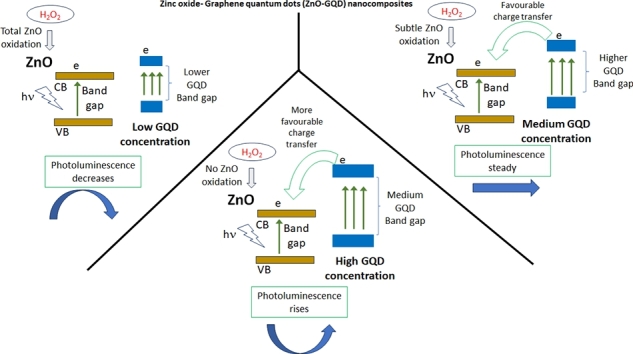
Abstract
Photoluminescence (PL) spectroscopy is one of the best methods to detect molecules due to its easiness, fast time of analysis and high sensitivity. In addition, zinc oxide (ZnO) possesses good optical properties and particularly PL emission in these materials have been exploited for their potential use as photocatalyst, light harvesting and photosensor. These PL properties enhance when graphene quantum dots (GQD) are added to ZnO. For these reasons, we investigated the PL performance of ZnO-GQD nanocomposites. In one experiment we evaluated the PL emission of solid samples ZnO and ZnO-GQD. In a second experiment, these samples were also evaluated in aqueous phase to investigate the H2O2 effect during an experiment lasting 170 minutes. Both experiments displayed six peaks and they were related to the same PL emission source. The PL emission peak around 415 nm was found to be principal source where GQD are interacting. By varying the GQD amount to low, medium, and high concentration, the effect of H2O2 acted consequently, altering the PL emission during experiment in aqueous phase. An oxygen rich environment (ORE) occurred due to H2O2 which oxides the ZnO surface. Low GQD concentration resulted affected by an ORE weakening the GQD-ZnO contact, decreasing PL emission. In high GQD concentration, H2O2 induced GQD to reach the ZnO surface, increasing the PL emission. Only medium GQD concentration prevented oxidation of ZnO and maintained the PL emission intensity constant. When H2O2 concentration increased, for the medium GQD concentration, an excess of charge by peroxides inhibited the charge transfer from GQD to ZnO. This inhibition produces a quenching of the PL emission.
Keywords: Zinc oxide, Graphene quantum dots, Photoluminescence, H2O2 detection, Oxygen-vacancies ZnO
Graphical abstract
Highlights
-
•
GQD hindered nonradiative electron traps on the ZnO.
-
•
H2O2 oxides ZnO or assists to GQD to increase PL emission.
-
•
A precise GQD concentration permits to maintain a steady PL emission.
-
•
GQD are attached at specific sites of ZnO where H2O2 can be photodetected.
1. Introduction
Zinc oxide is an important chemical compound since it has a myriad of applications ranging from antifungal to semiconductor-devices. These applications are accompanied by its benefits concerning the facile and green synthesis [1], [2], [3], low cost [4], low toxicity [5] and its wide band gap [6], [7]. This last particularity allows the use of ZnO as photosensor by means of photoluminescence (PL) spectroscopy. The PL property of ZnO, arises principally due to oxygen vacancies as the most common source of PL emission in the visible (vis) region [8]. In the ultraviolet (UV) region emission is ascribed principally to the formation of excitons [6], [9]; The excitation of ZnO in the UV-vis regions is a function of its application; for instance, photocatalytic application requires that semiconductors, work in the entire UV-vis spectrum, including infrared, due to most of these materials shall be exposed to sun irradiation [10]. Applications of ZnO as a light harvesting or as photoelectronic sensor normally requires only the UV region [11], [12]. Consequently, ZnO presents multiple emissions and with the prospect to modify its band gap and tune PL emissions several strategies have been realized, such as the incorporation of a dopant, surface passivation or the fabrication of nanocomposites. In this sense, graphene quantum dots (GQD) have been extensively used to produce ZnO-GQD nanocomposites, because they are chemically stable and possess high electron mobility [13]. For these reasons these nanocomposites have been intensively studied in the last ten years [10], [14], [15], [16], [17]. In general, particles with sizes higher than 50 nm where mostly ZnO serves as substrate in ZnO-GQD nanocomposites, present a notable emission in the visible region and charge transfer from GQD to ZnO enhancing its conductivity [12], [18], [19], [20]. There is also a generalization, that after GQD are incorporated to ZnO its band gap decreases [13], [19], [21]. This decrease has been ascribed to oxygen vacancies that create new energy levels below the ZnO conduction band [10]. However, a decrease in ZnO particle size might lead to an increase in the band gap and by GQD incorporation enhance the photocurrent induced facilitating its use as photocatalyst [22]. We have found that this photocurrent is not enhanced by high GQD concentration and might also be affected by the presence of a strong oxidant during a PL experiment. Similarly, it is especially important to expand such analysis by studying the role of the interaction among GQD and ZnO and its influence in PL emissions. Therefore, in this work we studied the GQD-ZnO interaction and how is affected under H2O2 exposure by means of PL. To achieve this task, PL studies of solids ZnO and ZnO-GQD were performed by treatment with H2O2 or calcination, to assign and relate emission peaks with a chemical environment in these compounds; furthermore, we extrapolated this to the same material in aqueous phase in order to explain how the system ZnO-GQD works in H2O2 solutions. Despite of a strong oxidant as H2O2, GQD were found to enhance or to conserve the PL emission of ZnO as nanocomposite through time. Specifically, GQD exert an enhancement of PL emission around 415 nm and this is done at oxygen vacancies contained in ZnO where hydrogen from GQD, is placed; moreover, we demonstrate that emissions detected at this wavelength, are the principal source of PL quenching as it was proved to detect H2O2. Detecting this molecule is of highly medical interest due to it is related with cellular oxidative stress, cellular ageing and cancer, among others [5], [23]. We propose a mechanism of the PL enhancement and quenching from ZnO-GQD nanocomposites by studying the band gap. Finally, this study covers a possible application of photodevice to detect H2O2 considering the time life of the materials under this oxidant. This is crucial to evaluate if these devices perish rapidly opening a field to prepare durable or recyclable materials.
2. Materials and methods
2.1. Chemical substances
In this study the following chemical substances were used, all reagent grade and high purity: Zn(CH3COO)2⋅2H2O (99.3%), 28 w% NH4OH (Fermont, 98%), NaOH solid (Fermont, 98%), 30 w% H2O2 (Sigma Aldrich, 98%) and deionized water (Karyeth, 0.76 μS cm−1).
2.2. Preparation of samples
2.2.1. Synthesis of graphene quantum dots and zinc oxide nanoparticles
Details of the preparation of solid graphene oxide are presented elsewhere [24] and from this solid, graphene quantum dots (GQD) were prepared by hydrothermal (HT) process. For the sake of clarity all samples prepared are shown in Table 1. The first step consisted of placing graphene oxide (0.01 g) inside a teflon autoclave containing 10 mL of deionized water and 3 mL of H2O2; this autoclave was introduced to an oven at 170 °C during 6 h. A volume of 6.5 mL of the resulting sample (named HD) was poured into a teflon autoclave and 400 μL of NH4OH (28 w%) were added. Finally, this mixture was treated by HT process for 6 h at 170 °C. From the obtained sample only the liquid phase was used, and this was named D. Volumes of 20, 100 and 500 μL were taken from D and diluted in 10 mL of deionized water to prepare the solutions Dl, Dm and Dh, respectively, in which suffixes l, m and h stand for low, medium and high concentrations.
Table 1.
Sources and amount of components used for preparation of samples.
| Prepared sample | Source of sample | Amount of components |
Method of preparation | ||
|---|---|---|---|---|---|
| Source | Water (mL) | Reagent | |||
| HD | Graphene oxide | 0.01 g | 10 | H2O2 (3 mL) | HT |
| D | HD | 6.5 mL | NA | NH4OH (400 μL) | HT |
| Dl | D | 20 μL | 10 | NA | Water diluted |
| Dm | D | 100 μL | 10 | NA | |
| Dh | D | 500 μL | 10 | NA | |
| Zw | Zinc acetate | 50 mL of 0.125 M solution | NA | NaOH (0.5 M) | Room temp. reaction |
| Zd(s) | Zw | 1 g | NA | NA | Dried at 80 °C |
| Zd(l) | Zd(s) | 0.01 g | 10 | NA | Magnetic stirring |
| Zc(s) | Zw | 1 g | NA | NA | Calcined at 500 °C |
| Zc(l) | Zc(s) | 0.01 g | 10 | NA | Magnetic stirring |
NA : not applicable
Zinc oxide nanoparticles (ZnNP), were synthesized by the reaction between Zn(CH3COO)2⋅2H2O and NaOH. First, 50 mL of zinc acetate solution (0.125 M) was transferred to a beaker and by gentle stirring, drops of NaOH solution (0.5 M) were added until the pH reached a value of 12. A bright white solid emerged and this suspension was stirred during 2 h. Finally, this solid was filtered and washed using 10 volumes of water with respect to the final suspension volume. One fraction of the obtained solid was named Zw where “w” implies as was synthesized. A mass of 1 g of Zw was dried at 80 °C for 20 minutes and 1 g of Zw was calcined at 500 °C for 2 h; the obtained solids were named Zd(s) and Zc(s) where “d” and “c” represent a dried and a calcined sample, respectively and “s” stands for solid. From Zd(s) and Zc(s) two suspensions were prepared using 0.01 g from each solid and 10 mL of deionized water by moderate stirring for 25 minutes and these were identified as Zd(l) and Zc(l), respectively, where (l) is for liquid (Table 1).
2.3. Preparation of samples and photoluminescence performance
Some samples shown in section 2.2.1 were directly measured by PL spectroscopy and evaluate the H2O2 effect. From these samples nanocomposites were also prepared to be measured also by PL means for the same purpose. A summary of all samples prepared for PL measurements is shown in Table 2. The PL spectra were taken in a Perkin Elmer LS55 spectrophotometer, for the solids ZdDl:1(s), Zd:1(s), Zd(s), Zc(s) and ZcDl-3(s) in order to explore the influence of the calcination, GQD and H2O2 effects in the PL emission (Fig. 1). From these spectra, with the aim to determine the performance of the nanocomposites with respect to time, samples Zc(l), ZcDl:0, ZcDm:0 and ZcDh:0 were taken to evaluation at the initial point (the far left point) and this was taken as reference in Fig. 3. After this measurement, 1 mL of the H2O2 (10−3 M) solution was added to each samples and they were renamed as mentioned in Table 2 (Zc-3, ZcDl-3, ZcDm-3 and ZcDh-3); the rest of the points were measured after 20, 50, 80, 110, 140 and 170 minutes (Fig. 3); for each point of these figures the emission intensity was considered.
Table 2.
Samples prepared for PL measurements.
| Prepared sample | Amount of substance |
Water (mL) | ||
|---|---|---|---|---|
| Zinc source | GQD source | H2O2 (volume concentration) | ||
| Dh-3 | NA | Dh (10 mL) | 1 mL (10−3M) | 1 |
| ZcDl-3 | Zc(l) (1 mL) | Dl (10 mL) | NA | |
| ZcDm-3 | Dm (10 mL) | NA | ||
| ZcDh-3 | Dh (10 mL) | NA | ||
| Zc-3 | NA | 10 | ||
| ZcDh:0 | Dh (10 mL) | NA | 1 | |
| Zc(l) | NA | NA | 11 | |
| Zd(s) | Zw (0.175 g) | NA | NA | NA |
| Zc(s) | NA | NA | NA | |
| Zd:1(s) | 10 mL (0.02 g Zc(s)/mL) | NA | 10 mL (1 M) | 100 |
| ZcD1-3(s) | Dl (100 mL) | 10 mL (10−3 M) | NA | |
| ZdD1:1(s) | 10 mL (0.02 g Zd(s)/mL) | Dl (100 mL) | 10 mL (1 M) | NA |
NA : not added;
(a) Water was added to conserve a volume ratio of 1/10/1 from Zn/GQD/H2O2 respectively, when a substance was not added;
(b) These samples were obtained in solid phase directly (see Table 1);
(c) These samples were prepared in liquid phase and filtered to obtain the solids.
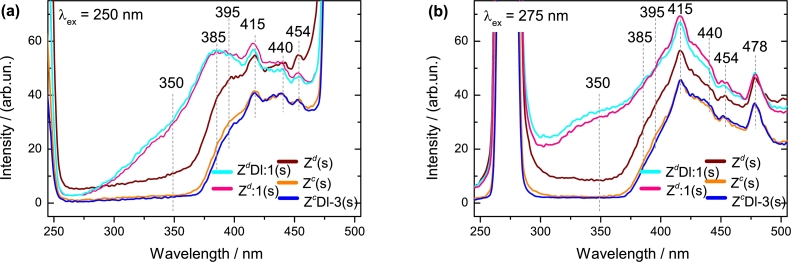
Figure 1.
PL spectra from solids samples taken as references and measured at λex (a) 250 nm and (b) 275 nm. These spectra correspond to calcined samples Zc(s) and ZcDl-3(s) and to non-calcined Zd(s), Zd:1(s) and ZdDl:1(s). The dashed gray lines trace the emission peaks (in nm) written at the top of each line.
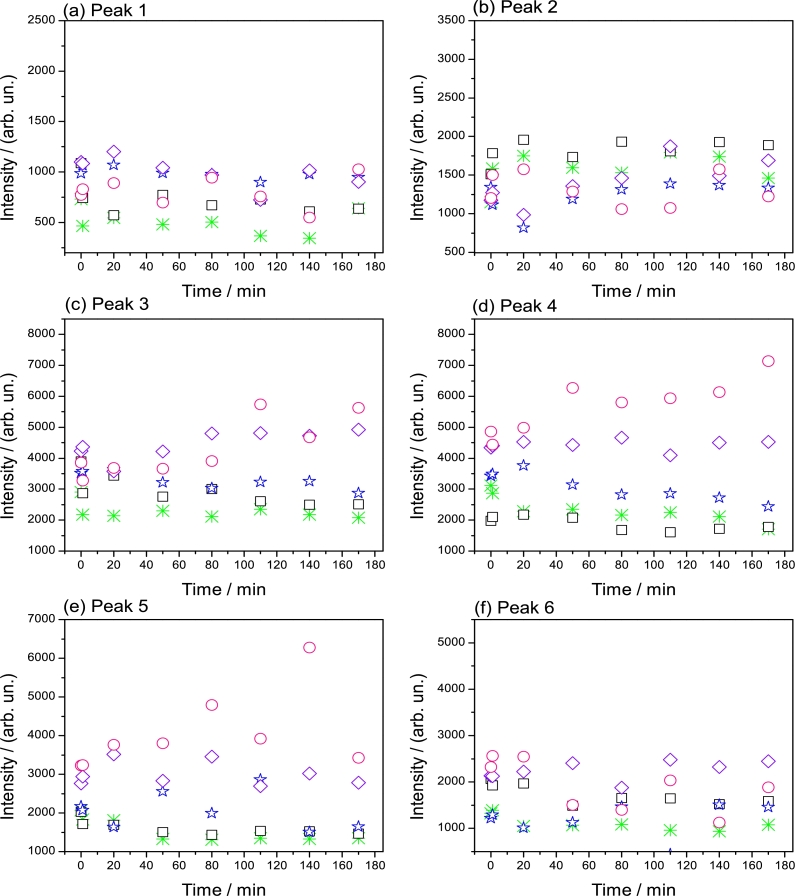
Figure 3.
Peaks intensities with respect to time from the spectra decomposition of samples Zc(l) (⁎), Zc-3 (□), ZcDl-3 (⋆), ZcDm-3 (⋄) and ZcDh-3 (∘). The far left points in each sample are the reference values (taken without H2O2) and the rest of measurements were done with a H2O2 solution (10−3 M); all evaluated at λex 275 nm.
2.4. Characterization
The following equipment were used to characterize the materials of this work. X rays diffraction (XRD) was measured in a Siemens D5000 equipment with a step size of 0.05° s−1 with a Cu X-rays source (λ = 1.5405 Å) and crystal phases were identified by the PROFEX 5.0.2 software. Scanning electron microscopy (SEM) was taken in a JEOL LSM 6701F. Ultraviolet and visible (UV-Vis) spectra were taken by a Shimadzu UV-1800 equipment. Fourier Transform Infrared spectroscopy (FTIR) was performed in a Perkin Elmer Spectrum One with attenuated total reflectance (ATR) complement. XPS spectra were taken with a Thermo Scientific K-Alpha instrument with a stripping capability of approximately 30 nm and Al source; the peak correction was performed using the Carbon adventitious signal (C 1s, 284.6 eV). Atomic Force Microscopy (AFM) was achieved using an AA3000 equipment. For DSC measurements a NETZSCH DSC 404F3 equipment was used with argon gas and a ramp of 10 °C/min
3. Results
3.1. Photoluminiscent (PL) emission sources from solid samples
The PL emissions were measured for solids samples treated in conditions as high H2O2 concentration or calcination and spectra are shown in Fig. 1. To study PL emissions, the peak at 415 nm, was taken as reference in order to detect variations on adjacent peaks and thus analyse the chemical environment where the emission emerges. By analysing the Fig. 1 detected peaks are designated as follow:
-
(A)
Peak at 350 nm. This peak is related to the free and bound exciton from the near band edge emissions [9]. These emissions have been found to display a very narrow shape at very low temperatures in ZnO structures; as temperature rises a broad band emerges leading to bound exciton emission [25]. Interestingly in our case, and also found in other work [26], this emission arises due to the presence of ZnO2. A comparison between Zd(s) and Zd:1(s) clearly indicates the addition of H2O2 implies the growth of this peak (Fig. 1a and Fig. 1b). Samples Zd:1(s) and ZdDl:1(s), treated with high concentration of H2O2, were mostly converted to ZnO2 and in Fig. S1 (see supplementary information), a representation of this process is shown in scheme 1; this is also confirmed by XRD analysis in Zd:1(s) (Fig. 4). It is important to note that this emission peak is not presented in ZcDl-3(s) due to the low H2O2 concentration used during the experiment. In spite this low concentration, H2O2 reacts with the ZnO surface to generate an oxygen rich environment (ORE) [27] as is proved in XPS analysis (Fig. S1 scheme 2 and Fig. 7); however, an increase in the exciton energy intensity is not observed in these solids.
-
(B)
Peak at 385 nm. This emission is also attributed to bound exciton, which hydrogen placed near the ZnO surface, enhances the emission intensity [28]. As H2O2 might be dissociated into H+ proton and HOO⋅ radical [29], hydrogen acceptor might be interacting with ZnO interstitials as in scheme 1 (Fig. S1). This is also inferred due to in our experiments, most of the emissions variations in the rest of the peaks detected, were attributed to ZnO and HOO⋅ interactions. Notwithstanding, when H2O2 is in higher concentration, hydrogen might enhance the emission intensity in Zd:1(s) and ZdDl:1(s), due to it could play roles as donor, acceptor and also might be passivating nonradiative recombination centres [28], [30].
-
(C)
Peak at 395 nm. For the calcined samples Zc(s) and ZcDl-3(s), this peak is modified, with respect to the peak at 415 nm for Zd(s). It is observed that for Zd:1(s) and ZdDl:1(s) the intensity is higher than Zd(s). In this case HOO⋅ radicals seem to reach intrinsics or native vacancies from the ZnO surface; this is manifested as the growth of oxygen onto the surface detected in XPS analysis (Fig. 7). The positioning of these radicals might increase the free carriers produced by electron-traps where free exciton emission emerges at this wavelength [25], [31]. Although this has been found to occur at very low temperatures, in our experiment, this phenomenon was likely forced due to the use of H2O2 even at low concentrations. The arrival of HOO⋅ radicals to produce an ORE, leads to a partial depletion layer where is possible that electron-hole recombine nonradiatively which finally inhibits exciton transition. It is inferred then that this process must have a limit where high H2O2 concentrations produce peroxide radical leading to zinc oxidation to form ZnO2 (Fig. 4). GQD is observed to reduce slightly the emission on both (Fig. 1a and Fig. 1b) suggesting that H+ from amine group, is interacting with ZnO vacancies. Replicas manifested as longitudinal phonon from bound exciton due to interstitial hydrogen donors, also might appear at this wavelength, however they were not present or have very low emission [25], [28].
-
(D)
Peak at 415 nm. After the calcination and the H2O2 exposure, a slightly higher PL emission in this peak is observed for Zc(s) and ZcDl-3(s) compared to Zd(s), and of course, this is more evident for Zd:1(s) and ZdDl:1(s) (Fig. 1b). This might be attributed to hydrogen placed onto the ZnO surface where it produces (1) a donor-acceptor pair, which is common at room temperatures [6], [25], [28] and/or (2) a possible surface passivation of nonradiative traps [30]. GQD slightly increase the PL emission, and contrary to peak at 395 nm, in this peak the amino group is not affected by the H2O2 presence. Therefore, in these sites GQD must be strongly anchored.
-
(E)
Peak at 440 nm. This emission peak corresponds to visible blue-region where oxygen vacancies (VO) are considered as the principal source. This has been ascribed due to VO, are energetically more favourable to be produced from the synthesis of ZnO and therefore we assign this peak to these point defects [32], [33]. These defects correspond to a neutral oxygen vacancy (V) where electron transition comes from these centres to the edge valence band [6], [25], [34]. For = 250 nm, Zd(s), Zc(s), and ZcDl-3(s), calcination, or the incorporation of H2O2 and GQD caused no great variations in the PL emission. This suggests that VO are intrinsic and correspond to deep-level centres [10], [21]. However, despite the low GQD concentration in ZcDl-3(s), a slightly increase in the emission is attained in both . In this case, after GQD incorporation, recombination from V is preferred indicating the possibility that some of these defects are fairly near the ZnO surface.
-
(F)
Peak at 454 nm. Intrinsic vacancies in Zd(s) produce an emission in the blue visible region. A difference between Zd(s) and Zc(s) spectra is registered and is associated to vacancies created by the calcination process. The decrease in PL emissions in Zc(s) and ZcDl-3(s) is associated with these vacancies which might be acting as electron-traps inhibiting electronic transitions. Moreover, when H2O2 is added an increase in the emission intensity is not observed. Opposite to peak at 440 nm, when high concentration of H2O2 is added, the possibility to cover the intrinsic and new vacancies is very low, as the PL intensity is lower in Zd:1(s) than Zd(s). Therefore, we consider that these vacancies are deeper than those found at 440 nm. Thus, this is the reason that these vacancies, are more stable, leading to red-shift emission [35]. Therefore, these vacancies should be at the ZnO bulk preventing diffusion of oxygen or other gases.
-
(G)
Peak at 478 nm. This peak remains after H2O2 addition to ZdDl:1(s) and Zd:1(s), relative to 415 nm peak (Fig. 1b). Therefore, neither oxygen nor hydrogen might affect these sites. Again, this peak is related to deep-level oxygen vacancies which are chemically stable to H2O2 and are related to green luminescent emissions [25].
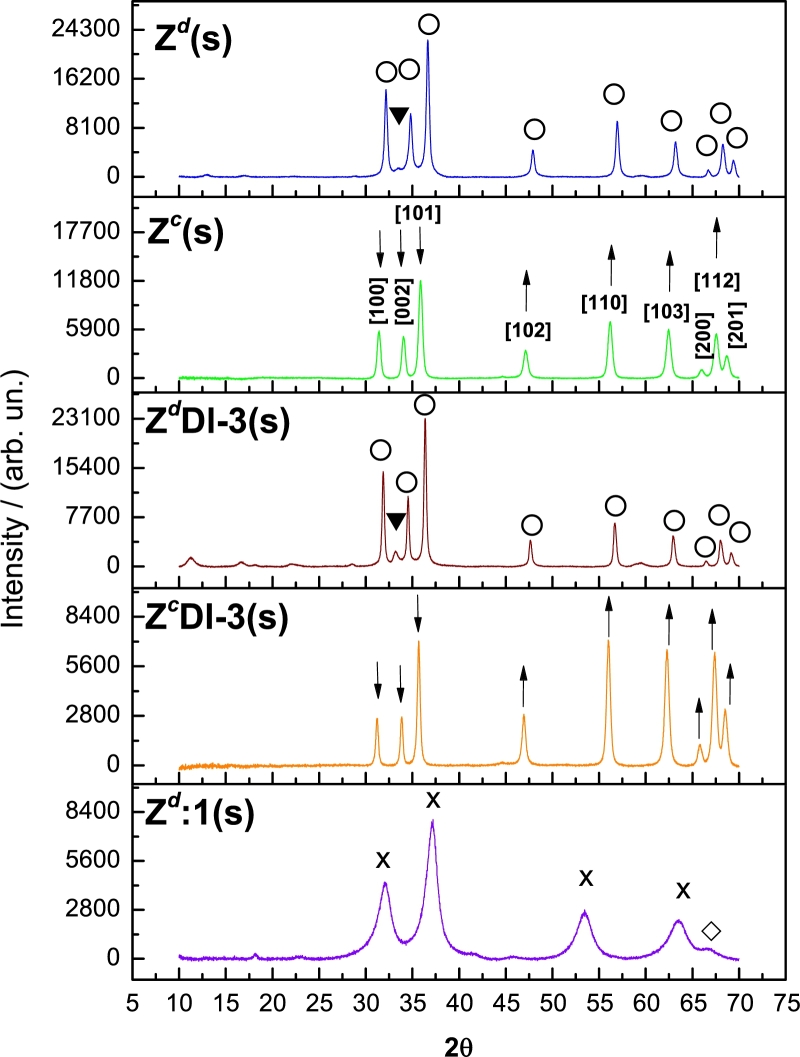
Figure 4.
XRD of Zd(s), Zc(s), nanocomposites ZdDl-3(s) and ZcDl-3(s) and Zd:1(s); the identified phases in the first four samples are, ZnO phase (∘) and hydrozincite(▾) and in Zd:1(s) phases are ZnO2 (×) and Zn(OH)2 (⋄).
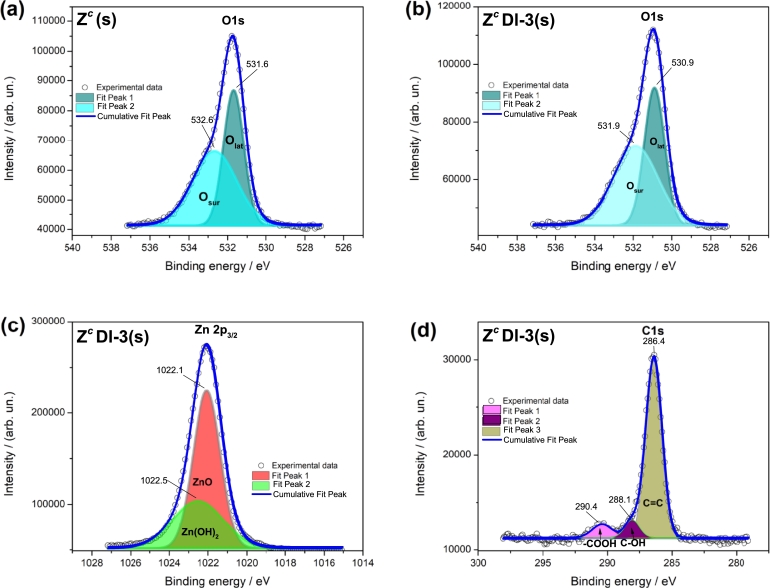
Figure 7.
XPS spectra from (a) Zc(s), (b) ZcDl-3(s) of the oxygen peak (O1s) and for the ZcDl-3(s) of the (c) zinc peak (Zn 2p3/2) and the (d) carbon peak (C1s).
3.2. Parallelism of emission source from solids to samples in liquid phase
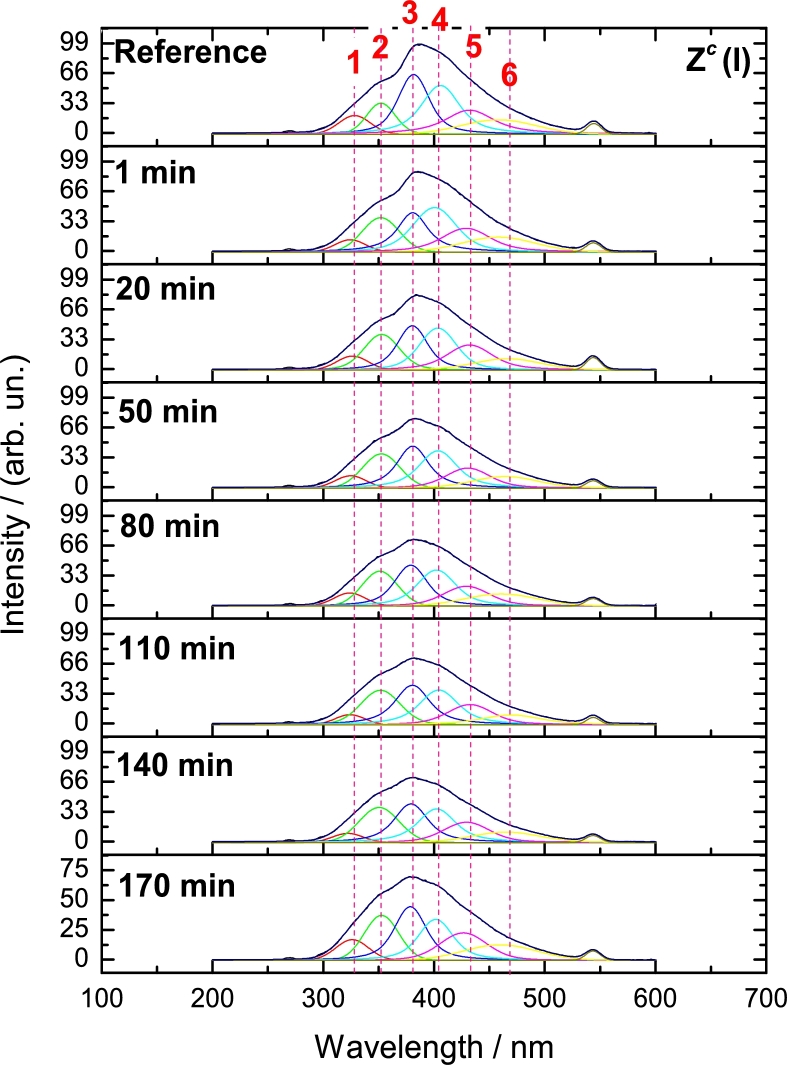
In this section we measured the PL in aqueous phase to observe stability of nanocomposites. In general terms, in this work stability includes photostability which defines if samples are not chemically modified by photons and chemically stable, which here is the extend that nanocomposites might last before being affected by H2O2. The emission spectra taken at = 275 nm, are shown in Fig. 2 for Zc(l) in liquid phase. For each spectrum, six peaks were found to fit well the experimental data. Therefore, from the solids spectra in Fig. 1, it is possible to assign the emission peaks from samples in liquid phase and they might be related with the same chemical environment from the ZnO structure treated in section 3.1. Because H2O2 was not used for Zc(l), it is important to note the irregularity of the PL emission for all peaks in all the measurements (Fig. 2). Long time exposure to water, provoked a mild chemical modification in Zc(l) which affects the PL emission.
Figure 2.
PL spectra from Zc(l) with respect to time evaluated at λex = 275 nm. The first measurement was achieved as reference by only measuring the PL emission from Zc(l) prepared as in section 2.2.1. After 1 minute 1 mL of water, in this case, was added to later measure spectra at every time showed in this figure. Each peak was identified with a red number at the top of figure, corresponding to description in this section.
It is noteworthy to mention that the same PL peaks found in Zc(l), were also displayed in the rest of samples and their plots are shown in Fig. S2. Results from Fig. S2 were condensed and are presented in Fig. 3, showing intensities for every peak found in these samples. If the first point (reference) is compared with the second point, which is after adding the H2O2 solution, a rapid effect is observed in each peak meaning that H2O2 acts immediately in almost all samples (where ZcDm-3 seems to be an exception). Then, variations in intensities over time, must be related with chemical environment variations provoked by H2O2, principally in ZnO structure. Thus, these six peaks from Fig. 3 are described as follows:
-
Peak 1
Opposite to PL emission in solid phase (Fig. 1), samples in liquid phase for peak 1 (Fig. 3a), present emissions even at low H2O2 concentrations. The H2O2 improves the PL intensity in all samples, whilst GQD have a minor effect. In ZcDh-3 the PL emission is mildly lower than ZcDl-3 probably due to GQD are impeding the H2O2 to reach the ZnO structure. For these reasons, the emission peak 1, is therefore ascribed principally to ZnO due to a possible formation of an ORE at these sites where the excitation is occurring. Notwithstanding, interestingly GQD might be inducing to slightly increase the amount of bound exciton emission in ZnO (Fig. 3a). In this sense, exciton binding energy might emerge due to the presence of water, H2O2 and GQD.
-
Peak 2
In Fig. 3b, the intensity of peak 2 remains mainly constant almost for all samples except for ZcDh-3. The emission emerging from this peak has low intensity but according to description of the emission peaks from Fig. 1, this peak is related also with the exciton-free and binding energy arising from interstitial hydrogen. It is clear that H2O2 tends to increase the emission in Zc-3, pointing the effect of this oxidant, due to hydrogen reaction with surfaces as presented in scheme 1 in Fig. S1. GQD in the nanocomposites have also a small effect as in peak 1. Note how ZcDl-3 and ZcDm-3 present a mildly higher PL emission intensity than ZcDh-3. In this situation, the ZnO surface is obstructed by GQD when is in higher concentration as in ZcDh-3.
-
Peak 3
This peak is related with the PL emission from oxygen vacancies, which are prone to be perturbed by the presence of GQD (Fig. 3c). It is noted that Zc(l), shows a steady behaviour during the experiment, however, Zc-3 presents a slightly higher intensity. This evidence suggests that ZnO is being attacked by H2O2 at low concentrations. Although Zc-3 presents higher PL emission than Zc(l), this decreases with respect to time. As the peak 3 from solids, HOO⋅ radicals adsorbed onto VO decrease exciton transitions; however, this process is slower in liquid phase due to desorption of these radicals is difficult and thus, the diminishing in PL emission is achieved gradually. As mentioned in section 3.1 (Fig. 1), GQD are prone to interact with these sites and this is confirmed as they initially increase the emission in all nanocomposites. Notwithstanding, the later performance depends on the GQD concentration. In ZcDl-3 a minor decrease in emission intensity is observed. On the contrary, ZcDm-3 and ZcDh-3, display an increase in intensity. As it was explained for the peak 2, a weak interaction from GQD in ZcDl-3 must be related with the decreasing of PL intensity, whilst in ZcDm-3 and ZcDh-3, interaction must be stronger, due to they possess higher GQD concentration and thus, greater amounts of carboxyls groups which might be interacting and preventing the H2O2 to reach ZnO surface (Fig. S3); moreover, amine groups (identified in XPS see section 3.4) must be interacting with H2O2. Therefore, the GQD action must be a function of its concentration and, when its concentration is higher, its positioning at the ZnO surface may be time consuming to be complete; in Fig. S4a, this is confirmed in an experiment with ZcDh:0 where no H2O2 was used and a similar performance although with lower intensity was displayed. This means that when H2O2 participates, a different configuration in GQD-ZnO-H2O2 interaction occurs due to an increase in intensity instead of decreasing effect is presented.
-
Peak 4
This peak is associated to an ORE at the surface of the ZnO crystal structure (Fig. 3d). From UV-Vis spectra in Fig. 6, Dh presents two bands at 210 and 255 nm, associated to amine and C=O groups, respectively. When GQD are in high concentration, these groups might reinforce the anchoring points at the surface of ZnO structure. Carboxyls groups are especially close to surface vacancies, serving as “protection” against H2O2, reducing the possibility of the ZnO surface oxidation by the attraction of amine groups to H2O2 (Fig. S3). Therefore, ZcDh-3 contains a great amount of these functional groups bonding over the ZnO surface increasing the PL emission intensity. Notwithstanding, a gradual decrease in ZcDl-3 is attributed to oxidation of ZnO surface by H2O2. With ZnO surface oxidation, the PL properties are lost due to a weak contact with GQD. In this sense, sample ZcDm-3 might possess the right amount of GQD functional groups which anchor to the ZnO surface remaining the nanocomposite chemically stable during the experiment. This is the reason why this sample is the ideal to be used as H2O2 photoluminescent sensor and the peak 4 is related to the effects of H2O2 that might cause a perturbation in the PL properties.
-
Peak 5
The peak 5, related to oxygen vacancies, is perturbed by H2O2 specially with long times, and low or high GQD concentration (Fig. 3e). It is observed that once GQD seem to be close at these sites PL emission increases for ZcDh-3 to decrease after 80 minutes. For ZcDl-3 and ZcDm-3, the behaviour is almost similar to ZcDh-3 however more unsteady. This decreasing in PL intensity, is ascribed to H2O2 which principally interacts with GQD instead with ZnO and this is proved as Zc-3 and Zc(l) have very similar performance.
-
Peak 6
Finally in peak 6, as well associated to oxygen vacancies at the bulk from ZnO, might be reached by radicals from H2O2 (Fig. 3f). Sample Zc-3 has higher PL emission intensity compared with Zc(l). This arises from the incorporation of HOO⋅ close to these sites. Increment of emission is only mildly dependent of GQD concentration. For ZcDh-3 and ZcDm-3, GQD must not be interfering with these sites, however in ZcDl-3 they might be impeding the diffusion of these radicals. In this way, GQD from ZcDl-3 might prefer these sites and this situation is not given for the other nanocomposites. In this respect, GQD which sizes are between 4 and 6 nm (Fig. S4b, Fig. S4c and Fig. S4d) are candidates to be interacting very close to these sites.
Figure 6.
(a) FTIR spectra where bands in general are associated to carboxyls or carbonates (from 1550 cm−1 to 1391 cm−1), to hydroxyls (from 1093 to 739 cm−1) and to ZnO (at 550 cm−1). (b) UV-Vis spectra for low, medium and high GQD concentrations, Dl, Dm and Dh, respectively, Zc(l), ZcDh:0 and (c) UV-Vis spectra for Zc-3, ZcDl-3, ZcDm-3 and ZcDh-3; insets in (b) and (c) are the Tauc plot for these samples. Note: the UV-vis spectra were measured after the PL time experiments from section 3.2 were achieved.
In general, it seems that in peaks 1 and 2 the effect of GQD is negligible. Moreover, as it was mentioned, water produces only a small effect on ZnO PL emission therefore differences between Zc(l) and Zc-3 must be ascribed due to the HOO⋅ and H+ at the interstitials which enhances exciton formation. For peaks 5 and 6, it seems that longer times influence to gases or H+ reach bulk vacancies faster. Whilst after calcination, vacancies created at the surface (or close to surface) of ZnO may interact with GQD. However, because these interactions are very weak GQD are attaching when PL emission increases and detaching when PL emission decreases. Because peaks 5 and 6 resembles emission peak at 454 nm for these solids samples, the similarities of Zc(s) and Zd(s), are that because vacancies at the bulk are presented in both samples they must show emission at the middle of visible spectrum; however close to the high energy of this spectrum, they show difference because Zd(s) possess less vacancies near the ZnO surface. This is because hydroxyls and in a lesser extension, carbonates, act as electron traps enhancing the PL emission.
3.3. Structural and morphological transformations of materials
During the synthesis of ZnO, the Zn(OH)2 phase might be also formed due to the process humidity and high pH. This can be corroborated in a XRD pattern for Zw in Fig. S5 where most of these peaks correspond to Zn(OH)2 [36]. After low drying temperature, spontaneous Zn(OH)2 decomposition into ZnO occurred as is shown for Zd(s) in XRD studies (Fig. 4); however, a portion of the initial solid was transformed into hydrozincite phase (Zn5(CO3)2(OH)6) which is marked with an inverted black triangle. Owing to the high temperature of calcination in Zw (500 °C for 2 h), it was possible to remove more hydrozincite phase as this peak is not present in XRD pattern of Zc(s) (Fig. 4). The sample Zc(s), in fact, shows characteristic peaks from ZnO which correspond to zincite phase; this specific pattern is typical from ZnO nanoparticles (ZnNP) and all planes correspond to ZnNP peaks [37]; the ZnO attainment was corroborated in a differential scanning calorimetric (DSC) shown in Fig. S6a similar to one from literature [38]. The removal of carbonates and hydroxyls by calcination, is the most feasible process to provoke a lattice reconstruction and favour the growth in the direction of planes with upside arrows (Fig. 4 Zc(s)). Besides of the loss of hydroxyls and carbonates, during this process, vacancies are also produced by the release of lattice oxygen from the Zn-O bond [39], rising the number of vacancies. With the generation of vacancies, a slight reduction of the crystal size was achieved in Zc(s) (Table S1). This coincides with a theoretical study where it was found a strong relation among the diminishing of crystal size and the easiness to form oxygen vacancies because of the low energy required [40]. However, as is observed in SEM images, for Zc(l) clusters of zinc oxide particles are produced whilst in Zc-3 are not. Therefore, H2O2 cause clusters to split (Fig. 5) and with exposure to a 1 M H2O2 solution, ZnO2 is produced, as is shown for Zd:1(s) in Fig. 4 and in a DSC analysis (Fig. S6b) which describes the ZnO2 decomposition to ZnO [27], [41]. Moreover, incorporation of interstitial hydrogen and oxygen into ZnO lattice by H2O2, increases the ZnO crystal size (Table S1). This reaction is disclosed by the decreasing intensity of the three first planes in nanocomposites ZdDl-3 and ZcDl-3. Comparing XRD patterns of ZdDl-3 and ZcDl-3 is clear that the diminishing of crystal size resulted from the calcination whilst H2O2 reduces clusters. When GQD are added to calcined ZnO and exposed to H2O2, a slightly increase in ZnO crystal size occurred (Fig. 4 ZcDl-3(s), Table S1). Due to GQD are placed at the zinc oxide surface, they help to maintain particles closer producing clusters although in a smaller size compared with Zc(l) (Fig. 5). Therefore, all modification to the ZnO crystal is mostly attributed to calcination and H2O2. This is in good agreement with the PL results previously presented, where the emissions from interstitials hydrogen and oxygen are independent of GQD; in other words, GQD are mostly placed at the sites corresponding to the emission from peak 4 (see section 3.2). This was confirmed with the experiment from sample ZcDh:0, measured without H2O2 (Fig. S4a); it is noticeable that this sample performs almost the same as ZcDh-3, however with a lower intensity due to H2O2 absence, limits GQD to reach the ZnO promptly (Fig. S3). Therefore, the H2O2 provokes newly, the crystal growth in planes from 2θ = 45° and thereafter, whilst the first three peaks again decrease in intensity. In this case, a slight possibility for carboxyls or amine groups from GQD to be placed at surface vacancies from ZnO might exist due to a further decrease in peak intensities at 2θ = 31.4°, 34.0° and 35.8° (Fig. 4 ZcDl-3(s)). In addition, the occupation of ZnO interstitials by oxygen or hydrogen during the contact with H2O2 also induces to a diminishing in these peaks intensities. The presence of a strong oxidant now might be oxidizing Zn creating an ORE in which small amounts of ZnO2-like species are producing; this leads to new vacancies which finally results in a decrease of crystal size (Table S1); even, this coincides with the diminishing of particle size when samples are exposed to H2O2 as is shown in the SEM images (Fig. 5).
Figure 5.
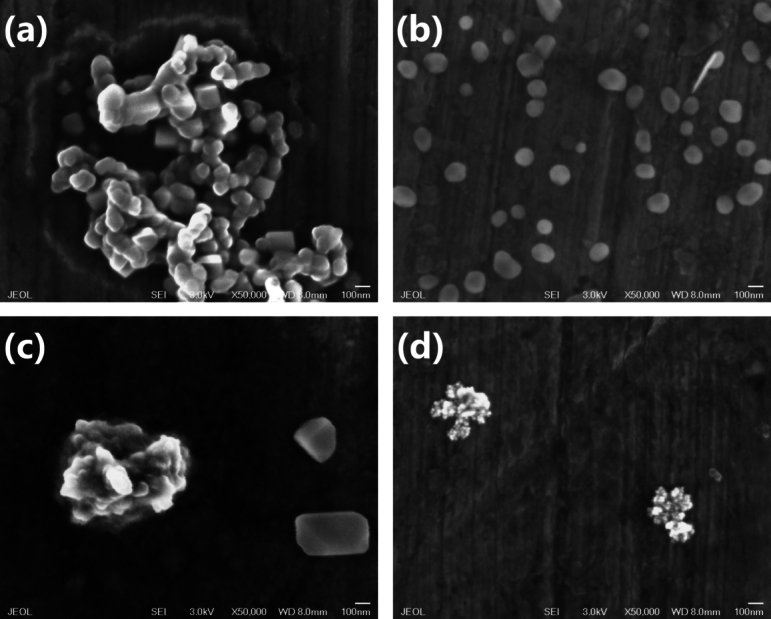
SEM images from (a) Zc(l) (b) Zc-3 (c) ZcDh:0 and (d) ZcDm-3. Images in (a) are from ZnO crystals with different shapes, while H2O2 apparently tend to reduce the cluster size into smaller particles (b); for ZcDh:0 in (c) H2O2 considerably reduced the clusters size which is shown in ZcDh-3 (d). All samples were collected in aqueous phase and were placed in an aluminium substrate.
In Fig. 5, SEM images are shown to study the morphology of fresh and H2O2 processed samples. In Zc(l) different shapes of ZnO particles are observed and due to the image scale, their sizes are around 100 nm (Fig. 5a). The growth process of ZnO consists in nucleation of ions and concentration of the zinc source where basic pH plays an important role. Inefficient stirring during the ZnO synthesis might be the causal to obtain particles of ZnO in prism and oval shapes [8] producing clusters of approximately 1 μm (Fig. S7a). From Fig. 5b smaller and well separated particles are noted for Zc-3. This is evidence of the H2O2 action by modifying the morphology of the ZnO particles and reducing its size; similar variations in the ZnO morphology have been ascribed to structural defects which influence in the PL properties [42]. These defects are performed by the calcination and a posterior H2O2 exposure leading to a general enhancement of the PL properties. Although carbon was found in this sample, this enhancement was due to combination of vacancies, zinc, and oxygen (see EDS analysis for Zc-3 from Fig. S7d). Contrary to Zc(l), the appreciation from the ZcDh:0 image (Fig. 5c) is that the clusters are smaller and enough separated. In this case GQD may prefer ovals-shaped particles and probably by a considerably attraction they maintain particles together and clusters totally separated; in Fig. S7f an EDS analysis of ZcDh:0 registered a low amount of zinc (Table S2) which might imply zinc is covered by GQD. Notwithstanding these particles merge, in ZcDm-3, the H2O2 break clusters reducing its size averaging 20 nm (Fig. 5d).
3.4. Chemical aspects from bulk and surface
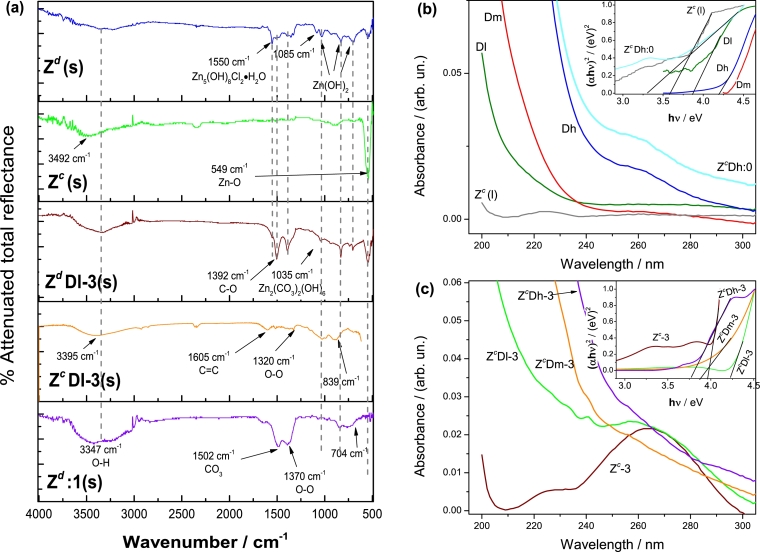
In Fig. 6a, IR spectra show the bands corresponding to several chemical groups which are modified due to H2O2 or calcination. For instance, the first band corresponds to the O-H tension bonding which appears at 3347 cm−1. In Fig. S11, the O-H band presented in Zw, is notably higher in intensity compared with Zd(s) and in fact this excess of OH, principally by water, avoids the spectrum sharpness. Only by drying the sample at 80 °C is possible to eliminate most of the superficially adsorbed O-H. This is ascribed to the chemical transformation from Zn(OH)2 to ZnO as was corroborated in XRD and DSC studies of Zw (Fig. 4 and Fig. S6). Once drying, O-H now are in a different arrangement and in this sense, the bands at 1550 and 1392 cm−1, correspond to the Zn5(OH)8Cl2⋅H2O complex in Zd(s), which has been registered in other studies [43], [44]. Now these bands are also exhibited in ZdDl-3(s), although a higher intensity is attained in the nanocomposite than in Zd(s). This is attributed to the HOO⋅, present in ZdDl-3(s), thanks to H2O2. In fact, for Zd:1(s) this band is even more intense confirming the action of these radicals (Fig. 6a). Moreover, in Zd:1(s) the presence of O-H is manifested also at 3347 cm−1; the amount of oxidant is too high in Zd:1(s) that not only might incorporate more O-H at the surface and as complexes, but also generates the peroxide group (O-O) to appear at 1370 cm−1. With this, it is possible an oxygen rich environment (ORE), which is related with the increase in emission when H2O2 is added during the PL experiments. Now, note that in ZdDl-3(s), H2O2 is not enough to put these O-H groups at the surface in bands at 3347 and 1630 cm−1, but interestingly the complexes corresponding to bands at 1550 and 1392 cm−1, are even also more intense. Owing to carboxyls are present in non-calcined samples, with the incorporation of HOO⋅ radicals, carboxyls must be bonding to these radicals to form carbonates. In this sense, the hydrozincite is produced when H2O2 is added to the dried sample [27], [45]. Winiarski et al. showed a similar IR spectrum corresponding to the hydrozincite phase [43] and coincides with the evolution of the hydrozincite peak for ZdDl-3(s) confirming the reaction of nanocomposites with H2O2 (Fig. 4). With calcination, all bands from carbonates and hydroxyls now are absent for Zc(s) and, as carbonates were eliminated, it is believed that for ZcDl-3(s) the bands at 1035 and 839 cm−1 correspond to an ORE in which now zinc must be surrounded by peroxide radicals; in fact the O-O band is barely shown in ZcDl-3(s) at 1320 cm−1. The vacancies due to carbonates elimination, might be now occupied by similar functional groups from GQD as the band at 1605 cm−1 is assigned to the C=C bond proving the interaction between ZnO and GQD [46], [47]. This last bond is related with the red shift of the O-H bond from 3492 cm−1 for Zc(s) to 3395 cm−1 in ZcDl-3(s). Note that both bands are at higher wavenumbers highlighting the calcination process, where only chemically bond O-H is on ZnO. The presence of C=C, produces the red shift of O-H for ZcDl-3(s) and we assign this to a strong ion-dipole attraction between carbon from GQD and oxygen from ZnO.
In Fig. 6b, UV-Vis spectrum for Dh shows an absorbance band at ≈ 260 nm which corresponds to transition originated by C=O groups [48]. In Dm and Dl this band has a very low absorbance value which is ascribed to the lower concentration of these samples. However, they present a band at ≈ 300 nm, assigned to an electronic transition occurring when nitrogen is present in GQD [48], [49] proving the presence of the nitrogen groups in GQD. In Fig. S4c and Fig. S4d an AFM profile shows that GQD consists of structures around 5 nm which might be stacked in layers, building clusters between 20 and 100 nm (Fig. S12). That GQD are grouped in layers is related with the amine and carboxyls groups interaction. Inset of Fig. 6b shows band gap estimated by Tauc plots for GQD samples and their values are related precisely with an average particle size where the shorter particle size the higher band gap [50]. In this sense, Dm and Dh seem to have a smaller particle size than Dl, and this is ascribed to a probable higher repulsion maintaining particles well separated when GQD are in greater concentration in a solution. The sample Zc(l) shows a very slight absorption at 265 nm which is assigned to Zn-O electronic transition [51]. For the nanocomposite ZcDh:0 we observe a stronger absorption compared to Dh at 265 nm and this is due to the interaction between these functional groups from GQD and Zn+2 [52]. Moreover, the greater ZnO particle size leads GQD to be more fixed when the nanocomposite is produced. Note that the band gap for Zc(l) is 3.57 eV which is a slightly higher value than those reported in literature (3.2 eV) and this is attributed to fact that UV spectrum was measured after the PL time experiments. For ZcDh:0 the band gap decreases to about 3.29 eV which is also observed in literature when GQD are added to ZnO [13], [19], [21]. For Zc-3 there is a notable absorption for the Zn-O (Fig. 6c) due to its exposure to H2O2. Note also that ZcDl-3 owing to the lowest GQD concentration, is similar to Zc-3 and it developed a band at 262 nm which is assigned also to Zn-O electronic transition. Samples ZcDm-3 and ZcDh-3 display a slight blue-shift respective ZcDl-3, although their bands are not as intense as in this last. When an ORE is occurring at the ZnO surface, the interaction between C=O and VO from ZnO, becomes weaker (Fig. S3); on the contrary for the other samples, C=O remain more strongly attached at the ZnO surface which diminish the absorbance from these groups. This is one of the reasons that PL properties in ZcDl-3 decrease with time in peaks 3 and 4 (Fig. 3). Similarly, the weaker intensity of this band, might be related with the formation of C-O-Zn bond which has been also proposed in other work [53]. Sample Zc-3 possess a higher band gap value of 3.95 eV compared with Zc(l) and as it was confirmed by XRD and SEM when particle diminishes, principally due to H2O2 incursion, band gap increases [22] (Inset of Fig. 6c). This is evidenced also for ZcDl-3 which is a similar sample and presents a band gap of 4.22 eV. The ZnO surface is more exposed in these samples and also, we confirmed this for Zc:1(s) which was completely oxidized to ZnO2 and its particle diminished to almost 6 nm as has been found in literature [54]. For ZcDm-3 and ZcDh-3 the band gaps diminished to 3.87 and 3.75 eV, respectively. Decrease in band gaps for these samples is attributed to ZnO surface protection granted by GQD.
Table 3 presents the atomic surface ratio (relative to oxygen) for samples Zc(s), ZcDl-3(s) and ZdDl-3(s), obtained from decomposition of the peaks detected by XPS measurement presented in Fig. 7 and Fig. S13. XPS spectra from oxygen peak (O1s) emerges around a binding energy (BE) of 531 eV corresponding to lattice oxygen (O) and a shoulder (at ≈ 532 eV) associated to surface oxygen (O) contained in water or other chemical compounds [1], [43] (Fig. 7a). In this figure, the red-shift in ZcDl-3(s), implies the oxygen distribution at the surface producing the new chemical environment in ZnO (Fig. 7b). Both peaks (O and O) show variations related with a decrease in the Zn/O ratio for ZcDl-3(s) compared to Zc(s) (Table 3). By this means, it is inferred that surface oxygen from ZnO, increases when H2O2 is added demonstrating the ORE condition.
Table 3.
Atomic surface ratio with respect to oxygen peaks detected by XPS measurements.
| Sample | O* | Zn2p3/2/O1s |
C1s/O1s |
|||
|---|---|---|---|---|---|---|
| ZnO | Zn(OH)2 | -COOH | C-OH | C=C/C-C | ||
| Zc(s) | O | 1.266 | 0.434 | 0.125 | 0.546 | 1.061 |
| O | 1.035 | 0.355 | 0.103 | 0.446 | 0.868 | |
| ZcDl-3(s) | O | 1.122 | 0.639 | 0.124 | 0.120 | 1.414 |
| O | 0.880 | 0.502 | 0.097 | 0.094 | 1.110 | |
| ZdDl-3(s) | O | 2.932 | 1.186 | 1.260 | 1.663 | 3.375 |
| O | 0.431 | 0.175 | 0.185 | 0.245 | 0.496 | |
* Lattice oxygen peak (O) and surface oxygen peak (O)
The peak at a BE of 1021 eV (Fig. 7c) corresponds to zinc (Zn2p3/2) and its decomposition is related to ZnO (≈ 1021.9 eV) and to zinc at BE of ≈ 1022 eV, where species as Zn(OH)2 are preferentially formed [36], [43]. Because the zinc-to-oxygen ratio in ZcDl-3(s) increases, the required oxygen to produce hydroxyl-zinc species is less than Zc(s) and ZdDl-3(s). This is associated with the calcination of sample, which permits a chemical structure where might exist a greater diffusion of H+ and HOO⋅, and therefore the calcination is very important. This peak also might be associated with oxygen vacancies because we deduced that owing to the minimum need of oxygen to produce hydroxylate species, radical, oxygen and H+, can be inserted at the surface vacancies of the ZnO structure. In this manner, we may note that the peak Zn2p3/2 from ZcDl-3(s) shift slightly to a higher BE suggesting that zinc is readily oxidized, than zinc from ZdDl-3(s). This is achieved thanks to easiest contact among H2O2 and the zinc surface in ZcDl-3(s).
The carbon peak (C1s) is detected in Zc(s), from calibration of XPS equipment, whilst in ZcDl-3(s) and ZdDl-3(s), GQD contribute to increase the amount (Table 3) at the surface; this indicates that with a simple mixing synthesis method it was possible to attach enough GQD to ZnO. This peak oscillates in the 284-286 eV range, where C=C from sp2 hybridization of GQD, besides C-C bond, might be overlapping (Fig. 7d); moreover, this carbon can be rich in oxygen which are presented as shoulders in C1s as -COOH and C-OH [10], [48], [49], [55]. Alike, it can be observed a lightly shift to higher BE in Zc(s) and ZcDl-3(s), owing to this last, possess more carbonates species because the hydrozincite phase. Therefore, in ZdDl-3(s) the carbon amount is higher due to contamination as it was demonstrated by XRD and SEM analysis.
Finally, the nitrogen detected by XPS measurements confirms the presence of GQD at the ZnO surface (Fig. S14). Due to a low signal-to-noise ratio the amount of nitrogen at the ZnO surface was too difficult to estimate. However, the atomic amount detected by the XPS equipment was around 0.28% at the surface for both samples, demonstrating a properly functionalization by amine groups.
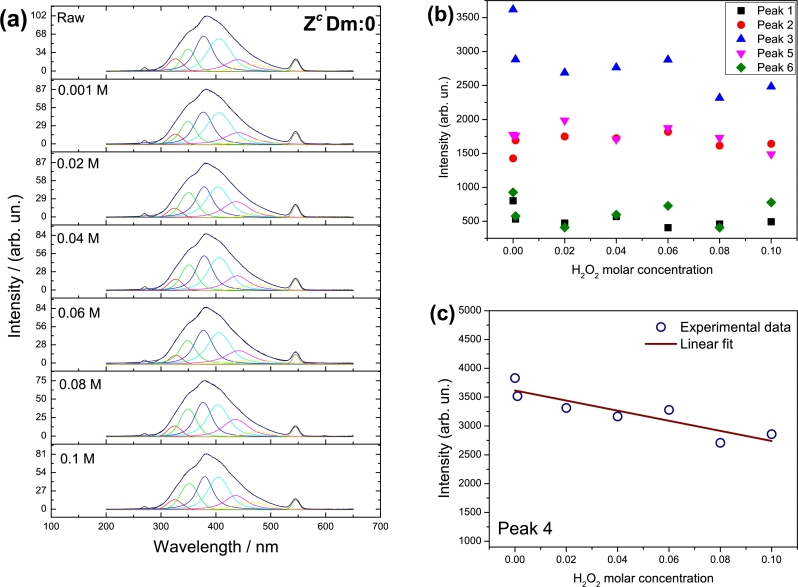
3.5. Analysis of H2O2 detection by photoluminescence
As it was determined in section 3.2, sample ZcDm-3 possess a steady performance in almost all peaks. For this reason, it was elected to prove the H2O2 effect in the PL, by varying its concentration. Fig. 8a, presents the effect of H2O2 in sample ZcDm:0 on the PL emission. In this experiment, a variation in H2O2 concentration was achieved and if ZcDm:0 and Zc(l) are compared (Fig. 8a with Fig. 2) it can be noted that ZcDm:0 presents negligible variation in PL spectra. GQD might be protecting the ZnO structure and for this reason a similar shape in spectra is attained even though the H2O2 concentration is increasing (Fig. 8a). Spectra from ZcDm:0 can be decomposed in six peaks which correspond to the same peaks found in the description from section 3.2. The behaviour of these peaks is shown in Fig. 8b. In this figure peaks 1 and 6, which are respectively assigned to an ORE and to oxygen vacancies, display the lowest PL intensities. Notwithstanding, the PL emission from these sites, is not strongly perturbed by H2O2 concentration; also, as it was determined in peak at 478 nm (G) from section 3.1, the sites for peak 6 seem to be chemically stable to H2O2. As in Fig. 3a and Fig. 3f, almost a steady performance is achieved, this means that these sites are not modified neither with time nor H2O2 concentration. Peak 2 is related with hydrogen at interstitials from ZnO structure. In this case, increasing the H2O2 concentration might also rise the proton generation; this situation would increase its diffusion into ZnO and should be a cause to increase the emission intensity. In fact, despite that this occurs, the growth of intensity is not as great as it was expected. This might be due to some of the H2O2 are already interacting with GQD placed at the sites of ZnO related to peak 4, which produce the quenching effect. Peak 5 is revealed when the calcination is done. Thus, vacancies generated by this process probably are near of the interstitials-hydrogen sites, but far enough of the ZnO surface; therefore, the vacancies are also chemically conserved as is observed that peak 5 in Fig. 8b behaves almost constant when H2O2 concentration increases. For peak 3 the H2O2 effect is more evident as when its concentration increases, emission intensity decreases. However, for concentrations of 0.001 M to 0.06 M in peak 3, the intensity is steady to decrease later again. This performance might be because of the ORE near the surface, where the oxygen provided mostly by H2O2, creates a new interaction with GQD by attracting amine groups instead of carboxyls. In this sense, a new chemical environment is produced where GQD are strongly bonded with ZnO surface and loss part of PL properties. In peak 4 sample ZcDm-3 has a steady performance and for this reason, it was elected to prove the H2O2 effect in the PL, by varying its concentration. Immediately is noted the difference among the interaction of H2O2 with this solid when the oxidant concentration is varied and when time is passing. With time, emission remains steady, denoting that is chemically not modified; on the contrary, when concentration of H2O2 varied, a diminishing in PL emission is noted in Fig. 8c. The first point, which is the PL emission when only ZcDm:0 and water is measured (raw) is far from the fitting line and from the next three points; notwithstanding, those following three points, along with the sixth measurement, fit well to a linear equation. The first and fifth point are not adjusted to the line probably due to a failure in the measurements. The last point, which was measured using a H2O2 concentration of 0.1 M, is well measured, however, might be the limit where a good quenching effect is achieved. In this sense, when H2O2 exceeds in concentration, a chemical modification in nanocomposite interferes with the analysis making it non-confident.
Figure 8.
(a) PL spectra from ZcDm:0 evaluated at λex = 275 nm; the first measurement (raw) was achieved without H2O2 and in posterior measurements consisted in varying the H2O2 concentration; the six identified peaks, correspond to description in section 3.2 (b) intensities from peaks displayed in (a) and (c) peak 4 experimental intensities (∘) and linear fit (wine line) which correspond to equation I = −8762.2c + 3616.2 with R2 = 0.80, where I is the intensity of PL emission and c is the H2O2 concentration.
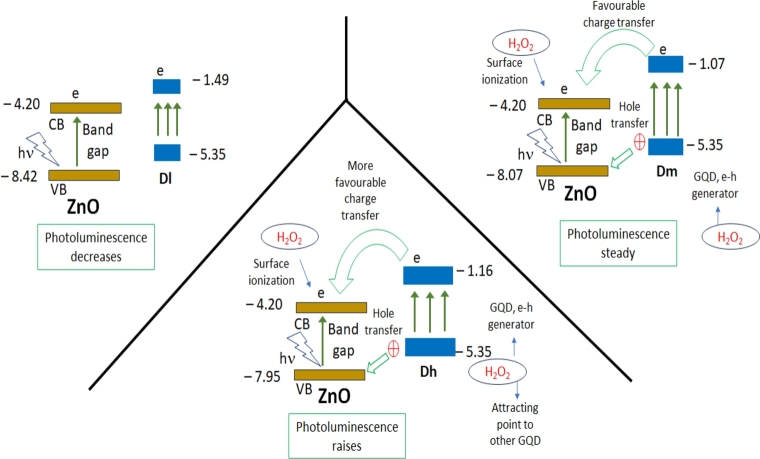
3.6. Interaction of ZnO and GQD by variations in band gap
Analysing the band gap from 6b and 6c insets, it is possible to conceive how the interaction among ZnO and GQD is taking place; this is represented in Fig. 9. Setting the conduction (CB) band of this solid with a value of - 4.20 eV [13], a value of the valence band (VB) of - 7.77 eV it is found. From the CB of ZnO we found a VB value of - 8.15 eV for Zc-3 which is ascribed to the generation of vacancies by H2O2 and to the diminishing of particle size. Also, by setting -5.35 eV the value for GQD [56] of the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO) values are found to be - 1.49, - 1.07 and - 1.16 eV for Dl, Dm and Dh, respectively (Fig. 9). Because the band gap of ZnO might change during experiment, in ZcDl-3 the VB is -8.42 eV. This value is lower than Zc-3 for which PL emission decreased. Moreover, charges produced in Dl are not energetically enough to be transferred to ZnO. In ZcDh-3 the ZnO band gap is the lowest (3.75 eV) in nanocomposites, and GQD might be contributing to increase the e-h recombination by transferring electrons to the CB of ZnO. From this perspective the highest LUMO value of Dm leads to conserve the e-h recombination for the nanocomposite ZcDm-3. Thus, in this process a compensation of electron loss by vacancies or non-radiative phenomena in ZnO is achieved with medium GQD concentration. Because of the electrostatic attraction between H2O2 (Fig. S3, scheme 3) PL intensity increases for ZcDh-3 displayed in Fig. 3d. Owing to sample Dl has the lower LUMO value in ZcDl-3, electrons of this sample might be only suffering an internal electronic transition. Besides that, vacancies in ZnO provoked by oxidation with H2O2 (in an ORE), inhibit the e-h recombination and thus PL emission is lost similarly as the Zc-3 process showed in Fig. 3d. Although from Fig. S4a for Dh-3, GQD might be not disturbed by H2O2 an interaction among these compounds exists. This is because neither for ZcDm-3 nor ZcDh-3 a decreasing in PL emission is presented as in Zc-3. In this manner, for Dm in ZcDm-3, e-h recombination is given in a similar form as in ZcDh-3; it is important to note that these recombinations are only favoured when the H2O2 concentration is maintained constant. In this respect GQD might also prevent the ZnO photoxidation which is associated with a strong bond between GQD and ZnO [53]. In literature, similar ZnO-GQD materials have been investigated and it has been found that charge is transferring from GQD to ZnO with a posterior e-h separation increasing the nanocomposite conductivity [12], [18], [19], [20], [22]. In our work is evident that this is not occurring due to e-h recombination has been enhanced in ZcDh-3 and it is conserved in ZcDm-3; we ascribe this to the small crystal size of ZnO. In these literature investigations the particle size ranges from 50 up to more than 1 μm. Besides the particle reduction, H2O2 exposure, provokes an anisotropic particle, which increases active sites where GQD are interacting [54]. These active sites are those related with peak 4 where GQD is interacting. Thus, we ascribed the enhancement of PL emission due to the small ZnO particle size.
Figure 9.
Representation of the possible ZnO and GQD band gaps position. As the band gap from GQD is higher, PL intensity increases favouring e-h recombinations in ZnO. Values of VB, CB, HOMO and LUMO are in eV.
During nanocomposites synthesis GQD are bonded principally by carboxyl groups to ZnO surface, as it has been found in others works [52], [53]. A DSC analysis was achieved in ZcDl-3 sample showing an exothermic peak at 367 °C (Fig. S6c). Due to zinc carbonates and nitrates show a decomposition peak staring from 290 °C up to 350 °C we conclude that this peak must be from GQD carboxyl groups chemically bonded to Zn [53], [57], [58]. Moreover, when these groups are placed at the ZnO surface, it is not discarded that more GQD might be stacked producing layers. Therefore water, but principally H2O2 molecules, are attracted to GQD. In this investigation, HOO⋅ radical from H2O2 is adsorbed on the ZnO surface decreasing the band gap in ZcDh-3 and in ZcDm-3, which is attributed to the fact that the ZnO was not attacked by H2O2 as in Zc-3. However, since the emission intensity increases with time for ZcDh-3 compared to ZDh:0, then H2O2 increases the number of recombination in ZcDh-3 and in a lesser extent in ZcDm-3. This is similar to other ZnO-GQD systems, where it has been found that an adsorbate (oxygen) at the ZnO surface, scarves free electrons from its surface [12], [13], [18], [20]. However, in these works, later the adsorbate and electrons are released, when a hole produced by light excitation reach the ZnO surface. These e-h recombination in our work, must occur in the ZnO and not in GQD, otherwise an increase in the emission intensity would be seen in Dh-3 and this is not the case (Fig. S4a). Therefore, H2O2 must be interacting at the ZnO – GQD interface in such a way, that when the H2O2 arrives, peroxide ion produced and attracted at the ZnO surface while hydronium ion remains on GQD. The peroxide attracts or could even split another small particle from the GQD, which allows the addition of a new GQD particle that would participate in the reaction. Because this occurs mostly in ZcDh-3 and less in ZcDm-3 this last is the more indicated to have a steady performance for the H2O2 sensing. Is important to note that although in our results GQD transfer charge to ZnO, photocurrent is not produced due to these charges recombine again. Holes from GQD must be also being transferring to VB of ZnO contributing to the enhancement of e-h recombination. Because H2O2might be decomposed into OH⋅ and OH−, they must be also aiding to recover electron and hole in GQD [46].
Once the H2O2 concentration increases, a quenching effect is produced (Fig. 8c) due to an excess of charges at the ZnO surface. This is an excess of peroxide, increasing electronic density. Thus, in the process of quenching for ZcDm-3 a proper GQD amount is protecting ZnO but when H2O2 concentration rises, ZnO surface is surrounded by more H+ and HOO⋅. In addition, the alteration might be a layer depletion of the band energy from the ZnO surface, resulting when molecules as oxygen are adsorbed at the surface which provokes a facile movement from the holes to the surface enhancing the e-h nonradiative recombination [25].
4. Conclusions
In this study, an investigation of the interaction of ZnO and GQD was achieved by means of photoluminescence (PL) measurements. GQD interacted by carboxyls chemically bonded to Zn. This interaction was a function of GQD concentration and depended on the ZnO vacancies. Vacancies were created principally by calcination and slightly by the H2O2 exposure when produced an oxygen rich environment (ORE) at the ZnO surface. This ORE altered GQD environment depending on its concentration in nanocomposite. In low GQD, an ORE oxidized the ZnO surface weakening interactions of GQD-ZnO which decreased the PL emission. GQD in high concentration remained attached to ZnO surface and H2O2 only aided to more GQD particles reach ZnO increasing the PL emission. H2O2 diminished the ZnO-GQD particle size creating more vacancies rising interactions between ZnO and GQD. In this respect, GQD might prefer oval-shapes ZnO particles as was deduced by SEM. Only medium GQD concentration displayed a stable PL emission during 170 min. GQD might be placed chemically at ZnO surface vacancies, where exciton transitions occurred around 400 nm; here, amine groups contained in GQD, passivated nonradiative traps. The growth of the GQD band gap, enhanced the charge transfer to ZnO increasing the PL emission intensity. Raising the H2O2 concentration conduced to an inhibition of the charge transfer produced by the depletion in layer of the band energy from the ZnO surface.
Declaration of generative AI and AI-assisted technologies in the writing process
Authors declare that no generative artificial intelligence was used in this manuscript.
CRediT authorship contribution statement
Rolando Efraín Ramírez Garza: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sara Luisa Rodríguez de Luna: Writing – review & editing, Methodology, Investigation, Conceptualization. Idalia Gómez: Supervision, Project administration, Investigation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to acknowledge the financial support from CONAHCYT (grant no. I1200/320/2022) and also from the UANL through the PROACTI (31-BQ-2023) program. We also thank to Yolanda Peña for her support with XRD and XPS measurements, Hugo Salas for SEM images and Edgar García for DSC measurements.
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31144.
Appendix A. Supplementary material
The following is the Supplementary material related to this article.
Supplementary information file contains schemes of surface nanocomposites, PL with peaks deconvolution of samples, estimation of GQD and nanocomposites particle size by AFM and XRD, respectively and DSC to confirm chemical changes in some nanocomposites. Also contains SEM and EDS to confirm changes in morphology and XPS to confirm detection of oxygen, carbon, zinc and nitrogen at the nanocomposites surface.
Data availability
Data will be fully available on request.
References
- 1.Hosny N.M., Gomaa I., Elmahgary M.G., Ibrahim M.A. ZnO doped C: facile synthesis, characterization and photocatalytic degradation of dyes. Sci. Rep. Aug. 2023;13(1) doi: 10.1038/s41598-023-41106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain I., Singh N.B., Singh A., Singh H., Singh S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2015;38(4):545–560. doi: 10.1007/s10529-015-2026-7. [DOI] [PubMed] [Google Scholar]
- 3.Fathima N., Pradeep N., Balakrishnan J. Green synthesis of graphene quantum dots and the dual application of graphene quantum dots-decorated flexible MSM p-type ZnO device as UV photodetector and piezotronic generator. Bull. Mater. Sci. Feb. 2021;44(1) doi: 10.1007/s12034-020-02326-w. [DOI] [Google Scholar]
- 4.Gurylev V., Perng T.P. Defect engineering of ZnO: review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021;41(10):4977–4996. doi: 10.1016/j.jeurceramsoc.2021.03.031. [DOI] [Google Scholar]
- 5.Khan F., Akhtar N., Jalal N., Hussain I., Szmigielski R., Hayat M.Q., Ahmad H.B., El-Said W.A., Yang M., Janjua H.A. Carbon-dot wrapped zno nanoparticle-based photoelectrochemical sensor for selective monitoring of h2o2 released from cancer cells. Mikrochim. Acta. Jan. 2019;186(2) doi: 10.1007/s00604-019-3227-x. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y.-J., Tsai C.-L., Lu Y.-M., Liu C.-J. Optical and electrical properties of undoped ZnO films. J. Appl. Phys. May 2006;99(9) doi: 10.1063/1.2193649. [DOI] [Google Scholar]
- 7.Mursal, Irhamni, Bukhari, Jalil Z. Structural and optical properties of zinc oxide (ZnO) based thin films deposited by sol-gel spin coating method. J. Phys. Conf. Ser. 2018;1116 doi: 10.1088/1742-6596/1116/3/032020. [DOI] [Google Scholar]
- 8.Li G.R., Hu T., Pan G.L., Yan T.Y., Gao X.P., Zhu H.Y. Morphology-function relationship of ZnO: polar planes, oxygen vacancies, and activity. J. Phys. Chem. C. 2008;112(31):11859–11864. doi: 10.1021/jp8038626. [DOI] [Google Scholar]
- 9.Grabowska J., Meaney A., Nanda K.K., Mosnier J.-P., Henry M.O., Duclère J.-R., McGlynn E. Surface excitonic emission and quenching effects in ZnO nanowire/nanowall systems: limiting effects on device potential. Phys. Rev. B. 2005;71(11) doi: 10.1103/physrevb.71.115439. [DOI] [Google Scholar]
- 10.Lin L.-Y., Kavadiya S., Karakocak B.B., Nie Y., Raliya R., Wang S.T., Berezin M.Y., Biswas P. ZnO/carbon dots composite hollow spheres: facile aerosol synthesis and superior CO2 photoreduction under UV, visible and near-infrared irradiation. Appl. Catal. B, Environ. 2018;230:36–48. doi: 10.1016/j.apcatb.2018.02.018. [DOI] [Google Scholar]
- 11.Barman M.K., Mitra P., Bera R., Das S., Pramanik A., Parta A. An efficient charge separation and photocurrent generation in the carbon dot–zinc oxide nanoparticle composite. Nanoscale. 2017;9(20):6791–6799. doi: 10.1039/c7nr01663h. [DOI] [PubMed] [Google Scholar]
- 12.Liu D., Li H.-J., Gao J., Zhao S., Zhu Y., Wang P., Wang D., Chen A., Wang X., Yang J. High-performance ultraviolet photodetector based on graphene quantum dots decorated zno nanorods/gan film isotype heterojunctions. Nanoscale Res. Lett. Aug. 2018;13(1) doi: 10.1186/s11671-018-2672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahimi K., Yazdani A., Ahmadirad M. Graphene quantum dots enhance UV photoresponsivity and surface-related sensing speed of zinc oxide nanorod thin films. Mater. Des. 2018;140:222–230. doi: 10.1016/j.matdes.2017.12.010. [DOI] [Google Scholar]
- 14.Hmar J.J.L., Majumder T., Dhar S., Mondal S.P. Sulfur and nitrogen co-doped graphene quantum dot decorated zno nanorod/polymer hybrid flexible device for photosensing applications. Thin Solid Films. 2016;612:274–283. doi: 10.1016/j.tsf.2016.06.014. [DOI] [Google Scholar]
- 15.Wang S., Zheng M., Jiang D., Yuan H., Chen H., Fan Y., Li F., Zhang W., Ma L., Shen W. Graphene quantum dot-sensitized gap@zno nanocomposite for high-performance uv photodetectors. J. Phys. D, Appl. Phys. 2022;55(39) doi: 10.1088/1361-6463/ac7fc8. [DOI] [Google Scholar]
- 16.Kathalingam A., Salman Ajmal H.M., Vikraman D., Kim S.-D., Park H.-C., Kim H.-S. Graphene quantum dots-wrapped vertically aligned zinc oxide nanorods arrays for photosensing application. J. Alloys Compd. 2021;853 doi: 10.1016/j.jallcom.2020.157025. [DOI] [Google Scholar]
- 17.Hoang Tran M., Park T., Hur J. Solution-processed zno: graphene quantum dot/poly-tpd heterojunction for high-performance uv photodetectors. Appl. Surf. Sci. 2021;539 doi: 10.1016/j.apsusc.2020.148222. [DOI] [Google Scholar]
- 18.Yang B., Chen J., Cui L., Liu W. Enhanced photocurrent of a zno nanorod array sensitized with graphene quantum dots. RSC Adv. 2015;5(73):59204–59207. doi: 10.1039/c5ra07836a. [DOI] [Google Scholar]
- 19.Kumar S., Dhiman A., Sudhagar P., Krishnan V. ZnO-graphene quantum dots heterojunctions for natural sunlight-driven photocatalytic environmental remediation. Appl. Surf. Sci. 2018;447:802–815. doi: 10.1016/j.apsusc.2018.04.045. [DOI] [Google Scholar]
- 20.Wongrat E., Nuengnit T., Panyathip R., Chanlek N., Hongsith N., Choopun S. Highly selective room temperature ammonia sensors based on ZnO nanostructures decorated with graphene quantum dots (GQDs) Sens. Actuators B, Chem. 2021;326 doi: 10.1016/j.snb.2020.128983. [DOI] [Google Scholar]
- 21.Roza L., Fauzia V., Rahman M., Isnaeni I., Putro P. ZnO nanorods decorated with carbon nanodots and its metal doping as efficient photocatalyst for degradation of methyl blue solution. Opt. Mater. 2020;109 doi: 10.1016/j.optmat.2020.110360. [DOI] [Google Scholar]
- 22.Efa M.T., Imae T. Hybridization of carbon-dots with ZnO nanoparticles of different sizes. J. Taiwan Inst. Chem. Eng. 2018;92:112–117. doi: 10.1016/j.jtice.2018.02.007. [DOI] [Google Scholar]
- 23.Zhang Z., Liu H., Zhai L., Wu J., Li L. Construction of BiOCl-TNTs photoelectrochemical sensor for detection of hydrogen peroxide. Chem. Phys. Lett. 2023;811 doi: 10.1016/j.cplett.2022.140177. [DOI] [Google Scholar]
- 24.Garza R.E.R., de Luna S.L.R., Padrón G.H., de la Fuente I.G. A “turn-off” photoluminescent H2O2 detection based on a zinc oxide–graphene quantum dot (ZnO–GQD) nanocomposite and the role of amine in the development of GQD. RSC Adv. 2023;13(32):21808–21819. doi: 10.1039/d3ra02355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao Z.-M., Zhang H.-Z., Zhou Y.-B., Xu J., Zhang J.-M., Yu D.-P. Surface effects on photoluminescence of single ZnO nanowires. Phys. Lett. A. 2008;372(24):4505–4509. doi: 10.1016/j.physleta.2008.04.013. [DOI] [Google Scholar]
- 26.Eliel M.M.J., Francisco P.D., Duarte M.J., Guillermo H.P., Nicolaza P. Structure and optical properties of ZnO and ZnO2 nanoparticles. J. Nano Res. 2019;56:49–62. doi: 10.4028/www.scientific.net/jnanor.56.49. [DOI] [Google Scholar]
- 27.Alvarado-Pérez V., Cabrera-Lara L.I., López-Téllez G., Mendoza-Anaya D., Hernández-López S., Camacho-López M. ZnO to ZnO2 transformation assisted by H2O2 at ambient conditions. Mater. Chem. Phys. 2019;233:180–184. doi: 10.1016/j.matchemphys.2019.05.066. [DOI] [Google Scholar]
- 28.Shin Y., Kim M., Oh J., Han M., Kim S., Chung K. Hydrogenation and annealing effects in n-type ZnO bulk samples. J. Korean Phys. Soc. 2008;53(9(5)):2504–2507. doi: 10.3938/jkps.53.2504. [DOI] [Google Scholar]
- 29.Balbuena P.B., Calvo S.R., Lamas E.J., Salazar P.F., Seminario J.M. Adsorption and dissociation of H2O2 on Pt and Pt −alloy clusters and surfaces. J. Phys. Chem. B. 2006;110(35):17452–17459. doi: 10.1021/jp063027z. [DOI] [PubMed] [Google Scholar]
- 30.Pavesi L., Martelli F., Martin D., Reinhart F.K. Photoluminescence enhancement in post-growth hydrogenated GaAlxAs (0≤x≤0.32) and GaAs/GaAlAs multilayer structures. Appl. Phys. Lett. 1989;54(16):1522–1524. doi: 10.1063/1.101339. [DOI] [Google Scholar]
- 31.Liao Z.-M., Liu K.-J., Zhang J.-M., Xu J., Yu D.-P. Effect of surface states on electron transport in individual ZnO nanowires. Phys. Lett. A. 2007;367(3):207–210. doi: 10.1016/j.physleta.2007.03.006. [DOI] [Google Scholar]
- 32.Erhart P., Albe K., Klein A. First-principles study of intrinsic point defects in ZnO: role of band structure, volume relaxation, and finite-size effects. Phys. Rev. B. 2006;73(20) doi: 10.1103/physrevb.73.205203. [DOI] [Google Scholar]
- 33.Wu J., Long F., Tang B., Tang X. Electronic structure and ferromagnetic properties of Zn vacancies in ZnO screw dislocations: first-principles calculations. AIP Adv. Jun. 2018;8(6) doi: 10.1063/1.5034501. [DOI] [Google Scholar]
- 34.Ye J., Gu S., Qin F., Zhu S., Liu S., Zhou X., Liu W., Hu L., Zhang R., Shi Y., Zheng Y. Correlation between green luminescence and morphology evolution of ZnO films. Appl. Phys. A. 2005;81(4):759–762. doi: 10.1007/s00339-004-2996-0. [DOI] [Google Scholar]
- 35.Wang J., Wang Z., Huang B., Ma Y., Liu Y., Qin X., Zhang X., Dai Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces. 2012;4(8):4024–4030. doi: 10.1021/am300835p. [DOI] [PubMed] [Google Scholar]
- 36.Wang M., Jiang L., Kim E.J., Hahn S.H. Electronic structure and optical properties of Zn(OH)2: LDA+U calculations and intense yellow luminescence. RSC Adv. 2015;5(106):87496–87503. doi: 10.1039/c5ra17024a. [DOI] [Google Scholar]
- 37.Muhammad W., Ullah N., Haroon M., Abbasi B.H. Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 2019;9(51):29541–29548. doi: 10.1039/c9ra04424h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordeeva A., Hsu Y.-J., Jenei I.Z., Brant Carvalho P.H.B., Simak S.I., Andersson O., Häussermann U. Layered zinc hydroxide dihydrate, Zn5(OH)10⋅2H2O, from hydrothermal conversion of ϵ-Zn(OH)2 at gigapascal pressures and its transformation to nanocrystalline ZnO. ACS Omega. 2020;5(28):17617–17627. doi: 10.1021/acsomega.0c02075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X., Hui K., Bin F., Hui K., Li L., Cho Y., Mane R.S., Zhou W. Effect of thermal annealing on the structural, electrical and optical properties of Al–Ni co-doped ZnO thin films prepared using a sol-gel method. Surf. Coat. Technol. 2015;261:149–155. doi: 10.1016/j.surfcoat.2014.11.043. [DOI] [Google Scholar]
- 40.Liu J., Gao F., Wu L., Zhang H., Hong W., Jin G., Zhai Z., Fu C. Size effect on oxygen vacancy formation and gaseous adsorption in ZnO nanocrystallites for gas sensors: a first principle calculation study. Appl. Phys. A. 2020;126(6):1–9. doi: 10.1007/s00339-020-03643-x. [DOI] [Google Scholar]
- 41.Ali S., Morsy R., El-Zawawy N., Fareed M., Bedaiwy M. Synthesized zinc peroxide nanoparticles (ZnO2; -NPs): a novel antimicrobial, anti-elastase, anti-keratinase, and anti-inflammatory approach toward polymicrobial burn wounds. Int. J. Nanomed. 2017;12:6059–6073. doi: 10.2147/ijn.s141201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andelman T., Gong Y., Polking M., Yin M., Kuskovsky I., Neumark G., O'Brien S. Morphological control and photoluminescence of zinc oxide nanocrystals. J. Phys. Chem. B. 2005;109(30):14314–14318. doi: 10.1021/jp050540o. [DOI] [PubMed] [Google Scholar]
- 43.Winiarski J., Tylus W., Winiarska K., Szczygieł I., Szczygieł B. XPS and FT-IR characterization of selected synthetic corrosion products of zinc expected in neutral environment containing chloride ions. J. Spectrosc. 2018;2018:1–14. doi: 10.1155/2018/2079278. [DOI] [Google Scholar]
- 44.Srivastava O.K., Secco E.A. Studies on metal hydroxy compounds. II. Infrared spectra of zinc derivatives ϵ-Zn(OH)2, β-ZnOHCl, ZnOHF, Zn5(OH)8Cl2, and Zn5(OH)8Cl2⋅H2O. Can. J. Chem. 1967;45(6):585–588. doi: 10.1139/v67-097. [DOI] [Google Scholar]
- 45.Rivas B.L., Seguel G.V., Ancatripai C. Polymer-metal complexes: synthesis, characterization, and properties of poly(maleic acid) metal complexes with Cu(II), Co(II), Ni(II), and Zn(II) Polym. Bull. 2000;44(5–6):445–452. doi: 10.1007/s002890070064. [DOI] [Google Scholar]
- 46.Phophayu S., Pimpang P., Wongrerkdee S., Sujinnapram S., Wongrerkdee S. Modified graphene quantum dots-zinc oxide nanocomposites for photocatalytic degradation of organic dyes and commercial herbicide. J. Reinf. Plast. Compos. 2019;39(3–4):81–94. doi: 10.1177/0731684419891245. [DOI] [Google Scholar]
- 47.Pavia D.L. Harcourt College Publishers; 2001. Introduction to Spectroscopy. [Google Scholar]
- 48.Hwang E., Hwang H.M., Shin Y., Yoon Y., Lee H., Yang J., Bak S., Lee H. Chemically modulated graphene quantum dot for tuning the photoluminescence as novel sensory probe. Sci. Rep. 2016;6(1):1–10. doi: 10.1038/srep39448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santiago S.R.M., Lin T.N., Chang C.H., Wong Y.A., Lin C.A.J., Yuan C.T., Shen J.L. Synthesis of n-doped graphene quantum dots by pulsed laser ablation with diethylenetriamine (deta) and their photoluminescence. Phys. Chem. Chem. Phys. 2017;19(33):22395–22400. doi: 10.1039/c7cp03993j. [DOI] [PubMed] [Google Scholar]
- 50.Kang H., Kim D.Y., Cho J. Top-down fabrication of luminescent graphene quantum dots using self-assembled Au nanoparticles. ACS Omega. 2023;8(6):5885–5892. doi: 10.1021/acsomega.2c07683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talam S., Karumuri S.R., Gunnam N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. ISRN Nanotechnol. 2012;2012:1–6. doi: 10.5402/2012/372505. [DOI] [Google Scholar]
- 52.Vempati S., Celebioglu A., Uyar T. Defect related emission versus intersystem crossing: blue emitting ZnO/graphene oxide quantum dots. Nanoscale. 2015;7(38):16110–16118. doi: 10.1039/c5nr04461h. [DOI] [PubMed] [Google Scholar]
- 53.Tayyebi A., outokesh M., Tayebi M., Shafikhani A., Şengör S.S. ZnO quantum dots-graphene composites: formation mechanism and enhanced photocatalytic activity for degradation of methyl orange dye. J. Alloys Compd. 2016;663:738–749. doi: 10.1016/j.jallcom.2015.12.169. [DOI] [Google Scholar]
- 54.V. L.P., Rajagopalan V. A new synergetic nanocomposite for dye degradation in dark and light. Sci. Rep. Dec. 2016;6(1) doi: 10.1038/srep38606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh D., Shin S., Lim C., Hwang D. Dopamine-mediated sclerotization of regenerated chitin in ionic liquid. Materials. 2013;6(9):3826–3839. doi: 10.3390/ma6093826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shang Y.D., Chen X.H., Ma W.H., Li S.Y., Wang Y.C., Xiang F.W. Preparation and optical properties research on graphene quantum dots. Key Eng. Mater. 2017;727:303–308. doi: 10.4028/www.scientific.net/kem.727.303. [DOI] [Google Scholar]
- 57.Pudukudy M., Yaakob Z., Rajendran R., Kandaramath T. Photodegradation of methylene blue over novel 3D ZnO microflowers with hexagonal pyramid-like petals. React. Kinet. Mech. Catal. 2014;112:527–542. doi: 10.1007/s11144-014-0703-5. [DOI] [Google Scholar]
- 58.Levine K.E., Collins B.J., Stout M.D., Wyde M., Afton S.E., Essader A.S., Ennis T.J., Amato K.E., McWilliams A.C., Fletcher B.L., Fernando R.A., Harrington J.M., Catlin N., Robinson V.G., Waidyanatha S. Characterization of Zinc Carbonate Basic as a Source of Zinc in a Rodent Study Investigating the Effects of Dietary Deficiency or Excess. Anal. Lett. 2017;50:2447–2464. doi: 10.1080/00032719.2017.1293073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information file contains schemes of surface nanocomposites, PL with peaks deconvolution of samples, estimation of GQD and nanocomposites particle size by AFM and XRD, respectively and DSC to confirm chemical changes in some nanocomposites. Also contains SEM and EDS to confirm changes in morphology and XPS to confirm detection of oxygen, carbon, zinc and nitrogen at the nanocomposites surface.
Data Availability Statement
Data will be fully available on request.