Abstract
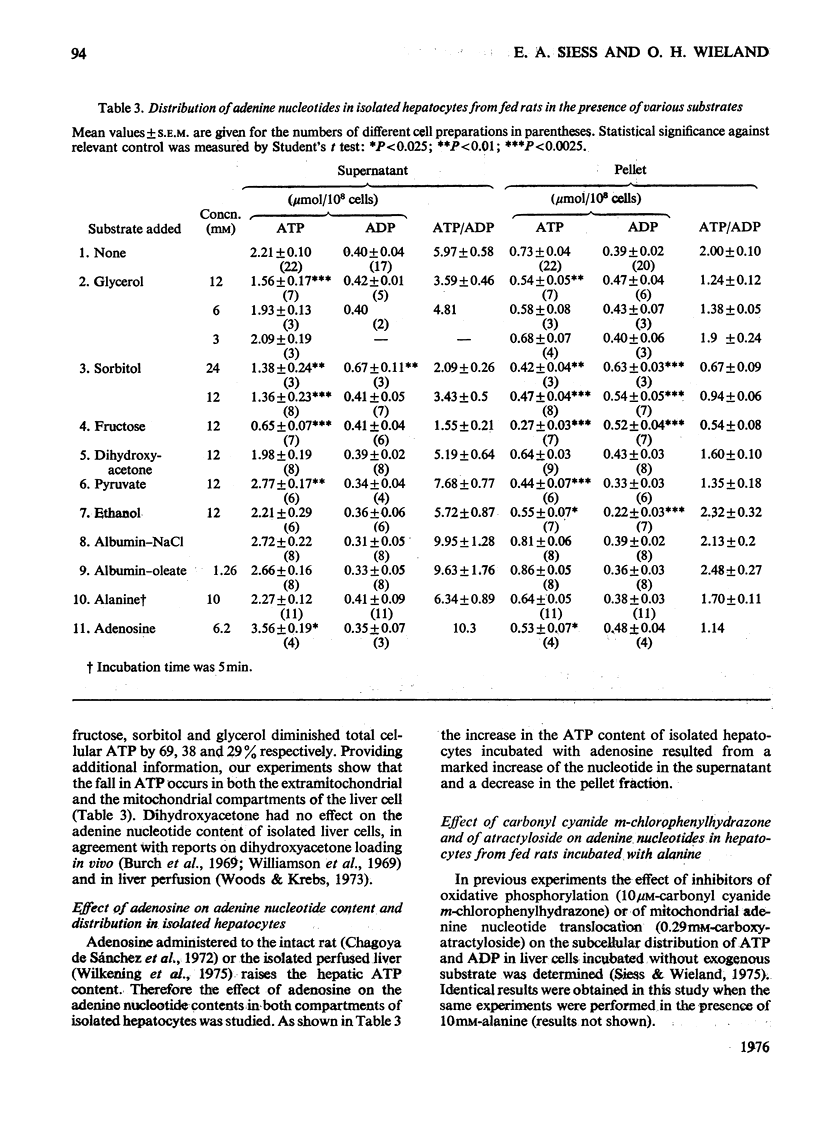
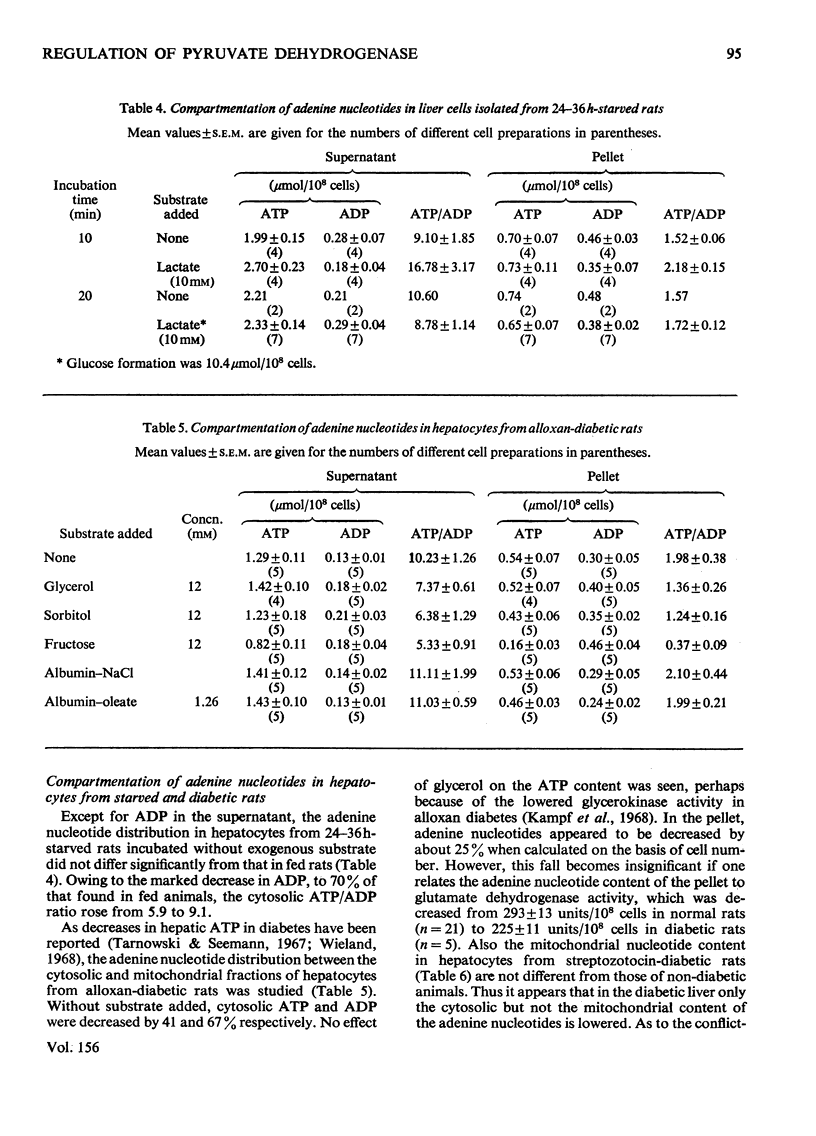
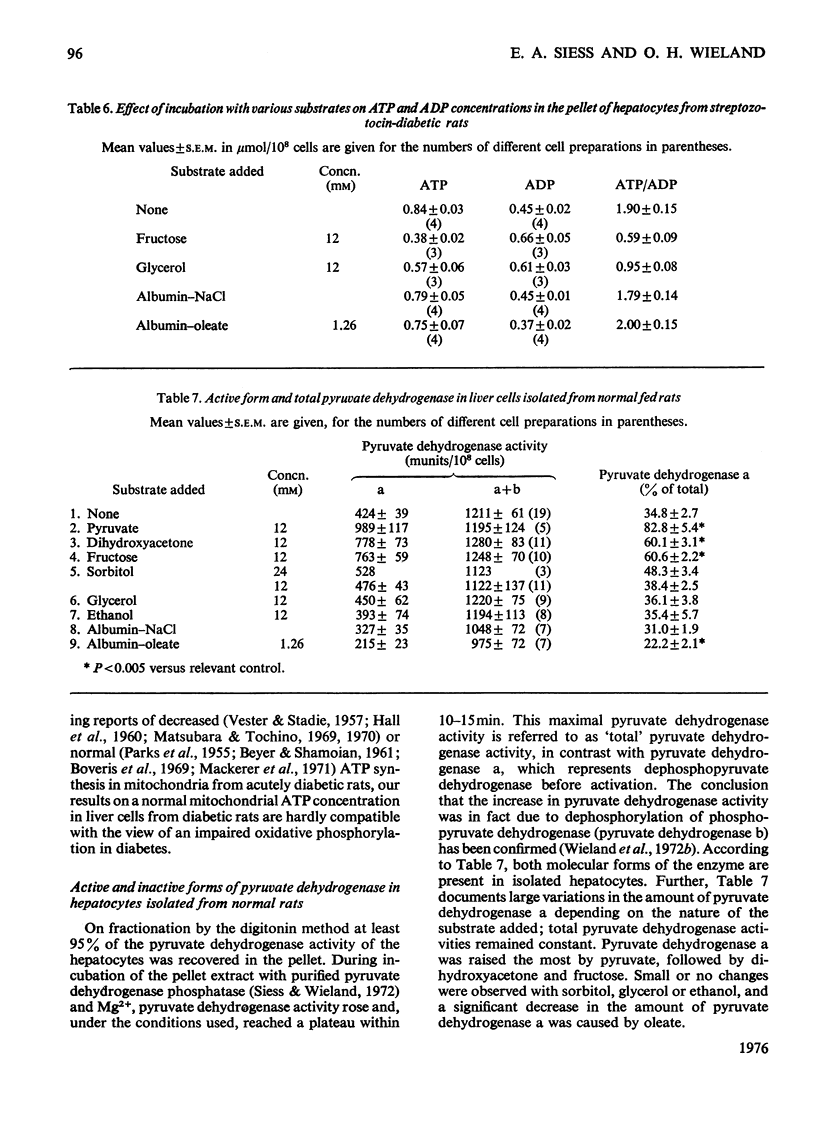
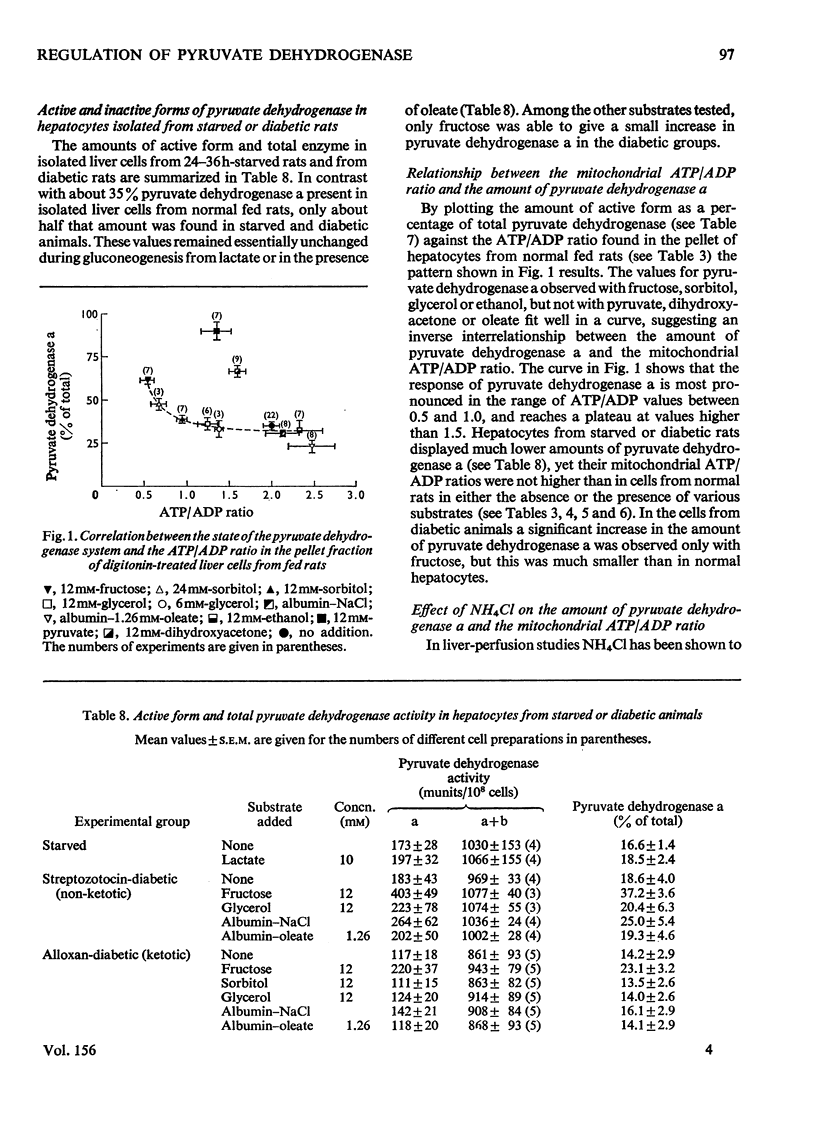
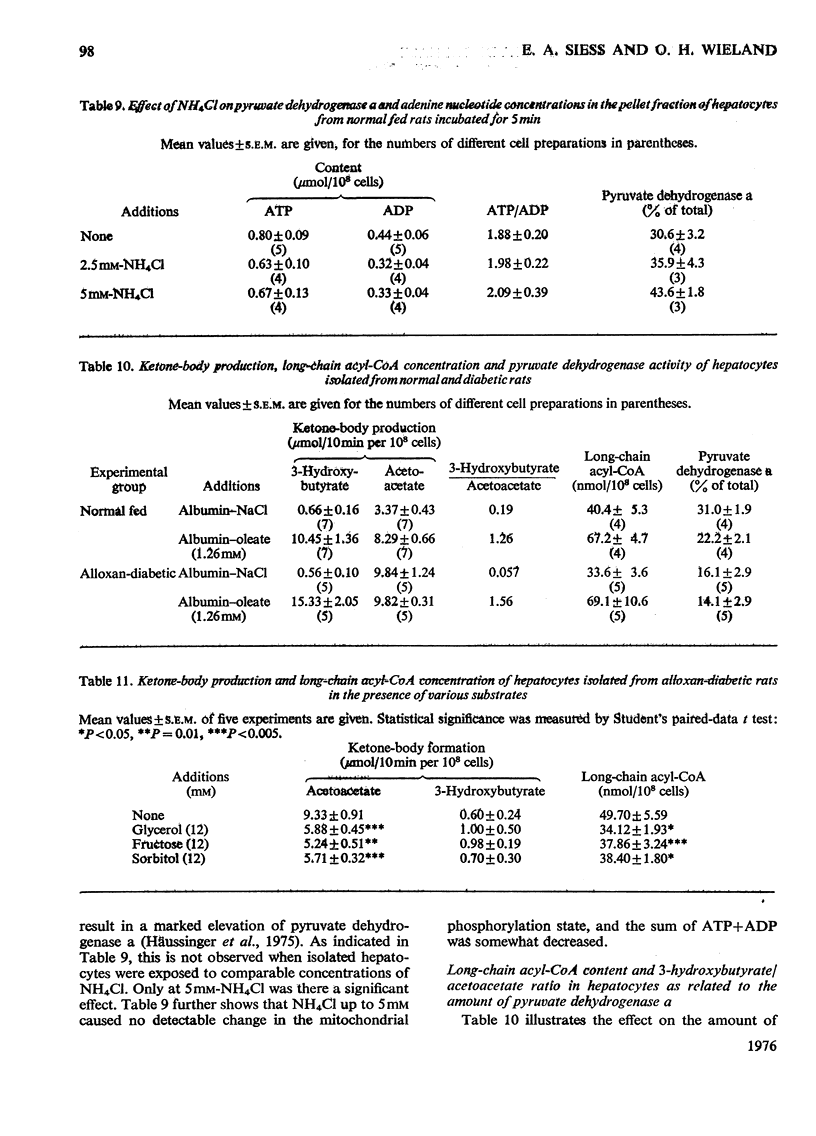
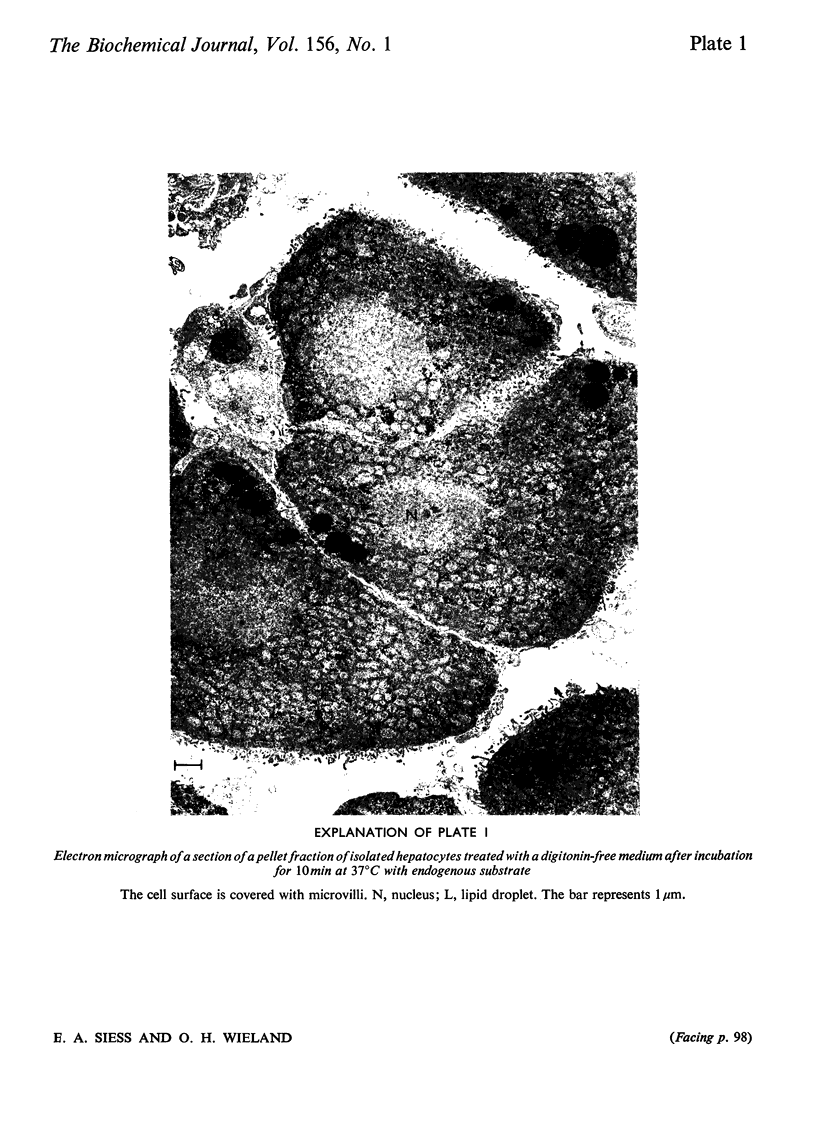
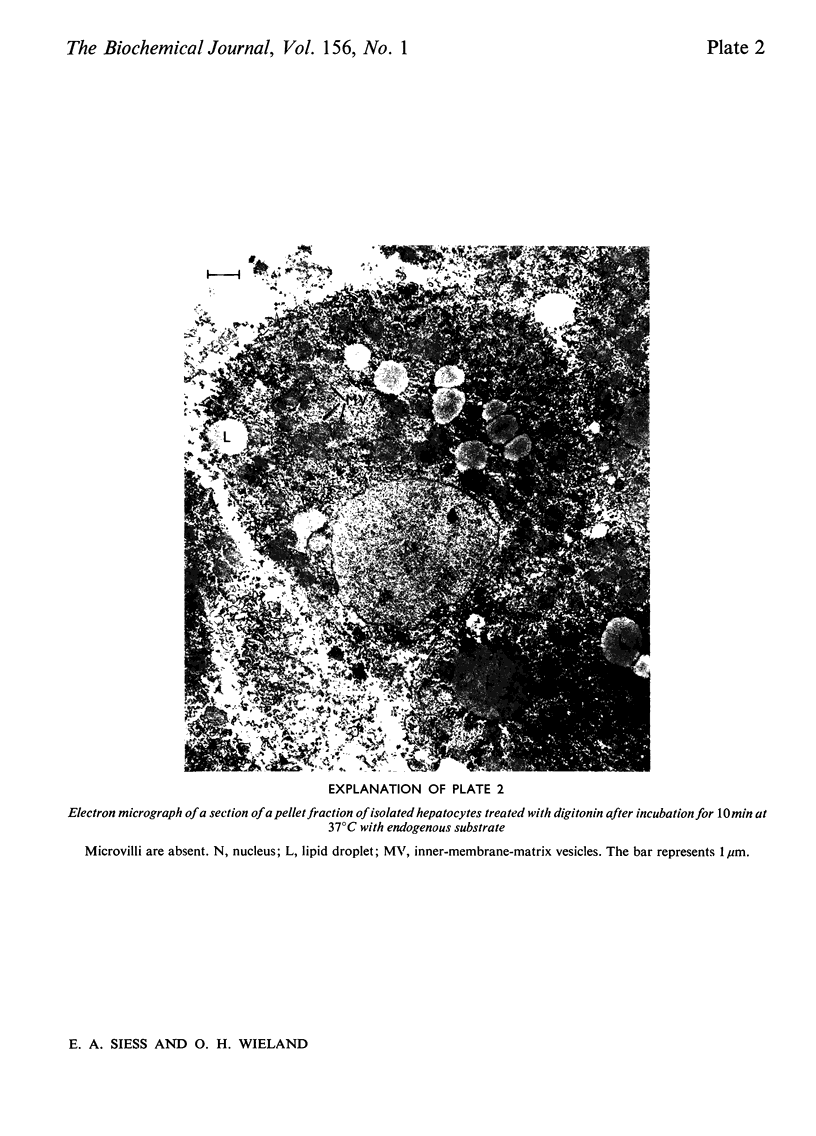
1. Cytosolic and mitochondrial ATP and ADP concentrations of liver cells isolated from normal fed, starved and diabetic rats were determined. 2. The cytosolic ATP/ADP ratio was 6,9 and 10 in normal fed, starved and diabetic rats respectively. 3. The mitochondrial ATP/ADP ratio was 2 in normal and diabetic rats and 1.6 in starved rats. 4. Adenosine increased the cytosolic and lowered the mitochondrial ATP/ADP ratio, whereas atractyloside had the opposite effect. 5. Incubation of the hepatocytes with fructose, glycerol or sorbitol led to a fall in the ATP/ADP ratio in both the cytosolic and the mitochondrial compartment. 6. The interrelationship between the mitochondrial ATP/ADP ratio and the phosphorylation state of pyruvate dehydrogenase in intact cells was studied. 7. In hepatocytes isolated from fed rats an inverse correlation between the mitochondrial ATP/ADP ratio and the active form of pyruvate dehydrogenase (pyruvate dehydrogenase a) was demonstrable on loading with fructose, glycerol or sorbitol. 8. No such correlation was obtained with pyruvate or dihydroxyacetone. For pyruvate, this can be explained by inhibition of pyruvate dehydrogenase kinase. 9. Liver cells isolated from fed animals displayed pyruvate dehydrogenase a activity twice that found in vivo. Physiological values were obtained when the hepatocytes were incubated with albumin-oleate, which also yielded the highest mitochondrial ATP/ADP ratio.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg J. J., Olson M. S. The inactivation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1975 Sep 16;66(2):533–540. doi: 10.1016/0006-291x(75)90543-4. [DOI] [PubMed] [Google Scholar]
- Bolender R. P., Weibel E. R. A morphometric study of the removal of phenobarbital-induced membranes from hepatocytes after cessation of threatment. J Cell Biol. 1973 Mar;56(3):746–761. doi: 10.1083/jcb.56.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. A., Cattaneo de Peralta Ram, Stoppani A. O., Foglia V. G. Phosphorylation, oxidation, and ubiquinone content in diabetic mitochondria. Proc Soc Exp Biol Med. 1969 Oct;132(1):171–174. doi: 10.3181/00379727-132-34174. [DOI] [PubMed] [Google Scholar]
- Burch H. B., Max P., Jr, Ghyu K., Lowry O. H. Metabolic intermediates in liver of rats given large amounts of fructose or dihydroxyacetone. Biochem Biophys Res Commun. 1969 Mar 10;34(5):619–626. doi: 10.1016/0006-291x(69)90783-9. [DOI] [PubMed] [Google Scholar]
- Chagoya de Sánchez V., Brunner A., Piña E. In vivo modification of the energy charge in the liver cell. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1441–1445. doi: 10.1016/s0006-291x(72)80138-4. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Chiang P. K., Sacktor B. Control of pyruvate dehydrogenase activity in intact cardiac mitochondria. Regulation of the inactivation and activation of the dehydrogenase. J Biol Chem. 1975 May 10;250(9):3399–3408. [PubMed] [Google Scholar]
- Cooper R. H., Randle P. J., Denton R. M. Regulation of heart muscle pyruvate dehydrogenase kinase. Biochem J. 1974 Dec;143(3):625–641. doi: 10.1042/bj1430625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers R., Heldt H. W., Schmucker P., Soboll S., Wiese H. Measurement of the ATP/ADP ratio in mitochondria and in the extramitochondrial compartment by fractionation of freeze-stopped liver tissue in non-aqueous media. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):378–393. doi: 10.1515/bchm2.1974.355.1.378. [DOI] [PubMed] [Google Scholar]
- Forsander O. A., Mäenpä P. H., Salaspuro M. P. Influence of ethanol on the lactate/pyruvate and beta-hydroxybutiate/acetoacetate ratios in rat liver experiments. Acta Chem Scand. 1965;19(7):1770–1771. doi: 10.3891/acta.chem.scand.19-1770. [DOI] [PubMed] [Google Scholar]
- Guder W. G., Wieland O. H. Metabolism of isolated kidney tubules. Regulation of pyruvate dehydrogenase by metabolic substrates. Eur J Biochem. 1974 Mar 1;42(2):529–538. doi: 10.1111/j.1432-1033.1974.tb03368.x. [DOI] [PubMed] [Google Scholar]
- HALL J. C., SORDAHL L. A., STEFKO P. L. The effect of insulin on oxidative phosphorylation in normal and diabetic mitochondria. J Biol Chem. 1960 May;235:1536–1539. [PubMed] [Google Scholar]
- Hennig G., Löffler G., Wieland O. H. Active and inactive forms of pyruvatedehydrogenase in skeletal muscle as related to the metabolic and functional state of the muscle cell. FEBS Lett. 1975 Nov 15;59(2):142–145. doi: 10.1016/0014-5793(75)80361-9. [DOI] [PubMed] [Google Scholar]
- Hucho F. Regulation of the mammalian pyruvate dehydrogenase multienzyme complex by Mg2+ and the adenine nucleotide pool. Eur J Biochem. 1974 Aug 1;46(3):499–505. doi: 10.1111/j.1432-1033.1974.tb03643.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Weiss L., Sies H. Activation of pyruvate dehydrogenase during metabolism of ammonium ions in hemoglobin-free perfused rat liver. Eur J Biochem. 1975 Apr 1;52(3):421–431. doi: 10.1111/j.1432-1033.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Kampf S. C., Seitz H. J., Tarnowski W. Alternation of glycerokinase activity in rat liver in different nutritional and hormonal states. Life Sci. 1968 Aug 15;7(16):815–825. doi: 10.1016/0024-3205(68)90112-4. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler G., Bard S., Wieland O. H. Control of pyruvate dehydrogenase interconversion by palmitoyl-coenzyme A as related to adenine nucleotide translocation in isolated fat cell mitochondria. FEBS Lett. 1975 Dec 15;60(2):269–274. doi: 10.1016/0014-5793(75)80729-0. [DOI] [PubMed] [Google Scholar]
- Mackerer C. R., Paquet R. J., Mehlman M. A., Tobin R. B. Oxidation and phosphorylation in live mitochondria from alloxan and steptozotocin diabetic rats. Proc Soc Exp Biol Med. 1971 Jul;137(3):992–995. doi: 10.3181/00379727-137-35712. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M., Pask H. T., Randle P. J. Mechanisms regulating adipose-tissue pyruvate dehydrogenase. Biochem J. 1972 Sep;129(3):763–773. doi: 10.1042/bj1290763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara T., Tochino Y. Depression of respiratory activities by fatty acids in liver mitochondria from diabetic rats and hormonal regulation of mitochondrial fatty acids. J Biochem. 1970 Nov;68(5):731–736. doi: 10.1093/oxfordjournals.jbchem.a129407. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Tochino Y. Depression of respiratory activities in the liver mitochondria of diabetic rats and the restorative action of insulin. J Biochem. 1969 Sep;66(3):397–404. doi: 10.1093/oxfordjournals.jbchem.a129158. [DOI] [PubMed] [Google Scholar]
- Mclean P., Gumaa K. A., Greenbaum A. L. Long chain acyl CoAs, adenine nucleotide translocase and the coordination of the redox states of the cytosolic and mitochondrial compartments. FEBS Lett. 1971 Oct 1;17(2):345–350. doi: 10.1016/0014-5793(71)80184-9. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- PARKS R. E., Jr, ADLER J., COPENHAVER J. H., Jr The efficiency of oxidative phosphorylation in mitochondria from diabetic rats. J Biol Chem. 1955 Jun;214(2):693–698. [PubMed] [Google Scholar]
- Patzelt C., Löffler G., Wieland O. H. Interconversion of pyruvate dehydrogenase in the isolated perfused rat liver. Eur J Biochem. 1973 Feb 15;33(1):117–122. doi: 10.1111/j.1432-1033.1973.tb02662.x. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Pelley J. W., Reed L. J. Regulation of pyruvate dehydrogenase kinase and phosphatase by acetyl-CoA/CoA and NADH/NAD ratios. Biochem Biophys Res Commun. 1975 Jul 22;65(2):575–582. doi: 10.1016/s0006-291x(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Portenhauser R., Wieland O. Regulation of pyruvate dehydrogenase in mitochondria of rat liver. Eur J Biochem. 1972 Dec 4;31(2):308–314. doi: 10.1111/j.1432-1033.1972.tb02534.x. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle P. J., Denton R. M. Rate control by insulin and its mechanism. Symp Soc Exp Biol. 1973;27:401–428. [PubMed] [Google Scholar]
- Roche T. E., Reed L. J. Monovalent cation requirement for ADP inhibition of pyruvate dehydrogenase kinase. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1341–1348. doi: 10.1016/0006-291x(74)90461-6. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Purification and characterization of pyruvate-dehydrogenase phosphatase from pig-heart muscle. Eur J Biochem. 1972 Mar 15;26(1):96–105. doi: 10.1111/j.1432-1033.1972.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Regulation of pyruvate dehydrogenase interconversion in isolated hepatocytes by the mitochondrial ATP/ADP ratio. FEBS Lett. 1975 Apr 1;52(2):226–230. doi: 10.1016/0014-5793(75)80811-8. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Bernhard G., Janson G. Interconversion of inactive to active pyruvate dehydrogenase in rat liver after fructose application in vivo. FEBS Lett. 1971 Mar 16;13(4):201–203. doi: 10.1016/0014-5793(71)80535-5. [DOI] [PubMed] [Google Scholar]
- Tarnowski W., Seeman M. Konzentrationsänderungen von Effektoren gluconeogenetischer Schlüsselenzyme in der Rattenleber unter gluconeogenetischen Bedingungen. Hoppe Seylers Z Physiol Chem. 1967 Jul;348(7):829–838. [PubMed] [Google Scholar]
- Taylor S. I., Mukherjee C., Jungas R. L. Regulation of pyruvate dehydrogenase in isolated rat liver mitochondria. Effects of octanoate, oxidation-reduction state, and adenosine triphosphate to adenosine diphosphate ratio. J Biol Chem. 1975 Mar 25;250(6):2028–2035. [PubMed] [Google Scholar]
- VESTER J. W., STADIE W. C. Studies of oxidative phosphorylation by hepatic mitochondria from the diabetic cat. J Biol Chem. 1957 Aug;227(2):669–676. [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Weibel E. R., Stäubli W., Gnägi H. R., Hess F. A. Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol. 1969 Jul;42(1):68–91. doi: 10.1083/jcb.42.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse S., Cooper R. H., Randle P. J. Mechanism of activation of pyruvate dehydrogenase by dichloroacetate and other halogenated carboxylic acids. Biochem J. 1974 Sep;141(3):761–774. doi: 10.1042/bj1410761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H., Patzelt C., Löffler G. Active and inactive forms of pyruvate dehydrogenase in rat liver. Effect of starvation and refeeding and of insulin treatment on pyruvate-dehydrogenase interconversion. Eur J Biochem. 1972 Apr 11;26(3):426–433. doi: 10.1111/j.1432-1033.1972.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Wieland O. H., Portenhauser R. Regulation of pyruvate-dehydrogenase interconversion in rat-liver mitochondria as related to the phosphorylation state of intramitochondrial adenine nucleotides. Eur J Biochem. 1974 Jun 15;45(2):577–588. doi: 10.1111/j.1432-1033.1974.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Wieland O., Funcke H. v., Löffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett. 1971 Jul 1;15(4):295–298. doi: 10.1016/0014-5793(71)80641-5. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E., Schulze-Wethmar F. H., von Funcke H. G., Winton B. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971 Apr;143(2):593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]
- Wilkening J., Nowack J., Decker K. The dependence of glucose formation from lactate on the adenosine triphosphate content in the isolated perfused rat liver. Biochim Biophys Acta. 1975 Jun 12;392(2):299–309. doi: 10.1016/0304-4165(75)90011-2. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Stubbs M., Veech R. L., Erecińska M., Krebs H. A. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem J. 1974 Apr;140(1):57–64. doi: 10.1042/bj1400057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods H. F., Krebs H. A. The effect of glycerol and dihydroxyacetone on hepatic adenine nucleotides. Biochem J. 1973 Jan;132(1):55–60. doi: 10.1042/bj1320055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W., Lardy H. A. Regulation of glucose synthesis in hormone-sensitive isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3213–3218. doi: 10.1073/pnas.70.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]