Abstract
Background
Influenza vaccines are available to help protect persons aged ≥65 years, who experience thousands of influenza hospitalizations annually. Because some influenza vaccines may work better than others, we sought to assess benefit of high‐dose (HD), adjuvanted (ADJ), and recombinant (RIV) influenza vaccines (“enhanced influenza vaccines”) compared with standard‐dose unadjuvanted influenza vaccines (SD) and with one another for prevention of influenza‐associated hospitalizations among persons aged ≥65 years.
Methods
We searched MEDLINE, Embase, CINAHL, Scopus, and Cochrane Library to identify randomized or observational studies published between January 1990 and October 2023 and reporting relative vaccine effectiveness (rVE) of HD, ADJ, or RIV for prevention of influenza‐associated hospitalizations among adults aged ≥65 years. We extracted study data, assessed risk of bias, and conducted random‐effects network meta‐analysis and meta‐regression.
Results
We identified 32 studies with 90 rVE estimates from five randomized and 27 observational studies (71,459,918 vaccinated participants). rVE estimates varied across studies and influenza seasons. Pooled rVE from randomized studies was 20% (95% CI −54 to 59) and 25% (95% CI −19 to 53) for ADJ and HD compared with SD, respectively; rVE was 6% (95% CI −109 to 58) for HD compared with ADJ; these differences were not statistically significant. In observational studies, ADJ, HD, and RIV conferred modestly increased protection compared with SD (rVE ranging from 10% to 19%), with no significant differences between HD, ADJ, and RIV. With enhanced vaccines combined, rVE versus SD was 18% (95% CI 3 to 32) from randomized and 11% (95% CI 8 to 14) from observational evidence. Meta‐regression of observational studies suggested that those requiring laboratory confirmation of influenza reported greater benefit of enhanced vaccines.
Conclusions
HD, ADJ, and RIV provided stronger protection than SD against influenza hospitalizations among older adults. No differences in benefit were observed in comparisons of enhanced influenza vaccines with one another.
Keywords: adjuvanted influenza vaccine, adjuvants, administration and dosage, aged, high‐dose trivalent influenza vaccine, immunologic, influenza vaccines, network meta‐analysis, recombinant influenza vaccine
Short abstract
See related articles by Andrew and McGeer.
Key points
Older adults face a wide choice of annual influenza vaccines, including conventional standard‐dose vaccines as well as enhanced influenza vaccines such as adjuvanted, high‐dose, and recombinant vaccines.
We found that enhanced vaccines conferred an 11%–18% reduction in risk of influenza hospitalization compared with standard influenza vaccines.
No one of the enhanced vaccines offered better protection compared with one another.
Why does this paper matter?
Given the substantial burden of influenza and elevated risk of serious complications of influenza among adults ≥65 years of age, it is important that these individuals receive annual influenza vaccination. Enhanced influenza vaccines—adjuvanted, high‐dose, and recombinant influenza vaccines—provide better protection against serious influenza outcomes, and these vaccines are preferred over standard influenza vaccines. Providers should offer an enhanced influenza vaccine to adults aged 65 and older at an opportunity for vaccine administration, and vaccination should not be delayed if a particular one of the enhanced influenza vaccines is unavailable. If none of these three vaccines is available at an opportunity for vaccine administration, then any other age‐appropriate influenza vaccine should be used.
INTRODUCTION
Severe influenza illness and its complications cause between 140,000 and 710,000 hospitalizations each year in the United States, the majority of which occur among persons aged ≥65 years. 1 , 2 , 3 , 4 About one in 10 older adults hospitalized with influenza will die from their illness 1 ; those who survive often experience poor outcomes after hospital discharge, including persistent diminished functional status 5 , 6 , 7 and need for readmission. 8 Annual vaccination remains the cornerstone of prevention of influenza illness; however, due to diminished humoral and cellular immune responses, 9 influenza vaccines are often less effective among older adults than among younger age groups. 10 , 11
Two influenza vaccines approved in the United States for adults aged ≥65 years were developed with features intended to promote an improved immune response among older adults: high‐dose inactivated influenza vaccine, which contains four times the hemagglutinin antigen dose present in standard influenza vaccines, and MF59‐adjuvanted inactivated vaccine. 12 In addition, recombinant influenza vaccine, approved in the U.S. for persons aged ≥18 years, contains three times the hemagglutinin antigen dose compared with standard influenza vaccines. 12 These vaccines (collectively referred to here as enhanced influenza vaccines) elicit stronger antibody responses compared with standard influenza vaccines 13 , 14 and have been associated with stronger protection against influenza‐associated outcomes in some studies. 15 , 16 , 17 Currently, these vaccines are recommended preferentially when available for persons aged ≥65 years in the United States and Canada. 18 , 19
While relative benefits of enhanced influenza vaccines compared with standard influenza vaccines for prevention of influenza‐associated hospitalizations have been evaluated in a number of observational studies, few have compared these vaccines directly with one another, and no randomized clinical trials have done so. Here, we report a systematic review and network meta‐analysis assessing effectiveness of high‐dose (HD), adjuvanted (ADJ), and recombinant (RIV) influenza vaccines compared with standard‐dose (SD) vaccines and with one another for prevention of influenza‐associated hospitalizations among persons aged ≥65 years.
METHODS
Objective
Our objective was to assess relative efficacy and effectiveness of enhanced influenza vaccines compared with SD and with one another. We followed PRISMA reporting guidelines (PRISMA checklist shown in Table S1). The study protocol is registered in PROSPERO (CRD42020161360).
Data sources and search strategies
In collaboration with a research librarian, we searched MEDLINE, Embase, CINAHL, Scopus, Cochrane Library, and ClinicalTrials.gov from January 1, 1990, through October 19, 2023 (Table S2) for reports of influenza vaccine efficacy/effectiveness among older adults without restrictions on influenza vaccine type or language.
Study selection
The PICO (population, intervention, comparison, and outcome) framework is summarized in Figure 1. Eligible reports were randomized (individually and cluster‐randomized designs) or comparative observational studies (traditional and test‐negative case–control and cohort designs) providing data for ≥1 intervention vaccine (HD, ADJ, or RIV in trivalent or quadrivalent formulations) among adults aged ≥65 years (including studies with additional age groups if results for adults aged ≥65 years were reported separately). We excluded reports of vaccines not used in the U.S. for persons aged ≥65 years (e.g., virosomal, intradermal, live attenuated vaccines, and vaccines containing adjuvant other than MF59); studies of monovalent or bivalent vaccines; animal studies; case reports; and interim reports superseded by final reports. Review articles, communications, and abstracts were excluded but used to identify additional eligible reports.
FIGURE 1.

PICO elements of this systematic review.
Data extraction
Two reviewers independently performed title‐abstract screening and full‐text screening using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia) and data collection using a pilot‐tested form to extract data including study design, methods, outcomes, seasons, and predominant circulating influenza (sub)types, funding source, and effect estimates and their confidence intervals. Discrepancies were resolved via consensus. Authors were contacted to clarify data and methods when needed.
Data synthesis
Effect estimates were measured as relative vaccine effectiveness (rVE), derived when necessary from reported measures of relative effect (hazard ratio, odds ratio, and risk ratio) as (1‐relative effect measure) * 100%. Variances were derived from 95% confidence intervals of effect estimates. Data from randomized trials and observational studies were assessed separately because study design, sources of potential bias, and tools for evaluating risk of bias differ substantially between randomized and observational studies. Pooled mean rVE and 95% confidence intervals (CIs) were calculated using a random‐effects network meta‐analysis with an inverse variance method. A random‐effects model was selected because heterogeneity among study participants and seasonal vaccine efficacy would be unlikely to result in the same vaccine treatment effect across studies.
Because VE of quadrivalent and trivalent SD are similar 20 , 21 and quadrivalent formulations of ADJ and HD were infrequently reported in the eligible studies (<1% of ADJ was quadrivalent in observational and randomized studies; <1% and <15% of HD was quadrivalent in observational and randomized studies, respectively), trivalent and quadrivalent vaccines of similar formulation were grouped, yielding four vaccine types (ADJ, HD, RIV, and SD). Studies reporting exclusively on cell‐cultured SD were excluded; however, studies that may have included cell‐cultured vaccines with other SD vaccines were included. A “no vaccine” group was included to accommodate data reporting absolute VE of enhanced vaccines, which can provide indirect information for estimating rVE in a network meta‐analysis.
We preferentially used season‐ and age‐group specific estimates rather than pooled estimates. Comparisons for which data were wholly contained in another comparison were excluded. When a study reported ≥1 estimate for the same outcome, estimates designated as primary outcomes and/or those with the most comprehensive adjustment for confounding were used. Studies using diagnostic code‐defined outcomes were included if codes contained ≥1 influenza code (ICD9 487 and/or ICD10 J10). If multiple sets of codes were available, codes using the most specific definition of influenza were used. Estimates derived during peak influenza season were used if available. Effect estimates from different influenza seasons or from nonoverlapping age groups reported within the same study were treated as independent. Effect estimates from studies reporting multiple vaccine comparisons in the same season were treated as correlated.
Frequentist network meta‐analysis using R version 4.2.2 and the netmeta package with restricted maximum likelihood was the primary method, supplemented by Bayesian methods for meta‐regression and vaccine rankings. Bayesian models used WinBUGS version 1.4.3 with vague priors, three chains of initial values, ≥50,000 burn‐in and ≥50,000 analytic iterations. Convergence was evaluated by Gelman‐Rubin statistics and model fit compared using the deviance information criterion. Heterogeneity was assessed using Cochran's Q Χ2 statistic and described by between‐studies standard deviation, I2 statistic (ratio of true variance to total variance), and 95% prediction interval (representing variation in effect estimates on an absolute scale 22 ). Consistency between direct and indirect evidence was examined using the Splitting Indirect and Direct Evidence (SIDE) method 23 and a full design‐by‐treatment interaction model. 24 Vaccine rankings were quantified by Surface Under the Cumulative RAnking curve (SUCRA). 25 Meta‐regression models assumed identical interaction effects for all vaccine types. P values <0.05 or CIs excluding the null were considered statistically significant.
Risk of bias
Risk of bias was assessed independently by two reviewers using Cochrane Risk of Bias version 1 for individually and cluster‐randomized studies 26 and ROBINS‐I for observational studies. 27 We determined a priori that control for confounding in observational studies required control for age, site, season, presence of chronic medical conditions, and calendar time of illness onset. Post hoc, we relaxed the requirement for control of calendar time of illness onset.
Sensitivity analyses
Sensitivity analyses included stratification by predominant circulating influenza A subtype; industry financial sponsorship; and use of laboratory‐confirmed outcomes. Sensitivity analysis included a “leave‐one‐out” analysis for the randomized trials. For observational evidence, sensitivity analyses included (1) restricting to studies with moderate or better risk of bias; (2) including five additional studies using a composite outcome of influenza‐associated hospitalizations and emergency department (ED) visits; (3) including one additional study that provided no effect estimates for interventions of interest in the primary report but referenced effect estimates in an ancillary project report that is no longer publicly available and for which peer review status is unknown.
RESULTS
A total of 11,850 citations were identified (Figure S1). Among these, 32 studies (5 individually randomized, 2 cluster‐randomized, and 25 observational studies; Table 1) reported estimates of efficacy/effectiveness for ≥1 intervention and comparison of interest. Study characteristics are provided in Table 1. Tables S3 and S6 present study‐specific effect estimates for randomized and observational studies, respectively.
TABLE 1.
Characteristics of included studies assessing association between enhanced influenza vaccines and risk of influenza‐associated hospitalizations among adults aged ≥65 years (n = 32).
| Study | Location | Season(s) | Vaccine groups (n) | Outcomes | Data source | Funding source |
|---|---|---|---|---|---|---|
| Individually randomized studies (n = 3) | ||||||
| DiazGranados 2015 28 |
United States Canada |
2011–2012 2012–2013 |
HD‐IIV3 (15,990) SD‐IIV3 (15,993) |
Serious events (primarily hospitalizations) adjudicated as probably due to influenza |
RCT | Manufacturer (Sanofi) |
| Vardeny 2021 29 |
United States Canada |
2016–2017 to 2018–2019 |
HD‐IIV3 (2606) SD‐IIV3 (2604) |
Hospitalizations adjudicated as related to influenza or pneumonia | RCT | Government and manufacturer (Sanofi) |
| Johansen 2023 30 | Denmark | 2021–2022 |
HD‐IIV4 (6245) SD‐IIV4 (6232) |
Hospitalizations for pneumonia or influenza (ICD10 J09‐J18) | Danish Health Data Authority | Manufacturer (Sanofi) |
| Cluster‐randomized studies (n = 2) | ||||||
| Gravenstein 2017 31 | United States | 2013–2014 |
HD‐IIV3 (19,127) SD‐IIV3 (19,129) |
Hospitalizations for pneumonia or influenza from Medicare Part A claims (ICD9 460–466, 480–488, 490–496, 500–518) | RCT and US Medicare | Manufacturer (Sanofi) |
| McConeghy 2020 32 | United States | 2016–2017 |
aIIV3 (24,926) SD‐IIV3 (25,286) |
Hospitalizations for pneumonia or influenza from Medicare Part A claims (ICD10 J09‐J18) | RCT and US Medicare | Manufacturer (Seqiris) |
| Cohort studies (n = 12) | ||||||
| Cocchio 2020 35 | Italy |
2011–2012 to 2016–2017 |
aIIV3 (68,660) SD‐IIV4 (410,737) |
Hospitalizations for pneumonia or influenza (ICD9 482.9, 485, 486, 487) a | Italian National Health Service | Government |
| Izurieta 2019 39 | United States | 2017–2018 |
HD‐IIV3 (8,488,136) aIIV3 (1,466,918) SD‐IIV4 (1,822,162) SD‐IIV3 (994,763) |
Hospitalizations for influenza (ICD10 J09, J10, J11, J129) | US Medicare | Government |
| Izurieta 2020 40 | United States | 2018–2019 |
HD‐IIV3 (7,905,252) aIIV3 (2,100,592) SD‐IIV4 (1,454,340) |
Hospitalizations for influenza (ICD10 J09, J10, J11, and J129) | US Medicare | Government |
| Izurieta 2021 17 | United States | 2019–2020 |
HD‐IIV3 (7,173,433) aIIV3 (2,565,513) RIV4 (608,433) SD‐IIV4 (1,584,451) |
Hospitalizations for influenza (ICD10 J09, J10, J11, J129) |
US Medicare | Government |
| Lu 2019 41 , b | United States |
2012–2013 to 2017–2018 |
HD‐IIV3 (10,990,902) SD‐IIV3 (2,449,025) |
Hospitalizations for influenza (ICD10 J09, J10, J11, J129; ICD9 487, 488) | US Medicare | Government |
| Mannino 2012 42 | Italy |
2006–2007 to 2008–2009 |
aIIV3 (84,665) SD‐IIV3 (79,589) |
Hospitalizations for pneumonia or influenza (ICD9 480–487) |
Italian National Health Care System | Manufacturer (Novartis) |
| Paudel 2020 45 | United States |
2011–2012 to 2014–2015 |
HD‐IIV3 (5,737,876) SD‐IIV3 (5,737,876) |
Hospitalizations for pneumonia or influenza (ICD9 codes, any position, not specified) | US Medicare | Manufacturer (Sanofi) |
| Richardson 2015 50 | United States | 2010–2011 |
HD‐IIV3 (25,714) SD‐IIV3 (139,511) |
Hospitalizations for pneumonia or influenza (ICD9 480–487 as primary diagnosis) |
US VHA | Government |
| Robison 2018 51 | United States | 2016–2017 |
HD‐IIV3 (23,712) SD‐IIV3 (23,712) |
Hospitalizations (PCR‐confirmed) |
CDC Influenza Surveillance Network (FluSurvNet) |
Government |
| van Aalst 2020 55 | United States |
2016–2017 to 2017–2018 |
HD‐IIV3 (1,900,920) aIIV3 (223,793) |
Hospitalizations for respiratory illness (ICD10 Jxx, primary discharge diagnosis) | Optum Clinformatics Data Mart c | Manufacturer (Sanofi) |
| Young‐Xu 2018 56 | United States | 2015–2016 |
HD‐IIV3 (49,091) SD‐IIV3 (24,682) |
Hospitalizations for pneumonia or influenza (ICD9 480–488 as a principal or secondary diagnosis) | US VHA | Manufacturer (Sanofi) |
| Young‐Xu 2019 57 | United States |
2010–2011 to 2014–2015 |
HD‐IIV3 (158,636) SD‐IIV3 (3,480,288) person‐seasons |
Hospitalizations for pneumonia or influenza (ICD9 480–488) | US VHA | Manufacturer (Sanofi) |
| Case–control studies (n = 15) | ||||||
| Bella 2019 33 | Italy | 2017–2018 |
aIIV3 (228) No vaccine (252) |
Hospitalizations (PCR‐confirmed) | Government | |
| Cheng 2019 34 | Australia | 2018 |
aIIV3 (NR) HD‐IIV3 (NR) No vaccine (NR) |
Hospitalizations (nucleic acid test‐confirmed) | Government | |
| Doyle 2021 36 | United States |
2015–2016 to 2016–2017 |
HD‐IIV3 (622) SD‐IIV (485) |
Hospitalizations (PCR‐confirmed) | CDC Hospitalized Adult Influenza Vaccine Effectiveness Network | Government |
| Gasparini 2013 37 | Italy | 2010–2011 |
aIIV3 (88) No vaccine (139) |
Hospitalizations for pneumonia or influenza (ICD9 480–487) | University | |
| Grijalva 2021 38 | United States | 2019–2020 |
HD‐IIV3 (142) No vaccine (63) |
Hospitalizations (PCR‐confirmed) | CDC IVY network | Government |
| Mira‐Iglesias 2019 43 | Spain | 2017–2018 |
aIIV3 (339) No vaccine (727) |
Hospitalizations (PCR‐confirmed) | Government and manufacturer (Sanofi) | |
| Mira‐Iglesias 2021 44 | Spain | 2018–2019 |
aIIV3 (305) No vaccine (245) |
Hospitalizations (PCR‐confirmed) | Government and manufacturer (Sanofi) | |
| Pebody 2020 46 | England | 2018–2019 |
aIIV3 (818) SD‐IIV (21) |
Hospitalizations (PCR‐confirmed) | Government | |
| Pott 2023 47 | Canada |
2012–2013 to 2014–2015 |
aIIV3 (526) SD‐IIV3 (3364) |
Hospitalizations (PCR‐confirmed) | Canadian SOS platform | Government and manufacturer (GlaxoSmithKline) |
| Puig‐Barbera 2004 48 | Spain | 2002–2003 |
aIIV3 (486) No vaccine (329) |
Hospitalizations for pneumonia, including for influenza (ICD9 480–487) | Case–control study hospital records | Not reported |
| Puig‐Barbera 2007 49 | Spain | 2004–2005 |
aIIV3 (401) No vaccine (118) |
Hospitalizations for pneumonia, including for influenza (ICD9 480–487) | Case–control study hospital records | Not reported |
| Spadea 2014 52 | Italy |
2010–2011 to 2011–2012 |
aIIV3 (519) SD‐IIV3 (678) No vaccine (1911) |
Hospitalizations for pneumonia or influenza (ICD9 480–487) | Not reported | |
| Stuurman 2021 53 | Finland, Romania, France, Italy, Spain | 2019–2020 |
aIIV3 (432) SD‐IIV3 (11) SD‐IIV4 (312) d No vaccine (791) |
Hospitalizations (PCR‐confirmed) | European DRIVE platform | Government and manufacturer (Sanofi) |
| Stuurman 2023 54 | Iceland, Italy, France, Romania, Spain | 2021–2022 |
aIIV3 (241) aIIV4 (193) HD‐IIV4 (19) SD‐IIV4 (318) e No vaccine (591) |
Hospitalizations (PCR‐confirmed) | European DRIVE platform | Government and manufacturer (Sanofi) |
| Zimmerman 2023 58 | United States |
2018–2019 to 2019–2020 |
RIV4 (3338) SD‐IIV4 (976) |
Hospitalizations (PCR‐confirmed) | EMR and Theradoc database f | Manufacturer (Sanofi) |
Abbreviations: aIIV3, trivalent MF‐59 adjuvanted inactivated influenza vaccine; aIIV4, quadrivalent MF‐59 adjuvanted inactivated influenza vaccine; CPT, Current Procedural Technology; EMR, electronic medical record; ER, emergency room; HD‐IIV3, trivalent high‐dose inactivated influenza vaccine; HD‐IIV4, quadrivalent high‐dose inactivated influenza vaccine; ICD, International Classification of Diseases code; ILI, influenza‐like illness; LCI, laboratory‐confirmed influenza; NR, not reported; P&I, pneumonia and influenza; PCR, polymerase chain reaction; RCT, randomized clinical trial; RIV4, quadrivalent recombinant influenza vaccine; SD‐IIV, standard‐dose inactivated influenza vaccine of combined or unknown valence; SD‐IIV3, trivalent standard‐dose inactivated influenza vaccine; SD‐IIV4, quadrivalent standard‐dose inactivated influenza vaccine; VE, vaccine efficacy/effectiveness; VHA, Veterans' Health Administration.
Cocchio 2020 includes hospital discharge records with ICD‐9‐CM 482.9 for bacterial pneumonia, unspecified; 485 for bronchopneumonia, unspecified organism; 486 for pneumonia, unspecified organism; and 487 for influenza; definition of “hospitalization” not specified.
Sample size for Lu 2019 does not include estimates from the 2017 to 18 influenza season, which were excluded because they are redundant with data reported in Izurieta 2019.
CDM is a database of administrative health claims from members of a large national U.S. managed care company with 17–19 million annually covered members and includes data from both commercial and Medicare Advantage health plans.
SD‐IIV4 from Stuurman 2021 includes Fluarix Tetra, Influvac Tetra, and VaxiGrip Tetra, with reported estimates for each of these vaccines pooled with pairwise fixed effects meta‐analysis to yield one estimate for SD‐IIV4 versus no vaccine.
SD‐IIV4 from Stuurman 2023 includes Fluarix Tetra, Influvac Tetra, and VaxiGrip Tetra from reported sensitivity analysis combining all SD‐IIV4 vaccines (vs no vaccine).
Theradoc® is an infection control software used to identify patients tested at clinician's discretion for influenza.
Randomized studies
Five randomized studies reported five effect estimates comprising 137,938 vaccinated participants 28 , 29 , 30 , 31 , 32 and six influenza seasons (four influenza A(H3N2)‐predominant and two with mixed viral strain predominance). Three studies were individually randomized 28 , 29 , 30 and two were cluster‐randomized 31 , 32 ; all were vaccine industry‐sponsored. Reports included four direct comparisons between HD and SD and one direct comparison between ADJ and SD. There were no direct comparisons of ADJ with HD or comparisons including RIV. The network included 69,044 recipients of SD (13% quadrivalent); 43,968 recipients of HD (14% quadrivalent), and 24,926 recipients of ADJ (0% quadrivalent) (Figure 2).
FIGURE 2.
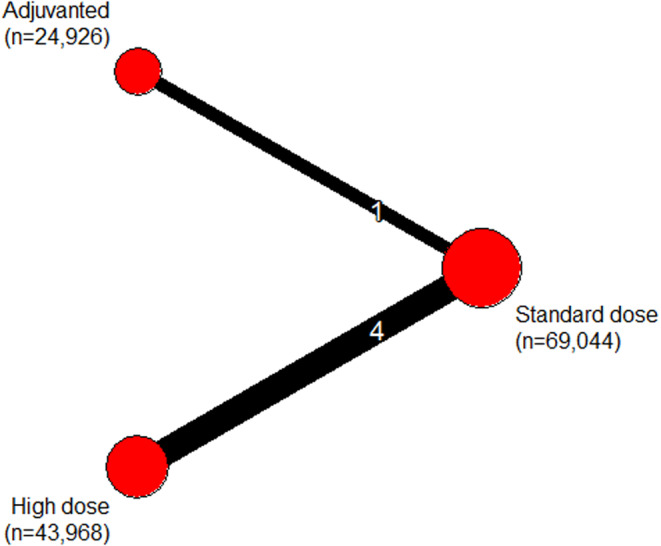
Network meta‐analysis plot of randomized evidence. The thickness of the line connecting a pair of vaccines is proportional to the number of direct comparisons between them and the size of the node is proportional to the total number of study participants in each vaccine group.
Figure 3a provides pooled network mean rVE estimates and 95% prediction intervals for prevention of influenza‐associated hospitalizations due to all influenza (sub)types for pairwise comparisons of vaccine types (corresponding netleague table given in Table S4). Although ADJ and HD were, on average, more effective than SD (rVE of 25% for HD vs. SD and 20% for ADJ vs. SD), differences were not statistically significant. HD performed slightly better than ADJ (rVE of 6%), but this difference was not statistically significant. We observed heterogeneity among studies, as indicated by wide prediction intervals and I 2 statistic of 46% (95% CI 0 to 82). Homogeneity was not rejected statistically (p = 0.14), but between‐study standard deviation was large. No test of inconsistency between direct and indirect evidence was possible because no comparisons included both direct and indirect evidence. SUCRAs indicated that HD had the largest fraction of competitors to which it was superior (67%), followed by ADJ (58%), and SD (25%). When combined, estimated mean rVE of enhanced vaccines versus SD was 18% (95% CI 3 to 32).
FIGURE 3.
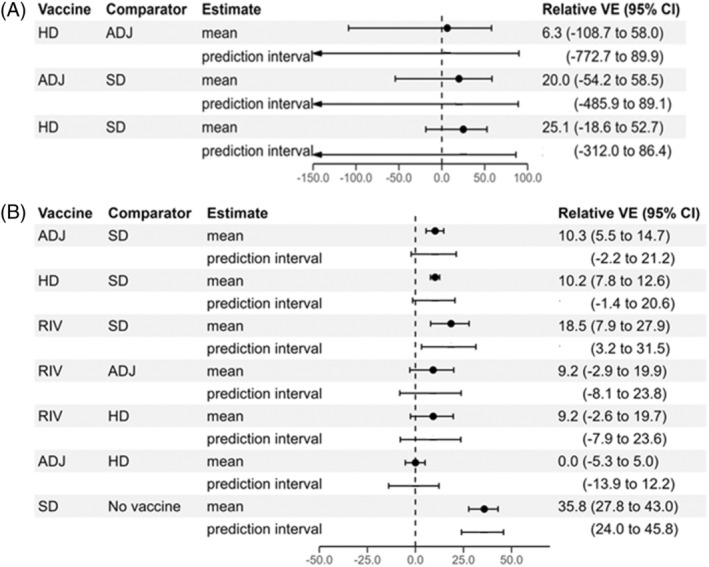
Forest plot of random‐effects network meta‐analysis results from (A) five randomized trials and (B) 27 observational studies, showing estimated mean relative vaccine effectiveness for prevention of influenza‐associated hospitalizations for pairwise comparisons of vaccine types. The 95% prediction intervals for each comparison are also shown. ADJ, adjuvanted inactivated influenza vaccine; HD, high‐dose inactivated influenza vaccine; RIV, recombinant influenza vaccine; SD, standard‐dose inactivated influenza vaccine; VE, vaccine efficacy/effectiveness.
Risk of bias
Figure S2 presents the risk of bias judgments for individually and cluster‐randomized studies. All trials had concern for risk of bias due to potential bias arising primarily from use of clinical diagnostic codes or other nonspecific outcome definitions rather than laboratory confirmation of influenza.
Sensitivity analyses
“Leave‐one‐out” analyses suggested that Johansen 2023 30 was influential, with overall ranking of vaccines changing upon its exclusion (Figures S3–S7). Johansen et al. observed higher rVE (64%, 95% CI 24 to 85) for HD versus SD than other studies; excluding Johansen 2023, rVE for HD versus SD was 12% (95% CI 2 to 22). Unlike the other randomized studies, Johansen 2023 was a pragmatic, open‐label trial using administrative health registry data; also, its participants had fewer comorbidities (Table S5). Johansen 2023 evaluated quadrivalent HD vaccine, but valency is unlikely to have contributed to higher effectiveness given lack of circulation of influenza B/Yamagata viruses during the Johansen 2023 study period. All studies except Gravenstein 2017 31 were conducted during seasons with substantial influenza A(H3N2) circulation.
Observational studies
Twenty‐seven observational studies reported 85 estimates including 71,321,980 vaccinated participants and 16 influenza seasons (Table S6). 17 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 The network comprised five nodes (four vaccine classes and a no vaccine group), 12 designs, and one subnetwork (Figure 4; no direct evidence was retrieved for RIV vs. no vaccine). Our review included approximately 42,000,000 recipients of HD; 22,000,000 recipients of SD; 6,500,000 recipients of ADJ; and 612,000 recipients of RIV. Among SD vaccines, 53% were trivalent, 22% quadrivalent, and 24% of unknown valence. Among HD and ADJ vaccines, >99% were trivalent.
FIGURE 4.
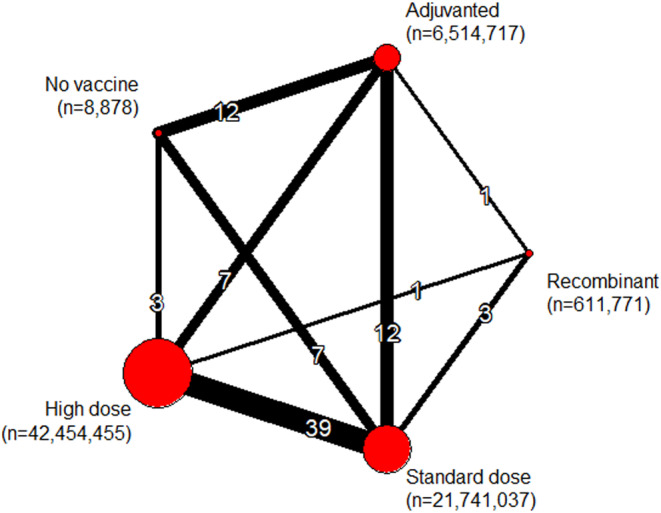
Network meta‐analysis plot for observational evidence. The thickness of the line connecting a pair of vaccines is proportional to the number of direct comparisons between them and the size of the node is proportional to the total number of study participants in each vaccine group.
Figure 3b gives network rVE estimates for prevention of influenza‐associated hospitalizations due to all influenza (sub)types for pairwise comparisons of vaccines (corresponding netleague table is given in Table S7). With SD as comparator, pooled mean rVE of ADJ was 10% (95% CI 6 to 15), of HD was 10% (8 to 13); and of RIV was 19% (8 to 28). Estimated mean rVE comparing RIV with ADJ and to HD was 9% (−3 to 20) and 9% (−3 to 20), respectively. Estimated mean rVE comparing ADJ with HD was 0% (−5 to 5). Prediction intervals overlapped the null value for all pairwise vaccine comparisons except for RIV versus SD. RIV had the highest SUCRA value (Table S8). Combining enhanced vaccines, estimated rVE of enhanced vaccines versus SD was 11% (95% CI 8 to 14).
Large between‐studies standard deviation indicated substantial heterogeneity, consistent with I 2 of 68% (95% CI 59% to 75%) and wide prediction intervals. Heterogeneity and inconsistency were present (p < 0.001 for both), with the largest contribution to heterogeneity arising from comparisons of HD versus SD. Consistency between direct and indirect comparisons was rejected when tested by a full design‐by‐treatment interaction model (p = 0.0003). Figure S8 presents comparisons of direct and indirect estimates and provides proportions of direct evidence contributing to each comparison.
Risk of bias
Nineteen (70%) studies had serious and eight (30%) had moderate risk of bias. After relaxing the requirement for control of calendar time, 9 (33%) studies had serious and 18 (67%) had moderate risk of bias. Most risk of bias concerns arose for bias due to confounding and risk of bias in selection of reported result (Figure S9). Bias judgment explanations are given in Table S9.
Subgroup analyses
With regard to predominant influenza (sub)type, thirty‐three (40%) of 83 effect estimates for which predominant circulating influenza (sub)type was known represented influenza A(H3N2)‐predominant seasons. We detected no interaction between influenza A(H3N2)‐predominance and rVE, although heterogeneity was less pronounced among the non‐influenza A(H3N2)‐predominant seasons. Regarding vaccine industry sponsorship, 38 (45%) of 85 effect estimates were obtained from vaccine industry‐sponsored studies. We observed no interaction between vaccine industry sponsorship and rVE. Heterogeneity and inconsistency were present in industry‐sponsored and nonindustry‐sponsored subgroups. Regarding use of laboratory‐confirmed outcomes, 30 of 85 (35%) effect estimates (from 12 studies) used laboratory‐confirmed outcomes. We observed a significant interaction between use of laboratory‐confirmed outcomes and rVE, with mean pooled rVE from laboratory‐confirmed studies tending to have greater magnitude than estimates derived using non‐laboratory‐confirmed outcomes (log scale regression coefficient of −0.21 [95% CI −0.35 to −0.06]; Figures S10 and S11). Studies with laboratory‐confirmed outcomes exhibited less heterogeneity and inconsistency (p > 0.05 for both; I 2 = 27% [95% CI 0 to 58]), whereas studies without laboratory‐confirmed outcomes showed both heterogeneity and inconsistency (p < 0.001 for both; I 2 = 74% [95% CI 65 to 80]). Comparisons involving RIV were particularly sensitive to use of laboratory‐confirmed outcomes (e.g., rVE of RIV vs HD was −26% among studies with laboratory‐confirmed outcomes and 10% among studies without laboratory‐confirmed outcomes; however, none of these rVE estimates was statistically significant). When restricting to laboratory‐confirmed outcomes, vaccine rankings changed, giving HD the highest SUCRA value (Table S8) and estimated mean rVE of enhanced vaccines (combined) versus SD of 24% (95% CI 10 to 35).
Evaluation of inconsistency
HD versus SD and ADJ versus HD comparisons had statistically significant differences between estimates derived from direct versus indirect evidence; however, because indirect evidence comprised only 3% of evidence for the HD versus SD comparison, the most consequential inconsistency arose from the ADJ versus HD comparison. Approximately two‐thirds of evidence contributing to the ADJ versus HD comparison came from direct evidence, which was composed almost entirely of cohort studies without laboratory‐confirmed outcomes, whereas the remaining one‐third was indirect evidence comprising primarily test‐negative case‐control studies with laboratory‐confirmed outcomes.
Sensitivity analyses
In analysis restricted to observational studies with moderate or better risk of bias, results were generally similar but pooled rVE estimates had slightly smaller magnitude (e.g., rVE of 7% for comparison of ADJ vs. SD; rVE of 9% for HD vs. SD; rVE of 18% for RIV vs. SD; Figure S12). SUCRA rankings changed slightly, although RIV remained the top‐ranked vaccine. In analysis including five studies that used a composite hospitalization/ED outcome, 59 , 60 , 61 , 62 , 63 results were similar to the main analysis (Figure S13; Table S10). Results were also similar when including an additional study that provided effect estimates only in an online supplement no longer publicly available (Table S11). 64
DISCUSSION
Our network meta‐analysis of 32 randomized and observational studies, including over 71 million vaccinated individuals and 16 influenza seasons, found that enhanced influenza vaccines conferred modestly better protection against influenza‐associated hospitalizations than standard inactivated influenza vaccines among adults aged ≥65 years. Randomized evidence was limited and of unclear risk of bias, and in these studies, the incremental benefit of enhanced vaccines over standard vaccines was not statistically significant. Observational studies, more prevalent and including far more vaccinated individuals, suggested that adjuvanted and HD influenza vaccines were about 10% more effective than standard vaccines, whereas the recombinant vaccine was about 19% more effective than standard vaccines in preventing influenza hospitalizations among older adults. Taken together, the enhanced vaccines conferred about an 11% risk reduction compared with SD based on evidence from observational studies. On average, studies using laboratory confirmation of influenza illness had higher relative effectiveness estimates. These findings remained robust when limiting to observational studies with moderate or better risk of bias. No significant differences in incremental benefit were observed in comparisons of enhanced influenza vaccines with one another.
No previous network meta‐analysis focusing on influenza‐associated hospitalizations and including randomized and observational evidence could be found with which to compare our results. With regard to relative effectiveness of HD versus SD, our findings are similar to those of Lee et al., 65 who reported a relative effectiveness of 11.7% against influenza‐associated hospitalizations. In a recent network meta‐analysis, Minozzi et al. 20 reported similar vaccine rankings based on laboratory‐confirmed influenza infection (SUCRAs of 80% for trivalent HD, 69% for trivalent ADJ, and 56% for trivalent SD).
Our analysis has several limitations. As with any meta‐analysis, certainty is limited by the number and methodological quality of underlying studies. Randomized study data were limited (particularly in regard to number of influenza seasons), as were data for some vaccine comparisons. There was a high degree of statistical heterogeneity among both randomized and observational evidence, and between‐study heterogeneity resulted in wide prediction intervals that sometimes spanned the null (suggesting that the expected range of true effects in similar studies could favor either vaccine, even if the confidence interval of the mean estimate effect did not include the null value). Although we examined results stratified by influenza A(H3N2) predominance, more precise assessment of viral subtype‐specific effects was limited because subtype‐specific estimates were not available for most studies. Similarly, we were unable to evaluate season‐specific characteristics such as degree of match between vaccine and circulating viruses, antigenic drift, and egg adaptation of vaccine viruses or host‐specific features such as immunologic imprinting and age cohort effects. We were unable to evaluate relative effectiveness in relation to prior influenza vaccination (generally not examined in included studies), age (given insufficient variability), or serologic correlates of protection. Analyses did not account for potential correlations across seasons for populations that may have at least partial common membership across seasons (such as the Veterans Administration or Medicare). We were unable to evaluate publication bias because methods for identifying publication bias in the context of network meta‐analysis are not yet widely used or available in statistical software. In addition, there were too few studies to evaluate publication bias among the randomized trials. Among the observational studies, visual examination of funnel plots for pairwise comparisons of HD versus SD and ADJ versus SD did not suggest publication bias. However, this finding should be interpreted with caution because evaluation of publication bias relies on study as the unit of analysis, and we were unable to account for the fact that several studies contributed more than one effect estimate for the same pairwise comparison. This work does not include assessment of vaccine safety. However, safety of influenza vaccines for older adults has been previously addressed through clinical studies; for enhanced vaccines specifically, some studies have noted increased reactogenicity associated with HD and ADJ as compared with SD. 20 Finally, we did not evaluate the overall certainty of the body of evidence using the GRADE framework. 66
In recent influenza seasons, several countries have adopted preferential recommendations for specific vaccine types for older adults. Canada recently adopted a preferential recommendation for use of any of the three enhanced influenza vaccine products without a preference among the products for adults aged ≥65 years. 19 Germany expresses a preference for HD for persons aged ≥69 years. 67 In Australia, 68 either ADJ or HD is preferred (although only ADJ is publicly funded), whereas in the United Kingdom, 69 either ADJ or RIV is preferred, with cell culture‐based inactivated vaccine recommended if neither of these is available, for those aged ≥65 years. In the United States, HD, ADJ, and RIV have been preferentially recommended over SD when available since the 2022–2023 influenza season. 70 Policy considerations are complicated by the observed variability of influenza vaccine effectiveness each season, and the small numbers of randomized trials representing relatively few influenza seasons. These factors foster complexity in estimating potential impact of preferential recommendations on the burden of severe influenza illness among older adults. In one model, 71 population benefits of a preferential recommendation for use of enhanced influenza vaccines provided only modest benefit in terms of preventing hospitalizations; this benefit was obviated in scenarios in which vaccination coverage decreased (e.g., due to a delay in vaccine availability). While delays in vaccine release at the start of the influenza season are uncommon, such findings highlight the need for recommendations to be structured such that as many potentially advantageous vaccines are recommended as evidence supports and to indicate that other age‐appropriate vaccines should be used if a preferred vaccine is not available. In addition to vaccine effectiveness and safety data, recommendations must be informed by considerations such as programmatic constraints, feasibility and acceptability to stakeholders, and resource use considerations. 72
Even as more effective vaccines have come to the market, influenza continues to account for substantial morbidity and mortality among older adults. Moving forward with current vaccines and as new ones arrive, it will be important to optimize use of these tools. Vaccine‐specific effectiveness data are of great value, but there are important tradeoffs in the selection of study designs. Given the variability of influenza, multiple seasons of data are needed to inform conclusions that might be generalizable. As randomized trials are resource intensive, optimization of observational designs to minimize bias to the extent possible may offer greater opportunities to compare vaccines head‐to‐head across multiple seasons.
AUTHOR CONTRIBUTIONS
JF, LG, and AF conceptualized the project. JF, LB, JC, LT, EA, and LG conducted literature screening and data extraction. JF conducted statistical analyses. JT developed and conducted literature searches. RM provided subject matter expertise for evidence synthesis and evaluation methods. JF and LG drafted the manuscript. All authors reviewed and edited the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
SPONSOR'S ROLE
This work was funded and conducted by the U.S. Centers for Disease Control & Prevention.
Supporting information
Figure S1. PRISMA flow diagram and reasons for exclusion.
Figure S2. Stop light diagram showing risk of bias by study and domain for randomized evidence.
Figures S3–S7. Leave‐one‐out sensitivity analysis for randomized evidence.
Figure S8. Comparison of direct, indirect, and network estimates, and proportion of evidence from direct evidence for observational studies.
Figure S9. Stop light diagram showing risk of bias by domain and study for observational studies.
Figure S10. Network relative vaccine effectiveness estimates for pairwise vaccine comparisons from observational studies without laboratory‐confirmed outcomes.
Figure S11. Network relative vaccine effectiveness estimates from observational studies with laboratory‐confirmed outcomes.
Figure S12. Network relative vaccine effectiveness estimates from studies with moderate (or better) risk of bias.
Figure S13. Network relative vaccine effectiveness estimates including studies using a composite hospitalization/emergency department visit outcome.
Table S1. PRISMA NMA checklist of items to include when reporting a systematic review involving a network meta‐analysis.
Table S2. Literature search terms.
Table S3. Effect estimates from five randomized trials used in the primary analysis.
Table S4. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from randomized evidence.
Table S5. Characteristics of randomized studies showing mean age and comorbid conditions.
Table S6. Effect estimates from 27 observational studies used in the primary analysis.
Table S7. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from observational evidence.
Table S8. Vaccine rankings as defined by the Surface Under the Cumulative RAnking (SUCRA) score based on observational evidence.
Table S9. Risk of bias by study and domain for 27 observational studies used in the primary analysis.
Table S10. Effect estimates from five observational studies with a composite hospitalization/emergency department visit outcome used in sensitivity analysis.
Table S11. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from observational evidence when including Stuurman 2020 64 as a sensitivity analysis.
Ferdinands JM, Blanton LH, Alyanak E, et al. Protection against influenza hospitalizations from enhanced influenza vaccines among older adults: A systematic review and network meta‐analysis. J Am Geriatr Soc. 2024;72(12):3875‐3889. doi: 10.1111/jgs.19176
The findings and conclusions in this presentation are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.
Portions of this work were presented at Options XI for Control of Influenza, Belfast, 2022, and at the meeting of the U.S. Advisory Committee on Immunization Practices, June 22, 2022.
See related editorial by Andrew and McGeer.
DATA AVAILABILITY STATEMENT
All data used to produce results presented herein are publicly available with the exception of effect estimates referenced in the ancillary report of Stuurman 2020, which can be provided by the authors upon request.
REFERENCES
- 1. Reed C, Chaves SS, Daily Kirley P, et al. Estimating influenza disease burden from population‐based surveillance data in the United States. PLoS One. 2015;10(3):e0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rolfes MA, Foppa IM, Garg S, et al. Annual estimates of the burden of seasonal influenza in the United States: a tool for strengthening influenza surveillance and preparedness. Influenza Other Respi Viruses. 2018;12(1):132‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. CDC . FluView–Outpatient illness surveillance. Atlanta, GA: U.S. Department of Health and Human Services, CDC. www.cdc.gov/flu/weekly
- 4. CDC . Disease burden of flu. https://www.cdc.gov/flu/about/burden/index.html
- 5. Andrew MK, MacDonald S, Godin J, et al. Persistent functional decline following hospitalization with influenza or acute respiratory illness. J Am Geriatr Soc. 2021;69(3):696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macias AE, McElhaney JE, Chaves SS, et al. The disease burden of influenza beyond respiratory illness. Vaccine. 2021;39(Suppl 1):A6‐A14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loyd C, Markland AD, Zhang Y, Fowler M, Harper S, Wright NC. Prevalence of hospital‐associated disability in older adults: a meta‐analysis. J Am Med Dir Assoc. 2020;21(4):455‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobrzynski DM, Ndi DN, Zhu Y, Markus T, Schaffner W, Talbot HK. Hospital readmissions after laboratory‐confirmed influenza hospitalization. J Infect Dis. 2020;222(4):583‐589. [DOI] [PubMed] [Google Scholar]
- 9. Haq K, McElhaney JE. Immunosenescence: influenza vaccination and the elderly. Curr Opin Immunol. 2014;29:38‐42. [DOI] [PubMed] [Google Scholar]
- 10. Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta‐analysis of test‐negative design case‐control studies. J Infect. 2017;75(5):381‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belongia EA, Simpson MD, King JP, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta‐analysis of test‐negative design studies. Lancet Infect Dis. 2016;16(8):942‐951. [DOI] [PubMed] [Google Scholar]
- 12. FDA . Vaccines licensed for use in the United States. fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- 13. Ng TWY, Cowling BJ, Gao HZ, Thompson MG. Comparative immunogenicity of enhanced seasonal influenza vaccines in older adults: a systematic review and meta‐analysis. J Infect Dis. 2019;219(10):1525‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox MM, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015;3(4):97‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high‐dose versus standard‐dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635‐645. [DOI] [PubMed] [Google Scholar]
- 16. Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med. 2017;376(25):2427‐2436. [DOI] [PubMed] [Google Scholar]
- 17. Izurieta HS, Lu M, Kelman J, et al. Comparative effectiveness of influenza vaccines among US Medicare beneficiaries ages 65 years and older during the 2019‐2020 season. Clin Infect Dis. 2021;73(11):e4251‐e4259. [DOI] [PubMed] [Google Scholar]
- 18. Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 iInfluenza season. MMWR Recomm Rep. 2023;72(RR‐2):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Government of Canada National Advisory Committee on Immunization . Statement on seasonal influenza vaccine for 2024–2025. 2024. [DOI] [PMC free article] [PubMed]
- 20. Minozzi S, Lytras T, Gianola S, et al. Comparative efficacy and safety of vaccines to prevent seasonal influenza: a systematic review and network meta‐analysis. EClinicalMedicine. 2022;46:101331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaglani M, Vasudevan A, Raiyani C, et al. Effectiveness of trivalent and quadrivalent inactivated vaccines against influenza B in the United States, 2011‐2012 to 2016‐2017. Clin Infect Dis. 2021;72(7):1147‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borenstein M. Avoiding common mistakes in meta‐analysis: understanding the distinct roles of Q, I‐squared, tau‐squared, and the prediction interval in reporting heterogeneity. Res Syn Meth. 2023;15:354‐368. [DOI] [PubMed] [Google Scholar]
- 23. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Stat Med. 2010;29(7–8):932‐944. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Res Synth Methods. 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salanti G, Nikolakopoulou A, Efthimiou O, Mavridis D, Egger M, White IR. Introducing the treatment hierarchy question in network meta‐analysis. Am J Epidemiol. 2022;191(5):930‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, eds. Cochrane Methods. Cochrane Database of Systematic Reviews; 2016. [Google Scholar]
- 27. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DiazGranados CA, Robertson CA, Talbot HK, Landolfi V, Dunning AJ, Greenberg DP. Prevention of serious events in adults 65 years of age or older: a comparison between high‐dose and standard‐dose inactivated influenza vaccines. Vaccine. 2015;33(38):4988‐4993. [DOI] [PubMed] [Google Scholar]
- 29. Vardeny O, Kim K, Udell JA, et al. Effect of high‐dose trivalent vs standard‐dose quadrivalent influenza vaccine on mortality or cardiopulmonary hospitalization in patients with high‐risk cardiovascular disease: a randomized clinical trial. JAMA. 2021;325(1):39‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansen ND, Modin D, Nealon J, et al. Feasibility of randomizing Danish citizens aged 65–79 years to high‐dose quadrivalent influenza vaccine vs. standard‐dose quadrivalent influenza vaccine in a pragmatic registry‐based setting: rationale and design of the DANFLU‐1 trial. Pilot Feasibility Stud. 2022;8(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high‐dose versus standard‐dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster‐randomised trial. Lancet Respir Med. 2017;5(9):738‐746. [DOI] [PubMed] [Google Scholar]
- 32. McConeghy KW, Davidson HE, Canaday DH, et al. Cluster‐randomized trial of adjuvanted vs. non‐adjuvanted trivalent influenza vaccine in 823 U.S. nursing homes. Clin Infect Dis. 2020;73:e4237‐e4243. [DOI] [PubMed] [Google Scholar]
- 33. Bella A, Gesualdo F, Orsi A, et al. Effectiveness of the trivalent MF59 adjuvated influenza vaccine in preventing hospitalization due to influenza B and A(H1N1)pdm09 viruses in the elderly in Italy, 2017–2018 season. Expert Rev Vaccines. 2019;18(6):671‐679. [DOI] [PubMed] [Google Scholar]
- 34. Cheng AC, Holmes M, Dwyer DE, et al. Influenza epidemiology in patients admitted to sentinel Australian hospitals in 2018: the Influenza Complications Alert Network (FluCAN). Commun Dis Intell. 2019;18:43. [DOI] [PubMed] [Google Scholar]
- 35. Cocchio S, Gallo T, Del Zotto S, et al. Preventing the risk of hospitalization for respiratory complications of influenza among the elderly: is there a better influenza vaccination strategy? A retrospective population study. Vaccines. 2020;8(3):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doyle JD, Beacham L, Martin ET, et al. Relative and absolute effectiveness of high‐dose and standard‐dose influenza vaccine against influenza‐related hospitalization among older adults‐United States, 2015‐2017. Clin Infect Dis. 2021;72(6):995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gasparini R, Amicizia D, Lai PL, Rossi S, Panatto D. Effectiveness of adjuvanted seasonal influenza vaccines (Inflexal V® and Fluad®) in preventing hospitalization for influenza and pneumonia in the elderly: a matched case‐control study. Hum Vaccin Immunother. 2013;9(1):144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grijalva CG, Feldstein LR, Talbot HK, et al. Influenza vaccine effectiveness for prevention of severe influenza‐associated illness among adults in the United States, 2019‐2020: a test‐negative study. Clin Infect Dis. 2021;73(8):1459‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of cell‐cultured and egg‐based influenza vaccines among elderly persons in the United States, 2017‐2018. J Infect Dis. 2019;220(8):1255‐1264. [DOI] [PubMed] [Google Scholar]
- 40. Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018‐2019. J Infect Dis. 2020;222(2):278‐287. [DOI] [PubMed] [Google Scholar]
- 41. Lu Y, Chillarige Y, Izurieta HS, et al. Effect of age on relative effectiveness of high‐dose versus standard‐dose influenza vaccines among US Medicare beneficiaries aged ≥65 years. J Infect Dis. 2019;220(9):1511‐1520. [DOI] [PubMed] [Google Scholar]
- 42. Mannino S, Villa M, Apolone G, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012;176(6):527‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mira‐Iglesias A, Lopez‐Labrador FX, Baselga‐Moreno V, et al. Influenza vaccine effectiveness against laboratory‐confirmed influenza in hospitalised adults aged 60 years or older, Valencia Region, Spain, 2017/18 influenza season. Euro Surveill. 2019;24(31):1800461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mira‐Iglesias A, Lopez‐Labrador FX, Garcia‐Rubio J, et al. Influenza vaccine effectiveness and waning effect in hospitalized older adults. Valencia Region, Spain, 2018/2019 Season. Int J Environ Res Public Health. 2021;18(3):1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paudel M, Mahmud S, Buikema A, et al. Relative vaccine efficacy of high‐dose versus standard‐dose influenza vaccines in preventing probable influenza in a Medicare Fee‐for‐Service population. Vaccine. 2020;38(29):4548‐4556. [DOI] [PubMed] [Google Scholar]
- 46. Pebody R, Whitaker H, Zhao H, et al. Protection provided by influenza vaccine against influenza‐related hospitalisation in ≥65 year olds: early experience of introduction of a newly licensed adjuvanted vaccine in England in 2018/19. Vaccine. 2020;38(2):173‐179. [DOI] [PubMed] [Google Scholar]
- 47. Pott H, Andrew MK, Shaffelburg Z, et al. Vaccine effectiveness of non‐adjuvanted and adjuvanted trivalent inactivated influenza vaccines in the prevention of influenza‐related hospitalization in older adults: a pooled analysis from the Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN). Vaccine. 2023;41(42):6359‐6365. [DOI] [PubMed] [Google Scholar]
- 48. Puig‐Barberà J, Diez‐Domingo J, Pérez Hoyos S, Belenguer Varea A, GonzálezVidal D. Effectiveness of the MF59‐adjuvanted influenza vaccine in preventing emergency admissions for pneumonia in the elderly over 64 years of age. Vaccine. 2004;23(3):283‐289. [DOI] [PubMed] [Google Scholar]
- 49. Puig‐Barbera J, Diez‐Domingo J, Varea AB, et al. Effectiveness of MF59‐adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. Vaccine. 2007;25(42):7313‐7321. [DOI] [PubMed] [Google Scholar]
- 50. Richardson DM, Medvedeva EL, Roberts CB, Linkin DR, Centers for Disease Control and Prevention Epicenter Program . Comparative effectiveness of high‐dose versus standard‐dose influenza vaccination in community‐dwelling veterans. Clin Infect Dis. 2015;61(2):171‐176. [DOI] [PubMed] [Google Scholar]
- 51. Robison SG, Thomas AR. Assessing the effectiveness of high‐dose influenza vaccine in preventing hospitalization among seniors, and observations on the limitations of effectiveness study design. Vaccine. 2018;36(45):6683‐6687. [DOI] [PubMed] [Google Scholar]
- 52. Spadea A, Unim B, Colamesta V, et al. Is the adjuvanted influenza vaccine more effective than the trivalent inactivated vaccine in the elderly population? Results of a case‐control study. Vaccine. 2014;32(41):5290‐5294. [DOI] [PubMed] [Google Scholar]
- 53. Stuurman AL, Biccler J, Carmona A, et al. Brand‐specific influenza vaccine effectiveness estimates during 2019/20 season in Europe – Results from the DRIVE EU study platform. Vaccine. 2021;39(29):3964‐3973. [DOI] [PubMed] [Google Scholar]
- 54. Stuurman AL, Carmona A, Biccler J, et al. Brand‐specific estimates of influenza vaccine effectiveness for the 2021‐2022 season in Europe: results from the DRIVE multi‐stakeholder study platform. Front Public Health. 2023;20(11):1195409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Aalst R, Gravenstein S, Mor V, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: a retrospective cohort study. Vaccine. 2020;38(2):372‐379. [DOI] [PubMed] [Google Scholar]
- 56. Young‐Xu Y, Van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high‐dose versus standard‐dose influenza vaccines among Veterans Health Administration patients. J Infect Dis. 2018;217(11):1718‐1727. [DOI] [PubMed] [Google Scholar]
- 57. Young‐Xu Y, Snider JT, van Aalst R, et al. Analysis of relative effectiveness of high‐dose versus standard‐dose influenza vaccines using an instrumental variable method. Vaccine. 2019;37(11):1484‐1490. [DOI] [PubMed] [Google Scholar]
- 58. Zimmerman RK, Dauer K, Clarke L, Nowalk MP, Raviotta JM, Balasubramani GK. Vaccine effectiveness of recombinant and standard dose influenza vaccines against outpatient illness during 2018‐2019 and 2019‐2020 calculated using a retrospective test‐negative design. Hum Vaccin Immunother. 2023;19(1):2177461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Domnich A, Panatto D, Pariani E, et al. Relative effectiveness of the adjuvanted vs non‐adjuvanted seasonal influenza vaccines against severe laboratory‐confirmed influenza among hospitalized Italian older adults. Int J Infect Dis. 2022;125:164‐169. [DOI] [PubMed] [Google Scholar]
- 60. Imran M, Puig‐Barbera J, Ortiz JR, et al. Relative effectiveness of MF59 adjuvanted trivalent influenza vaccine vs nonadjuvanted vaccines during the 2019–2020 influenza season. Open Forum Infect Dis. 2022;9(5):ofac167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Machado MAA, Moura CS, Abrahamowicz M, Ward BJ, Pilote L, Bernatsky S. Relative effectiveness of influenza vaccines in elderly persons in the United States, 2012/2013‐2017/2018 seasons. NPJ Vac. 2021;6(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pelton SI, Divino V, Postma MJ, et al. A retrospective cohort study assessing relative effectiveness of adjuvanted versus high‐dose trivalent influenza vaccines among older adults in the United States during the 2018‐19 influenza season. Vaccine. 2021;39(17):2396‐2407. [DOI] [PubMed] [Google Scholar]
- 63. Pelton SI, Divino V, Shah D, et al. Evaluating the relative vaccine effectiveness of adjuvanted trivalent influenza vaccine compared to high‐dose trivalent and other egg‐based influenza vaccines among older adults in the US during the 2017‐2018 influenza season. Vaccines. 2020;8(3):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stuurman AL, Bollaerts K, Alexandridou M, et al. Vaccine effectiveness against laboratory‐confirmed influenza in Europe – results from the DRIVE network during season 2018/19. Vaccine. 2020;38(41):6455‐6463. [DOI] [PubMed] [Google Scholar]
- 65. Lee JKH, Lam GKL, Shin T, et al. Efficacy and effectiveness of high‐dose versus standard‐dose influenza vaccination for older adults: a systematic review and meta‐analysis. Expert Rev Vaccines. 2018;17(5):435‐443. [DOI] [PubMed] [Google Scholar]
- 66. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383‐394. [DOI] [PubMed] [Google Scholar]
- 67. Robert Koch Institute Standing Committee on Vaccination . Recommendations by the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute. 2023. Accessed March 8, 2024. https://www.rki.de/EN/Content/infections/Vaccination/recommandations/04_23_englisch.pdf?__blob=publicationFile
- 68. Australian Government Department of Health and Aged Care . National Immunisation Program 2024 Influenza Vaccination. Accessed March 8, 2024. https://www.health.gov.au/sites/default/files/2024‐02/2024‐influenza‐vaccination‐program‐advice‐for‐health‐professionals_0.pdf
- 69. UK Health Security Agency/National Health Service England . National flu immunisation programme 2023 to 2024 letter. Accessed March 8, 2024. https://www.gov.uk/government/publications/national‐flu‐immunisation‐programme‐plan/national‐flu‐immunisation‐programme‐2023‐to‐2024‐letter
- 70. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71(RR‐1):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Morris SE, Grohskopf LA, Ferdinands JM, Reed C, Biggerstaff M. Evaluating potential impacts of a preferential vaccine recommendation for adults 65 years of age and older on US influenza burden. Epidemiology. 2023;34:345‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. CDC/Advisory Committee on Immunization Practices (ACIP) . Evidence to recommendations frameworks. Accessed March 8, 2024. https://www.cdc.gov/vaccines/acip/recs/grade/etr.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. PRISMA flow diagram and reasons for exclusion.
Figure S2. Stop light diagram showing risk of bias by study and domain for randomized evidence.
Figures S3–S7. Leave‐one‐out sensitivity analysis for randomized evidence.
Figure S8. Comparison of direct, indirect, and network estimates, and proportion of evidence from direct evidence for observational studies.
Figure S9. Stop light diagram showing risk of bias by domain and study for observational studies.
Figure S10. Network relative vaccine effectiveness estimates for pairwise vaccine comparisons from observational studies without laboratory‐confirmed outcomes.
Figure S11. Network relative vaccine effectiveness estimates from observational studies with laboratory‐confirmed outcomes.
Figure S12. Network relative vaccine effectiveness estimates from studies with moderate (or better) risk of bias.
Figure S13. Network relative vaccine effectiveness estimates including studies using a composite hospitalization/emergency department visit outcome.
Table S1. PRISMA NMA checklist of items to include when reporting a systematic review involving a network meta‐analysis.
Table S2. Literature search terms.
Table S3. Effect estimates from five randomized trials used in the primary analysis.
Table S4. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from randomized evidence.
Table S5. Characteristics of randomized studies showing mean age and comorbid conditions.
Table S6. Effect estimates from 27 observational studies used in the primary analysis.
Table S7. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from observational evidence.
Table S8. Vaccine rankings as defined by the Surface Under the Cumulative RAnking (SUCRA) score based on observational evidence.
Table S9. Risk of bias by study and domain for 27 observational studies used in the primary analysis.
Table S10. Effect estimates from five observational studies with a composite hospitalization/emergency department visit outcome used in sensitivity analysis.
Table S11. Netleague table of all pairwise comparisons of network relative vaccine effectiveness estimates from observational evidence when including Stuurman 2020 64 as a sensitivity analysis.
Data Availability Statement
All data used to produce results presented herein are publicly available with the exception of effect estimates referenced in the ancillary report of Stuurman 2020, which can be provided by the authors upon request.


