Abstract
The mechanism by which murine polyomavirus penetrates cells and arrives at the nucleus, the site of viral replication, is not well understood. Simian virus 40 and JC virus, two closely related members of the polyomavirus subfamily, use caveola- and clathrin-mediated uptake pathways for entry, respectively. The data presented here indicate that compounds that block endocytosis of both caveola- and clathrin-derived vesicles have no effect on polyomavirus infectivity. Polyomavirus does not appear to colocalize with either clathrin light chain or caveolin-1 by immunofluorescence microscopy. Additionally, expression of a dominant-negative form of dynamin I has no effect on polyomavirus uptake and infectivity. Therefore, polyomavirus uptake occurs through a class of uncoated vesicles in a clathrin-, caveolin-1-, and dynamin I-independent manner.
Polyomavirus is a nonenveloped DNA tumor virus that efficiently transforms cells in culture and induces tumors in a wide variety of tissues in the mouse (11). A great deal is known about the molecular biology of virus replication and cell transformation (7), but the events leading to virus internalization, routing to the nucleus, and virus disassembly remain poorly understood. In its natural host, polyomavirus infects more than 30 distinct cells types (11). This wide host range depends, in part, on the ability of the virus to bind nonselectively to cell surface glycoproteins with terminal α-2,3-linked sialic acid, which are broadly and abundantly expressed in the mouse (5, 6). The virus is composed of 360 copies of the major capsid protein VP1, which binds to cell surface sialyloligosaccharides. Discrimination between different sialic acid residues determines the ability of different polyomavirus strains to successfully spread in the animal (5). To date, specific, cell surface sialic acid-containing protein receptors have not been identified (5).
Early electron microscopic (EM) entry studies on polyomavirus and the related virus simian virus 40 (SV40) demonstrated that shortly after uptake, the majority of the virus was seen as single particles in small, monopinocytic vesicles (∼50 to 80 nm in diameter) that lack an apparent protein coat (19, 23, 25). Virus was also seen as multiple particles in larger vesicles variously described as phagocytic vesicles, endocytotic vesicles, and, after longer incubation, tubular membrane-bounded structures (13, 19, 20, 23, 25, 26). The exact nature of the vesicles was not determined, but immunolocalization indicated that the tubular membrane-bounded compartments containing SV40 were endoplasmic reticulum (ER) derived (20).
More recent studies on the entry pathway of SV40 have shown that the virus first binds to major histocompatibility complex class I molecules and is then localized to specialized cell surface domains called caveolae (37). SV40 is then taken up into caveola-derived vesicles as the first step in entry (2). Caveolae constitute specialized membrane domains composed of unique lipids, mainly sphingolipids and cholesterol, and of caveolins, the major protein component which binds to cholesterol. Caveolae form a large, dynamic membranous system that is important not only for transcytosis and potocytosis but also for signal transduction (reviewed in references 3 and 36). Once SV40 is taken up into cells, the SV40-containing vesicles may enter ER-derived tubules, as described by Kartenbeck et al. (20), as part of the potocytotic pathway between the ER and the plasma membrane via caveolae (3). Whether polyomavirus is taken up into caveola-derived vesicles and targeted to the ER in a fashion similar to that of SV40 has not been determined.
Interestingly, a recent study on another polyomavirus, the human JC virus, demonstrated that entry of this virus into human glial cells is disrupted by treatment with chlorpromazine (32). This indicates that JC virus requires a functional clathrin-coated pit endocytic pathway for successful uptake. With the obvious differences between the internalization pathways of these two related viruses, SV40 and JC virus, it is not apparent what pathway polyomavirus uses to enter cells. We have employed a combination of pharmacological and biochemical approaches to begin to dissect the early steps in the entry of polyomavirus into cells.
MATERIALS AND METHODS
Cells and viruses.
Primary baby mouse kidney (BMK) cells were prepared and used 3 to 4 days after culturing. NIH 3T3 cells were purchased from the American Type Culture Collection. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Company, St. Louis, Mo.) containing 4.5 g of glucose per liter, 10% heat-inactivated calf serum (CS; Life Technologies, Gaithersburg, Md.), 100 IU of penicillin per ml, and 100 IU of streptomycin (Life Technologies) per ml in a 5% CO2 humidified incubator at 37°C. Plasmids containing the cDNA for either hemagglutinin (HA)-tagged wild-type dynamin I, or HA-tagged dominant-negative dynamin I (K44A), under the control of a tetracycline-responsive element, were a gift of S. Schmid (Scripps Research Institute, La Jolla, Calif.). The pTEToff vector was purchased from Clontech (Palo Alto, Calif.). A tetracycline-responsive, stable NIH 3T3 cell line was established with this vector in accordance with the manufacturer's instructions. Cells were then transfected with either the wild-type or mutant plasmid and cotransfected with a plasmid encoding resistance to puromycin (pBABE). Puromycin-resistant clones were selected and examined for dynamin expression 48 h after removal of tetracycline from the medium by indirect immunofluorescence assay (IFA) against the HA antigen as described below.
The RA strain of polyomavirus (small-plaque strain) used in this study was propagated on BMK cells. For infectivity studies, a crude viral lysate prepared by freeze-thawing and centrifugation of cellular debris was used. For preparation of purified virus, CsCl equilibrium centrifugation, done as previously described (9, 27), was employed. Protein concentration was determined by the MicroBCA assay (Pierce Chemical Company, Rockford, Ill.) and by measuring the ratio of optical densities at 280 and 260 nm. Virus was titer was determined by plaque assay.
Purified polyomavirus was labeled using a FluoReporter Oregon Green 488 Protein Labeling Kit (Molecular Probes, Eugene, Oreg.) in accordance with the manufacturer's instructions. Oregon Green-labeled polyomavirus (OG-Py) was labeled with 208 fluorophores per virion as calculated in accordance with the manufacturer's instruction. The virus titer was examined by plaque assay and HA assay and was similar to that of the parental virus.
Antibodies and reagents.
Fluorescein isothiocyanate (FITC)-conjugated cholera toxin B subunit (FITC-Ctx), filipin, tetracycline, puromycin, and 4′,6′-diamidino-2-phenylindole (DAPI) were purchased from Sigma. Oregon Green-conjugated transferrin (OG-Tfrn) was purchased from Molecular Probes. Nystatin and chlorpromazine were purchased from Calbiochem (San Diego, Calif.). The rabbit polyclonal antibody to caveolin-1 was purchased from Transduction Laboratories (Lexington, Ky.). The mouse monoclonal antibody to the HA antigen (12CA5) was purchased from BAbCo (Berkeley, Calif.). The mouse monoclonal antibody against the light chain of clathrin was a gift of T. Kirchhausen (Harvard Medical School). Rabbit polyclonal antibodies to polyomavirus large T antigen (PyLTAg) and VP1 were generated within the laboratory. Oregon Green-conjugated goat anti-rabbit or anti-mouse immunoglobulin G and rhodamine-conjugated goat anti-rabbit or anti-mouse immunoglobulin G were purchased from Molecular Probes.
Indirect IFA.
After the desired incubation time, cells were fixed in 3.5% paraformaldehyde (PFA; Electron Microscopy Sciences, Ft. Washington, Pa.). Samples were permeabilized by treatment with either 0.1% Triton X-100 (Sigma) in phosphate-buffered saline containing 1% CS for examination of either caveolin-1 or clathrin or by incubation in ethanol-acetic acid (2:1) for examination of PyLTAg. Samples were incubated with the primary antibody in phosphate-buffered saline with 1% CS for 1 h at room temperature. Samples were incubated with either Oregon Green- or rhodamine-labeled secondary antibodies and DAPI and incubated for 1 h at room temperature. The washed coverslips were mounted with Moviol, sealed with nail polish, and examined by fluorescence microscopy using a Leica MSP60 DMLB microscope with a 100× Plan oil objective coupled to a Sony DCK-5000 camera. Images were then imported and prepared in Adobe Photoshop 5.5.
Infectivity assay.
For analysis of polyomavirus infectivity, cells were plated on 12-mm glass coverslips and grown to approximately 80% confluency at 37°C in a CO2 incubator. Cells were pretreated with various compounds, described below, as indicated. Stocks of polyomavirus (RA strain) whose titers had been determined by plaque assay were diluted in DMEM without bicarbonate (HCO3−) containing 2% CS and buffered with 10 mM HEPES (pH 5.4) or another appropriate buffer as indicated below. Cells and virus were incubated from 60 min to 3 h for BMK and NIH 3T3 cells, respectively, at 37°C in a CO2 incubator, and then virus was removed by aspiration. Extracellular virus was neutralized by the addition of anti-VP1 antibody A3 in DMEM with 2% CS, with or without the indicated compounds, and incubated for an additional 30 min at 37°C. Virus was allowed to replicate for 24 h at 37°C. Successful entry was assessed by nuclear expression of PyLTAg by IFA as described above. Data are presented as the percentage of the total nuclei counted that were PyLTAg positive. Duplicate samples were tested, and 500 nuclei were counted per sample.
To disrupt caveola-mediated uptake, cells were treated with the indicated concentrations of either nystatin or filipin for 1 h to 3 h at 37°C in a CO2 incubator prior to infection. To neutralize the endosomal pH, cells were pretreated with increasing concentrations of NH4Cl (1 to 25 mM) for 30 min at 37°C. Effective neutralization was determined by treatment of samples with acridine orange and fluorescence microscopic examination (4) prior to infection with virus. Cells were also pretreated with increasing amounts of chlorpromazine for 60 min at 37°C in a CO2 incubator. Virus was diluted in medium with or without chlorpromazine and incubated with cells for 60 min to 3 h at 37°C prior to neutralization of extracellular virus. To disrupt clathrin-mediated uptake by incubation in hypertonic medium, cells were incubated with medium alone or 0.45 M sucrose in DMEM for 10 min at 37°C in a CO2 incubator. Virus was diluted into either medium alone or sucrose-containing medium and allowed to infect cells at 37°C for 60 min to 3 h. Disruption of clathrin-mediated endocytosis by cytosol acidification was achieved by the protocol described by Sandvig et al. (34). Cells were pretreated with or without 50 mM NH4Cl for 30 min at 37°C and then incubated in amiloride-K+-containing buffer or buffer without amiloride for 30 min at 37°C in a CO2 incubator. Virus was diluted into either buffer alone or amiloride buffer and then incubated for 60 min to 3 h at 37°C, and then extracellular virus was neutralized.
Uptake assays.
To analyze the uptake of FITC-Ctx, cells on coverslips pretreated as described above for the infectivity studies were chilled on ice for 10 min and then incubated with 4 μg of toxin per ml on ice for 30 min. The cells were washed three times with cold medium and then incubated at 37°C in a CO2 incubator for 30 min. At the assay endpoint, samples were fixed in 3.5% PFA and then processed for IFA against caveolin-1 as described above. To analyze uptake of OG-Tfrn, cells that had been plated and grown to 80% confluency on 12-mm glass coverslips were preincubated in DMEM containing 1% defatted bovine serum albumin (DMEM-BSA; Sigma) for 1 h at 37°C in a CO2 incubator to deplete cells of extracellular transferrin. The agents filipin, nystatin, NH4Cl, and chlorpromazine were included in the DMEM-BSA as indicated. For cytosol acidification, the protocol was initiated on cells after transferrin depletion. Incubation with sucrose or NaCl was performed in DMEM-BSA for 10 min after the initial 1-h incubation for transferrin depletion. Cells were chilled on ice for 10 min, and then 40 μg of OG-Tfrn per ml in DMEM-BSA, in the presence or absence of the indicated compounds, was added, and the mixture was incubated on ice for 30 min. Cells were washed three times with cold medium and then incubated at 37°C in a CO2 incubator in DMEM-BSA, with or with compounds, for 30 min. The coverslips were then fixed in 3.5% PFA and analyzed for uptake and clathrin light-chain protein by IFA as described above.
Entry assay.
To assess entry of labeled polyomavirus into cells, OG-Py in DMEM–2% CS without HCO3− was added to prechilled cells. Virus was allowed to bind for 60 min at 4°C. Unbound virus was removed by aspiration, and cells were washed with cold DMEM–2% CS with HCO3− and then incubated at 37°C in a CO2 incubator. Samples were then fixed in 3.5% PFA and processed for IFA as described above at the indicated times.
RESULTS
Role of caveolae in polyomavirus internalization.
In order to dissect the primary steps in the pathway of polyomavirus entry into cells, we first examined the nature of the vesicles in which polyomavirus particles are taken up. Since the closely related virus SV40 employs caveolae for entry into cells, we wanted to determine whether caveolae play a role in polyomavirus entry as well. The sterol-binding compounds nystatin and filipin disrupt caveola function by selectively removing cholesterol from the membrane, resulting in caveolin-1 dissociation from caveolae (33). Nystatin treatment blocks SV40 infectivity (1), presumably by disrupting caveola function and preventing virus uptake. The effect of nystatin on the caveolin-1 in BMK and NIH 3T3 cells was first assessed by indirect IFA. Cells treated with nystatin showed that caveolin-1 withdrew from the cell surface, as indicated by the loss of the sharp staining at the edges of the cells (Fig. 1a and b, A versus C). The caveolin-1 appeared to relocalize to an intracellular site, possibly the ER (8, 35) or the trans-Golgi network (21, 28, 35), as indicated by the stronger intracellular staining seen in NIH 3T3 cells (Fig. 1a, A versus C) and the more punctate staining seen in the BMK cells (Fig. 1b, A versus C). The effect on endocytosis of this relocalization of caveolin-1 upon treatment with nystatin was examined by measuring internalization of either OG-Tfrn to determine clathrin-coated pit uptake or FITC-Ctx to determine caveola uptake. Cholera toxin B is targeted to caveolae by its receptor, the ganglioside GM1 (31), and its uptake is blocked by these cholesterol-binding compounds (30). Both NIH 3T3 and BMK cells treated with nystatin could readily take up OG-Tfrn, indicating that this compound had no effect on clathrin-mediated endocytosis (Fig. 1a and b, F). Mock-treated NIH 3T3 and BMK cells took up FITC-Ctx, but cells treated with nystatin did not contain large amounts of intracellular FITC-Ctx, demonstrating the specificity of nystatin's effect on caveolin-mediated uptake (Fig. 1a and b, B versus D).
FIG. 1.
(a) Uptake of cholera toxin and transferrin into control and nystatin-treated NIH 3T3 cells. NIH 3T3 cells were either mock treated (A and B) or pretreated with nystatin (C to F) and then incubated with either FITC-Ctx (A to D) or OG-Tfrn (E and F). Uptake of FITC-Ctx is shown in panels B and D, and the corresponding α-caveolin-1 uptake is shown in panels A and C. Uptake of OG-Tfrn is shown in panel F, and the corresponding α-clathrin light-chain uptake is shown in panel E. (b) Uptake of cholera toxin and transferrin into control and nystatin-treated BMK cells. BMK cells were either mock treated (A and B) or pretreated with nystatin (C to F) and then incubated with either FITC-Ctx (A to D) or OG-Tfrn (E and F). Uptake of FITC-Ctx is shown in panels B and D, and the corresponding α-caveolin-1 uptake is shown in panels A and C. Uptake of OG-Tfrn is shown in panel F, and the corresponding α-clathrin light-chain uptake is shown in panel E.
In a similar manner, BMK and NIH 3T3 cells were either mock treated or treated with increasing concentrations of either nystatin or filipin and incubated for 1 h at 37°C in a CO2 incubator. Cells were then infected with polyomavirus in the presence or absence of the cholesterol-binding agents, as appropriate. After incubation at 37°C, extracellular virus was neutralized with antibody and further incubated for 24 h at 37°C. Previous neutralization experiments with rabbit polyclonal antibody A3 indicated that approximately 90% and of the virus has been endocytosed from the surface of BMK cells within 30 min and 90% of the virus is off the surface of NIH 3T3 cells within 3 h (data not shown). Therefore, BMK cells were neutralized after a 30-min incubation with virus and NIH 3T3 cells were neutralized after a 3-h incubation with virus. The effectiveness of polyomavirus entry was assessed by an indirect IFA for nuclear PyLTAg. Neither compound, even at the highest concentrations used (100 μg/ml for nystatin and 10 μg/ml for filipin), had any effect on the ability of polyomavirus to infect cells (Table 1). Additionally, infecting the cells at a low multiplicity of infection to lessen the risk of entry through a lower-specificity pathway demonstrated that these compounds had no effect on polyomavirus uptake (data not shown). Therefore, unlike SV40, polyomavirus does not require functional caveolae to enter either BMK or NIH 3T3 cells.
TABLE 1.
Effects of nystatin and filipin on the ability of polyomavirus to infect cells
| Treatment | % PyTAg-positive cells
|
|
|---|---|---|
| BMK | NIH 3T3 | |
| Mock | 45 | 64 |
| Nystatin at: | ||
| 100 μg/ml | 47 | 62 |
| 50 μg/ml | 41 | 65 |
| 25 μg/ml | 43 | 57 |
| Filipin at: | ||
| 10 μg/ml | 44 | 65 |
| 5 μg/ml | 42 | 58 |
| 2.5 μg/ml | 42 | 64 |
Role of clathrin-coated pits in internalization of polyomavirus.
To determine whether an acidic compartment is required for polyomavirus infectivity, BMK and NIH 3T3 cells were pretreated with increasing concentrations of NH4Cl to neutralize the endosomal pH. Cells were infected in the presence of these compounds for 1 to 3 h at 37°C. Any remaining extracellular virus was neutralized, and the cells were further incubated at 37°C for 24 h. Samples were assayed for productive polyomavirus entry by examination of nuclear PyLTAg expression (Table 2). From these data, it is apparent that polyomavirus does not require an acidic compartment, as NH4Cl treatment had no effect on polyomavirus infectivity. These concentrations of NH4Cl are known to inhibit the infectivity of low-pH-dependent viruses (24).
TABLE 2.
Effect of NH4Cl treatment on polyomavirus infectivity
| Treatment | % PyTAg-positive cells
|
|
|---|---|---|
| NIH 3T3 | BMK | |
| Mock | 72.4 | 49.2 |
| NH4Cl at: | ||
| 1 mM | 73.2 | 49.4 |
| 5 mM | 70.5 | 51.6 |
| 10 mM | 71.0 | 50.3 |
| 25 mM | 72.1 | 49.8 |
The major endocytic vesicle pathway in most cells is the clathrin-coated pit route. Although previous EM studies on polyomavirus entry have never observed virus particles in coated pits, a recent study indicates that the closely related JC virus uses clathrin to penetrate its host cells (32). To examine whether clathrin-coated pit-mediated endocytosis plays a role in the entry of polymavirus into cells, several approaches were taken. Unlike treatment of cells with the cholesterol-binding agents, treatments that block uptake via clathrin have pleiotropic effects. To avoid misinterpretation, we have used multiple approaches to disturb clathrin-mediated endocytosis to determine whether clathrin is required for polyomavirus entry. Firstly, BMK and NIH 3T3 cells were untreated or treated with chlorpromazine, a cationic amphiphilic agent that causes dissociation of both clathrin and the adapter proteins from the plasma membrane by preventing clathrin recycling (40), and examined for the ability to take up OG-Tfrn. BMK cells tolerated the drug only up to 5 μg/ml without lifting off the dish. Both untreated BMK and NIH 3T3 cells could take up OG-Tfrn (Fig. 2A and B), and chlorpromazine treatment had no effect on the uptake of FITC-Ctx (data not shown). In the cells that were treated with chlorpromazine, the OG-Tfrn had a different, punctate appearance (Fig. 2a and b, D) with less signal overall. Similarly, clathrin light chain appears punctate in the treated samples compared with the untreated samples (Fig. 2a and b, C versus A). This is similar to what was reported by Pho et al. (32) and indicates that chlorpromazine has interrupted normal uptake of transferrin.
FIG. 2.
(a) Uptake of transferrin into control and treated NIH 3T3 cells. NIH 3T3 cells were either mock treated (A and B) or pretreated with either chlorpromazine (C and D), hypertonic medium (E and F), or acidified cytosol (G and H) and then incubated with OG-Tfrn. OG-Tfrn uptake is shown in panels B, D, F, and H, and the corresponding α-clathrin light-chain uptake is shown in panels A, C, E, and G. (b) Uptake of transferrin into control and treated BMK cells. BMK cells were either mock treated (A and B) or pretreated with either chlorpromazine (C and D), hypertonic medium (E and F), or acidified cytosol (G and H) and then incubated with OG-Tfrn. OG-Tfrn uptake is shown in panels B, D, F, and H, and the corresponding α-clathrin light-chain uptake is shown in panels A, C, E, and G.
To examine whether chlorpromazine can disrupt the ability of BMK and NIH 3T3 cells to allow polyomavirus entry, cells were pretreated with increasing concentrations of the compound prior to infection. Treatment with up to 10 μg/ml had no effect on the entry of polyomavirus into NIH 3T3 cells, but this highest concentration appeared to be toxic to BMK cells (Table 3). The next highest concentration of chlorpromazine, 5 μg/ml, had no effect on polyomavirus infectivity in BMK cells (Table 3), as measured by PyLTAg staining, indicating that disruption of clathrin-mediated endocytosis has no affect on polyomavirus entry.
TABLE 3.
Effect of chlorpromazine on polyomavirus infectivity
| Treatment | % PyTAg-positive cells
|
|
|---|---|---|
| NIH 3T3 | BMK | |
| Mock | 72.2 | 43.2 |
| Chlorpromazine at: | ||
| 1 μg/ml | 71.1 | 44.2 |
| 5 μg/ml | 69.2 | 44.9 |
| 10 μg/ml | 70.9 | NDa |
ND, not done.
Another approach to disruption of the clathrin endocytic pathway is incubation of cells in hypertonic medium. This results in the dissociation of clathrin from the plasma membrane (14, 18). BMK and NIH 3T3 cells were pretreated with medium alone or 0.45 M sucrose in medium for 10 min, and then the ability of these cells to endocytose OG-Tfrn was examined. Both NIH 3T3 and BMK cells were blocked from uptake of OG-Tfrn compared with the untreated controls (Fig. 2a and b, F versus B). Therefore, similar to what was seen with chlorpromazine treatment, incubation of cells with hypertonic medium blocked OG-Tfrn uptake. The sucrose hypertonicity treatment had no apparent effect on the ability of polyomavirus to infect either NIH 3T3 or BMK cells (Table 4).
TABLE 4.
Effect of sucrose hypartonicity treatment on polyomavirus infectivity
| Treatment | % PyTAg-positive cells
|
|
|---|---|---|
| NIH 3T3 | BMK | |
| Mock | 71.9 | 72.3 |
| Sucrose (0.445 M) | 68.9 | 69.1 |
| Mock | 16 | 36 |
| Acidification | 30 | 50 |
We next examined whether acidification of the cytosol can affect polyomavirus infectivity. Cytosol acidification is thought to inhibit clathrin-coated pit uptake (14, 17) while concomitantly upregulating other clathrin-independent endocytic pathways (22). Both NIH 3T3 and BMK cells had a decrease in uptake of OG-Tfrn, indicating a diminution in clathrin-mediated uptake (Fig. 2a and b, H versus B). Neither NIH 3T3 nor BMK cells were blocked for polyomavirus infectivity (Table 4). Interestingly, both NIH 3T3 and BMK cells showed modest increases in infectivity upon treatment that acidified the cytosol (Table 4). This further confirms that clathrin-mediated endocytosis plays no role in polyomavirus entry. Additionally, it indicates that the vesicles that take up polyomavirus can be upregulated in response to cytosol acidification in these cells.
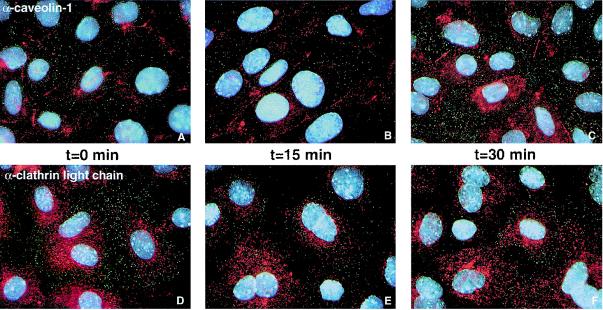
To visually examine the uptake of polyomavirus into cells using fluorescence microscopy and whether polyomavirus colocalizes with either clathrin or caveolin-1, purified, high-titer polyomavirus was labeled with the fluorophore Oregon Green. The labeled virus (OG-Py) retained a plaque titer equivalent to that of the unlabeled virus. Examination of OG-Py by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorescence analysis indicated that 67% (approximately 140 fluorophores per virus) of the label was incorporated into the outer layer protein VP1, with the core proteins VP2 and VP3 containing only 8% of the label. The remainder of the Oregon green fluorophore appears to have labeled the histones (approximately 50 fluorophores per virus; data not shown). The number of fluorophores on VP1 should allow detection of single viral particles by fluorescence microscopy, whereas the number of fluorophores on histones would be just barely at the level of detection (Molecular Probes, personal communication). OG-Py was bound to BMK cells in the cold and was then taken up into cells after warming at 37°C. At the indicated time points, cells on coverslips were fixed and the samples were processed for IFA against either clathrin light chain or caveolin-1. There was little or no obvious colocalization between OG-Py and caveolin-1 or clathrin light chain (Fig. 3) at any of the time points examined. Since antibody neutralization studies indicated that after 30 min at 37°C, greater than 90% of the virus was endocytosed (data not shown), if the virus was taken up in either clathrin-coated pits or caveolae, we would expect to see colocalization after the 30-min incubation. Later time points did not shown any colocalization (data not shown). These data further confirm the pharmacological data that polyomavirus uses neither clathrin-mediated nor caveola-mediated vesicles for entry.
FIG. 3.
OG-Py uptake into BMK cells. OG-Py was bound to cells at 4°C for 30 min. Unbound virus was removed by washing, and cells were incubated at 37°C for the indicated times and then fixed and processed for IFA against either α-caveolin 1 (A to C) or α-clathrin light chain (D to F).
Role of dynamin I in polyomavirus internalization.
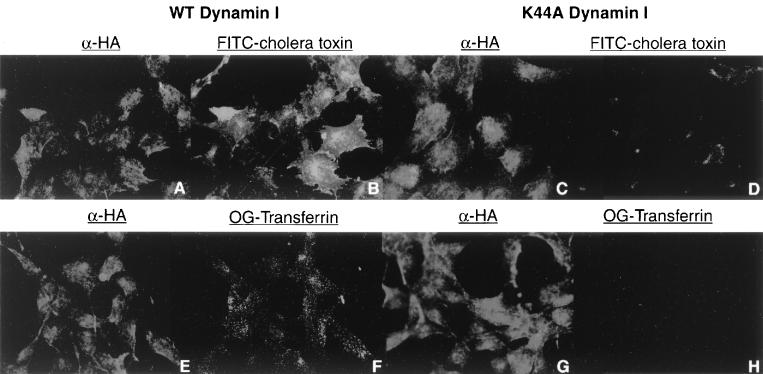
Dynamin is a molecule that is required for the formation and budding of clathrin-coated and caveola-derived vesicles (15, 16, 29, 38). It has been shown to be required for the uptake of several viruses that enter cells via clathrin-coated pits, Semliki Forest virus, human rhinovirus type 14 (12), and adenovirus (39), but not required for the uptake of poliovirus (12). A dominant-negative mutant form of dynamin I that cannot bind or hydrolyze GTP (K44A) (10) has been shown to block uptake of ligands using either clathrin-coated pits or caveolae (10, 29). To examine whether dynamin plays a role in the entry pathway of polyomavirus, stable NIH 3T3 cells expressing an HA-tagged form of either dominant-negative K44A mutant dynamin or its wild-type counterpart under the control of the Tet promoter were established. At 48 h after removal of tetracycline, the ability of NIH 3T3 cells expressing either wild-type or mutant dynamin I to take up either OG-Tfrn or FITC-Ctx was examined. Cells expressing the wild-type form of dynamin were able to take up both FITC-Ctx and OG-Tfrn (Fig. 4B and D). The cells that were expressing the K44A mutant dynamin had significantly reduced levels of uptake of both FITC-Ctx and OG-Tfrn (Fig. 4F and H). This indicated that the K44A mutant dynamin was functioning in the appropriate dominant-negative fashion, blocking uptake from both the clathrin- and caveola-mediated pathways. When the ability of these cells to be infected with polyomavirus was examined (Table 5), there was clearly little or no difference between cells expressing the wild-type and mutant forms of dynamin, as measured by PyLTAg staining. Even infecting cells with a lower multiplicity of infection, in an effort to minimize uptake by any possible less-specific entry pathway, did not alter the ability of cells expressing the K44A mutant dynamin to be productively infected by polyomavirus. This indicates that, similar to poliovirus (12), polyomavirus uses some type of not well-characterized vesicles to enter cells, the uptake of which is not disrupted by the effect of a dominant-negative dynamin I.
FIG. 4.
Uptake of cholera toxin and transferrin into wild-type (WT) and K44A mutant dynamin I-expressing NIH 3T3 cells. NIH 3T3 cells expressing either wild-type (A, B, E, and F) or K44A mutant (C, D, G, and H) dynamin I were incubated with either FITC-Ctx (A to D) or OG-Tfrn (E to H). Uptake of FITC-Ctx is shown in panels B and D, and the corresponding α-caveolin 1 uptake is shown in panels A and C. Uptake of OG-Tfrn is shown in panels F and H, and the corresponding α-clathrin light-chain uptake is shown in panels E and G.
TABLE 5.
Ability of polyomavirus to infect cells expressing wild-type or mutant dynamin I
| Form of dynamin I expressed | % PyTAg-positive cells
|
|
|---|---|---|
| 30 PFU/cell | 6 PFU/cell | |
| Wild type | 65 | 26 |
| K44A mutant | 69 | 24 |
DISCUSSION
The mechanism by which polyomavirus penetrates cells is not clearly understood. Since the polyomaviruses, as a subfamily, are closely related on a structural level and replicate within the nucleus, it has usually been inferred that these viruses enter cells in a similar fashion. Early EM studies with SV40 and polyomavirus indicated that virus was taken up in small vesicles and possibly targeted to the ER (13, 19, 20, 23, 25, 26). More recent studies imply that caveola-derived vesicles are the vesicles required for SV40 uptake (2). Surprisingly, studies on the human JC virus indicate that this polyomavirus uses the clathrin-mediated endocytic pathway for uptake into glial cells (32). From the data presented in this paper, it now appears that murine polyomavirus uses yet another type of vesicle pathway to enter cells. In both primary BMK cells and NIH 3T3 cells, pharmacological experiments demonstrated that neither clathrin-coated pits nor caveolae are required for polyomavirus infectivity, implying that some other vesicle pathway is used for uptake. Fluorescently labeled polyomavirus did not colocalize with either caveolin-1 or clathrin light chain, giving further credence to the idea that this virus does not use either type of coated vesicle for entry into cells. This was validated by the demonstration that a dominant-negative form of dynamin I, a K44A mutant form which is required for the endocytosis of clathrin and caveola-derived vesicles, had no effect on the ability of polyomavirus entry into cells. These data, combined with studies concerning the entry of SV40 (2, 37) and human JC virus (32) into cells, indicate that polyomaviruses, as a family, use a divergent set of endocytic vesicles to accomplish the same goal of penetrating cells and reaching the nucleus, the site of viral replication. How polyomavirus is routed to the nucleus after entry remains an important subject of investigation.
ACKNOWLEDGMENTS
We thank T. Kirchhausen for the antibody against clathrin light chain, R. Dowgiert for preparation of the primary BMK cells, and T. Lis for help in the preparation of Fig. 3.
This work was supported by National Institutes of Health grants R35 CA44343 and PO1 CA50661. J. M. Gilbert is supported by training grant 5T32CA72320 from the National Institutes of Health and by the Bunting Fellowship Program at the Radcliffe Institute for Advanced Study at Harvard University.
REFERENCES
- 1.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin-enriched membrane domains and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson H A, Chen Y, Norkin L C. MHC class I molecules are enriched in caveolae but do not enter with simian virus 40. J Gen Virol. 1998;79:1469–1477. doi: 10.1099/0022-1317-79-6-1469. [DOI] [PubMed] [Google Scholar]
- 3.Anderson R G W. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Arvan P, Rudnick G, Castle J. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984;259:13567–13572. [PubMed] [Google Scholar]
- 5.Bauer P H, Cui C, Stehle T, Harrison S C, DeCaprio J A, Benjamin T L. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J Virol. 1999;73:5826–5832. doi: 10.1128/jvi.73.7.5826-5832.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Benjamin T. Roles of N-glycans with α-2,6 as well as α-2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:400–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 7.Cole C N. Polyomavirinae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P A, editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1997–2025. [Google Scholar]
- 8.Conrad P A, Smart E J, Ying Y S, Anderson R G, Bloom G S. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Consigli R A, Zabielski J, Weil R. Plaque assay of polyoma virus in primary mouse kidney cell cultures. Appl Microbiol. 1973;26:627–628. doi: 10.1128/am.26.4.627-628.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawe C J, Freund R, Mandel G, Ballmer-Hofer K, Talmage D A, Benjamin T L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987;127:243–261. [PMC free article] [PubMed] [Google Scholar]
- 12.De Tulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. EMBO J. 1998;17:4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith G R, Marriott S J, Rintoul D A, Consigli R A. Early events in polyomavirus infection: fusion of monopinocytotic vesicles containing virions with mouse kidney cell nuclei. Virus Res. 1988;10:41–52. doi: 10.1016/0168-1702(88)90056-1. [DOI] [PubMed] [Google Scholar]
- 14.Hansen S H, Sandvig K, van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium and cytosol acidification. J Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henley J R, Cao H, McNiven M A. Participation of dynamin in the biogenesis of cytoplasmic vesicles. FASEB J. 1999;13(Suppl. 2):S243–S247. doi: 10.1096/fasebj.13.9002.s243. [DOI] [PubMed] [Google Scholar]
- 16.Henley J R, Krueger E W A, Oswald B J, McNiven M A. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989;108:401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuser J E, Anderson R G W. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;198:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummeler K, Tomassini N, Sokol F. Morphological aspects of the uptake of simian virus 40 by permissive cells. J Virol. 1970;6:87–93. doi: 10.1128/jvi.6.1.87-93.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurzchalia T, Dupree P, Parton R G, Kellner R, Virta H, Lehnart M, Simons K. VIP 21, a 21-kD membrane protein, is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 23.MacKay R L, Consigli R A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976;19:620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattern C F T, Takemoto K K, Daniel W A. Replication of polyoma virus in mouse embryo cells: electron microscopic observations. Virology. 1966;30:242–256. doi: 10.1016/0042-6822(66)90099-7. [DOI] [PubMed] [Google Scholar]
- 26.Maul G G, Rovera G, Vorbrodt A, Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978;28:936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillen J, Consigli R A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977;21:1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monier S, Parton R G, Vogel F, Behlke J, Henske A, Kurzchalia T V. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh P, McIntosh D P, Schnitzer J E. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlandi P A, Fishman P H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parton R G, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pho M T, Ashok A, Atwood W J. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74:2288–2292. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothberg K G, Ying Y-S, Kamen B A, Anderson R G W. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandvig K, Olsnes S, Petersen O W, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smart E, Ying Y-S, Conrad P, Anderson R G W. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart E J, Graf G A, McNiven M A, Sessa W C, Engelman J A, Scherer P E, Okamoto T, Lisanti M P. Caveolins, liquid-ordered domains and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stang E, Kartenbeck J, Parton R G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol Biol Cell. 1997;8:47–57. doi: 10.1091/mbc.8.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Bliek A M, Redelmeier T E, Damke H, Tisdale E J, Meyerowitz E M, Schmid S l. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:552–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang K, Huang S, Kappor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L H, Rothberg K G, Anderson R G W. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:107–117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]