 | 2.680 | 0.241 | 12.951 | 99.99 |
| J_04 | 0.155 | 0.424 | 22.762 | 55.386 | 0.087 | 380.559 | 0.604 | 9.829 | 5.248 | 0.272 | 5.233 | 100.00 |
| J_05 | 0.159 | 0.742 | 19.755 | 44.277 | 0.088 | 385.796 | 0.405 | 9.053 | 2.790 | 0.333 | 22.399 | 100.00 |
| J_06 | 0.529 | 0.734 | 24.213 | 54.267 | 0.068 | 294.584 | 1.468 | 7.132 | 4.460 | 0.210 | 6.919 | 100.00 |
| J_07 | 0.087 | 0.614 | 23.049 | 62.724 | 0.103 | 448.204 | 1.295 | 5.578 | 3.418 | 0.095 | 3.038 | 100.00 |
| J_08 | 0.065 | 0.614 | 21.376 | 44.862 | 0.073 | 318.587 | 0.555 | 3.544 | 2.834 | 0.254 | 25.822 | 99.99 |
| J_09 | 0.114 | 0.873 | 24.418 | 57.573 | 0.094 | 408.926 | 1.533 | 5.192 | 3.885 | 0.097 | 6.222 | 100.00 |
| J_10 | 0.178 | 0.374 | 22.406 | 59.077 | 0.090 | 392.342 | 0.550 | 7.935 | 6.132 | 0.180 | 3.078 | 99.99 |
| J_11 | 0.122 | 0.571 | 20.061 | 40.503 | 0.091 | 397.579 | 0.308 | 7.219 | 2.279 | 0.263 | 28.582 | 100.00 |
| J_12 | 0.329 | 1.389 | 25.293 | 42.923 | 0.055 | 238.286 | 0.836 | 6.390 | 1.975 | 0.249 | 20.562 | 100.00 |
| J_13 | 0.136 | 0.892 | 24.659 | 56.556 | 0.102 | 444.713 | 0.309 | 8.598 | 3.643 | 0.212 | 4.895 | 99.99 |
| J_14 | 0.289 | 1.708 | 23.729 | 42.479 | 0.131 | 569.966 | 0.695 | 16.905 | 3.504 | 0.373 | 10.188 | 99.99 |
| J_15 | 0.129 | 0.395 | 23.431 | 59.212 | 0.078 | 339.099 | 0.526 | 7.615 | 5.511 | 0.182 | 2.921 | 100.00 |
| J_16 | 0.117 | 0.809 | 23.801 | 52.465 | 0.126 | 550.327 | 0.283 | 8.355 | 3.220 | 0.320 | 10.504 | 99.99 |
Table 2.
XRF spectroscopy analysis report providing the elemental composition of coal ash.
| XRF Spectroscopy (mass %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Na2O | MgO | Al2O3 | SiO2 | P2O5 | P (ppm) | K2O | CaO | TiO2 | MnO | Fe2O3 | Total |
| J_01 | 0.836 | 2.565 | 29.685 | 49.670 | 0.031 | 135.290 | 0.458 | 4.454 | 1.867 | 0.099 | 7.354 | 97.02 |
| J_02 | 0.268 | 1.130 | 28.836 | 58.183 | 0.077 | 336.044 | 0.249 | 1.522 | 1.930 | 0.041 | 17.681 | 109.92 |
| J_03 | 0.482 | 3.050 | 27.793 | 53.232 | 0.066 | 288.038 | 0.289 | 6.059 | 1.996 | 0.048 | 5.123 | 98.14 |
| J_04 | 0.363 | 0.862 | 27.993 | 61.903 | 0.048 | 209.482 | 0.180 | 3.834 | 2.751 | 0.018 | 1.243 | 99.20 |
| J_05 | 0.385 | 1.784 | 24.371 | 56.118 | 0.092 | 401.507 | 0.220 | 3.789 | 2.390 | 0.224 | 9.367 | 98.74 |
| J_06 | 0.710 | 1.382 | 31.115 | 60.211 | 0.043 | 187.661 | 0.772 | 3.630 | 2.595 | 0.024 | 2.506 | 102.98 |
| J_07 | 0.382 | 1.278 | 29.170 | 68.258 | 0.080 | 349.137 | 0.601 | 2.819 | 1.933 | 0.000 | 1.194 | 105.71 |
| J_08 | 0.255 | 1.424 | 27.653 | 57.406 | 0.066 | 288.038 | 0.275 | 2.239 | 2.080 | 0.107 | 11.301 | 102.80 |
| J_09 | 0.347 | 1.828 | 28.087 | 62.636 | 0.087 | 379.686 | 0.787 | 3.129 | 2.377 | 0.000 | 2.949 | 102.22 |
| J_10 | 0.390 | 0.921 | 26.924 | 61.757 | 0.074 | 322.951 | 0.222 | 4.047 | 3.127 | 0.011 | 1.149 | 98.62 |
| J_11 | 0.348 | 1.372 | 24.315 | 52.596 | 0.100 | 436.421 | 0.170 | 4.135 | 1.894 | 0.147 | 13.360 | 98.44 |
| J_12 | 0.749 | 2.698 | 30.355 | 50.874 | 0.031 | 135.290 | 0.480 | 4.078 | 1.562 | 0.100 | 8.842 | 99.77 |
| J_13 | 0.313 | 1.685 | 30.759 | 63.053 | 0.090 | 392.779 | 0.071 | 4.082 | 1.600 | 0.042 | 2.028 | 103.72 |
| J_14 | 0.617 | 4.751 | 20.735 | 40.324 | 0.055 | 240.031 | 0.379 | 10.040 | 2.090 | 0.088 | 2.839 | 81.92 |
| J_15 | 0.347 | 0.853 | 27.531 | 61.009 | 0.045 | 196.389 | 0.161 | 3.491 | 2.810 | 0.001 | 0.961 | 97.21 |
| J_16 | 0.334 | 1.568 | 30.020 | 60.791 | 0.107 | 466.970 | 0.071 | 4.298 | 1.454 | 0.116 | 4.147 | 102.90 |
Selection of MIR bands suitable for P determination
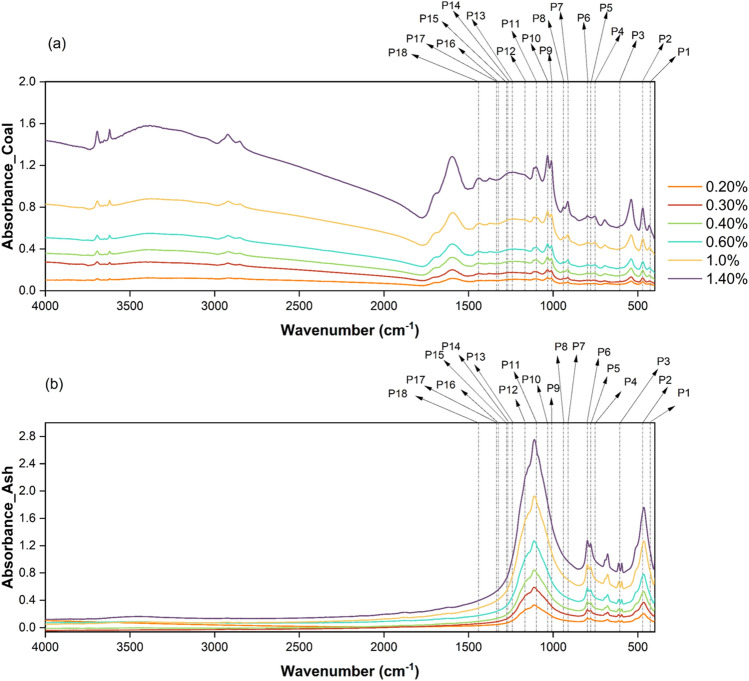
FTIR is used to identify, analyze and quantify functional groups and chemical bonds in compounds through its ability to measure the absorbance of infrared light at different wavelengths. Phosphorus-containing compounds exhibit characteristic IR absorption bands depending on the specific functional groups present. Phosphorus-containing compounds typically exhibit absorption bands in the IR spectrum at around 1200–950 cm-1, which are associated with the stretching vibrations of P–O bonds. Additionally, other functional groups associated with phosphorus, like phosphates, phosphonates, phosphines, phosphine oxides, and phosphate esters, and their infrared active regions, were identified. Besides that, the IR-sensitive frequency range of the PO43- anionic complex common to phosphorus-containing minerals was also identified. A set of 18 peaks was identified and utilized for the current study44–47. The 18 mid-IR sensitive peaks of phosphorus for the coal and ash sample J_03 (set of 06 pellets with known sample concentrations showing incremental intensity in absorbance) are shown in Fig. 2. Using the functional group and chemical bond assignments provided in Table 3, the area under the curve (AUC) was determined for all 18 peaks.
Figure 2.
Mid-IR absorbance spectra of (a) 06 coal sample pellets and (b) 06 coal ash sample pellets of known incremental concentration of sample J_04 showing the identified phosphorus sensitive ranges or peaks as given in table 3.
Table 3.
Details of identified MIR absorption peaks of phosphorus compounds and their assigned functional and ionic groups used for the study44–47.
| Peak | Onset (cm-1) | Offset (cm-1) | Center (cm-1) | Assignment |
|---|---|---|---|---|
| P_1 | 419 | 444 | 427 | O–P–O (scissors deformation) |
| P_2 | 444 | 495 | 470 | |
| P_3 | 600 | 612 | 607 | PO43-asymmetric deformation |
| P_4 | 730 | 767 | 752 | P–O (stretch) |
| P_5 | 767 | 787 | 777 | |
| P_6 | 787 | 833 | 797 | |
| P_7 | 885 | 930 | 912 | P–H (bend) |
| P_8 | 930 | 952 | 939 | |
| P_9 | 990 | 1022 | 1009 | P–O–C (asymmetric stretching) |
| P_10 | 1022 | 1065 | 1032 | |
| P_11 | 1069 | 1108 | 1099 | PO43-asymmetric valence oscillations |
| P_12 | 1140 | 1191 | 1165 | P=O (stretch) |
| P_13 | 1238 | 1260 | 1242 | |
| P_14 | 1260 | 1272 | 1268 | |
| P_15 | 1272 | 1288 | 1275 | |
| P_16 | 1318 | 1328 | 1324 | |
| P_17 | 1332 | 1338 | 1334 | |
| P_18 | 1430 | 1445 | 1441 | P–C (stretch) |
Model estimation and assessment
For model estimation, a set of six sample pellets was prepared for each of the sixteen coal (coal + KBr) and its ash samples (coal ash + KBr). The six samples were prepared at known dilution factors by fixing the KBr weight at 220 mg and the weight of coal in the sample pellet varying in the order of 0.44, 0.66, 0.88, 1.33, 2.22, and 3.12 mg. The same dilution factors were applied for the preparation of coal ash sample pellets. The spectral response of all 96 coal sample pellets and coal ash sample pellets was recorded individually using Bruker FTIR. The recorded spectra also contained signatures from the KBr spectra, which were mixed with the samples to create pellets. To eliminate the KBr signature from the sample spectra, the spectra of pure KBr pellets were recorded and used as a reference spectrum. This reference spectrum was then subtracted from each pellet’s signature, resulting in a new spectrum that was used for subsequent analysis.
The quantitative analysis of phosphorus content in coal and coal ash samples through FTIR spectroscopy involved the application of piecewise linear regression (PFTIR_PLR), partial least square regression (PFTIR_PLSR), random forest (PFTIR_RF) and support vector regression (PFTIR_SVR) models. All models were established with the area under the curve of the eighteen identified absorption peaks associated with the functional groups of phosphorus calculated and used as the independent input variable set. For model development and validation, the K fold cross-validation technique was utilized to test the performance of the FTIR data-based model in determining the phosphorus content in the sample pellets. This cross-validation technique permits the use of samples to build and validate the model using independent sets (“training set” and “test set”) created using a 15:01 split. The K-Fold divides all the samples into k (k = 16) groups of samples. A total of 96 sample pellets for each coal and its ash were used to create 16 groups, where each group had 06 pellets prepared from coal and its ash samples individually at known concentrations. One fold is utilized for testing during each run, while the other folds (K − 1) are used for training so that during the entire process, each fold will be used for testing at least once and is independent of the “training set”.
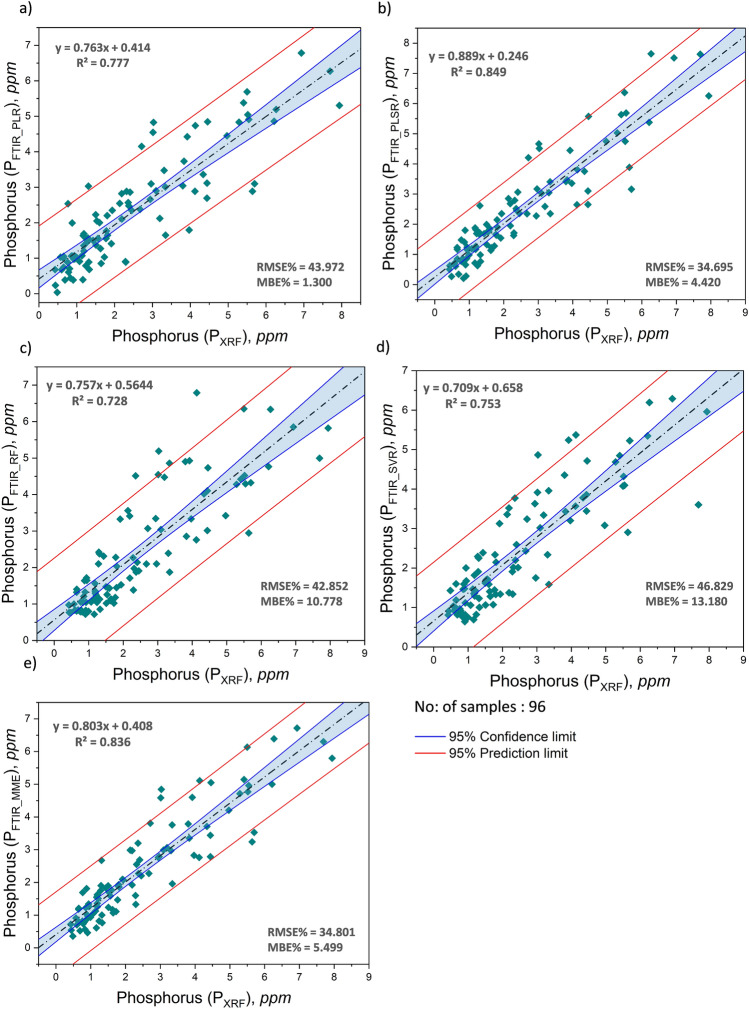
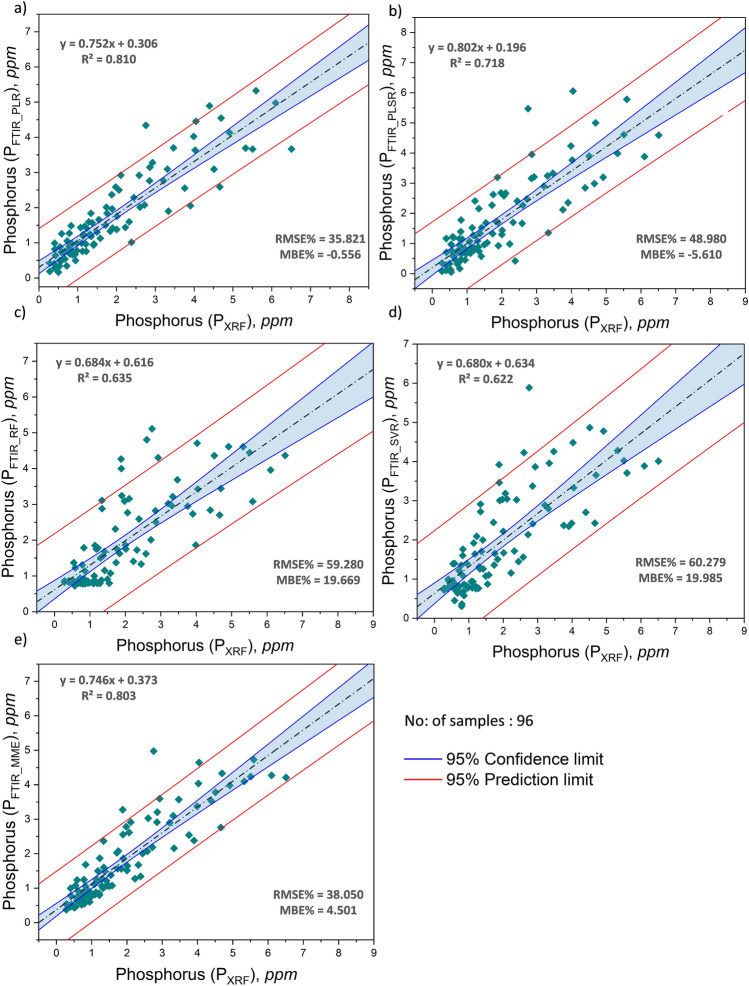
PFTIR_PLR, PFTIR_PLSR, and PFTIR_RF exhibited enhanced robustness, with predicted values closer to the actual values in the dataset compared to PFTIR_SVR. Occasionally, one or more negative values were detected in the PLR and PLSR models, which were replaced by the average of the 3 best performing models. The estimation of phosphorus is found to be more reliable and consistent using the multi-model estimation technique (PFTIR_MME) taking the average of the 3 best performing models (PFTIR_PLR, PFTIR_PLSR, PFTIR_RF). The model-estimated phosphorus content for 96 coal samples and 96 coal ash samples can be located in Supplementary Table S1 and Supplementary Table S2, respectively. The model predicted values were compared to the XRF measured phosphorus content to observe the correlation between the two values and the quality of the model (Figs. 3, 4).
Figure 3.
Correlation between phosphorus content in coal measured by XRF (PXRF) and modeled using FTIR (a) PFTIR_PLR (b) PFTIR_PLSR (c) PFTIR_RF (d) PFTIR_SVR and (e) PFTIR_MME using independent “test set” (validation set) using K fold cross-validation.
Figure 4.
Correlation between phosphorus content in coal ash measured by XRF (PXRF) and modeled using FTIR (a) PFTIR_PLR (b) PFTIR_PLSR (c) PFTIR_RF (d) PFTIR_SVR and (e) PFTIR_MME using independent “test set” (validation set) using K fold cross-validation.
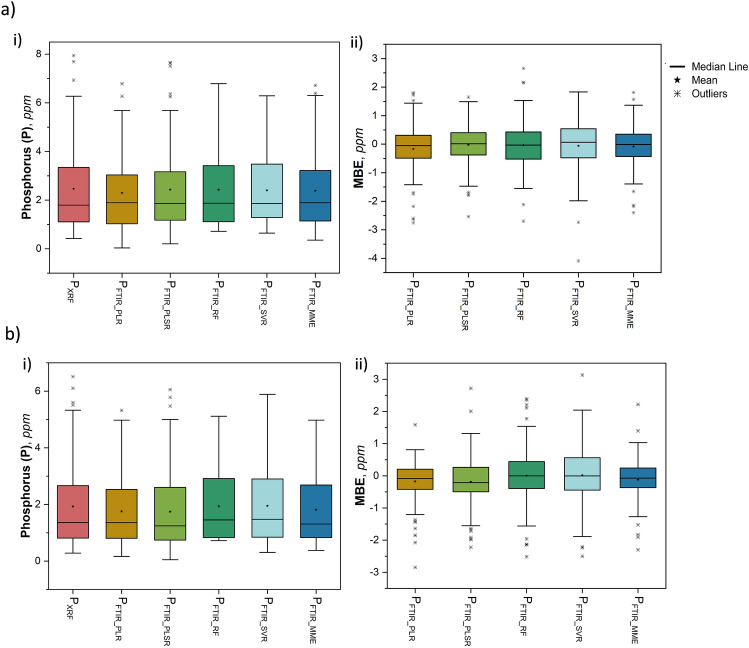
Statistical parameters including the mean (), standard deviation (), coefficient of determination (R2), root mean squared error (RMSE), mean bias error (MBE), and mean absolute error (MAE) in absolute and percentage are chosen as the performance measures48 of the established models. PFTIR_MME showed the highest correlation with PXRF with all R-squared (R2) values exceeding 0.80 for both coal and its ash, suggesting a good fit of the model to the data. A comprehensive statistical analysis has been conducted to evaluate the efficacy of the proposed model utilizing the FTIR spectral response of phosphorus functional groups. The comparison between model predicted (PFTIR_PLR, PFTIR_PLSR, PFTIR_RF, PFTIR_SVR, PFTIR_MME) and XRF measured (PXRF) phosphorus content and their correlation for coal and its ash is given in Figs. 3 and 4 respectively. The boxplot representing the distribution of phosphorus determined through XRF and using model predicted FTIR spectroscopy in coal and its ash (Fig. 5a (i),b (i)) shows no significant difference in means. Similarly, the MBE of the presented models was plotted to estimate the average bias in each of the models for coal and ash (Fig. 5a (ii),b (ii)). The MBE of the multi-model estimated phosphorus using FTIR (PFTIR_MME) data is found to be low for coal and its ash (− 0.077 ppm and − 0.118 ppm), depicting that both the model performance is consistent and unbiased on average.
Figure 5.
Boxplots corresponding to (a) coal i) P content determined in coal by XRF and modeled using FTIR in the experiment, (ii) MBE of the modeled P content in coal using FTIR, and (b) coal ash i) phosphorus content determined in ash by XRF and modeled using FTIR in the experiment, (ii) MBE of the modeled phosphorus content in ash using FTIR.
Standard statistical significance tests were utilized to test if there is any substantial difference between the means (d) and variance (2) of the phosphorus content obtained from XRF (PXRF) and model estimated FTIR spectroscopy (PFTIR_MME) of both coal and ash. The paired t-test for means, conducted at a 99% confidence level ( = 0.01), comparing the phosphorus content in coal and its ash measured by XRF and predicted by FTIR yielded p-values greater than =0.01, indicating the inability to reject the null hypothesis (H0: d = 0). This suggests no significant difference between the mean values of PXRF and PFTIR_PLR, PFTIR_PLSR, PFTIR_RF, PFTIR_SVR, PFTIR_MME, in both coal and its ash (Table 4). Similarly, a two-sample F-test for variance at a 99% confidence level also showed p value greater than = 0.01, supporting the acceptance of the null hypothesis (H0: 02 = p2), indicating no significant difference in variance between measured (02) and model predicted (p2) phosphorus values for both coal and coal ash (Table 5). In conclusion, there is no statistically significant difference in mean or variance between measured and predicted phosphorus values at a 99% confidence level for coal and its ash.
Table 4.
Results of paired two-sample t-test for means (two-tailed at 99 % CI, = 0.01) to compare the difference in means of measured (PXRF) and model estimated P content (PFTIR_PLR, PFTIR_PLSR, PFTIR_RF, PFTIR_SVR, PFTIR_MME) in (a) coal and (b) coal ash.
| Pair | 2 | tstat | p-value | tcritical | H0: d = 0 | |
|---|---|---|---|---|---|---|
| (a) Coal: t-test: Paired Two Sample for Means, n = 96; df = 95 | ||||||
| PFTIR_PLR (ppm) | 2.294 | 2.446 | 1.938 | 0.056 | 2.629 | T |
| PFTIR_PLSR (ppm) | 2.434 | 3.034 | 0.394 | 0.695 | 2.629 | T |
| PFTIR_RF (ppm) | 2.428 | 2.566 | 0.362 | 0.718 | 2.629 | T |
| PFTIR_SVR (ppm) | 2.405 | 2.181 | 0.628 | 0.532 | 2.629 | T |
| PFTIR_MME (ppm) | 2.385 | 2.518 | 1.03 | 0.305 | 2.629 | T |
| PXRF (ppm) | 2.462 | 3.265 | ||||
| (b) Ash: t-test: Paired Two Sample for Means, n = 96; df = 95 | ||||||
| PFTIR_PLR (ppm) | 1.757 | 1.552 | 2.593 | 0.011 | 2.629 | T |
| PFTIR_PLSR (ppm) | 1.745 | 1.991 | 2.268 | 0.026 | 2.629 | T |
| PFTIR_RF (ppm) | 1.937 | 1.638 | -0.061 | 0.951 | 2.629 | T |
| PFTIR_SVR (ppm) | 1.948 | 1.653 | -0.176 | 0.860 | 2.629 | T |
| PFTIR_MME (ppm) | 1.813 | 1.540 | 1.733 | 0.086 | 2.629 | T |
| PXRF (ppm) | 1.931 | 2.223 | ||||
Table 5.
Results of paired two-sample F-test for variance (two-tailed at 99 % CI, = 0.01) to compare the difference in means of measured (PXRF) and model estimated P content (PFTIR_PLR, PFTIR_PLSR, PFTIR_RF, PFTIR_SVR, PFTIR_MME) in (a) coal and (b) coal ash.
| Pair | 2 | Fstat | p-value | CI | H0: 02 = p2 | |
|---|---|---|---|---|---|---|
| (a) Coal: F-test: Two sample for Variances, n = 96; df = 95 | ||||||
| P FTIR_PLR (ppm) | 2.294 | 2.446 | 1.334 | 0.162 | 0.783, 2.274 | T |
| PFTIR_PLSR (ppm) | 2.434 | 3.034 | 1.076 | 0.723 | 0.631, 1.833 | T |
| PFTIR_RF (ppm) | 2.428 | 2.566 | 1.271 | 0.244 | 0.746, 2.167 | T |
| PFTIR_SVR (ppm) | 2.405 | 2.181 | 1.496 | 0.051 | 0.878, 2.549 | T |
| PFTIR_MME (ppm) | 2.385 | 2.518 | 1.297 | 0.208 | 0.761, 2.209 | T |
| PXRF (ppm) | 2.462 | 3.265 | ||||
| (b) Ash: F-test: Two sample for Variances, n = 96; df = 95 | ||||||
| PFTIR_PLR (ppm) | 1.757 | 1.552 | 1.433 | 0.081 | 0.841, 2.442 | T |
| PFTIR_PLSR (ppm) | 1.745 | 1.991 | 1.117 | 0.591 | 0.655, 1.903 | T |
| PFTIR_RF (ppm) | 1.937 | 1.638 | 1.358 | 0.138 | 0.797, 2.314 | T |
| PFTIR_SVR (ppm) | 1.948 | 1.653 | 1.344 | 0.151 | 0.789, 2.290 | T |
| PFTIR_MME (ppm) | 1.813 | 1.540 | 1.444 | 0.075 | 0.847, 2.460 | T |
| PXRF (ppm) | 1.931 | 2.223 | ||||
The comparison between the phosphorus content measured by XRF (PXRF) and predicted using multi-model FTIR spectroscopy (PFTIR_MME) for coal and its ash samples is given in Fig. 6a,b respectively, which clearly depict that the multi-model estimated phosphorus content is similar and comparable to the measured phosphorus content in both the samples.
Figure 6.
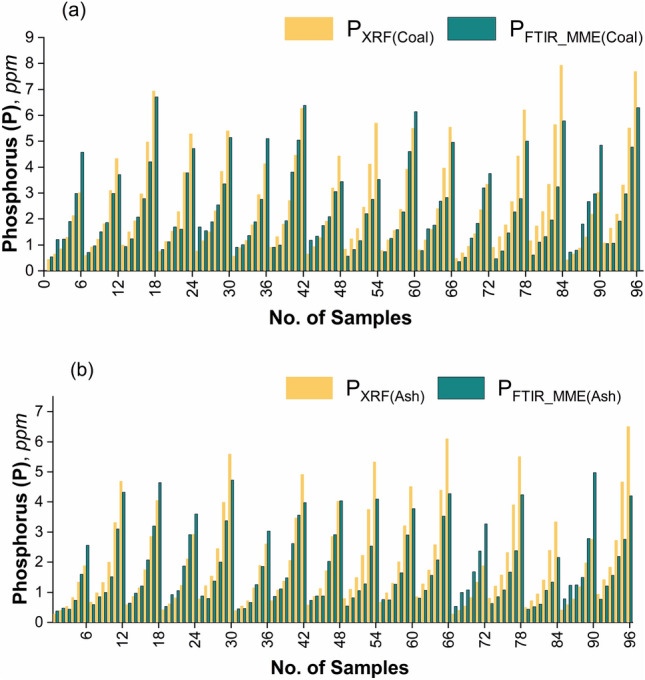
Comparison chart of the P content (ppm) measured by XRF (PXRF) and by multi-model FTIR (PFTIR_MME) technique for (a) coal, and (b) coal ash samples.
All the results collectively suggest that multi-model FTIR spectroscopy is a novel, sensitive, and reliable alternative to predict low levels of phosphorus content in coal and its ash.
Discussion
Researchers have investigated several methods to determine the phosphorus content in soils, compound fertilizers, sludge waste, seafood, etc., using different spectroscopic analytical techniques like laser-induced breakdown spectroscopy (LIBS), prompt gamma neutron activation analysis (PGNAA), XRF, X-ray absorption spectroscopy (XAS), ICP-OES, ICP-MS, near-infrared spectroscopy (NIRS), mid-infrared spectroscopy (MIRS), and visible-near infrared spectroscopy (vis-NIRS) (Table 6). PGNAA delivers continuous online monitoring of coal composition but comes with a high cost and radiation hazards. XRF offers immediate analysis capabilities but is limited to detecting elements with atomic numbers lower than 1149. NIRS is an established technique utilized in many industries to ensure quality, but the low dipole moment between the phosphorus and oxygen atoms hinders the direct detection of phosphorus or phosphate through NIRS. Nevertheless, the quantification of phosphorus is feasible through NIRS if it is organically bound or closely linked to other soil properties. The limited availability and accuracy of NIRS models for predicting phosphorus content in soil can thus be justified50,51. Researchers have employed MIRS to estimate the levels of plant-available phosphorus50,52,53. Studies show that MIRS outperformed NIRS, especially for available phosphorus analyses52. However, despite these advantages, the calibration functions for both IR methods still had relatively low R2 values. The capacity for minimal invasiveness and high-quality soil phosphorus sensing through IR (NIRS and MIRS) spectroscopy is anticipated to improve significantly with the incorporation of more efficient mathematical spectra analysis using machine learning techniques50.
Table 6.
Comparison of the statistical parameter values achieved for models developed in previous studies and present model to estimate P content in different samples (ISAM, Improved Standard Addition Method; FTIR-PAS, Fourier transform mid-infrared photoacoustic spectroscopy; GA-PLS, Genetic algorithm partial least squares; ANN, Artificial neural networks; WET, Water extractable phosphorus; PAP, Plant available phosphorus).
| Sl. no. | Method | Nature & sample count | R2 RMSE (RMSE%) | MBE (MBE%) | MAE RSD% | Location reference |
|---|---|---|---|---|---|---|
| 1 | LIBS with linear regression |
Soil; N = 90 |
0.99 NA |
NA NA |
NA NA |
Denmark74 |
| 2 | LIBS with PLSR |
Soil; N = 30 |
0.59 NA |
NA NA |
NA NA |
China33 |
| 3 | LIBS with SVR |
Soil; N = 30 |
0.99 NA |
NA NA |
184.71 0.0006 |
China33 |
| 4 | LIBS with linear fitting |
Soil; N = 30 |
0.24 NA |
NA NA |
NA NA |
China33 |
| 5 | LIBS with SVM |
Seafood; N = 21 |
0.99 NA |
NA NA |
NA 5.18 |
China54 |
| 6 | LIBS with linear regression |
Soil; N = 10 |
0.86 NA |
NA NA |
0.0068 NA |
China75 |
| 7 | ICP-OES with ISAM |
Soil; N = 40 |
NA NA |
NA NA |
NA 1.23 |
China76 |
| 8 | LIBS with PLSR |
Soil; N = 147 |
0.76 NA |
1.9 NA |
NA NA |
Denmark55 |
| 9 | LIBS with linear correlation |
Fertilizer; N = 26 |
0.9 NA |
NA NA |
NA NA |
Brazil77 |
| 10 | vis-NIRS with PLSR |
Soil; N = 147 |
0.55 NA |
0.54 NA |
NA NA |
Denmark78 |
| 11 | LIBS-vis-NIRS with PLSR |
Soil; N = 147 |
0.74 NA |
-2.9 NA |
NA NA |
Denmark55 |
| 12 | vis-NIRS with PLSR |
Soil; N = 60 |
0.91 0.16 |
NA NA |
NA NA |
China78 |
| 13 |
FTIR-PAS with PLSR (for WEP) |
Soil; N = 60 |
0.80 0.78 |
NA NA |
NA NA |
Denmark79 |
| 14 |
FTIR-PAS with PLSR (for PAP) |
Soil; N = 60 |
0.7 134.1 |
NA NA |
NA NA |
Denmark79 |
| 15 | Vis-NIRS with GA-PLS |
Soil; N = 103 |
0.79 NA |
NA NA |
NA NA |
Japan80 |
| 16 | Vis-NIRS with ANN |
Soil; N = 41 |
0.81 NA |
NA NA |
NA NA |
Thailand53 |
| 17 | FTIR with PLR |
Coal; N = 96 |
0.777 0.865 (43.972) |
− 0.169 (1.30) |
0.6 NA |
Present study India |
|
Coal ash; N = 96 |
0.810 0.677 (35.821) |
− 0.174 (− 0.556) |
0.450 NA |
|||
| 18 | FTIR with PLSR |
Coal; N = 96 |
0.849 0.703 (34.695) |
− 0.028 (4.420) |
0.511 NA |
|
|
Coal ash; N = 96 |
0.718 0.822 (48.980) |
− 0.186 (− 5.610) |
0.596 NA |
|||
| 19 | FTIR with RF |
Coal; N = 96 |
0.728 0.940 (42.852) |
− 0.035 (10.778) |
0.701 NA |
|
|
Coal ash; N = 96 |
0.635 0.901 (59.280) |
0.006 (19.669) |
0.639 NA |
|||
| 20 | FTIR with SVR |
Coal; N = 96 |
0.753 0.900 (46.829) |
− 0.058 (13.180) |
0.657 NA |
|
|
Coal ash; N = 96 |
0.622 0.918 (60.279) |
0.017 (19.985) |
0.696 NA |
|||
| 21 |
FTIR with MME (multi-model estimation using PLR,PLSR,RF) |
Coal; N = 96 |
0.836 0.735 (34.801) |
− 0.077 (5.500) |
0.528 NA |
|
|
Coal ash; N = 96 |
0.803 0.676 (38.050) |
− 0.118 (4.501) |
0.474 NA |
In the field of prediction modeling, several statistical evaluation metrics are commonly used to evaluate the quality of these models. These metrics include the correlation between measured and predicted values like R2, RMSE, MBE, and MAE. In general, models with higher R2 and lower RMSE and MBE values are considered to have better prediction quality. It is commonly accepted that models with an R2 value greater than 0.70 with lower MAE and RMSE are deemed to have acceptable predictive power, while models with an R2 value below 0.50 and a relatively higher MAE and RMSE value are considered to have poor prediction capability50.
It can be noticed from Table 6 that a few models using LIBS with multivariate analysis based on machine learning achieve good prediction capability for phosphorus content in the soil compared to other methods. The literature review shows that LIBS with the SVM prediction model provides the best results in soil samples. However, the diverse characteristics of soil samples, including their heterogeneity, roughness, and particle size, create uncertainties when implementing LIBS, particularly due to the matrix effect impacting quantification accuracy54,55. LIBS technology has been used to identify and quantify several major elements (C, H, O, S, Si) along with very few minor elements (Fe, Ti, Al, Ca, Na, Cu)28,49,56–58. To the best of our knowledge, no substantial literature or models estimate the phosphorus content of coal and ash using mid-infrared FTIR spectroscopy. Thus, the present attempt to determine the phosphorus content of coal and ash using mid-infrared FTIR spectroscopy is a novel method. Table 6 shows that the multi-model estimation of phosphorus proposed in this study using FTIR data for coal samples presents a good R2 of 0.835 and R2 of 0.803 for the coal ash samples (with relatively low RMSE and MBE) and is comparable to other models reviewed. Compared to FTIR, LIBS tends to be relatively more expensive and complex due to its intricate laser systems, leading to higher initial purchase expenses. Additionally, maintenance and calibration needs are more frequent in LIBS due to the complexities associated with laser operation and plasma generation. Therefore, the proposed model using FTIR with multivariate analysis based on machine learning techniques shows promising potential as a real-time, cost-effective alternative analytical approach for rapidly and accurately quantifying phosphorus in coal and coal ash. The model’s accuracy can be further enhanced by incorporating a greater number of samples from other coal field basins. The model presented here may be developed further to facilitate improvements in the monitoring and analysis of phosphorus content in coal and ash. Phosphorus content in a variety of other natural materials, such as soil, shale, and others, can be potentially estimated by making suitable adjustments to the mid-infrared FTIR data-based model.
Methods
Coal and ash samples
All the coal samples used in the study were collected from the Johilla coalfield of the Son-Mahanadi Basin, present in the Umaria district of Madhya Pradesh, India. The samples were collected from five underground projects at Kundri, Pali, Pinoura, Umaria, Vindhya, and one opencast project at Kanchan in the coalfield, as per ASTM D-223459 guidelines. A total of sixteen samples used in this study were collected from each of these locations. The collected coal samples were crushed and sieved to a size of 212 m following the ASTM D-474960 standard procedure for XRF and FTIR analysis. As per the ASTM standard test method, 1 g of the same samples (212 m) was weighted and combusted at temperatures of for 1 hour for ash yield61.
X-ray fluorescence spectroscopy
The X-ray fluorescence spectroscopy of whole coal samples and their coal ash was conducted in the Rigaku ZSX Primus IV Wavelength Dispersive X-Ray Fluorescence (WDXRF) spectroscopy at the Central Research Facility, Indian Institute of Technology (Indian School of Mines) Dhanbad (IIT(ISM) Dhanbad), Jharkhand, India. The analysis of coal samples has the advantage of being able to detect volatile elements that may be lost during ashing or fusion. A tube-above sequential wavelength dispersive X-ray fluorescence (WDXRF) spectrometer is used to determine the concentrations of major and minor atomic elements in samples. The accuracy of the WDXRF spectrometric measurements of elements depends on various factors such as concentration, particle size, matrix effects, surface roughness, and the quality of standard materials. The samples were filled in an aluminium mold and pressed for roughly one minute at 200 kN to prepare pellets. In this study, the WDXRF technique was used to determine the total phosphorus concentration, which served as the reference for phosphorus levels.
Fourier-transform infrared (FTIR) spectroscopy
FTIR spectroscopy is the most common form of infrared spectroscopy used for identifying the vibrations of functional groups and anionic groups. The FTIR spectra of the coal and coal ash samples were recorded using an INVENIO S, BRUKER OPTIK, GmBH (model & make) at the Department of Applied Geology, IIT(ISM) Dhanbad, Jharkhand, India. Specimen pellet preparation for FTIR analysis involves weighing and homogenously mixing the ground sample powder (212 m) with potassium bromide (KBr) powder (IR spectroscopy grade, Uvasol, Kaliumbromid, Germany). This composite (coal + KBr & coal ash + KBr) is filled on the anvil surface of the KBr die and spread by inserting the plunger. The assembled die was placed on the cover plate of the cylinder of the hydraulic press, and pressure was applied ( tons for 5 min) to make circular, thin pellets of diameter 1.3 cm. To reduce the risk of moisture and other gases affecting the experiment, the FTIR optical bench was purged with N2 gas flowing at 200 l per hour for a duration of 2 h before conducting the analysis. The KBr pellets that had been prepared were placed in a quick lock base plate positioned in the FTIR sample chamber. The sample pellets were then subjected to infrared radiation, allowing for the measurement of the resulting absorption spectrum. Subsequently, the absorbance versus frequency plot (Y–X plot) was generated based on the acquired spectra. For the coal samples, the absorption spectra were limited to the wavenumber range of 4000–400 cm-1.
Piecewise linear regression (PLR)
The estimation model has been used to predict the phosphorus content in coal and coal ash by computing the relationship between a set of independent variables (derived from the FTIR spectra) and a dependent variable (observed P content from XRF). A piecewise linear empirical equation with a breakpoint and quasi-newton method, along with a least squares loss function, was used to solve the coefficients of the model using the training data42. Through the iterative convergence of an empirical equation that has been predefined, this non-linear method can be utilised to achieve the goal of minimizing the least square’s function. The coefficients of the empirical equation are typically reliant on the pool of input data that is available62. To achieve optimal values for the coefficients, the process of optimization could involve numerous iterative convergences on the empirical equation and the data that was chosen. It is possible to compute the loss function in such a way that, at each iteration, the goal is to reduce the square of the difference between the observed and predicted phosphorus content using an empirical equation that has been predefined63. The optimization method utilizes the 1st order derivative of a function to determine the function’s slope at a given point and the 2nd order derivative to determine the rate and direction of change of the slope. This method evaluates the function at different points during each step to approximate the first- and second-order derivatives, further utilized to identify the minimum loss function. By minimizing the sum of squared errors between observed data and model predictions, the method can estimate statistical model parameters for estimation problems64.
When analyzing with a breakpoint in the model, two sets of coefficients are produced for the variables (QNbp_L and QNbp_R where bp_L and bp_R are model generated with breakpoint corresponding to the left and right equations, respectively. A single form of the coefficient (QNnbp) was obtained for the model with no breakpoint. QNbp(avg) is also found by averaging the phosphorus content obtained from the left and right equations. Notably, the estimated value of phosphorus (ppm) in coal obtained from QNnbp using the no breakpoint is often the closest estimate to the actual experimental value. At times, the non-breakpoint estimated phosphorus (ppm) values are outside the expected range of ± 1.5 IQR (inter-quartile range). To address this issue, the interquartile range (IQR) was determined to identify and eliminate any out-of-range values. The proposed model works on the following conditions: the value from the QNnbp model is taken into consideration if the projected phosphorus content from QNnbp is between the low and high ranges (Q1 − 1.5 IQR or Q3 + 1.5 IQR, respectively). Else, if the modeled phosphorus content in coal from QNnbp is beyond the low or high range (Q1 − 1.5 IQR or Q3 + 1.5 IQR, respectively), the P value obtained from the QNbp_avg model is taken into consideration.
In the case of ash samples, the model with a breakpoint corresponding to the left equation (QNbp_L) provides the closest estimate to the actual experimental value of phosphorus in ash. If the projected phosphorus content in ash from QNbp_L is not between the low and high ranges (Q1 − 1.5 IQR or Q3 + 1.5 IQR, respectively), the P value obtained from QNnbp model is taken into consideration. The accuracy of phosphorus content estimation in coal using PLR is improved by this method of defining a threshold to identify out-of-range results. It helps to restrict errors in the estimation of phosphorus content in unknown samples.
Partial least square regression (PLSR)
Partial least squares, also referred to as “projection to Latent Structures,” is a category of learning techniques developed to model the relationship of observed variables using latent variables. PLSR calculates components by maximizing covariance between feature and response matrices, making it particularly effective for problems featuring many highly correlated features and multiple responses. Developed for predicting, PLSR finds application in various spectroscopies, such as near-infrared reflectance (NIR) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, and Fourier transform-Raman (FT-Raman) spectroscopy65.
Implementation of PLSR involves simultaneous decomposition of the spectral matrix and concentration matrix to eliminate redundant information, fully considering their relationship to derive an optimized calibration model. The outcome is a linear relationship establishing the basis for quantitative analysis of material elements66. PLSR resembles principal component regression but differs in using target variables to identify scores highly covariant with predictor variables. PLSR excels with datasets demonstrating the multicollinearity of predictor variables, and its advantage over PCR lies in the reduced optimal number of components67,68. The model is created using the cross_decomposition module of the scikit-learn library in the Python programming environment.
Random forest (RF)
Random forest is a supervised ensemble learning method employed for both regression and classification tasks. Ensemble learning integrates predictions from multiple models to yield more accurate results than a single model. It produces numerous decision trees and develops their predictions to make final predictions. Decision trees are fundamental models that predict outcomes by executing splits based on predictors that maximally reduce mean squared error69. The approach is rooted in integrating multiple decision trees, offering robustness to non-linearity, and is best suited for modeling nonlinear data. The combination of individual decision trees mitigates their high variance, addressing the overfitting issue without requiring pruning70,71. In this study, a random forest regressor model from the ensemble module of the scikit-learn library in the Python programming environment is utilized. Hyperparameter tuning is conducted through RandomizedSearchCV and GridSearchCV. After comparing with the default hyperparameter settings, the best estimator is selected for modeling.
Support vector regression (SVR)
Support Vector Machine (SVM) is a robust supervised machine learning paradigm rooted in a multivariate nonlinear correction approach introduced in the 1990s with a problem-solving capacity involving nonlinearity and high dimensionality. SVM has evolved through ongoing algorithmic optimization, showcasing impressive learning performance in nonlinear regression and function approximation, leading to widespread adoption in quantitative research, especially in spectral analysis, and the development of support vector regression33,65. The model simplifies conventional regression processes by efficiently predicting outcomes on training data using a technique known as “transduction inference”72. Its core function involves identifying the optimal hyperplane that separates data in a multi-dimensional space, minimizing errors across all training samples73. For model optimization and training, the GridSearchCV function and the SVM module within the scikit-learn library in the Python programming environment were employed.
Conclusions
Accurate and rapid measurement of phosphorus content in coal and coal ash is crucial for efficient and sustainable utilization. In this investigation, we evaluated the ability of Fourier transform infrared spectroscopy (FTIR) in conjunction with machine learning models like piecewise linear regression (PLR), partial least square regression (PLSR), random forest (RF), and support vector regression (SVR) for quantifying the phosphorus content in coal and coal ash. The study proposes a novel FTIR-based multi-model approach combining PLR, PLSR, and RF regression models to be a reliable alternative to traditional analytical methods for the rapid and near real-time measurement of phosphorus in coal and coal ash. The major findings of the study are:
The FTIR-based model is sensitive enough to estimate very low levels of phosphorus in both coal and coal ash samples.
PLR, PLSR, and RF methods exhibited enhanced robustness compared to the SVR method. The estimation of phosphorus is found to be more consistent using a multi-model estimation technique (FTIR_MME), taking the average of the three best-performing models. The accuracy of the proposed model to estimate the phosphorus content in coal and ash is relatively good (R of 0.836, RMSE of 0.735 ppm, RMSE (%) of 34.801, MBE of − 0.077 ppm, MBE (%) of 5.499, and MAE of 0.528 ppm in coal samples, and R of 0.803, RMSE of 0.676 ppm, RMSE (%) of 38.050, MBE of − 0.118 ppm, MBE (%) of 4.501, and MAE of 0.474 ppm in coal ash samples).
The determination of phosphorus in coal and coal ash using mid-infrared FTIR (PFTIR_MME) is a promising cost-effective alternative compared to conventional methods such as XRF (PXRF).
Statistical tests of significance using two-tailed paired t-test for means and F-test for variances prove that there is no difference in means and variances, respectively, between XRF measured phosphorus content (PXRF) and multi-model-estimated phosphorus content (PFTIR_MME) in coal and coal ash.
The model’s accuracy can be further enhanced by incorporating a greater number of samples from other coal field basins. The model presented here may be developed further to facilitate improvements in the monitoring and analysis of phosphorus content in coal and ash. Phosphorus content in a variety of other natural materials, such as soil, shale, and others, can be potentially estimated by making suitable adjustments to the mid-infrared FTIR-data based model.
Supplementary Information
Acknowledgements
The authors are thankful to South Eastern Coalfield Limited (SECL) for providing the necessary support related to the mine visit, sample collection, fieldwork, and geological literature. The authors are grateful to the DST, India (http://www.dst.gov.in) for providing financial support to set up the “DST-FIST Level-II Facility” at the Department of Applied Geology (AGL), IIT (ISM) Dhanbad (http://www.iitism.ac.in). For sample preparation, analyses, and visualization used in this study, authors are thankful to the AGL, IIT (ISM), Dhanbad for providing necessary access to the equipment (particularly, FTIR Spectroscopy), technical support, and laboratory facilities through DST-FIST Level-II Program [No. SR/FST/ESII-014/2012(C)]. The X-ray fluorescence spectroscopy of whole coal samples was conducted in the Rigaku ZSX Primus IV Wavelength Dispersive X-Ray Fluorescence (WDXRF) spectroscopy at the Central Research Facility (CRF), IIT (ISM), Dhanbad, Jharkhand, India. Author (AKP) is grateful to SERB, DST for partial support (grant) through Project No. MTR/2023/001086.
Author contributions
A.K.P. led the conceptualization and methodology. A.V., S.M., B.P., S.H.M., A.S. and A.K.P. conducted the formal analysis. Investigation and resource allocation were shared among A.V., S.M., B.P., S.H.M., A.K.P. and N.D. A.V. prepard the first draft. Review and editing were done by A.K.P., S.M., A.S., N.D., A.K.V. and B.C.S. A.K.P. supervised the project, administered resources, and secured funding and equipment acquisition, along with A.K.V. and B.C.S. All authors reviewed the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-63672-x.
References
- 1.Dai, S. et al. Modes of occurrence of elements in coal: A critical evaluation. Earth Sci. Rev.222, 103815. 10.1016/j.earscirev.2021.103815 (2021). [Google Scholar]
- 2.Ward, C. R., Corcoran, J., Saxby, J. & Read, H. Occurrence of phosphorus minerals in Australian coal seams. Int. J. Coal Geol.30, 185–210. 10.1016/0166-5162(95)00055-0 (1996). [Google Scholar]
- 3.Onifade, M. & Genc, B. Spontaneous combustion liability of coal and coal-shale: A review of prediction methods. Int. J. Coal Sci. Technol.6, 151–168. 10.1007/s40789-019-0242-9 (2019). [Google Scholar]
- 4.Onifade, M. & Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol.30, 303–311. 10.1016/j.ijmst.2020.03.001 (2020). [Google Scholar]
- 5.Gbadamosi, A. R., Onifade, M., Genc, B. & Rupprecht, S. Spontaneous combustion liability indices of coal. Combust. Sci. Technol.193, 2659–2671. 10.1080/00102202.2020.1754208 (2021). [Google Scholar]
- 6.Onifade, M. Countermeasures against coal spontaneous combustion: A review. Int. J. Coal Prep. Util.42, 2953–2975. 10.1080/19392699.2021.1920933 (2022). [Google Scholar]
- 7.Oskarsson, P., Nielsen, K. B., Lahiri-Dutt, K. & Roy, B. India’s new coal geography: Coastal transformations, imported fuel and state-business collaboration in the transition to more fossil fuel energy. Energy Res. Soc. Sci.73, 101903. 10.1016/j.erss.2020.101903 (2021). [Google Scholar]
- 8.Davis, B., Esterle, J. & Rodrigues, S. Towards understanding phosphorus distribution in coal: A case study from the Bowen Basin. ASEG Ext. Abstr.2018, 1–8. 10.1071/ASEG2018abM3_3A (2018). [Google Scholar]
- 9.Finkelman, R. B., Palmer, C. A. & Wang, P. Quantification of the modes of occurrence of 42 elements in coal. Int. J. Coal Geol.185, 138–160. 10.1016/j.coal.2017.09.005 (2018). [Google Scholar]
- 10.Bertine, K. K. & Goldberg, E. D. Fossil fuel combustion and the major sedimentary cycle. Science173, 233–235. 10.1126/science.173.3993.233 (1971). [DOI] [PubMed] [Google Scholar]
- 11.Burchill, P., Howarth, O. W., Richards, D. & Sword, B. J. Solid-state nuclear magnetic resonance studies of phosphorus and boron in coals and combustion residues. Fuel69, 421–428. 10.1016/0016-2361(90)90308-D (1990). [Google Scholar]
- 12.Goodarzi, F., Grieve, D. & Labonté, M. Mineralogical and elemental composition of tonsteins from the east kootenay coalfields. Southeastern British Columbia. Energy Sources12, 265–295. 10.1080/00908319008960206 (1990). [Google Scholar]
- 13.Orem, W. & Finkelman, R. Coal formation and geochemistry. In Treatise on Geochemistry, 191–222 (Elsevier, 2003). 10.1016/B0-08-043751-6/07097-3.
- 14.Ketris, M. & Yudovich, Y. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol.78, 135–148. 10.1016/j.coal.2009.01.002 (2009). [Google Scholar]
- 15.Varma, A. K. et al. Petrographic controls on phosphorous distribution in coal seams of the Jharia basin. India. J. Earth Syst. Sci.128, 103. 10.1007/s12040-019-1128-3 (2019). [Google Scholar]
- 16.Tshipa, R. S. & Mulaba-Bafubiandi, A. F. Coal cleaning through removal of phosphorus mineral from morupule main seam coal. Int. J. Coal Prep. Util.42, 3117–3136. 10.1080/19392699.2021.1933458 (2022). [Google Scholar]
- 17.Basu, S., Seetharaman, S. & Lahiri, A. K. Thermodynamics of phosphorus and sulphur removal during basic oxygen steelmaking. Steel Res. Int.81, 932–939. 10.1002/srin.201000086 (2010). [Google Scholar]
- 18.Díaz-Faes, E., Barriocanal, C., Díez, M. & Alvarez, R. Applying TGA parameters in coke quality prediction models. J. Anal. Appl. Pyrol.79, 154–160. 10.1016/j.jaap.2006.11.001 (2007). [Google Scholar]
- 19.Howard, J. L., Amos, D. F. & Daniels, W. L. Phosphorus and potassium relationships in southwestern virginia coal-mine spoils. J. Environ. Qual.17, 695–700. 10.2134/jeq1988.00472425001700040029x (1988). [Google Scholar]
- 20.Li, X. et al. Application of a spectrum standardization method for carbon analysis in coal using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc.68, 955–962. 10.1366/13-07345 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Experience at Tata Steel. Mukherjee, T. & chatterjee, Amit. Production of low phosphorus steels from high phosphorus Indian hot metal. Bull. Mater. Sci.19, 893–903 (1996). [Google Scholar]
- 22.Tang, H., Qin, Y. & Qi, T. Phosphorus removal and iron recovery from high-phosphorus hematite using direct reduction followed by melting separation. Miner. Process. Extr. Metall. Rev.37, 236–245. 10.1080/08827508.2016.1181628 (2016). [Google Scholar]
- 23.Zhang, L. & Ninomiya, Y. Transformation of phosphorus during combustion of coal and sewage sludge and its contributions to PM10. Proc. Combust. Inst.31, 2847–2854. 10.1016/j.proci.2006.07.113 (2007). [Google Scholar]
- 24.Haris, M., Ansari, M. S. & Khan, A. A. Supplementation of fly ash improves growth, yield, biochemical, and enzymatic antioxidant response of chickpea (Cicer arietinum L.). Hortic. Environ. Biotechnol.62, 715–724. 10.1007/s13580-021-00351-0 (2021). [Google Scholar]
- 25.Tian, C., Gong, B., Wang, X., Zhao, Y. & Zhang, J. Ash formation and trace elements associations with fine particles in an ultra-low emission coal-fired power plant. Fuel288, 119718. 10.1016/j.fuel.2020.119718 (2021). [Google Scholar]
- 26.Sajid Ansari, M., Ahmad, G., Khan, A. A., Mohamed, H. I. & Elhakem, A. Coal fly ash and nitrogen application as eco-friendly approaches for modulating the growth, yield, and biochemical constituents of radish plants. Saudi J. Biol. Sci.29, 103306. 10.1016/j.sjbs.2022.103306 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dogan, O. & Kobya, M. Elemental analysis of trace elements in fly ash sample of Yatağan thermal power plants using EDXRF. J. Quant. Spectrosc. Radiat. Transf.101, 146–150. 10.1016/j.jqsrt.2005.11.072 (2006). [Google Scholar]
- 28.Evans, E. H. & Giglio, J. J. Interferences in inductively coupled plasma mass spectrometry. A review. J. Anal. At. Spectrom.8, 1–18. 10.1039/JA9930800001 (1993). [Google Scholar]
- 29.Grotti, M., Leardi, R. & Frache, R. Combined effects of inorganic acids in inductively coupled plasma optical emission spectrometry. Spectrochim. Acta Part B At. Spectrosc57, 1915–1924. 10.1016/S0584-8547(02)00161-1 (2002) (7th RIO symposium on atomic spectrometry). [Google Scholar]
- 30.Hu, Z. & Qi, L. 15.5-Sample digestion methods. Treatise Geochem.1, 87–109 (2014). [Google Scholar]
-
31.Bizzi, C. A. et al. Effect of simultaneous cooling on microwave-assisted wet digestion of biological samples with diluted nitric acid and O
 pressure. Anal. Chim. Acta837, 16–22. 10.1016/j.aca.2014.05.051 (2014).
[DOI] [PubMed] [Google Scholar]
pressure. Anal. Chim. Acta837, 16–22. 10.1016/j.aca.2014.05.051 (2014).
[DOI] [PubMed] [Google Scholar] - 32.Mketo, N., Nomngongo, P. N. & Ngila, J. C. An overview on analytical methods for quantitative determination of multi-element in coal samples. TrAC Trends Anal. Chem.85, 107–116. 10.1016/j.trac.2016.09.002 (2016). [Google Scholar]
- 33.Guo, G. et al. Multi-element quantitative analysis of soils by laser induced breakdown spectroscopy (LIBS) coupled with univariate and multivariate regression methods. Anal. Methods11, 3006–3013. 10.1039/C9AY00890J (2019). [Google Scholar]
- 34.Spears, D., Borrego, A., Cox, A. & Martinez-Tarazona, R. Use of laser ablation ICP-MS to determine trace element distributions in coals, with special reference to V, Ge and Al. Int. J. Coal Geol.72, 165–176. 10.1016/j.coal.2007.02.001 (2007). [Google Scholar]
- 35.Dong, M. et al. Elemental analysis of coal by tandem laser induced breakdown spectroscopy and laser ablation inductively coupled plasma time of flight mass spectrometry. Spectrochim. Acta Part B109, 44–50. 10.1016/j.sab.2015.04.008 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Feng, J., Wang, Z., West, L., Li, Z. & Ni, W. A PLS model based on dominant factor for coal analysis using laser-induced breakdown spectroscopy. Anal. Bioanal. Chem.400, 3261–3271. 10.1007/s00216-011-4865-y (2011). [DOI] [PubMed] [Google Scholar]
- 37.Feng, J., Wang, Z., Li, L., Li, Z. & Ni, W. A nonlinearized multivariate dominant factor-based partial least squares (PLS) model for coal analysis by using laser-induced breakdown spectroscopy. Appl. Spectrosc.67, 291–300. 10.1366/11-06393 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Li, X. et al. Application of a spectrum standardization method for carbon analysis in coal using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc.68, 955–962. 10.1366/13-07345 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Li, X., Wang, Z., Fu, Y., Li, Z. & Ni, W. A model combining spectrum standardization and dominant factor based partial least square method for carbon analysis in coal using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B99, 82–86. 10.1016/j.sab.2014.06.017 (2014). [Google Scholar]
- 40.Ma, Y. et al. Accurate sulfur determination of coal using double-pulse laser-induced breakdown spectroscopy. J. Anal. At. Spectrom.35, 1458–1463. 10.1039/C9JA00448C (2020). [Google Scholar]
- 41.Yan, C. et al. Determination of carbon and sulfur content in coal by laser induced breakdown spectroscopy combined with kernel-based extreme learning machine. Chemom. Intell. Lab. Syst.167, 226–231. 10.1016/j.chemolab.2017.06.006 (2017). [Google Scholar]
- 42.Shukla, A., Prasad, A. K., Mishra, S., Vinod, A. & Varma, A. K. Rapid estimation of sulfur content in high-ash Indian coal using mid-infrared FTIR data. Minerals13, 634. 10.3390/min13050634 (2023). [Google Scholar]
- 43.Mishra, S. et al. Estimation of carbon content in high-ash coal using mid-infrared Fourier-transform infrared spectroscopy. Minerals13, 938. 10.3390/min13070938 (2023). [Google Scholar]
- 44.Veiderma, M., Knubovets, R. & Tõnsuaadu, K. Structural properties of apatites from Finland studied by FTIR spectroscopy. Bull. Geol. Soc. Finl.70, 69–75. 10.17741/bgsf/70.1-2.005 (1998). [Google Scholar]
- 45.Stuart, B. H. Infrared Spectroscopy: Fundamentals and Applications (Wiley, Chichester, 2005) (OCLC: 690408260). [Google Scholar]
- 46.Pavia, D., Lampman, G., Kriz, G. & Vyvyan, J. Introduction to Spectroscopy (Cengage Learning, 2014). [Google Scholar]
- 47.Klee, W. & Engel, G. IR spectra of the phosphate ions in various apatites. J. Inorg. Nucl. Chem.32, 1837–1843. 10.1016/0022-1902(70)80590-5 (1970). [Google Scholar]
- 48.Preety, K., Prasad, A. K., Varma, A. K. & El-Askary, H. Accuracy assessment, comparative performance, and enhancement of public domain digital elevation models (ASTER 30 m, SRTM 30 m, CARTOSAT 30 m, SRTM 90 m, MERIT 90 m, and TanDEM-X 90 m) Using DGPS. Remote Sens.14, 1334. 10.3390/rs14061334 (2022). [Google Scholar]
- 49.Liu, K. et al. A review of laser-induced breakdown spectroscopy for coal analysis. TrAC Trends Anal. Chem.143, 116357. 10.1016/j.trac.2021.116357 (2021). [Google Scholar]
- 50.Kruse, J. et al. Innovative methods in soil phosphorus research: A review. J. Plant Nutr. Soil Sci.178, 43–88. 10.1002/jpln.201400327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang, C.-W., Laird, D. A., Mausbach, M. J. & Hurburgh, C. R. Near-infrared reflectance spectroscopy-principal components regression analyses of soil properties. Soil Sci. Soc. Am. J.65, 480–490. 10.2136/sssaj2001.652480x (2001). [Google Scholar]
- 52.Viscarra Rossel, R., Walvoort, D., McBratney, A., Janik, L. & Skjemstad, J. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma131, 59–75. 10.1016/j.geoderma.2005.03.007 (2006). [Google Scholar]
- 53.Daniel, K. W., Tripathi, N. K. & Honda, K. Artificial neural network analysis of laboratory and in situ spectra for the estimation of macronutrients in soils of Lop Buri (Thailand). Soil Res.41, 47. 10.1071/SR02027 (2003). [Google Scholar]
- 54.Tian, Y. et al. Quantitative determination of phosphorus in seafood using laser-induced breakdown spectroscopy combined with machine learning. Spectrochim. Acta Part B175, 106027. 10.1016/j.sab.2020.106027 (2021). [Google Scholar]
- 55.Sánchez-Esteva, S. et al. Combining laser-induced breakdown spectroscopy (LIBS) and visible near-infrared spectroscopy (Vis-NIRS) for soil phosphorus determination. Sensors20, 5419. 10.3390/s20185419 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, Y. et al. Improved measurement in quantitative analysis of coal properties using laser induced breakdown spectroscopy. J. Anal. At. Spectrom.35, 810–818. 10.1039/C9JA00429G (2020). [Google Scholar]
- 57.Dong, M. et al. A comparative model combining carbon atomic and molecular emissions based on partial least squares and support vector regression correction for carbon analysis in coal using LIBS. J. Anal. At. Spectrom.34, 480–488. 10.1039/C8JA00414E (2019). [Google Scholar]
- 58.Yao, S. et al. Development of a rapid coal analyzer using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc.72, 1225–1233. 10.1177/0003702818772856 (2018). [DOI] [PubMed] [Google Scholar]
- 59.American Society for Testing and Materials (ASTM). Standard Practice for Collection of a Gross Sample of Coal ASTM D2234/D2234M 17 (2017).
- 60.American Society for Testing and Materials (ASTM). Standard Test Method for Performing the Sieve Analysis of Coal and Designating Coal Size ASTM D4749 12 (2012).
- 61.A. A. ASTM D3174 â 12. Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal (2019).
- 62.Prasad, A. K., Chai, L., Singh, R. P. & Kafatos, M. Crop yield estimation model for Iowa using remote sensing and surface parameters. Int. J. Appl. Earth Obs. Geoinf.8, 26–33. 10.1016/j.jag.2005.06.002 (2006). [Google Scholar]
- 63.Prasad, A. K., Singh, R. P., Tare, V. & Kafatos, M. Use of vegetation index and meteorological parameters for the prediction of crop yield in India. Int. J. Remote Sens.28, 5207–5235. 10.1080/01431160601105843 (2007). [Google Scholar]
- 64.Singh, R. P., Prasad, A. K., Tare, V. & Kafatos, M. Crop yield prediction using piecewise linear regression with a break point and weather and agricultural parameters (2010).
- 65.Boucher, T. F. et al. A study of machine learning regression methods for major elemental analysis of rocks using laser-induced breakdown spectroscopy. Spectrochim. Acta Part B107, 1–10. 10.1016/j.sab.2015.02.003 (2015). [Google Scholar]
- 66.Se, K. W., Ghoshal, S. K. & Wahab, R. A. Laser-induced breakdown spectroscopy unified partial least squares regression: An easy and speedy strategy for predicting Ca, Mg and Na content in honey. Measurement136, 1–10. 10.1016/j.measurement.2018.12.052 (2019). [Google Scholar]
- 67.Baumann, P. et al. Estimation of soil properties with mid-infrared soil spectroscopy across yam production landscapes in West Africa. SOIL7, 717–731. 10.5194/soil-7-717-2021 (2021). [Google Scholar]
- 68.Geladi, P. & Dåbakk, E. Computational methods and chemometrics in near infrared spectroscopy. In Encyclopedia of Spectroscopy and Spectrometry 350–355 (Elsevier, 2017). 10.1016/B978-0-12-803224-4.00352-6.
- 69.Hwang, S.-W. et al. Feature importance measures from random forest regressor using near-infrared spectra for predicting carbonization characteristics of kraft lignin-derived hydrochar. J. Wood Sci.69, 1. 10.1186/s10086-022-02073-y (2023). [Google Scholar]
- 70.Dhiman, G., Bhattacharya, J. & Roy, S. Soil textures and nutrients estimation using remote sensing data in North India-Punjab region. Procedia Comput. Sci.218, 2041–2048. 10.1016/j.procs.2023.01.180 (2023). [Google Scholar]
- 71.Kaur, G., Das, K. & Hazra, J. Soil nutrients prediction using remote sensing data in Western India: An evaluation of machine learning models. In IGARSS 2020—2020 IEEE International Geoscience and Remote Sensing Symposium 4677–4680 (IEEE, Waikoloa, 2020). 10.1109/IGARSS39084.2020.9324201.
- 72.Peng, Y. et al. Estimation of soil nutrient content using hyperspectral data. Agriculture11, 1129. 10.3390/agriculture11111129 (2021). [Google Scholar]
- 73.Song, Y.-Q. et al. Predicting spatial variations in soil nutrients with hyperspectral remote sensing at regional scale. Sensors18, 3086. 10.3390/s18093086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sánchez-Esteva, S., Knadel, M., Labouriau, R., Rubæk, G. H. & Heckrath, G. Total phosphorus determination in soils using laser-induced breakdown spectroscopy: Evaluating different sources of matrix effects. Appl. Spectrosc.75, 22–33. 10.1177/0003702820949560 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Cuiping Lu, C. L. et al. Analysis of total nitrogen and total phosphorus in soil using laser-induced breakdown spectroscopy. Chin. Opt. Lett.11, 053004–053007. 10.3788/COL201311.053004 (2013).
- 76.Yang, J. et al. Determination of phosphorus in soil by ICP-OES using an improved standard addition method. J. Anal. Methods Chem.2018, 1–8. 10.1155/2018/1324751 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marangoni, B. S. et al. Phosphorus quantification in fertilizers using laser induced breakdown spectroscopy (LIBS): A methodology of analysis to correct physical matrix effects. Anal. Methods8, 78–82. 10.1039/C5AY01615K (2016). [Google Scholar]
- 78.Fan, P., Li, X., Qiu, H. & Hou, G.-L. Spectral analysis of total phosphorus in soils based on its diagnostic reflectance spectra. Results Chem.3, 100145. 10.1016/j.rechem.2021.100145 (2021). [Google Scholar]
- 79.Huang, J. et al. Application of Fourier transform mid-infrared photoacoustic spectroscopy for rapid assessment of phosphorus availability in digestates and digestate-amended soils. Sci. Total Environ.832, 155040. 10.1016/j.scitotenv.2022.155040 (2022). [DOI] [PubMed] [Google Scholar]
- 80.Kawamura, K. et al. Laboratory visible and near-infrared spectroscopy with genetic algorithm-based partial least squares regression for assessing the soil phosphorus content of upland and lowland rice fields in Madagascar. Remote Sens.11, 506. 10.3390/rs11050506 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).