Abstract
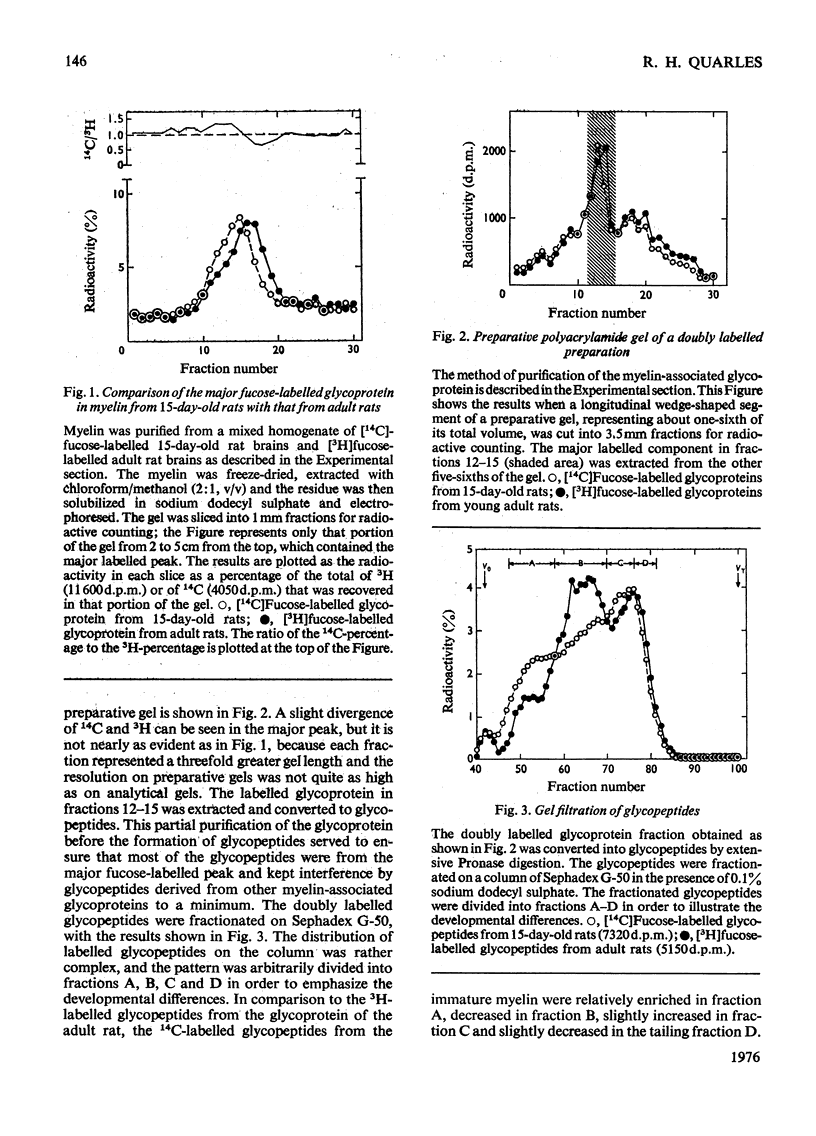
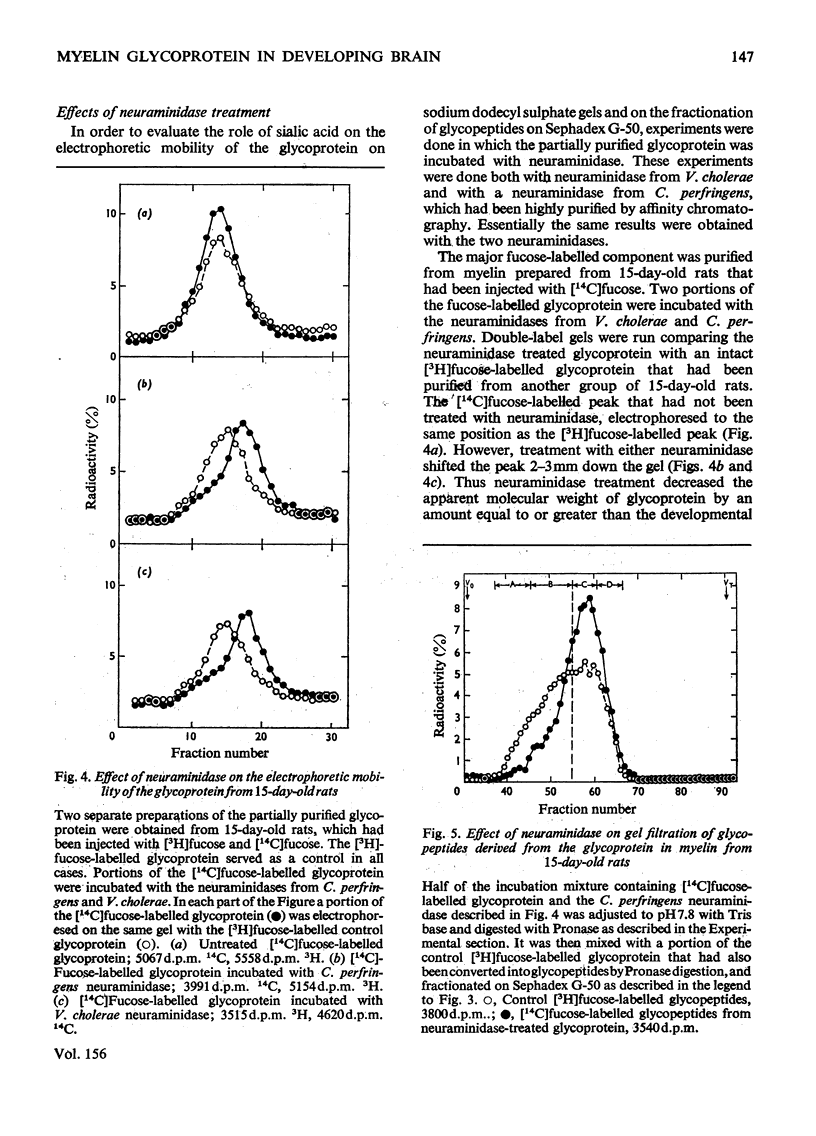
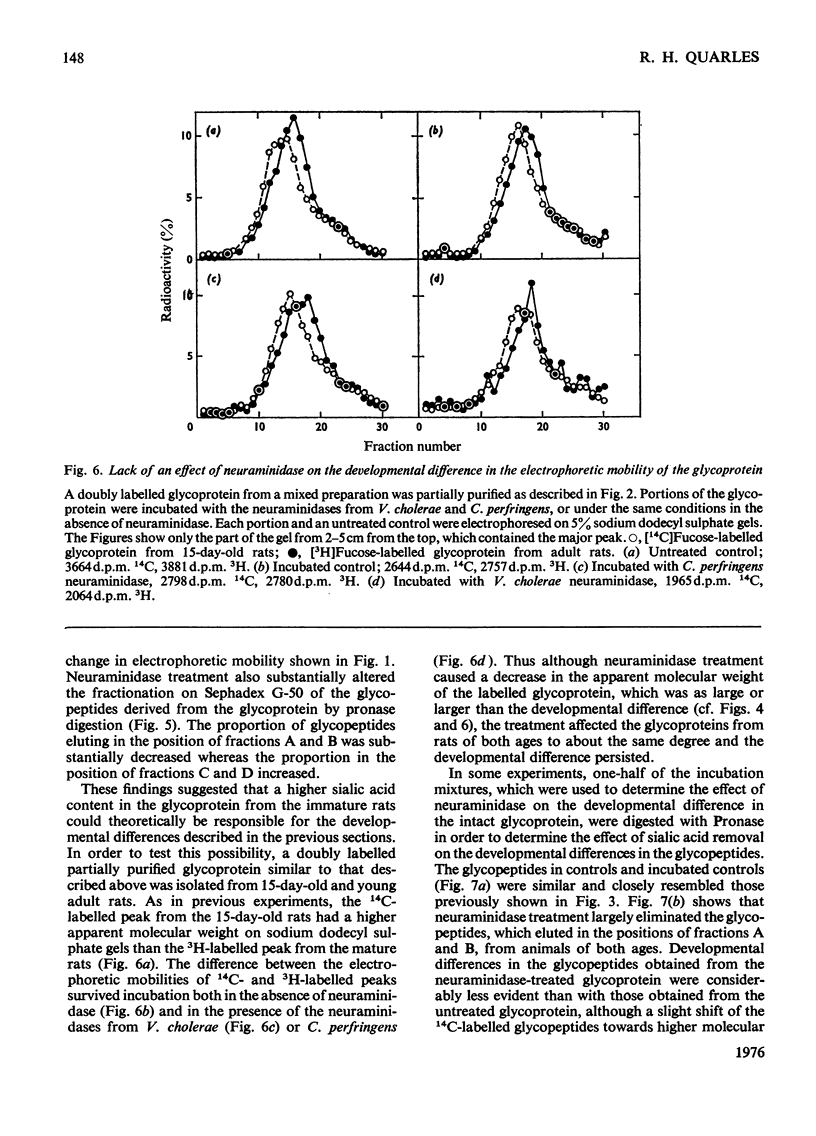
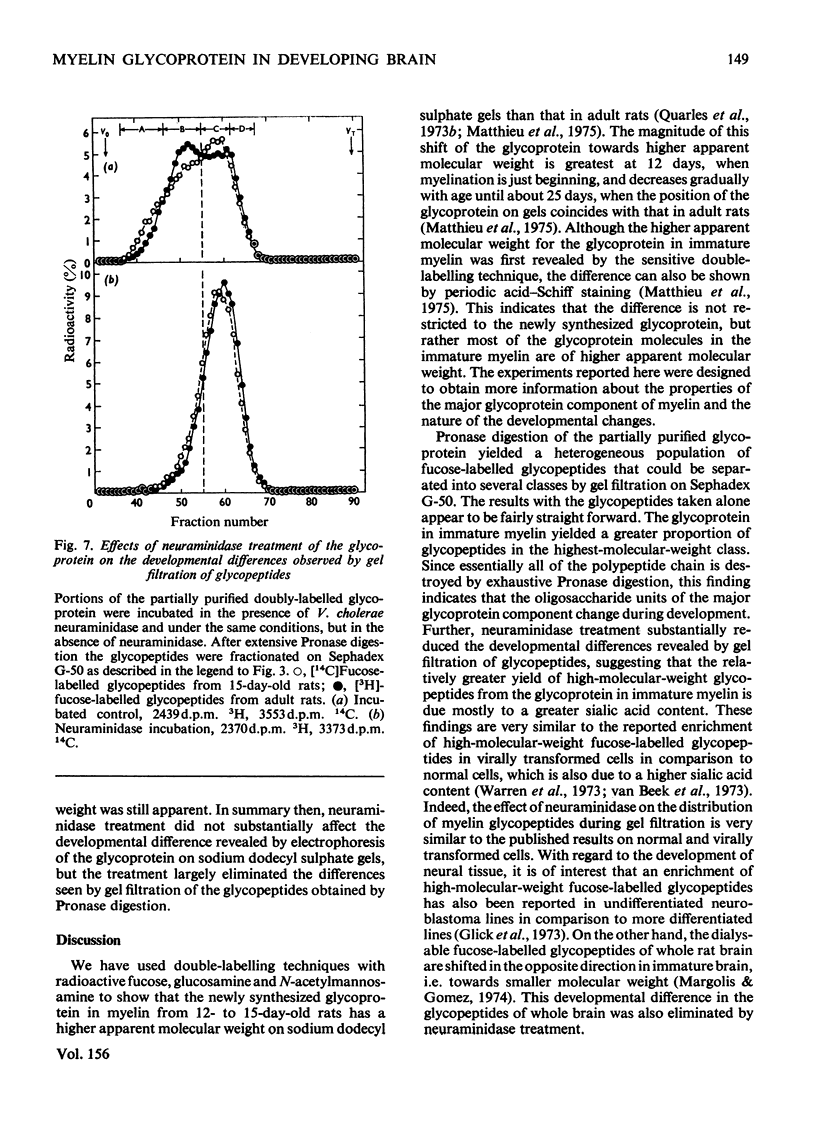
Rats (14 days old) were injected with [14c]fucose and young adult rats with [3H]fucose in order to label the myelin-associated glycoproteins. As previously reported, the major [14C]fucose-labelled glycoprotein in the immature myelin had a higher apparent molecular weight on sodium dodecyl sulphate/polyacrylamide gels that the [3H]fucose-labelled glycoprotein in mature myelin. This predominant doubly labelled glycoprotein component was partially purified by preparative gel electrophoresis and converted to glycopeptides by extensive Pronase digestion. Gel filtration on Sephadex G-50 separated the glycopeptides into several clases, which were designted A,B, C AND D, from high to low molecular weight. The 14C-labelled glycopeptides from immature myeline were enriched in the highest-molecular-weight class A relative to the 3H-labelled glycopeptides from mature myelin. Neuraminidase treatment of the glycoprotein before Pronase digestion greatly decreased the proportion of glycopeptides fractionating in the higher-molecular-weight classes and largely eliminated the developmental differences that were apparent by gel filtration. However, neuraminidase treatment did not decrease the magnitude of the developmental difference revealed by electrophoresing the intact glycoprotein on sodium dodecyl sulphate gels, although it did decrease the apparent molecular weight of the glycoprotein from both the 15-day-old and adult rats by an amount comparable in magnitude to that developmental difference. The results from gel filtration of glycopeptides indicate that there is a higher content of large molecular weight, sialic acid-rich oligosaccharide units in the glycoprotein of immature myelin. However, the higher apparent molecular weight for the glycoprotein from 15-day-old rats on sodium dodcyl sulphate gels is not due primarily to its higher sialic acid content.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunngraber E. G. The possible role of glycoproteins in neural function. Perspect Biol Med. 1969 Spring;12(3):467–470. doi: 10.1353/pbm.1969.0012. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. A comparative study of glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1970 Nov 10;9(23):4567–4576. doi: 10.1021/bi00825a016. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Effect of growth on the glycoproteins from the surface of control and Rous sarcoma virus transformed hamster cells. Biochemistry. 1971 May 25;10(11):2176–2180. doi: 10.1021/bi00787a034. [DOI] [PubMed] [Google Scholar]
- Burger M. M. Surface changes in transformed cells detected by lectins. Fed Proc. 1973 Jan;32(1):91–101. [PubMed] [Google Scholar]
- Cuatrecasas P., Illiano G. Membrane sialic acid and the mechanism of insulin action in adipose tissue cells. Effects of digestion with neuraminidase. J Biol Chem. 1971 Aug 25;246(16):4938–4946. [PubMed] [Google Scholar]
- Glick M. C., Kimhi Y., Littauer U. Z. Glycopeptides from surface membranes of neuroblastoma cells. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1682–1687. doi: 10.1073/pnas.70.6.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S., Norton W. T., Morell P. Quaking mouse: isolation and characterization of myelin protein. J Neurochem. 1971 Nov;18(11):2119–2128. doi: 10.1111/j.1471-4159.1971.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Jackson R. L., Segrest J. P., Kahane I. Molecular features of the major glycoprotein of the human erythrocyte membrane. Fed Proc. 1973 Aug;32(8):1833–1837. [PubMed] [Google Scholar]
- Margolis R. K., Gomez Z. Structural changes in brain glycoproteins during development. Brain Res. 1974 Jul 12;74(2):370–372. doi: 10.1016/0006-8993(74)90594-0. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Brady R. O., Quarles R. H. Change in a myelin-associated glycoprotein in rat brain during development: metabolic aspects. Brain Res. 1975 Mar 14;86(1):55–65. doi: 10.1016/0006-8993(75)90637-x. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Quarles R. H., Brady R. O., Webster H. de F. Variation of proteins, enzyme markers and gangliosides in myelin subfractions. Biochim Biophys Acta. 1973 Dec 5;329(2):305–317. doi: 10.1016/0304-4165(73)90295-x. [DOI] [PubMed] [Google Scholar]
- Matthieu J. M., Quarles R. H., de Webster H. F., Hogan E. L., Brady R. O. Characterization of the fraction obtained from the CNS of Jimpy mice by a procedure for myelin isolation. J Neurochem. 1974 Sep;23(3):517–523. doi: 10.1111/j.1471-4159.1974.tb06054.x. [DOI] [PubMed] [Google Scholar]
- Moscona A. A. Embryonic and neoplastic cell surfaces: availability of receptors for concanavalin A and wheat germ agglutinin. Science. 1971 Mar 5;171(3974):905–907. doi: 10.1126/science.171.3974.905. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973 Oct;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Poduslo J. F., Quarles R. H., Brady R. O. External labeling of galactose in surface membrane glycoproteins of the intact myelin sheath. J Biol Chem. 1976 Jan 10;251(1):153–158. [PubMed] [Google Scholar]
- Quarles R. H., Brady R. O. Synthesis of glycoproteins and gangliosides in developing rat brain. J Neurochem. 1971 Oct;18(10):1809–1820. doi: 10.1111/j.1471-4159.1971.tb09586.x. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Everly J. L., Brady R. O. Demonstration of a glycoprotein which is associated with a purified myelin fraction from rat brain. Biochem Biophys Res Commun. 1972 Apr 28;47(2):491–497. doi: 10.1016/0006-291x(72)90741-3. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Everly J. L., Brady R. O. Evidence for the close association of a glycoprotein with myelin in rat brain. J Neurochem. 1973 Nov;21(5):1177–1191. doi: 10.1111/j.1471-4159.1973.tb07573.x. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Everly J. L., Brady R. O. Myelin-associated glycoprotein: a developmental change. Brain Res. 1973 Aug 30;58(2):506–509. doi: 10.1016/0006-8993(73)90022-x. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of cells before and after transformation by oncogenic viruses. Fed Proc. 1973 Jan;32(1):80–85. [PubMed] [Google Scholar]
- van Beek W. P., Smets L. A., Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells--a general phenomenon? Cancer Res. 1973 Nov;33(11):2913–2922. [PubMed] [Google Scholar]


