Abstract
Background
The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) Study was designed to determine the effects of a best‐practice hearing intervention on cognitive decline among community‐dwelling older adults. Here, we conducted a secondary analysis of the ACHIEVE Study to investigate the effect of hearing intervention on self‐reported communicative function.
Methods
The ACHIEVE Study is a parallel‐group, unmasked, randomized controlled trial of adults aged 70–84 years with untreated mild‐to‐moderate hearing loss and without substantial cognitive impairment. Participants were randomly assigned (1:1) to a hearing intervention (audiological counseling and provision of hearing aids) or a control intervention of health education (individual sessions with a health educator covering topics on chronic disease prevention) and followed semiannually for 3 years. Self‐reported communicative function was measured with the Hearing Handicap Inventory—Elderly Screening version (HHIE‐S, range 0–40, higher scores indicate greater impairment). Effect of hearing intervention versus control on HHIE‐S was analyzed through an intention‐to‐treat model controlling for known covariates.
Results
HHIE‐S improved after 6‐months with hearing intervention compared to control, and continued to be better through 3‐year follow‐up. We estimated a difference of −8.9 (95% CI: −10.4, −7.5) points between intervention and control groups in change in HHIE‐S score from baseline to 6 months, −9.3 (95% CI: −10.8, −7.9) to Year 1, −8.4 (95% CI: −9.8, −6.9) to Year 2, and − 9.5 (95% CI: −11.0, −8.0) to Year 3. Other prespecified sensitivity analyses that varied analytical parameters did not change the observed results.
Conclusions
Hearing intervention improved self‐reported communicative function compared to a control intervention within 6 months and with effects sustained through 3 years. These findings suggest that clinical recommendations for older adults with hearing loss should encourage hearing intervention that could benefit communicative function and potentially have positive downstream effects on other aspects of health.
Keywords: clinical trial, cognition, functional disability, hearing handicap, hearing loss
Key points
Hearing loss is highly prevalent among older adults and associated with negative health outcomes, yet under‐diagnosed and under‐treated.
Hearing intervention with hearing aids improved self‐perceived communicative function within 6 months and with sustained effects through 3 years.
Healthcare providers should routinely encourage patients to seek treatment for hearing loss given potential beneficial effects on communication and other health outcomes.
Why does this paper matter?
As hearing loss is highly prevalent in older adults, with adverse effects on communicative function and other health outcomes, our results definitively demonstrate that hearing intervention improves communicative function within 6 months of intervention onset with sustained effects at 3 years. These findings are important for understanding whether future strategies and policies to increase rates of hearing care could broadly benefit older adults' health.
INTRODUCTION
Hearing loss is prevalent in approximately 65% of adults over age 60 and 80% of adults over age 80. 1 , 2 Regardless of age‐of‐onset, hearing loss is significantly related to decreased quality of life 3 and increased risks of age‐related health conditions including cognitive decline and dementia, falls, and social isolation. 4 , 5 , 6 , 7 A key mechanism through which hearing loss may exert adverse effects on health outcomes is through impaired functional communication. Hearing loss affects both the audibility and clarity of speech signals, thereby challenging communication in daily conversations and social situations. Described by the World Health Organization International Classification of Functioning, Disability and Health (WHO‐ICF), 8 hearing loss is not just the reduction of sound perception. The ICF framework is based on an inclusive bio‐psycho‐social model of functioning, thus, hearing loss is better defined as impaired bodily structure and functioning, activity limitations, and participation restrictions in daily activity; all of which has psychosocial and emotional consequences that are influenced by environmental and personal factors. Normal hearing then would be defined as having positive psychosocial and emotional wellbeing related to the ability to hear sound accurately with ease and to fully participate in daily activities with no limitations or restrictions. 9 , 10 For simplicity, we refer to the multi‐dimensions of hearing loss (anatomical and perceptional dysfunction, psychosocial and emotional distress, and activity limitations/restrictions), as communicative function.
Poor communicative function can lead to harmful effects across many areas of health, such as social isolation, 11 depression, 12 cognition, 13 increased hospitalization, 14 worse patient‐provider communication, 15 and worse management of health conditions. 16 Treatment of hearing loss is a potentially effective intervention for improving communicative function among older adults with hearing loss. Evidence from observational and quasi‐experimental studies show a positive effect of hearing intervention with hearing aids on self‐perceived communicative function. 17 , 18 However, inferences about the effects of hearing intervention in non‐experimental studies may be confounded by the individual characteristics of those who choose to obtain hearing aids (e.g., education, income, health behaviors) and may not be generalizable to other populations. 17 In the quasi‐experimental studies conducted to date, limitations included small sample size, lack of a control group, poorly defined hearing loss and/or hearing aid use (often a single question), with no measurement of either hearing aid benefit or frequency of use. 18 A systematic review of and meta‐analysis of 16 studies suggested that hearing interventions had a medium‐to‐large effect on communicative functioning, 19 yet the authors concluded there was limited evidence from RCTs, calling for need for future rigorous RCTs. Additional reviews of prior randomized controlled trials 20 , 21 , 22 have generally suggested a benefit of hearing intervention on self‐perceived communicative function, but these trials continue to be limited by being restricted to clinic‐based populations, 21 , 23 use of non‐prescriptive hearing aids, 24 small sample size, 20 , 23 lack of a control group, and/or limited follow‐up time. 23
In this study, we conduct a secondary analysis of the Aging and Cognitive Health Evaluation in Elders (ACHIEVE) Study 25 , 26 which was funded by the National Institute on Aging, to describe the effect of hearing intervention versus health education control on self‐perceived communicative function over 3 years, an exploratory outcome of the ACHIEVE Study. The ACHIEVE Study is the longest‐duration and largest randomized trial of hearing intervention ever conducted and was designed to investigate whether hearing intervention versus health education control reduced cognitive decline (primary outcome) over 3 years in community‐dwelling older adults with untreated hearing loss. The primary report of the ACHIEVE Study indicated that hearing intervention did not reduce 3‐year cognitive decline in the primary analysis of the total cohort; however, a prespecified sensitivity analysis showed that the effect differed between the two study populations that comprised the cohort. 25 These findings suggest that a hearing intervention might reduce cognitive change over 3 years in populations of older adults at increased risk for cognitive decline and better understanding of the study sample's benefits from the hearing intervention are needed. Determining if a well‐defined hearing intervention has sustained effects on improving communicative function is important for understanding whether strategies and policies to increase rates of hearing aid uptake could broadly benefit community‐dwelling older adults with hearing loss.
METHODS
Data source—The ACHIEVE Study
The ACHIEVE Study was a randomized controlled trial testing the effect of a best‐practice hearing intervention versus health education control on cognitive decline over 3 years. The premise underlying the ACHIEVE Study was based on evidence suggesting that hearing loss in older adults is independently associated with accelerated cognitive decline and incident dementia, 5 and may be amenable to hearing intervention. 13
The study was conducted at four sites in the United States: Forsyth County, North Carolina (NC); Jackson, Mississippi (MS); Minneapolis suburbs, Minnesota (MN); and Washington County, Maryland (MD). At the study sites, participants were recruited either from the ongoing Atherosclerosis Risk in Communities (ARIC) Study, 27 or de novo from the local study site communities. The ACHIEVE Study was approved by the institutional review boards of all study sites and associated academic centers. Participants provided written informed consent. An independent data and safety monitoring board (DSMB) met semi‐annually to review study progress, adverse events, and changes to the study protocol and statistical analysis plan. The ACHIEVE Study protocol, outcomes, and analysis plan were registered at ClinicalTrials.gov ID = NCT03243422, before the unmasking of any trial data.
ACHIEVE Study design and inclusion criteria details are reported elsewhere, 25 , 26 as well as details of the recruitment methodology 28 and baseline characteristics of the participants. 29 , 30 In brief, participants included in the ACHIEVE Study were aged 70–84 years, had adult‐onset bilateral hearing loss (better‐ear 4‐frequency (0.5–4 kHz) pure tone average [PTA] ≥30 dB HL [decibel hearing level] and <70 dB HL), no current hearing aid use, and no substantial cognitive impairment at the time of enrollment (Mini‐Mental State Examination [MMSE] 31 score ≥23 for participants with a high school degree or less, ≥25 for participants with some college education or more). While the overarching goal of the ACHIEVE Study was to test the effect of hearing intervention on 3‐year cognitive decline, pre‐defined exploratory outcomes included evaluating the effects of hearing intervention on other health outcomes, including self‐perceived communicative function, which was examined in the current analysis.
Measures
Self‐perceived communicative function
The Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) 32 , 33 served as a measure of communicative function. The HHIE‐S represents the WHO‐ICF model of individual health and wellness, 9 , 34 and captures the impact of hearing loss on communicative function inclusive of activity, participation, and personal/environmental contexts. The HHIE‐S is a validated and highly reliable (r = 0.97) 10‐item questionnaire that measures the psychosocial and emotional impacts of hearing loss in adults aged 65 years and older. The HHIE‐S is frequently used to measure hearing aid intervention benefit. 19 , 23 , 35 , 36 , 37 , 38 , 39
Questions are phrased so the functional impairment is directly attributable to hearing loss; that is, “Does a hearing problem cause you to … attend religious services less often than you would like?” and “… feel embarrassed when meeting new people?” HHIE‐S scores range from 0 to 40 with higher scores indicating greater self‐perceived handicap, and can be categorized into None (0–8), Mild–Moderate (10–24), and Severe (26–40). 33 HHIE‐S scores ≥10 are considered clinically actionable indicating a clinically significant communicative impairment. In the current analysis, the HHIE‐S score was modeled both as a continuous score and as a categorical score.
The HHIE‐S was collected by a trained interviewer at baseline and during the 6‐month, year‐1, year‐2, and year‐3 follow‐up visits. Due to the COVID‐19 pandemic, all study sites were closed for in‐person assessment from March 2020 to June 2021, so during this time, HHIE‐S data were collected via telephone. Prior to telephone interviews, participants were mailed response cards for visual support to aid in collecting responses. Final year‐3 data collection was completed face‐to‐face at all study sites from June 2021 to November 2022.
Covariates
Baseline covariates were selected based on their known association with hearing loss. Sociodemographic variables collected via face‐to‐face interview included age (years), race (White, Black, other), sex (male, female), educational attainment (less than high school, high school diploma or equivalent, greater than high school), marital status (married, not married), living arrangements (alone, with others), annual income (<$25,000, $25,000‐49,999, $50,000‐74,999, $75,000–$100,000, >$100,000), if data were collected in person or via telephone, and recruitment characteristics such as recruitment route (ARIC vs de novo cohort) and whether the participant was part of a recruited spousal pair.
Health‐related covariates included hypertension (measured systolic blood pressure ≥140, diastolic blood pressure ≥90, or self‐reported medication use for lowering blood pressure); diabetes status (measured fasting blood glucose ≥126 mg/dL, non‐fasting level ≥200 mg/dL, self‐reported diagnosis of diabetes by a physician, or self‐reported medication use for diabetes); smoking and drinking history (never, former, and current); symptoms of depression (Center for Epidemiological Studies—Depression [CES‐D] total score) 40 and loneliness (UCLA Loneliness scale 41 ) 4‐frequency pure tone average (0.5, 1, 2, and 4 kHz; PTA) for the better‐hearing ear; and global cognitive factor score. The global cognition factor score was derived from a comprehensive neurocognitive battery. Tests included delayed word recall, digit symbol substitution, incidental learning, trail making parts A and B, logical memory, digit span backwards, Boston naming, word fluency, and animal naming. Standardized factor scores were developed using a latent variable modeling approach that has been previously used and validated. 25
Procedures
Detailed procedures for the ACHIEVE Study were previously described. 25 , 26 Briefly, participants completed screening and baseline visits, four intervention visits, and then were followed semi‐annually for the next 3 years. During annual assessments, a full neurocognitive test battery was administered by psychometrists trained and supervised by a neuropsychologist. Tests included delayed word recall, digit symbol substitution, incidental learning, trail making parts A and B, logical memory, digit span backwards, Boston naming, word fluency, and animal naming. Standardized factor scores were developed using a latent variable modeling approach that has been previously used and validated. The ACHIEVE hearing intervention, 42 was delivered over 4 sessions across 8–10 weeks. During the intervention period, each participant received a comprehensive hearing evaluation, hearing aids and other assistive devices, and ongoing hearing‐related counseling driven by prioritized listening goals set by each participant. Re‐instruction in use of devices and hearing rehabilitative strategies was provided during booster visits held every 6 months.
Participants randomized to the health education control intervention used content from the “10 Keys™ to Healthy Aging” program, 43 which provided opportunities to engage in self‐management of medical conditions and overall health. 43 This control intervention was previously implemented as a control intervention in other trials. 44 , 45 The format of the control intervention was designed to control for degree of staff and participant interaction time and to parallel the intensity of the hearing intervention. Participants met with a health educator every 1 to 3 weeks for a total of four visits after randomization. Session content was tailored to each participant and included a standardized didactic education component and a 5‐ to 10‐min upper body stretching program. Participants returned for booster sessions every 6 months.
Analytic plan
We described participant characteristics at baseline by randomization group and by recruitment route using chi‐square tests for categorical variables and Kruskal–Wallis analysis of variance for continuous variables, and we calculated the proportion of individuals with mild or greater handicap (HHIE total score ≥10) between randomization groups at each follow‐up visit.
We estimated the effect of the hearing intervention on perceived communicative function over 3 years under the intention‐to‐treat principle using a two‐level (a random intercept and a random time slope for each participant) linear mixed effect model with an identity covariance matrix. Estimated changes, 95% confidence intervals (95% CI), and p values were obtained using restricted maximum likelihood with a Kenward‐Roger correction. We estimated both unadjusted and fully adjusted models treating time as a discrete variable. The unadjusted model included a binary variable for the intervention (hearing intervention vs health education control) and the interaction of time with the intervention group variable. The fully adjusted model additionally included baseline age, race, sex, education, marital status, living arrangements, income, recruitment route, being part of a recruited pair, hypertension, diabetes, smoking and drinking status, depression, loneliness, global cognitive factor score, better‐ear PTA, whether the outcome was assessed over the phone or in person, and the interaction between time and all additional covariates.
We performed a series of sensitivity analyses including stratifying by study population. We imputed missing covariates and HHIE‐S scores for each study visit using multiple imputation by chained equations. Only missing HHIE‐S scores due to incomplete items or participants lost to follow up were imputed. All potential assessments after death were excluded from the imputation process. This strategy imputed every HHIE‐S item separately and included all covariates from the fully adjusted model plus age squared. Twenty imputed datasets were generated following a 100‐iteration burn‐in period. For each of the HHIE‐S items and baseline covariates, future HHIE‐S assessments were excluded from the equations. Second, the COVID‐19 global pandemic and related lockdowns might have impacted participants' perception of their communicative function. In sensitivity analyses, we included a binary variable that takes the value of one if the HHIE‐S instrument was collected after March 16, 2020, and zero otherwise. As COVID‐19 guidelines and perceptions might have varied by state, these secondary analyses also included the interaction between recruitment site and the COVID‐19 variable. We also completed a restricted analysis between baseline and Year‐3 when all data collection took place in‐person only.
We also assessed the per‐protocol and complier average causal effect using a two‐stage least squares approach and stratified analyses by recruitment route (ARIC, de novo). HHIE‐S was an exploratory outcome of the ACHIEVE Study and analyses were considered hypothesis‐generating rather than hypothesis‐testing. Thus, we focus on the patterns of effect across outcomes instead of evaluating statistical significance. p‐values were provided for descriptive purposes only. All analyses were done in Stata/SE 18.0.
RESULTS
Participant demographics and clinical characteristics
A total of 977 participants were randomized in the ACHIEVE trial: 490 to the hearing intervention and 487 to the health education control. Baseline characteristics of the full sample can be found elsewhere. 28 , 29 The current analysis excluded all participants with missing covariates (n = 47 excluded for missing covariates: education, n = 1; income, n = 27; hypertension status, n = 3; and UCLA loneliness score, n = 16) or missing HHIE‐S total score (n = 7), yielding a final analytic sample of N = 923 participants, 461 from the hearing intervention and 462 from the health education control group. (See Figure 1).
FIGURE 1.
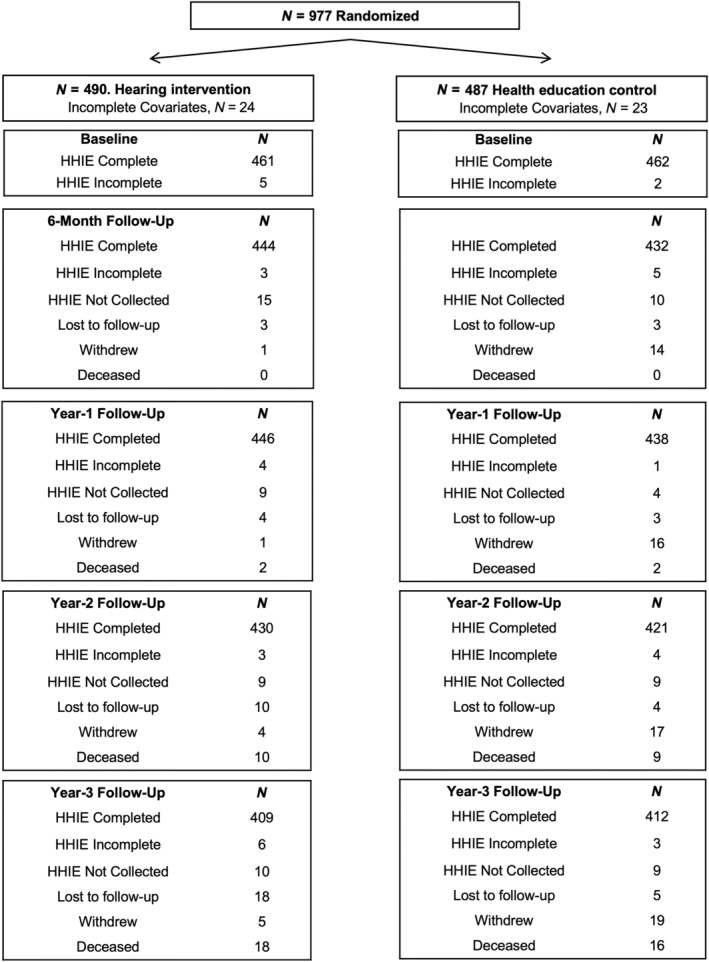
Modified consort flow diagram showing how many participants were enrolled (n = 977); randomized to either the hearing intervention (n = 490) or the health education control intervention (n = 487); and the complete datasets from baseline through Year 3 follow‐up. Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) was the primary outcome of interest and detailed completed, incomplete or missing scores are noted at all assessment time points.
At baseline, participants' mean age was 76.7 years (standard deviation [SD] = 4.0) and 53.0% were female. Most participants self‐identified as being White (87.9%), while 30.2% reported living alone, and 61.9% reported being currently married. In terms of educational attainment, over half of participants (53.0%) reported having a bachelor's degree or higher and 43.1% had a high school diploma or had completed some college. Only 3.9% of participants in our sample did not complete high school. (Table 1).
TABLE 1.
Baseline demographic, functional, and clinical characteristics including hearing aid usage and communication function of ACHIEVE participants stratified by randomly assigned treatment.
| Total | Control | Hearing intervention | ||
|---|---|---|---|---|
| N = 923 | N = 462 | N = 461 | p‐value | |
| Demographics | ||||
| Age, years, mean (SD) | 76.7 (4.0) | 77.0 (4.0) | 77.5 (4.0) | 0.07 |
| Sex, N (%) | 0.92 | |||
| Male | 434 (47.0) | 218 (47.2) | 216 (46.9) | |
| Female | 489 (53.0) | 244 (52.8) | 245 (53.2) | |
| Race, N (%) | 0.60 | |||
| White | 811 (87.9) | 401 (86.8) | 410 (88.9) | |
| Black | 105 (11.4) | 57 (12.3) | 48 (10.4) | |
| Other | 7 (0.8) | 4 (0.9) | 3 (0.7) | |
| Education, N (%) | 0.96 | |||
| Less than high school | 36 (3.9) | 18 (3.9) | 18 (3.9) | |
| High school or some college | 398 (43.1) | 197 (42.6) | 201 (43.6) | |
| College or more | 489 (53.0) | 247 (53.5) | 242 (52.5) | |
| Marital status, N (%) | 0.27 | |||
| Married | 571 (61.9) | 294 (63.6) | 277 (60.1) | |
| Not married | 352 (38.1) | 168 (36.4) | 184 (39.9) | |
| Living alone, N (%) | 279 (30.2) | 131 (28.4) | 148 (32.1) | 0.21 |
| Household income, N (%) | 0.08 | |||
| <$25,000 | 143 (15.5) | 72 (15.6) | 71 (15.4) | |
| $25,000–$49,999 | 275 (29.8) | 122 (26.4) | 153 (33.2) | |
| $50,000–$74,999 | 207 (22.4) | 119 (25.8) | 88 (19.1) | |
| $75,000–$100,000 | 137 (14.8) | 71 (15.4) | 66 (14.3) | |
| >$100,000 | 161 (17.4) | 78 (16.9) | 83 (18.0) | |
| Recruitment route descriptions | ||||
| Field center, N (%) | 0.89 | |||
| Forsyth County, NC | 221 (23.9) | 113 (24.5) | 108 (23.4) | |
| Jackson, MS | 235 (25.5) | 121 (26.2) | 114 (24.7) | |
| Minneapolis suburbs, MN | 215 (23.3) | 106 (22.9) | 109 (23.6) | |
| Washington County, MD | 252 (27.3) | 122 (26.4) | 130 (28.2) | |
| Recruitment route, N (%) | 0.74 | |||
| ARIC | 216 (23.4) | 106 (22.9) | 110 (23.9) | |
| De novo | 707 (76.6) | 356 (77.1) | 351 (76.1) | |
| Participant part of a spousal pair, N (%) | 86 (9.3) | 43 (9.3) | 43 (9.3) | 0.99 |
| Baseline functional and clinical characteristics | ||||
| Hypertension, a N (%) | 530 (57.4) | 271 (58.7) | 259 (56.2) | 0.45 |
| Diabetes, b N (%) | 183 (19.8) | 85 (18.4) | 98 (21.3) | 0.28 |
| Drinking status, N (%) | 0.85 | |||
| Current | 524 (56.8) | 260 (56.3) | 264 (57.3) | |
| Former | 230 (24.9) | 114 (24.7) | 116 (25.2) | |
| Never | 169 (18.3) | 88 (19.1) | 81 (17.6) | |
| Cigarette smoking, N (%) | 0.25 | |||
| Current | 24 (2.6) | 8 (1.7) | 16 (3.5) | |
| Former | 421 (45.6) | 213 (46.1) | 208 (45.1) | |
| Never | 478 (51.8) | 241 (52.2) | 237 (51.4) | |
| CES‐depression, c mean (SD) | 2.5 (2.5) | 2.4 (2.4) | 2.5 (2.7) | 0.68 |
| UCLA loneliness, mean (SD) | 32.8 (8.5) | 32.6 (8.5) | 32.9 (8.5) | 0.60 |
| Prorated MMSE, d mean (SD) | 28.2 (1.6) | 28.2 (1.6) | 28.2 (1.6) | 0.93 |
| Global cognition factor score, e mean (SD) | 0.0 (0.9) | 0.0 (0.9) | 0.0 (1.0) | 0.92 |
| Better‐ear PTA, f mean (SD) | 39.4 (6.9) | 39.3 (6.7) | 39.6 (7.1) | 0.55 |
| Baseline HHIE‐S score (continuous), mean (SD) | 15.4 (9.8) | 15.0 (9.4) | 15.8 (10.2) | 0.24 |
| Baseline HHIE‐S score (categorical), g N (%) | 0.85 | |||
| No hearing handicap (HHIE‐S <10) | 287 (31.1) | 145 (31.4) | 142 (30.8) | |
| Mild or greater handicap (HHIE‐S ≥10) | 636 (68.9) | 317 (68.6) | 319 (69.2) | |
| Hearing aid usage, h mean (SD) | 6.6 (4.5) | – | 6.6 (4.5) | |
Abbreviations: ARIC, Atherosclerosis Risk in Communities Study; MD, Maryland; MN, Minnesota; MS, Mississippi; NC, North Carolina; SD, standard deviation; UCLA, University of California, Los Angeles.
Hypertension was defined as measured systolic blood pressure ≥140, diastolic blood pressure ≥90, or self‐reported medication use for lowering blood pressure.
Diabetes was defined as measured fasting blood glucose ≥126 mg/dL, non‐fasting level ≥200 mg/dL, self‐reported diagnosis of diabetes by a physician, or self‐reported medication use for diabetes.
Depression was defined with the 11‐item Center for Epidemiologic Studies Depression (CES‐D) Scale which measures frequency of depressive symptoms in the past week. No depressive symptomology: score range: 0–9; Depressive symptomology: score range: 9.
Mini‐Mental State Examination [MMSE] was used to measure baseline cognition. A MMSE score ≥23 for participants with a high school degree or less, ≥25 for participants with some college education or more was used to define normal cognition.
Global cognition factor score was derived from a comprehensive neurocognitive battery. Tests included delayed word recall, digit symbol substitution, incidental learning, trail making parts A and B, logical memory, digit span backwards, Boston naming, word fluency, and animal naming. Standardized factor scores were developed using a latent variable modeling approach that has been previously used and validated.
Hearing was measured audiometrically and summarized with a 4‐frequency pure tone average (0.5, 1, 2, and 4 kHz; pure‐tone average [PTA]) for the better‐hearing ear.
HHIE‐S = Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) scores has the following clinical scaling No Handicap (0–8), Mild–Moderate (10–24), and Severe (26–40).
Hearing aid usage was defined by a time‐weighted average of datalogging of the hearing aid use per day between baseline and Year 3. A total of 392 participants had information for average number of hearing aid use between baseline and Year 3.
The mean better‐ear PTA was 39.4 dB HL, consistent with mild‐to‐moderate hearing loss (Table 1). There was no difference in PTA between intervention groups. Participants randomized to the hearing intervention were compliant with a time‐weighted average of datalogging of 6.6 hours (SD = 4.5) of hearing aid use per day between baseline and Year 3.
Of the 923 participants in the analytic sample, 23.4% of participants were recruited from the ARIC Study and the remainder were recruited de novo from the surrounding communities of the four study sites. Table S1 displays the baseline characteristics of the analytic sample by recruitment route (ARIC vs de novo) where it can be seen there were differences between the recruitment cohorts with respect to several demographic features (e.g., age, sex, race, education, income, health conditions, and HHIE‐S score). There were differences on HHIE‐S scores at baseline (p < 0.001); a larger proportion (73.3%) of participants who were recruited de novo from surrounding communities had mild–moderate or greater perceived communicative difficulty (HHIE‐S score ≥10) compared to the ARIC cohort (54.6%), despite nearly equivalent average level of audiometric hearing between ARIC (39.1 dB HL, SD = 6.7) and de novo (39.6 dB HL, SD = 6.9) groups.
Graphically presented in Figures 2A,B and 3, nearly 70% of the total participants had a clinically significant communicative impairment (HHIE‐S score ≥10), and the proportion of participants with mild or greater perceived communicative difficulty was similar by intervention group at baseline. At the 6‐month assessment, the proportion of participants with clinically‐significant communicative impairment (HHIE‐S score ≥10) was lower for hearing intervention (22.3%) compared to health education control (65.0%). Figure 3 and Table S2 show this improvement was maintained at 1 year (hearing intervention: 22.0%, health education control: 64.8%), 2‐year (hearing intervention: 25.8%, health education control: 62.5%), and 3‐year follow‐up assessments (hearing intervention: 34.2%, health education control: 74.8%). Individual change from baseline to 3‐year follow‐up assessment in HHIE‐S score are graphically presented in Figure 2B; nearly all participants in the hearing intervention had lower HHIE‐S scores (improved perceived communicative function) at 3‐year follow‐up, while change in HHIE‐S score in the health education control was mixed.
FIGURE 2.
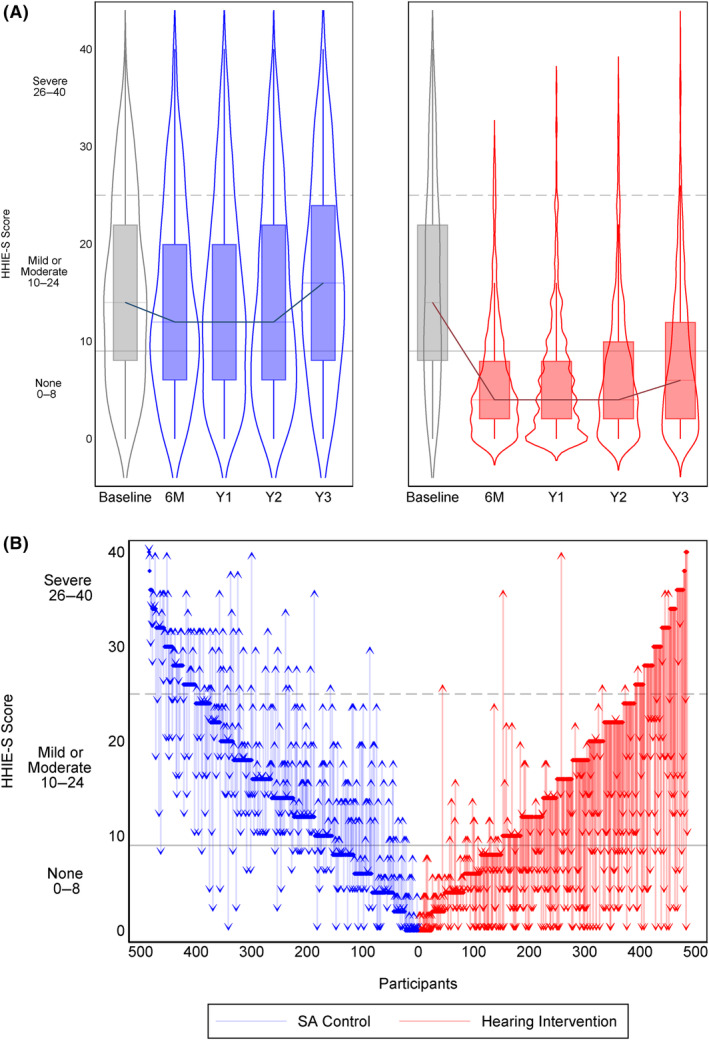
(A and B) Blue represents the control intervention while red represents the hearing intervention group. In the top panel (A), Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) score for each group is shown at Baseline (gray) and at 6 months (6 M) and Years 1–3 (Y1, Y2, Y3). Clinical scaling of the HHIE‐S is shown with gray horizontal lines indicating No Handicap (0–8), Mild–Moderate (10–24), and Severe (26–40). The bottom panel (B) shows a waterfall plot for each participant indicating their starting HHIE‐S score at baseline to their final HHIE‐S score at Year 3.
FIGURE 3.
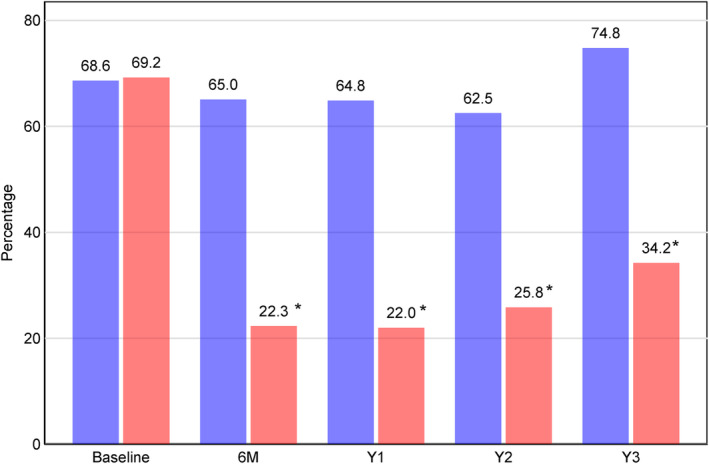
Proportion of participants with clinically significant Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) scores (greater than mild handicap, score ≥10) by intervention assignment. Blue represents the control intervention while red represents the hearing intervention group. *p < 0.001.
Table 2 reports the estimated effect of the hearing intervention on change in HHIE‐S score from baseline to Year 3. Hearing intervention had a positive effect on perceived communicative function in both models at all time points. In fully adjusted models, we estimated a difference of −8.9 (95% CI: −10.4, −7.5) points between intervention and control groups in change in HHIE‐S score from baseline to 6 months, −9.3 (95% CI: −10.8, −7.9) to Year 1, −8.4 (95% CI: −9.8, −6.9) to Year 2, and − 9.5 (95% CI: −11.0, −8.0) to Year 3. In sensitivity analyses (Table S3), the positive effect of hearing intervention on perceived communicative function were statistically similar by recruitment route (ARIC, de novo), but patterns of effect suggest a stronger intervention effect in the de novo cohort compared to the ARIC cohort at 6 months (−9.9 [95% CI: −11.6, −8.3] vs −5.7 [95% CI: −8.6, −2.8]), Year 1 (−10.4 vs −6.0), Year 2 (−9.0 vs −5.9), and Year 3 (−10.3 vs −6.4). Figure S1A,B graphically display change in HHIE‐S among the whole sample and then separated by recruitment source. The magnitude of the intervention effect was larger in per‐protocol (Table S4) and complier average causal effect sensitivity analyses (Table S5). The magnitude of the intervention effect in both sensitivity analyses that included multiple imputation of missing data (Table S6) and inclusion of COVID‐19 covariates (Table S7) was similar to the magnitude of effect observed in primary analyses. The magnitude of the intervention effect in restricted sensitivity analyses where only baseline and Year‐3 were compared (in‐person data collection only) was similar to the analyses with all Years included (Table S8).
TABLE 2.
Estimated effect of the ACHIEVE intervention on HHIE‐S scores over 3 years under the intention to treat principle (N = 923).
| Unadjusted model | Fully adjusted model | |||
|---|---|---|---|---|
| Coefficient (95% CI) | p‐value | Coefficient (95% CI) | p‐value | |
| Baseline | ||||
| Control | 15.0 (14.3, 15.8) | 14.7 (14.0, 15.5) | ||
| Hearing intervention | 15.8 (15.0, 16.6) | 15.3 (14.5, 16.0) | ||
| 6 months | ||||
| Control | 14.1 (13.2, 14.9) | 13.9 (13.2, 14.7) | ||
| Difference from baseline | −1.0 (−2.1, 0.1) | −0.8 (−1.8, 0.3) | ||
| Hearing intervention | 5.8 (5.0, 6.6) | 5.6 (4.8, 6.3) | ||
| Difference from baseline | −10.0 (−11.1, −8.9) | −9.7 (−10.7, −8.7) | ||
| Difference between groups | −9.0 (−10.6, −7.4) | <0.001 | −8.9 (−10.4, −7.5) | <0.001 |
| 1 year | ||||
| Control | 14.2 (13.4, 15.0) | 14.4 (13.6, 15.1) | ||
| Difference from baseline | −0.9 (−2.0, 0.3) | −0.4 (−1.4, 0.7) | ||
| Hearing intervention | 5.7 (4.9, 6.5) | 5.6 (4.9, 6.4) | ||
| Difference from baseline | −10.1 (−11.2, −9.0) | −9.7 (−10.7, −8.6) | ||
| Difference between groups | −9.3 (−10.8, −7.7) | <0.001 | −9.3 (−10.7, −7.9) | <0.001 |
| 2 years | ||||
| Control | 14.2 (13.4, 15.0) | 14.9 (14.0, 15.8) | ||
| Difference from baseline | −0.8 (−2.0, 0.3) | 0.2 (−1.1, 1.5) | ||
| Hearing intervention | 6.6 (5.8, 7.4) | 7.2 (6.2, 8.1) | ||
| Difference from baseline | −9.2 (−10.4, −8.1) | −8.1 (−9.4, −6.9) | ||
| Difference between groups | −8.4 (−10.0, −6.8) | <0.001 | −8.3 (−9.8, −6.8) | <0.001 |
| 3 years | ||||
| Control | 16.3 (15.5, 17.2) | 16.4 (15.6, 17.2) | ||
| Difference from baseline | 1.3 (0.1, 2.4) | 1.7 (0.6, 2.7) | ||
| Hearing intervention | 7.8 (6.9, 8.6) | 7.5 (6.7, 8.3) | ||
| Difference from baseline | −8.1 (−9.2, −6.9) | −7.8 (−8.9, −6.7) | ||
| Difference between groups | −9.4 (−11.0, −7.7) | <0.001 | −9.5 (−11.0, −8.0) | <0.001 |
Note: Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) scores (means and 95% CI) for each group (control vs hearing intervention) is reported at each assessment points and the difference from baseline and between the groups is reported after randomization (6 months and Years 1–3). The clinical scaling of the HHIE‐S is No Handicap (0–8), Mild–Moderate (10–24), and Severe (26–40), thus, a reduction from baseline or between groups indicates reduced impairment. The unadjusted model included a binary variable for the intervention (hearing intervention vs health education control) and the interaction of time with the intervention group variable. The fully adjusted model additionally included baseline age, race, sex, education, marital status, living arrangements, income, recruitment route, being part of a recruited spousal pair, hypertension, diabetes, smoking and drinking status, depression, loneliness, global cognition factor score, better‐ear pure tone average, whether the outcome was assessed over the phone or in person, and the interaction between study visit and all additional covariates.
DISCUSSION
The ACHIEVE Study is the largest and longest randomized controlled trial investigating the effects of hearing intervention on self‐perceived communicative function. Hearing intervention (compared to health education control) had a strong, positive, and clinically significant effect on self‐perceived communication that was evident at 6‐months post‐intervention and was sustained through the end of study follow‐up (3‐years post‐intervention). Findings suggest hearing intervention is effective for improving self‐perceived communicative function among older adults with hearing loss. Greater clinical awareness of hearing and increased uptake of hearing treatment could have benefits for many older adults with hearing loss.
Approximately 70% of participants reported clinically actionable functional impairment at baseline (HHIE‐S score ≥10, mild or greater impairment); with nearly half of the participants in the hearing intervention group reducing impairment to no hearing handicap (HHIE‐S score 0–8). This finding shows that these participants with a significant impairment at baseline later reported good communicative functioning indicating ability to process speech sounds sufficiently to enable the individuals to participate in a wide range of desired daily social listening activities without limitations or restrictions. Findings from the ACHIEVE Study are consistent with prior evidence and expand our current understanding of the sustained benefit of hearing intervention on communicative function. Among 194 veterans, Mulrow conducted a randomized controlled trial to evaluate the effects of hearing intervention versus waitlist control on cognition and also reported improved HHIE scores at 6‐weeks and 4‐month follow‐up. 46 Our results expand to larger clinical population and provide long‐term sustained evidence. More recently, in a prospective observational study 18 of 99 adults aged 60–84 years, compared to an observational control group, almost two‐thirds of those in the hearing intervention group reported significantly improved communicative functioning, with a greater proportion of males reporting significantly more improvement. That study used different tools to capture communicative function but had similar best practices audiological care with detailed hearing aid usage. In a longitudinal cross‐sectional evaluation of hearing aid users (n = 69) and non‐users (n = 597), communicative function benefit (also measured with the HHIE) with hearing aids was reported at 5 and 11 years of follow‐up. 47 We now confirm the improvement with hearing intervention with a much larger sample in a randomized trial design that helps reduce group differences that were either not measured or not fully accounted for by statistical adjustment.
Differences in baseline HHIE‐S scores observed between the ARIC and de novo cohorts highlight the importance for clinicians of actively screening for hearing and encouraging patients to consider hearing intervention. Unlike the de novo cohort, who were self‐selected volunteers specifically interested in a healthy aging study, participants from the ARIC Study represent a randomly‐sampled cohort of the population who joined ACHIEVE based on their ongoing participation in the ARIC Study. The ARIC cohort reported less problems with communication impairment at baseline than the de novo cohort (HHIE‐S 12.1 vs 16.4, respectively) despite equivalent levels of audiometric hearing level. Self‐perceived hearing difficulties is a key factor for hearing aid uptake and use 48 , 49 ; and interestingly, even with the ARIC participants reporting less communicative limitations, they showed improvements in communicative function. The self‐perceived communicative functioning is likely explained by the interest among the self‐selected de novo participants in pursing hearing intervention, while the lower HHIE‐S scores in the ARIC cohort suggest these participants likely would not have voluntarily pursued hearing intervention on their own or reported concerns about hearing to their healthcare provider. However, both cohorts experienced an improvement in communicative functioning with hearing intervention at 6‐months that was sustained at 3‐year follow‐up. The ARIC cohort also experienced the greatest benefit with hearing intervention in reduction of 3‐year cognitive decline, as reported previously in the primary results of the ACHIEVE Study. 25 These findings suggest that clinicians should consider screening for hearing and discussing the role of hearing intervention regardless of participant self‐report of communicative difficultly. Although we recommend the use of the HHIE‐S or comparable versions, 33 , 36 , 50 as a tool to assess communicative function and benefit of hearing intervention, perhaps the combination of self‐perceived function and objective measurements of hearing are needed. Referral to an audiologist is one way for a patient to obtain objective status of their hearing, but clinicians can also encourage patients with a smartphone to test their hearing themselves using apps that can accurately provide a user's 4‐frequency pure tone average (PTA4, also termed the Hearing Number, 51 ). Inclusion criteria for participants in the ACHIEVE Study included a PTA4/Hearing Number between 30 and 70 dB corresponding generally to a mild to moderately‐severe level of hearing loss.
Our study had limitations. Participants and study staff were not masked to intervention assignment, given the nature of the intervention, which may have influenced responses; however, interventionists did not collect outcome data, and participants were masked to the study hypotheses. Participants were informed that both interventions were designed to promote healthy aging and that they would be offered the other intervention after the 3‐year study period. Further, the HHIE‐S was asked verbally by study staff and participants with untreated hearing loss in the health education control may have had more difficulty responding. Although the HHIE‐S is the most widely used and accepted measure of self‐reported communicative function, a single instrument may not fully capture all communicative function domains. We have other planned analyses that will look at other objective and subjective measures of communication function which will be important to compare to the present findings. There are known effects of age and gender on HHIE‐S 52 that were not the focus of this analysis and would require specific analytic approaches to thoroughly evaluate. While we acknowledge the role of ethnicity on hearing care and health related outcomes among Hispanic individuals, 53 the availability of ethnicity information in the ACHIEVE study is limited. Out of the N = 977 recruited participants, ethnicity information is available only for N = 860 (88%). In total only N = 9 (1%) individuals self‐identified as Hispanic, all of which were recruited de novo. Due to these data limitations, our analyses do not account for ethnicity, and we recommend that future research efforts include a more diverse population sample. Our results may not be generalizable to populations outside the ACHIEVE Study from the four field centers, and the best practice hearing intervention may not be scalable to deliver in all clinical environments.
Findings from this study add to the existing evidence supporting a positive effect of hearing intervention on multiple components of health. However, hearing loss remains underdiagnosed and unaddressed. 54 , 55 As the communicative functioning, social engagement, and psychosocial wellbeing of the aging population is important, 9 , 10 , 56 we recommend greater clinical as well as patient awareness of hearing and hearing intervention for older adults with hearing loss. Patients who experience or may not yet perceive hearing‐related limitations in communicative function can both benefit from hearing intervention. We recommend healthcare practitioners provide hearing screening or referral services which can lead to critical identification, diagnosis, education, support, and treatment for older adults with hearing loss. Findings also support efforts to increase affordable access to hearing care through Medicare coverage of quality hearing care and treatment. In the ACHIEVE Study, hearing intervention was effective for improving self‐perceived communicative function over 3 years among older adults with hearing loss. These findings have implications for greater awareness of hearing and increased uptake of hearing treatment which could have benefits for many older adults with hearing loss.
AUTHOR CONTRIBUTIONS
Study concept and design: VAS, EGM, MLA, NSR, JC, JAD, AG, NWG, KH, THM, JSP, JAS, FRL, THC. Acquisition of subjects and/or data: VAS, EGM, MLA, NSR, SF, SB, JC, JAD, LGM, KH, CMM, THM, HNC, JSP, JAS, LS, FRL, THC. Analysis and interpretation of data: VAS, EGM, MLA, NSR, SF, SB, JAD, AG, LGM, ARH, CMM, HNC, JRP, LS, FRL, THC. Preparation of manuscript: VAS, EGM, MLA, ARH, FRL.
CONFLICT OF INTEREST STATEMENT
Dr. Sanchez reported industry funding related to consulting or research support from Otonomy Inc., Autifony Therapeutics Ltd., Boehringer Ingelheim, Frequency Therapeutics Ltd., Pipeline Therapeutics, Aerin Medical, Oticon Medical, Helen of Troy Ltd., Sonova Holding AG, and Phonak USA. Dr. Reed reported serving on the scientific advisory boards of Neosensory. Dr. Schrack is a consultant to Edwards Lifesciences. Dr. Lin reported being a consultant to Frequency Therapeutics and Apple and being the director of a research center funded in part by a philanthropic gift from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. Dr. Lin is also a board member of the nonprofit Access HEARS. All other authors report no relevant disclosures.
SPONSOR'S ROLE
The investigators thank Phonak LLC. Hearing aids, hearing assistive technologies, and related materials were provided at no cost to the researchers or the participants from Phonak LLC; however, the sponsoring manufacturer did not participate in the design, data collection or analysis, nor the reporting within this manuscript.
FINANCIAL DISCLOSURE
The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) Study is supported by the National Institute on Aging (NIA) grant R01AG055426, with magnetic brain resonance examination funded by NIA R01AG060502 and with previous pilot study support NIAR34AG046548 and the Eleanor Schwartz Charitable Foundation, in collaboration with the Atherosclerosis Risk in Communities (ARIC) Study, supported by National Heart, Lung and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data in ARIC is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA, and NIDCD), and with previous brain MRI examinations funded by R01HL70825 from the NHLBI. Hearing aids, hearing assistive technologies, and related materials used in the ACHIEVE Study were provided at no cost to the researchers or the participants from Sonova/Phonak LLC. The funders of the study, nor the sponsoring manufacturer, had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Supporting information
Figure S1. (A and B) Blue represents the control intervention while red represents the hearing intervention group. In the top panel (S1A), Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) score for each group is shown at baseline (0 years from randomization) and at 6 months and Years 1–3 (1, 2, 3 years from randomization). Bottom panel (S1B) shows de novo and ARIC recruitment sources separated out. De novo is represented in solid line (blue control, red intervention) and ARIC is represented with dashed line (blue control, red intervention). Clinical scaling of the HHIE‐S is the following: No Handicap (0–8), Mild–Moderate (10–24), and Severe (26–40).
Table S1. Baseline characteristics of analytic sample by recruitment type.
Table S2. Proportion of study participants with no handicap (HHIE‐S ≤8) and mild or worse hearing handicap (HHIE‐S ≥10) by randomization group.
Table S3. Estimated effect of the ACHIEVE intervention on HHIE‐S scores under the intention to treat principle stratified by recruitment type.
Table S4. Estimated effect of the ACHIEVE intervention on HHIE‐S scores analysis restricted to participants who completed their assigned interventions (per‐protocol analyses) (N = 775).
Table S5. Estimated effect of the ACHIEVE intervention on HHIE‐S scores using complier average causal effect. Two‐stage least squares (N = 923).
Table S6. Estimated effect of the ACHIEVE intervention on HHIE‐S scores using two‐level mixed effect linear model. Multiple imputation analyses (N = 977).
Table S7. Estimated effect of the ACHIEVE intervention on HHIE‐S scores. Two‐level mixed effect linear model. Analyses including COVID‐19 timing related covariates (N = 923).
Table S8. Estimated effect of the ACHIEVE intervention on HHIE‐S scores. Two‐level mixed effect linear model. Analyses restricting our sample to only baseline and Year 3 measures, all of which took place in‐person (N = 923).
ACKNOWLEDGMENTS
Members of the ACHIEVE Collaborative Research Group are listed at achievestudy.org. The authors thank the staff and participants of the ACHIEVE and ARIC studies for their important contributions.
The investigators thank the members of the ACHIEVE DSMB (Doug Galasko, Julie Buring, Judy Dubno, Tom Greene, and Larry Lustig) for their guidance and insights during the course of the study.
Sanchez VA, Arnold ML, Garcia Morales EE, et al. Effect of hearing intervention on communicative function: A secondary analysis of the ACHIEVE randomized controlled trial. J Am Geriatr Soc. 2024;72(12):3784‐3799. doi: 10.1111/jgs.19185
This trial was registered at ClinicalTrials.gov, NCT03243422.
DATA AVAILABILITY STATEMENT
A de‐identified dataset and data dictionary will be made available in 2024 on a publicly available U.S. data repository pending approval by the funding sponsor (National Institute on Aging). Additional details on data access policies will be made available at https://www.achievestudy.org at that time. The study protocol and statistical analysis plan are available at https://www.clinicaltrials.gov. Access to ACHIEVE study manuals and forms are available by contacting the corresponding author.
REFERENCES
- 1. Goman AM, Lin FR. Prevalence of hearing loss by severity in the United States. Am J Public Health. 2016;106:1820‐1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dixon PR, Feeny D, Tomlinson G, et al. Health‐related quality of life changes associated with hearing loss. JAMA Otolaryngol Head Neck Surg. 2020;146:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. National Academies of Sciences Engineering and Medicine . Hearing Health Care for Adults: Priorities for Improving Access and Affordability. The National Academies Press; 2016. [PubMed] [Google Scholar]
- 5. Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol Ser A Biol Sci Med Sci. 2011;66A:1131‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mick P, Pichora‐Fuller MK. Is hearing loss associated with poorer health in older adults who might benefit from hearing screening? Ear Hear. 2016;37:e194‐e201. [DOI] [PubMed] [Google Scholar]
- 7. Martinez‐Amezcua P, Kuo P‐L, Reed NS, et al. Association of hearing impairment with higher‐level physical functioning and walking endurance: results from the Baltimore Longitudinal Study of Aging. J Gerontol Ser A. 2021;76:e290‐e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staff WHO . International Classification of Functioning. World Health Organization; 2001. [Google Scholar]
- 9. Humes LE. An approach to self‐assessed auditory wellness in older adults. Ear Hear. 2021;42:745‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timmer BHB, Bennett RJ, Montano J, et al. Social‐emotional well‐being and adult hearing loss: clinical recommendations. Int J Audiol. 2023;63(6):381‐392. [DOI] [PubMed] [Google Scholar]
- 11. Bott A, Saunders G. A scoping review of studies investigating hearing loss, social isolation and/or loneliness in adults. Int J Audiol. 2021;60:30‐46. [DOI] [PubMed] [Google Scholar]
- 12. Sharma RK, Chern A, Golub JS. Age‐related hearing loss and the development of cognitive impairment and late‐life depression: a scoping overview. Semin Hear. 2021;42:10‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thai A, Khan SI, Choi J, et al. Associations of hearing loss severity and hearing aid use with hospitalization among older US adults. JAMA Otolaryngol Head Neck Surg. 2022;148:1005‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed NS, Gami A, Myers C, et al. Association of Self‐Reported Trouble Hearing and Patient–Provider Communication with Hospitalizations among Medicare Beneficiaries, Seminars in Hearing. Thieme Medical Publishers; 2021:26‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKee MM, Stransky ML, Reichard A. Hearing loss and associated medical conditions among individuals 65 years and older. Disabil Health J. 2018;11:122‐125. [DOI] [PubMed] [Google Scholar]
- 17. Dawes P, Cruickshanks KJ, Fischer ME, Klein BEK, Klein R, Nondahl DM. Hearing‐aid use and long‐term health outcomes: hearing handicap, mental health, social engagement, cognitive function, physical health, and mortality. Int J Audiol. 2015;54:838‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarant J, Harris D, Busby P, et al. The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J Clin Med. 2020;9(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chisolm TH, Johnson CE, Danhauer JL, et al. A systematic review of health‐related quality of life and hearing aids: final report of the American Academy of Audiology task force on the health‐related quality of life benefits of amplification in adults. J Am Acad Audiol. 2007;18:151‐183. [DOI] [PubMed] [Google Scholar]
- 20. Johnson CE, Danhauer JL, Ellis BB, Jilla AM. Hearing aid benefit in patients with mild sensorineural hearing loss: a systematic review. J Am Acad Audiol. 2016;27:293‐310. [DOI] [PubMed] [Google Scholar]
- 21. Feltner C, Wallace IF, Kistler CE, et al. creening for Hearing Loss in Older Adults: An Evidence Review for the U.S. Preventive Services Task Force. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews, Agency for Healthcare Research and Quality (US); 2021. [PubMed] [Google Scholar]
- 22. Ferguson M, Kitterick PT, Chong L, et al. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database Syst Rev. 2017;9(9):CD012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Humes LE, Rogers SE, Quigley TM, Main AK, Kinney DL, Herring C. The effects of service‐delivery model and purchase price on hearing‐aid outcomes in older adults: a randomized double‐blind placebo‐controlled clinical trial. Am J Audiol. 2017;26:53‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Sousa KC, Manchaiah V, Moore DR, et al. Effectiveness of an over‐the‐counter self‐fitting hearing aid compared with an audiologist‐fitted hearing aid: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2023;149:522‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin FR, Pike JR, Albert MS, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402:786‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deal JA, Goman AM, Albert MS, et al. Hearing treatment for reducing cognitive decline: design and methods of the aging and cognitive health evaluation in elders randomized controlled trial. Alzheimers Dement Diagn Assess Dis Monit. 2018;4:499‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ARIC I . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687‐702. [PubMed] [Google Scholar]
- 28. Reed NS, Gravens‐Mueller L, Huang AR, et al. Recruitment and baseline data of the aging and cognitive health evaluation in elders (ACHIEVE) study: a randomized trial of a hearing loss intervention for reducing cognitive decline. Alzheimers Dement Transl Res Clin Interv. 2024;10:e12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez VA, Arnold ML, Betz JF, et al. Description of the baseline audiologic characteristics of the participants enrolled in the aging and cognitive health evaluation in elders study. Am J Audiol. 2024;33(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang AR, Reed NS, Deal JA, et al. Depression and health‐related quality of life among older adults with hearing loss in the ACHIEVE Study. J Appl Gerontol. 2024;43(5):550‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arevalo‐Rodriguez I, Smailagic N, Roqué‐Figuls M, et al. Mini‐mental state examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2021;7:Cd010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. Asha. 1983;25:37‐42. [PubMed] [Google Scholar]
- 33. Lichtenstein MJ, Bess FH, Logan SA. Diagnostic performance of the hearing handicap inventory for the elderly (screening version) against differing definitions of hearing loss. Ear Hear. 1988;9:208‐211. [DOI] [PubMed] [Google Scholar]
- 34. Humes LE. The World Health Organization's hearing‐impairment grading system: an evaluation for unaided communication in age‐related hearing loss. Int J Audiol. 2019;58:12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Humes LE, Wilson DL, Barlow NN, Garner C. Changes in hearing‐aid benefit following 1 or 2 years of hearing‐aid use by older adults. J Speech Lang Hear Res. 2002;45:772‐782. [DOI] [PubMed] [Google Scholar]
- 36. Newman CW, Weinstein BE, Jacobson GP, Hug GA. Test–retest reliability of the hearing handicap inventory for adults. Ear Hear. 1991;12:355‐357. [DOI] [PubMed] [Google Scholar]
- 37. Newman CW, Weinstein BE, Jacobson GP, Hug GA. The hearing handicap inventory for adults: psychometric adequacy and audiometric correlates. Ear Hear. 1990;11:430‐433. [DOI] [PubMed] [Google Scholar]
- 38. Humes LE, Halling D, Coughlin M. Reliability and stability of various hearing‐aid outcome measures in a group of elderly hearing‐aid wearers. J Speech Hear Res. 1996;39:923‐935. [DOI] [PubMed] [Google Scholar]
- 39. Humes LE, Kinney DL, Main AK, Rogers SE. A follow‐up clinical trial evaluating the consumer‐decides service delivery model. Am J Audiol. 2019;28:69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kohout FJ, Berkman LF, Evans DA, Cornoni‐Huntley J. Two shorter forms of the CES‐D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179‐193. [DOI] [PubMed] [Google Scholar]
- 41. Russell D, Peplau LA, Ferguson ML. Developing a measure of loneliness. J Pers Assess. 1978;42:290‐294. [DOI] [PubMed] [Google Scholar]
- 42. Sanchez VA, Arnold ML, Reed NS, et al. The hearing intervention for the aging and cognitive health evaluation in elders randomized controlled trial: Manualization and feasibility study. Ear Hear. 2020;41:1333‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newman AB, Bayles CM, Milas CN, et al. The 10 keys to health aging: findings from an innovative prevention program in the community. J Aging Health. 2010;22:547‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morone NE, Greco CM, Moore CG, et al. A mind‐body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016;176:329‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Venditti EM, Zgibor JC, Vander Bilt J, et al. Mobility and vitality lifestyle program (MOVE UP): a community health worker intervention for older adults with obesity to improve weight, health, and physical function. Innov Aging. 2018;2:igy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mulrow CD. Quality‐of‐life changes and hearing impairment. Ann Intern Med. 1990;113:188. [DOI] [PubMed] [Google Scholar]
- 47. Dawes P, Fortnum H, Moore DR, et al. Hearing in middle age: a population snapshot of 40–69 year olds in the UK. Ear Hear. 2014;35:44‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Knudsen LV, Oberg M, Nielsen C e. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends Amplif. 2010;14:127‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyer C, Hickson L, Lovelock K, Lampert M, Khan A. An investigation of factors that influence help‐seeking for hearing impairment in older adults. Int J Audiol. 2014;53:S3‐S17. [DOI] [PubMed] [Google Scholar]
- 50. Cassarly C, Matthews LJ, Simpson AN, Dubno JR. The revised hearing handicap inventory and screening tool based on psychometric reevaluation of the hearing handicap inventories for the elderly and adults. Ear Hear. 2019;41:95‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lin FR. Age‐related hearing loss. N Engl J Med. 2024;390:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 52. Humes LE, Dubno JR. A comparison of the perceived hearing difficulties of community and clinical samples of older adults. J Speech Lang Hear Res. 2021;64:3653‐3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arnold ML, Hyer K, Small BJ, et al. Factors associated with self‐perceived hearing handicap in adults from Hispanic/Latino background: findings from the Hispanic community health study/study of Latinos. Ear Hear. 2021;42:762‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wallhagen MI, Pettengill E. Hearing impairment: significant but underassessed in primary care settings. J Gerontol Nurs. 2008;34:36‐42. [DOI] [PubMed] [Google Scholar]
- 55. Johnson CE, Danhauer JL, Koch LL, Celani KE, Lopez IP, Williams VA. Hearing and balance screening and referrals for Medicare patients: a national survey of primary care physicians. J Am Acad Audiol. 2008;19:171‐190. [DOI] [PubMed] [Google Scholar]
- 56. Saunders GH, Vercammen C, Timmer BH, et al. Changing the narrative for hearing health in the broader context of healthy living: a call to action. Int J Audiol. 2021;60:86‐91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (A and B) Blue represents the control intervention while red represents the hearing intervention group. In the top panel (S1A), Hearing Handicap Inventory for the Elderly—Screening version (HHIE‐S) score for each group is shown at baseline (0 years from randomization) and at 6 months and Years 1–3 (1, 2, 3 years from randomization). Bottom panel (S1B) shows de novo and ARIC recruitment sources separated out. De novo is represented in solid line (blue control, red intervention) and ARIC is represented with dashed line (blue control, red intervention). Clinical scaling of the HHIE‐S is the following: No Handicap (0–8), Mild–Moderate (10–24), and Severe (26–40).
Table S1. Baseline characteristics of analytic sample by recruitment type.
Table S2. Proportion of study participants with no handicap (HHIE‐S ≤8) and mild or worse hearing handicap (HHIE‐S ≥10) by randomization group.
Table S3. Estimated effect of the ACHIEVE intervention on HHIE‐S scores under the intention to treat principle stratified by recruitment type.
Table S4. Estimated effect of the ACHIEVE intervention on HHIE‐S scores analysis restricted to participants who completed their assigned interventions (per‐protocol analyses) (N = 775).
Table S5. Estimated effect of the ACHIEVE intervention on HHIE‐S scores using complier average causal effect. Two‐stage least squares (N = 923).
Table S6. Estimated effect of the ACHIEVE intervention on HHIE‐S scores using two‐level mixed effect linear model. Multiple imputation analyses (N = 977).
Table S7. Estimated effect of the ACHIEVE intervention on HHIE‐S scores. Two‐level mixed effect linear model. Analyses including COVID‐19 timing related covariates (N = 923).
Table S8. Estimated effect of the ACHIEVE intervention on HHIE‐S scores. Two‐level mixed effect linear model. Analyses restricting our sample to only baseline and Year 3 measures, all of which took place in‐person (N = 923).
Data Availability Statement
A de‐identified dataset and data dictionary will be made available in 2024 on a publicly available U.S. data repository pending approval by the funding sponsor (National Institute on Aging). Additional details on data access policies will be made available at https://www.achievestudy.org at that time. The study protocol and statistical analysis plan are available at https://www.clinicaltrials.gov. Access to ACHIEVE study manuals and forms are available by contacting the corresponding author.


