Abstract
Background
This study aims to evaluate the add‐on effects of oral Chinese herbal medicine (CHM) for mild cognitive impairment (MCI), when used in addition to donepezil compared to donepezil alone.
Methods
Randomized controlled trials comparing these treatments across all types of MCI were identified from nine databases and three registers until August 2023. Outcome measures were Mini‐Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and adverse events (AEs). Methodological quality was assessed using Cochrane risk‐of‐bias tool, and evidence certainty was evaluated using the GRADE method.
Results
Involving 1611 participants across 20 studies, meta‐analysis results indicate that oral CHM combined with donepezil significantly improved cognitive function in MCI patients compared to donepezil alone, as evidenced by MMSE (1.88 [1.52, 2.24], I 2 = 41%, 12 studies, 993 participants) and MoCA (MD: 2.01 [1.57, 2.44], I 2 = 52%, 11 studies, 854 participants). Eleven studies reported details of AEs, identifying gastrointestinal symptoms and insomnia as the most common symptoms. No significant difference in AEs frequency was found between the groups (RR: 0.91 [0.59, 1.39], I 2 = 4%, 11 studies, 808 participants). All 20 studies were evaluated as having “some concerns” regarding the overall risk of bias. The certainty of evidence for MMSE was “moderate” and “low” for MoCA. From frequently utilized herbs, two classical CHM formulae were identified: Kai xin san and Si wu decoction. The observed treatment effects of commonly used herbs may be exerted through multiple pharmacological mechanisms, including anti‐inflammatory, anti‐oxidative stress, anti‐apoptotic actions, promotion of neuronal survival and modulation of the cholinergic system.
Conclusions
The concurrent use of oral CHM and donepezil appears to be more effective than donepezil alone in improving the cognitive function of MCI, without leading to an increase in AEs. While recognizing concerns of overall methodological quality, this combined therapy should be considered as an alternative option for clinical practice.
Keywords: add‐on treatment, donepezil, mild cognitive impairment, oral Chinese herbal medicine, systematic review
Key points
The concurrent use of oral CHM and donepezil appears to be more effective than donepezil alone in improving the cognitive function of MCI patients.
The combined therapy of CHM and donepezil did not lead to an increase in adverse events compared to the use of donepezil alone.
Why does this paper matter?
This study investigates the add‐on effects of oral CHM for MCI. The findings of this research quantified the treatment effects of oral CHM combined with donepezil, which may assist in clinical practice and indicate future research topics in the area of CHM for MCI.
INTRODUCTION
Mild cognitive impairment (MCI) is the intermediate stage between normal age‐related memory and thinking decline and the more severe dementia. 1 MCI presents difficulties in memory, language or executive function. 2 The global prevalence of MCI among community dwellers aged 50 years and older was 15.56%. 3 Furthermore, during a 2‐year follow‐up period, 14.9% of individuals aged 65 or older with MCI developed dementia. 4 Given that MCI is considered a precursor to dementia, taking early action is crucial to delay the onset of dementia.
Acetylcholine is an essential neurotransmitter associated with cognitive function, and dysfunctions in cholinergic neurons are prominently observed in individuals with cognitive impairment. 5 One approach to alleviate these dysfunctions is by inhibiting the enzyme acetylcholinesterase, which prevents the breakdown of acetylcholine. 5 Cholinesterase inhibitors are approved for the treatment of dementia, with their efficacy distinctly established in enhancing cognitive domains and global functioning. 6 , 7 A systematic review examining the impact of cholinesterase inhibitors on MCI concluded that these inhibitors exhibited a slight efficacy in enhancing cognitive function scores and reducing the incidence of progression to dementia when compared to placebo. 8 However, the application of cholinesterase inhibitors for MCI is not recommended by the latest clinical guideline, 4 considering their common side effects and the minimal treatment effects margin of MCI. While due to the lack of approved medications for MCI, off‐label cholinesterase inhibitors are commonly prescribed to patients with MCI in clinical settings. 9 , 10 Moreover, despite their modest effects, many patients express a desire to receive the treatment. 9 It is worth noting that, clinicians are advised to discuss the off‐label status and the absence of empirical evidence with patients before prescribing cholinesterase inhibitors for MCI patients, as recommended by the latest guideline. 4
Donepezil, a cholinesterase inhibitor, is widely used to treat dementia and MCI in clinical practice. 11 , 12 , 13 Additionally, oral Chinese herbal medicine (CHM) has been used to manage cognitive impairment in China for a long time. 14 , 15 The cognition‐enhancing function of commonly used CHM may be attributed to various mechanisms, including antioxidant, anti‐apoptotic, anti‐neurotoxic, anti‐cytotoxic and anti‐inflammatory actions. 16 However, the effectiveness and safety of combining oral CHM and donepezil for managing MCI have not been thoroughly evaluated. Therefore, we conducted a comprehensive systematic review to evaluate the add‐on effects of oral CHM when used in combination with donepezil for managing MCI, compared to donepezil alone.
METHODS
This systematic review was conducted according to the Cochrane Handbook 17 and was reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline 18 (see PRISMA checklist in Table S1). The study protocol was registered at the PROSPERO international prospective register of systematic reviews (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=450702), and the registered ID is CRD42023450702.
Eligibility criteria
Studies that met all the following criteria were included in this systematic review:
Participants: Patients diagnosed with any type of MCI using standardized diagnostic criteria or based on the clinicians' assessment. Patients with accompanied conditions, such as cerebrovascular disease, were not a reason for study exclusion.
Interventions: Any orally administered CHM combined with donepezil. Studies evaluated single compounds extracted from certain herbs, such as the standardized extract of Ginkgo biloba leaves, were not included in this review since these herbs were not classified as traditional CHM. 19 Usual care for underlying diseases was allowed if the same treatments were applied to both the experimental and donepezil groups, except for any other types of Chinese medicine therapies, anti‐dementia drugs or other therapies aimed at improving cognitive function (e.g., cognitive training).
Controls: Only donepezil‐controlled trials were included. Co‐interventions were allowed if they were same as those used in the intervention group.
Outcomes: Studies reporting at least one of the following outcomes at the end of treatment were included: scores of Mini‐Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Adverse events (AEs) were also analyzed if the original RCTs reported this outcome.
Study design: Randomized controlled trials.
Search strategy
Two reviewers (LL and CSZ) independently searched nine databases and three registry platforms: PubMed, Excerpta Medica Database (Embase), Cochrane Central Register of Controlled Trials (CENTRAL) (including the Cochrane Library), Cumulative Index of Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED), China Biomedical Literature (CBM), China National Knowledge Infrastructure (CNKI) Database, Wanfang, Chongqing VIP (CQVIP) Database, International Clinical Trials Registry Platform (ICTRP), ClinicalTrials.gov, Chinese Clinical Trial Registry (ChiCTR) from their respective inceptions to August 2023. No restrictions were placed on the language of publication. The search terms were the keywords and their synonyms of MCI, CHM, and RCT (see search strategy in Table S2). In addition, Google Scholar and references from published systematic reviews on Chinese medicine for MCI were also searched.
Study selection and data extraction
Two reviewers (LL and CSZ) independently examined the titles and abstracts obtained from the comprehensive search, excluding irrelevant studies and duplicates, then thoroughly screened full‐text articles of potential studies against the predefined selection criteria. Any discrepancy between these two reviewers was resolved through discussion with a third reviewer (ALZ).
For data extraction, two independent reviewers (LL and CSZ) extracted information from each eligible study, including sample size, characteristics of participants, details of intervention and control, duration of treatment and follow‐up, and clinical outcomes data.
Risk of bias assessment
The methodological quality of each study was independently evaluated by two reviewers (LL and CSZ) using Version 2 of the Cochrane risk‐of‐bias tool for randomized trials (RoB 2) tool. 17 Any disagreement between these two reviewers was resolved by discussing with a third reviewer (ALZ). Judgments were summarized as “low” or “high” risk of bias or “some concerns.” 17
Statistical analysis
Review Manager 5.4 and Stata 15 were used for data analysis in this review. For continuous data, we calculated mean difference (MD) with 95% confidence intervals (CIs) when the outcome data were measured using consistent scales. For cases where different measurement scales were used for the same outcome, we calculated the standardized mean difference (SMD) with 95% CIs. For binary data, we used risk ratios (RRs) and 95% CIs to present the effect size. A random‐effect meta‐analysis model was used to calculate the pooled effect size. Heterogeneity between trials was assessed using the I 2 test, which was incorporated into the forest plots. When high heterogeneity was observed, we attempted to investigate its source by conducting subgroup analyses and meta‐regressions using variables such as MCI subtypes and treatment duration. To assess the robustness of the findings, we conducted sensitivity analyses. We assessed the potential for publication bias by constructing a funnel plot and using Egger's test.
Certainty of evidence
The certainty of the evidence for the primary outcomes (MMSE and MoCA) was assessed as high, moderate, low, or very low according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, 20 considering the risk of bias, inconsistency of results, indirectness of evidence, imprecision and publication bias.
RESULTS
Results of the search
A total of 8676 records were identified through database searching and an additional 1401 records were found via searching clinical trial registries. After removing duplicates, a screening process was conducted on 8840 records. Among these, 331 full‐text reports were obtained for further screening. Eventually, 20 studies (20 reports) that fulfilled the selection criteria were included. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40
Furthermore, a supplementary search was carried out on Google Scholar and citations from previously published systematic reviews were also examined. However, no additional reports were found, as five reports meeting the inclusion criteria were already identified by the initial search. 21 , 24 , 29 , 34 , 38 The selection process is presented in Figure 1.
FIGURE 1.
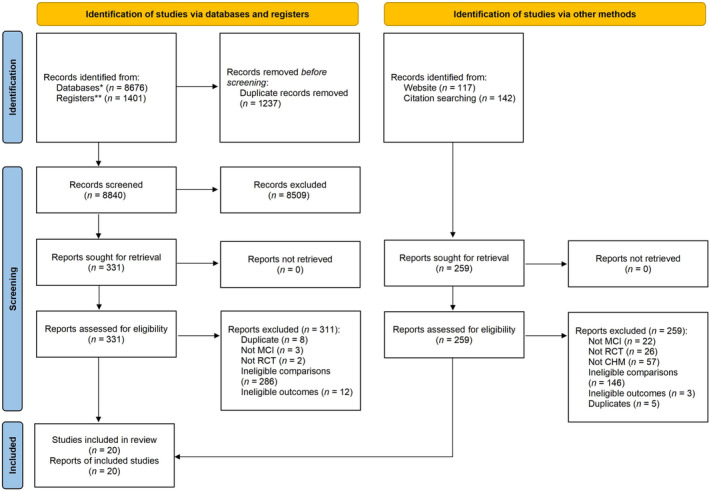
Flowchart of study selection. CHM, Chinese herbal medicine; MCI, mild cognitive impairment; RCT, randomized controlled trial. *Databases: AMED, Allied and Complementary Medicine Database; CBM, China Biomedical Literature; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulative Index of Nursing and Allied Health Literature; CNKI, China National Knowledge Infrastructure Database; CQVIP, Chongqing VIP Database; Embase, Excerpta Medica Database; PubMed; Wanfang. **Registers: Chinese Clinical Trial Registry (ChiCTR) (https://www.chictr.org.cn/), ClinicalTrials.gov (https://clinicaltrials.gov/), International Clinical Trials Registry Platform (ICTRP) (https://trialsearch.who.int/).
Characteristics of included studies
All 20 studies were conducted in China with participant numbers ranging from 52 to 198, totaling 1611 participants. Four studies reported a total of 14 dropouts, with eight from the CHM add‐on donepezil groups and six from the donepezil groups. Eight trials recruited MCI patients without indicating a specified subtype, 27 , 29 , 31 , 34 , 35 , 36 , 38 , 40 seven trials recruited vascular MCI. 22 , 23 , 24 , 25 , 26 , 32 , 39 Three trials recruited MCI due to Alzheimer's disease, degenerative etiology and Amnestic MCI, separately. 30 , 33 , 37 Three studies specifically focused on MCI with diabetes. 21 , 22 , 28 The main characteristics of the included studies are shown in Table S3.
All 20 included studies compared the combination of oral CHM and donepezil to donepezil. The treatment duration ranged from 1 to 6 months. More specifically, one study applied treatments for 1 month, 30 eight studies lasted for 2 months, 23 , 25 , 27 , 28 , 31 , 32 , 38 , 40 nine studies were conducted over 3 months. 21 , 24 , 26 , 33 , 34 , 35 , 36 , 37 , 39 Table S4 presents detailed information on the participants' baseline characteristics, including age, gender, education level and cognitive score.
Fifteen studies evaluated the treatment effects using MMSE 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 , 38 , 40 and 14 studies reported data on MoCA. 21 , 22 , 23 , 25 , 28 , 29 , 31 , 32 , 33 , 35 , 37 , 38 , 39 , 40 Eleven studies reported information on AEs. 21 , 22 , 24 , 26 , 27 , 28 , 32 , 33 , 34 , 37 , 40 Sixteen CHM formulae were identified from the 20 included studies. Detailed ingredients of the formulae of each study are presented in Table S5. A total of 81 Chinese medicine herbs were included in these 16 CHM formulae, with Wolfiporia cocos (Schw.) Ryv. & Cilbn. (fu ling) being the most commonly used one. Glycyrrhiza uralensis Fisch. (gan cao) is excluded from our frequency analysis on herbs, since it is believed to harmonize other medicinal ingredients according to traditional Chinese Medicine theory and is almost used in all formulae. Table S6 presents the top 10 most commonly used Chinese medicine herbs.
Risk of bias assessment
We conducted a comprehensive assessment of the risk of bias for each included study using the RoB 2 tool based on two outcome measures: MMSE and MoCA (Figure S1). An intention‐to‐treat analysis model was used in our assessment. Overall, all 20 studies were assessed as “some concerns.” In terms of randomization process, all studies were judged as “some concerns” because they did not provide information on the generation of allocation sequence and sequence concealment. For the domain of “deviations from the intended interventions,” considering that all the 20 studies were add‐on designed, it was likely that people delivering the interventions were aware of participants' assigned intervention during the trial. Therefore, all studies were assessed as “some concerns” in this domain. All studies were given a “low risk” judgment for “missing outcome data” since no bias due to missing outcome data was found. Considering that both MMSE and MoCA comprise a series of questions with clearly defined scoring criteria, making them less likely to be influenced by knowing which intervention the participants was given, all studies were classified as having a “low risk” judgment in the domain of “measurement of the outcome.” For the “selection of the reported outcome,” all studies were rated as “some concerns” due to the absence of preregistered trial protocols.
Effectiveness
Fifteen studies evaluated the treatment effects using MMSE 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 , 38 , 40 and 14 studies reported data on MoCA. 21 , 22 , 23 , 25 , 28 , 29 , 31 , 32 , 33 , 35 , 37 , 38 , 39 , 40 However, one study provided end‐of‐treatment MMSE scores based on varied education levels for the experimental and control groups, without providing an overall MMSE scores for each group. 33 Therefore, this study was excluded from the meta‐analysis. Furthermore, three studies reported baseline MoCA values between 12.88–16.29 in the experimental group and 13.65–16.12 in the control group. 22 , 25 , 29 According to the data released by the MoCA developer, the average MoCA score for MCI is 22 (range, 19–25). 41 In another study involving 8411 Chinese community, for detecting MCI and dementia, the optimal cutoff points of MoCA were 13/14 for illiterate individuals, 19/20 for those with 1–6 years of education, and 24/25 for individuals with seven or more years of education. 42 However, among the three studies reporting low baseline MoCA scores in our review, one study did not provide the participants' education information, 29 and the other two studies indicated that the average education level of the participants was at least more than 6 years. 22 , 25 This indicates the possibility that certain participants might be in a cognitive impairment stage more severe than MCI, even though the authors indicated in their trials that dementia were excluded. We contacted the authors for clarification but did not receive any response. Therefore, these three studies were also excluded from the meta‐analysis on MoCA. Detailed information about baseline, end‐of‐treatment (EoT), and change scores of MMSE and MoCA can be seen in Table S7. For the overall meta‐analysis of mean differences (MDs) on MMSE and MoCA, we utilized MDs based on both changes from baseline and EoT scores, to provide a comprehensive evaluation on the treatment effects.
MMSE
The pooled meta‐analysis on EoT MMSE scores indicate that the combination of CHM and donepezil was more effective than donepezil alone (MD: 1.88 [1.52, 2.24], I 2 = 41%, 12 studies, 993 participants) (Figure 2). 23 , 24 , 26 , 27 , 28 , 30 , 31 , 34 , 36 , 37 , 38 , 40 Moreover, the meta‐analysis based on change scores of MMSE also suggests that the combined therapy produced a greater improvement on MMSE scores compared to donepezil alone (MD: 1.77 [1.31, 2.23], I 2 = 64%, 12 studies, 993 participants) (Figure S2).
FIGURE 2.
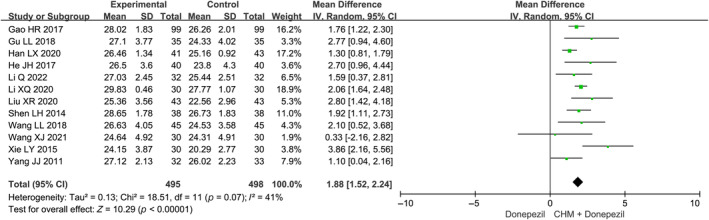
Overall meta‐analysis on MMSE at the end of treatment. CHM, Chinese herbal medicine.
Subgroup analysis on MMSE was conducted according to the MCI subtypes with different causes and treatment duration (Figures S3 and S4). The inclusion of different types of CHM remedies may also introduce treatment effect heterogeneity. However, with 12 CHM formulae identified from the 12 RCTs, discovering the source of the heterogeneity through meta‐regression or subgroup analysis based on CHM formulae was not feasible.
Notably, the subgroup analyses consistently demonstrate a favorable pattern across various subgroups, suggesting that the treatment effect in the group receiving CHM plus donepezil was superior to that observed in the donepezil‐only groups. In the subgroup analysis based on MCI subtypes, we observed a trend suggesting that MCI due to Alzheimer's disease (MD: 2.06 [1.64, 2.48], I 2 = Not applicable, 1 study, 60 participants) might have a greater treatment effect compared to MCI due to vascular disease (MD: 1.61 [1.10, 2.13], I 2 = 39, 3 study, 352 participants), while having a similar treatment effect to unspecified MCI (MD: 2.03 [1.42, 2.65], I 2 = 39, 8 studies, 581 participants) (Figure S3). Meanwhile, studies with shorter treatment durations (1 month and 2 month) demonstrate a slightly greater mean difference (1 month: MD: 2.06 [1.64, 2.48], I 2 = Not applicable, 1 study, 60 participants; 2 months: MD: 2.08 [1.41, 2.76], I 2 = 51%, 6 studies, 553 participants) compared to those with longer treatment durations (3 months) (MD: 1.62 [1.11, 2.12], I 2 = 18%, 5 studies, 380 participants) (Figure S4). However, these differences did not reach statistical significance (p > 0.05). The results of univariate meta‐regression analyses indicated that MCI subtypes (p = 0.867) and treatment duration (p = 0.330) might not be the factors influencing the heterogeneity (Figure S5).
MoCA
The meta‐analysis on EoT MoCA scores of 11 studies demonstrates that CHM in combination with donepezil showed superior effects regarding the MoCA score, as compared to donepezil alone (MD: 2.01 [1.57, 2.44], I 2 = 52%, 11 studies, 854 participants) (Figure 3). 21 , 23 , 28 , 31 , 32 , 33 , 35 , 37 , 38 , 39 , 40 Furthermore, the meta‐analysis utilizing the change scores of MoCA indicates that the combined therapy significantly improved the effects of donepezil alone (MD: 1.84 [1.42, 2.25], I 2 = 45%, 11 studies, 854 participants) (Figure S6).
FIGURE 3.
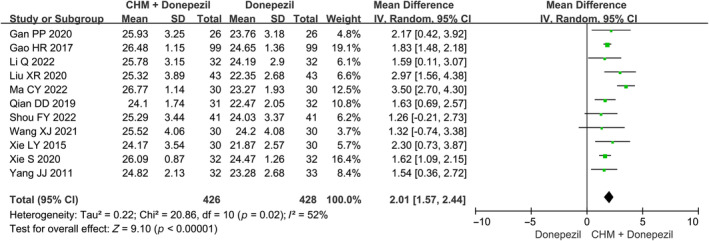
Overall meta‐analysis on MoCA at end of treatment. CHM, Chinese herbal medicine.
Subgroup analysis was also performed to evaluate the MoCA outcome, stratified by treatment duration (Figure S7). Similar to the results on MMSE, the results suggest a trend toward slightly larger treatment effects in studies with shorter durations compared to longer durations (2 months: MD: 2.30 [1.57, 3.04], 6 studies, 533 participants; 3 months: MD: 1.61 [1.19, 2.03], 5 studies, 321 participants). However, no statistically significant subgroup difference was detected (p = 0.11). Similarly, univariate meta‐regression showed that the treatment duration was unlikely to affect the heterogeneity of the treatment effects (p = 0.165) (Figure S8). For the outcome of MoCA, due to limited information on the causes of MCI and the inclusion of a total 11 formulae across the 11 RCTs, subgroup or meta‐regression analysis based on these factors could not be performed.
Sensitivity analysis
To assess the potential impact of individual studies on the pooled estimations for the outcomes of MMSE and MoCA, sensitivity analyses were conducted by iteratively excluding each included study for both outcomes. The findings reveal that the omission of any of the included RCTs had no substantial alteration on the overall effect estimate for MMSE (Figure S9) and MoCA (Figure S10). In summary, the sensitivity analysis underscores the robustness of the pooled results of MMSE and MoCA, regardless of the inclusion or exclusion of individual studies.
Publication bias
Publication bias assessment was conducted to evaluate the MMSE and MoCA outcomes, employing both the funnel plot and the Egger test. Examination of the funnel plot for both MMSE (Figure S11) and MoCA (Figure S12) outcomes revealed a distribution of studies displaying a relatively symmetrical pattern. Moreover, the findings derived from the Egger's test for both MMSE (p = 0.361) and MoCA (p = 0.708) indicate no statistical significance, thus signifying the absence of observable publication bias within the studies included in this review.
Certainty in evidence
Regarding the MMSE outcome, the certainty of evidence was downgraded to a “moderate” level due to the presence of risk of bias. As for the MoCA outcome, the certainty of evidence was assessed as “low” due to the presence of risk of bias and inconsistency (Table S8).
Adverse events
Eleven studies reported detailed information on AEs (Table S9). 21 , 22 , 24 , 26 , 27 , 28 , 32 , 33 , 34 , 37 , 40 Throughout the trials, common AEs included gastrointestinal symptoms such as nausea and diarrhea. Furthermore, insomnia was also frequently reported by the participants, with seven participants in the CHM plus donepezil groups and nine participants in the donepezil group (Table S10). Our meta‐analysis of the risk ratio for the number of participants reporting AEs at the end of treatment reveals no statistically significant differences between the combination groups and donepezil groups (RR: 0.91 [0.59, 1.39], 11 studies, I 2 = 4%, 808 participants) (Figure S13).
DISCUSSION
Summary of the key findings
The recent clinical guideline did not recommend specific pharmacological treatments for MCI management, but mentioning off‐label use of cholinesterase inhibitors due to their limited benefits and potential risks. 4 Hence, any strategy that could enhance the benefits or mitigate the risks associated with cholinesterase inhibitors might contribute to clinical practice. Donepezil is a frequently used cholinesterase inhibitor to enhance cognitive function in patients with cognitive impairment. In China, clinicians also commonly use oral CHM to manage MCI. In our previous systematic review aimed at evaluating the efficacy of CHM for MCI, we found that CHM significantly enhanced cognitive function in MCI patients compared to those in the placebo control group, as measured by MMSE and MoCA scores. 16 However, there is uncertainty regarding whether combining oral CHM with donepezil offers additional benefits or increases risks.
In this systematic review, we included 20 studies, encompassing a total number of 1611 MCI patients. Our meta‐analysis reveals that the combination of CHM and donepezil significantly enhanced cognitive function in MCI patients, as evidenced by improvements in MMSE and MoCA scores. Regarding safety, the reporting frequency of AEs did not significantly differ between the combined therapy and donepezil‐only groups. This indicates that although the combination of oral CHM with donepezil may not mitigate the adverse events attributed to donepezil, it does not cause any additional risk. Furthermore, oral CHM demonstrate potential in increasing therapeutic effect, offering a new strategy for managing MCI in clinical practice. However, it is essential to interpret these findings with caution given the potential limitations in reporting or capturing AEs. Moreover, all 20 studies in our reviews were evaluated as having “some concerns” regarding the overall risk of bias. The certainty of evidence for MMSE outcomes was rated as “moderate,” while for MoCA, it was assessed as “low.” Therefore, further well‐designed RCTs are imperative to validate our findings.
Pharmacological mechanisms
Among those frequently utilized herbs, two classical formulae were identified, they are: Kai xin san and Si wu decoction.
Kai xin san has been administered to address forgetfulness for centuries and comprises of four herbs: Panax ginseng C. A. Mey., Polygala tenuifolia Wild., Acorus calamus var. angustatus Besser and Wolfiporia cocos (Schw.) Ryv. & Cilbn. 43 Experimental studies show Kai xin san's potential in alleviating cognitive dysfunction in Alzheimer's disease models by modulating the cholinergic system, mitigating damage to synaptic plasticity, attenuating tau hyperphosphorylation and neuroinflammation, and suppressing neuronal apoptosis and oxidative stress. 44 , 45 , 46 , 47
Si wu decoction, a traditional herbal formula comprising Ligusticum striatum DC., Rehmannia glutinosa (Gaertn.) DC., Angelica sinensis (Oliv.) Diels and Paeonia lactiflora Pall., has demonstrated potential in treating cognitive impairment. 48 Ligusticum striatum with tetramethylpyrazine as its main active component, offering therapeutic effects against Alzheimer's disease by reducing amyloid beta deposition, tau phosphorylation and neuroinflammation. 49 Tetramethylpyrazine also aids in restoring cholinergic neuron function, while providing neuroprotection for vascular dementia by inhibiting apoptosis and improving synaptic plasticity. 49 As for Rehmannia glutinosa, Angelica sinensis and Paeonia lactiflora, they demonstrate neuroprotective, anti‐inflammatory, antioxidant effects and anti‐apoptotic properties, addressing multiple pathways associated with Alzheimer's disease. 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 Details of the pharmacological mechanisms has been summarized in Table S11.
Sleep disturbance in MCI patients
Our meta‐analysis examining the risk ratio for participants reporting AEs at the end of treatment found no significant between the combination and donepezil‐only groups. Notably, prevalent AEs across the studies were gastrointestinal symptoms like nausea and diarrhea. Insomnia was also recurrent, reported by seven participants in the CHM plus donepezil groups and nine in the donepezil‐only group.
However, limited sleep‐related information at baseline makes it challenging to determine whether sleep disturbance, a common symptom in patients with cognitive impairment, 2 is a preexisting condition in MCI patients or induced by medication use. Moreover, previous studies suggested that sleep disturbance and cognitive impairment can influence each other, and potentially lead to a worsening of both conditions. 58 , 59 , 60 There might be a bidirectional association between sleep disturbance and cognitive impairment, and several mechanisms may be involved: including impaired amyloid beta clearance, accumulated tau protein, aggravated neurodegeneration, neuroinflammation and disrupted neurogenesis, disrupted synaptic plasticity and change in neurotransmitters. 61 Therefore, it underscores the significance of investigating sleep disturbance in future studies within the field of MCI.
While our study does not determine a relationship between sleep disturbance and different medication use, previous studies indicated that the usage of donepezil might be related to the sleep disturbances. A meta‐analysis examining sleep architecture changes linked to donepezil use revealed that subjects on donepezil had a significantly increased percentage of rapid eye movement sleep compared to placebo/controls, while also showing reductions in stage 2 sleep percentage, sleep efficiency and total sleep duration. 62 Another meta‐analysis revealed that donepezil is associated with a higher incidence of vivid dreams and insomnia compared to placebo. 63 The impact of donepezil on sleep patterns could be attributed to its pharmacological regulation of acetylcholine metabolism and the resulting elevated brain concentration, as acetylcholine can promote or enhance wakefulness. 62 Study suggests the importance of monitoring sleep problems during donepezil titration and follow‐up, with potential adjustments to dosage or administration timing as needed. 62 Considering that the use of donepezil might impact sleep in patients with MCI, when combined with the use of CHM, improving sleep quality becomes an important consideration when prescribing the CHM prescription. Interestingly, among those frequently used herbs from our included study, some of them (Wolfiporia cocos, Rehmannia glutinosa, Acorus calamus, Angelica sinensis, Panax ginseng) share a common function in regulating sleep disturbance according to the Chinese medicine theory. Although we cannot conclusively determine that combined treatment with oral CHM reduces the incidence of sleep disturbance due to the low reporting rate of adverse events, it does offer insights for future research.
Implications for clinical practice
Our study suggests that combining CHM with donepezil offers additional benefits without increasing risks. This finding implies a potential avenue for managing MCI. Clinicians might consider the combination therapy as an alternative strategy, especially for MCI patients who might not experience optimal responses with donepezil alone but still prefer pharmacological treatment.
Notably, commonly used Chinese herbs such as Panax ginseng, Polygala tenuifolia, Wolfiporia cocos, Acorus calamus, Rehmannia glutinosa, Paeonia lactiflora, Angelica sinensis, and Ligusticum striatum. have shown cognitive‐enhancing effects in experimental studies. Moreover, these herbs are key components of classic Chinese medicinal formulae: Kai xin san and Si wu decoction. Clinically, selectively incorporating these herbs might offer a targeted and potentially effective therapeutic approach for managing MCI.
Furthermore, addressing sleep disturbance is a crucial aspect that requires heightened attention in MCI patients. Considering the inclusion of herbs known for their sleep‐enhancing properties in CHM prescriptions might also be a beneficial approach.
Implications for future study
MCI can be further classified into various subtypes based on different etiological contributors, such as Alzheimer's disease, vascular disease, Lewy bodies and frontotemporal degeneration, each with different developmental courses. 64 In this systematic review, subgroup and meta‐regression analyses were performed to investigate the potential heterogeneity caused by the underlying etiologies of MCI. Although no significant differences were found in either the subgroup or meta‐regression analyses, we observed a trend suggesting that MCI caused by Alzheimer's disease might receive better treatment effects compared to the subgroup with vascular disease. This trend could be related to their different developmental courses and response to donepezil. Regarding their response to donepezil, previous researches have shown that donepezil has some cognitive‐improving effects on MCI and dementia with various underlying etiologies. 65 , 66 However, majority of the evidence pertains to Alzheimer's disease, and there is a lack of clear evidence on the efficacy of donepezil for different subtypes of MCI. 65 , 66 Combining CHM with donepezil may be more effective in the subgroup of MCI caused by Alzheimer's disease. However, it should be noted that due to the limited information provided by the RCTs, a definitive conclusion could not be reached. For future clinical trials investigating treatment effects for MCI, focusing on specific subtypes is essential to provide more accurate clinical guidance. Given the common occurrence of mixed pathologies in MCI, 67 determining the precise etiology of MCI is not always feasible. 64 Biomarkers may help identify the underlying etiology, particularly for MCI related to Alzheimer's disease; nevertheless, biomarkers for other degenerative diseases are less definitive and warrant further evaluation. 68 This poses a challenge for both clinical practice and research in the area of MCI.
The subgroup analysis of MMSE and MoCA outcomes based on treatment duration yielded a noteworthy observation. While the differences between groups did not achieve statistical significance, studies with shorter treatment durations appeared to have a marginally enhanced treatment effect compared to their longer‐duration counterparts. This finding raises several questions. For instance, why might shorter treatment durations exhibit a more pronounced effect? Could there be a potential saturation effect where benefits plateau or even diminish over extended periods?
A possible explanation of our findings focuses on the brain pathology features of MCI. MCI is characterized by neuronal attrition, synaptic deterioration 69 and diminished cortical connectivity. 70 Initiating treatment in the early stages of cognitive impairment might be the key element in preserving synaptic and neuronal function. In contrast, extending treatment durations might not offer increased benefits after a certain point. It is important to note that no definite conclusions have been reached regarding this issue. To address this question, future studies should carefully investigate how treatment length affects its effectiveness. Randomized controlled trials comparing the effects of short‐term versus long‐term treatment durations could provide invaluable insights. Similarly, studies aimed at understanding why results vary between shorter and longer treatments will be crucial for improving our understanding of MCI treatment approaches.
Limitations of the study
Several limitations of this systematic review should be considered when interpreting the results. First, all the studies included in this review were conducted in China, this geographical confinement raises questions about the generalizability of our findings to broader populations outside China. Therefore, future research necessitates internationally collaborated multicenter trials to ascertain the generalizability of these outcomes.
Second, most of the studies had relatively short treatment duration, which limits our understanding of the long‐term benefits of the treatment. Given that MCI is a chronic condition, there is an urgent need for well‐designed RCTs with extended intervention durations, to assess the long‐term effectiveness and safety of combining CHM with donepezil. In the field of MCI research, clinical trials evaluating the effects of interventions on symptomatic progression generally necessitate a minimum trial duration of 6 months. 71
Further, the CHM formulae and CHM forms varied widely between the included studies. None of the studies mentioned the manufacturing process of the CHM adopted in the trials, making it unclear whether the patients received exactly the same treatment within the trial. Those heterogeneity poses challenges for interpreting the results and their clinical application. Future studies should focus on specific herbal formulations that exhibit promising benefits or delve deeper into certain promising herbs to offer more conclusive evidence. Consideration should also be given to standardized CHM forms with good manufacturing practice in future research.
CONCLUSIONS
The concurrent use of oral CHM and donepezil appears to be more effective than donepezil alone in improving the cognitive function of MCI patients, without leading to an increase in AEs. While recognizing concerns of the overall methodological quality, this combined therapy should be considered as an alternative option for clinical practice.
AUTHOR CONTRIBUTIONS
Lingling Liu: Conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft. Claire Shuiqing Zhang: Conceptualization, methodology, validation, investigation, data curation, writing—original draft. Anthony Lin Zhang: Conceptualization, methodology, supervision, project administration. Yefeng Cai: Writing—review & editing, supervision, funding acquisition. Charlie Changli Xue: Writing—review & editing, supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
SPONSOR'S ROLE
The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
FINANCIAL DISCLOSURE
This work was supported by the China‐Australia International Research Centre for Chinese Medicine, RMIT University, the NATCM's Project of High‐level Construction of Key TCM Disciplines (zyyzdxk‐2023154), the funding from Guangzhou University of Chinese medicine (No. 2021xk26), and Guangdong Provincial Key Laboratory of Research on Emergency in TCM (No. 2017B030314176).
Editor's Note.
As Western clinical geroscience marches toward mainstream application, there is new scientific interest in interventions with multifunctional effects on aging outcomes, such as physical activity, metformin, and GLP‐1 receptor agonists. Chinese herbal medicines are generally comprised of several herbs; developed empirically by traditional practitioners over centuries, they are the oldest recognized multifunctional therapeutics. Currently being avidly investigated using rigorous scientific methods—mainly in China—various formulations may strengthen general adaptive capacities and exert a wide range of symptomatic and organ‐ and receptor‐specific effects. Liu et al. have used rigorous systematic review and meta‐analytic methods to examine potential synergistic cognitive effects of Chinese herbal medicines and donepezil in mild cognitive impairment. Appropriately cautious in interpreting the evidence, the authors draw our attention to the need for a broad, inclusive, and international neuroscience of aging that unifies the complementary traditions of Western and Chinese medical research.
Soo Borson, MD
Supporting information
Table S1. PRISMA 2020 checklist.
Table S2. Search strategy.
Table S3. Characteristics of included studies.
Table S4. Participants characteristics of included studies.
Table S5. Ingredients of Chinese herbal medicine preparations of included studies.
Table S6. Most frequently used herbs in included studies.
Table S7. MMSE and MoCA: Baseline, end‐of‐treatment, and change scores.
Table S8. Grade assessment for MMSE and MoCA.
Table S9. Summary of adverse events.
Table S10. Frequency distribution of different adverse events.
Table S11. Pharmacological mechanisms of Kai xin san and Si wu decoction.
Figure S1. Risk of bias in included studies.
Figure S2. Overall meta‐analysis on change scores of MMSE.
Figure S3. Subgroup analysis on MMSE at the end of treatment (based on the MCI subtypes).
Figure S4. Subgroup analysis on MMSE at the end of treatment (based on the treatment duration).
Figure S5. Univariate meta‐regression analyses for the end‐of‐treatment effects on MMSE.
Figure S6. Overall meta‐analysis on change scores of MoCA.
Figure S7. Subgroup analysis on MoCA at the end of treatment (based on the treatment duration).
Figure S8. Univariate meta‐regression analysis for the end‐of‐treatment effects on MoCA.
Figure S9. Sensitivity analysis based on MMSE (one‐by‐one exclusion method).
Figure S10. Sensitivity analysis based on MoCA (one‐by‐one exclusion method).
Figure S11. Funnel plot of studies reporting MMSE at the end of treatment.
Figure S12. Funnel plot of studies reporting MoCA at the end of treatment.
Figure S13. Meta‐analysis on adverse events at the end of treatment.
ACKNOWLEDGMENT
Open access publishing facilitated by RMIT University, as part of the Wiley ‐ RMIT University agreement via the Council of Australian University Librarians.
Liu L, Zhang CS, Zhang AL, Cai Y, Xue CC. Oral Chinese herbal medicine combined with donepezil for mild cognitive impairment: A systematic review and meta‐analysis. J Am Geriatr Soc. 2024;72(12):3890‐3902. doi: 10.1111/jgs.19125
Lingling Liu and Claire Shuiqing Zhang contributed equally to this work and share the first authorship.
Contributor Information
Yefeng Cai, Email: caiyefeng@126.com.
Charlie Changli Xue, Email: charlie.xue@rmit.edu.au.
REFERENCES
- 1. Mild Cognitive Impairment (MCI). 2023. Accessed May 08, 2023. https://www.mayoclinic.org/diseases-conditions/mild-cognitive-impairment/symptoms-causes/syc-20354578
- 2. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington; 2013:591‐643. [Google Scholar]
- 3. Bai W, Chen P, Cai H, et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta‐analysis and systematic review of epidemiology studies. Age Ageing. 2022;51:afac173. [DOI] [PubMed] [Google Scholar]
- 4. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev. 2006;2006(3):CD006104. [DOI] [PubMed] [Google Scholar]
- 6. Pink J, O'Brien J, Robinson L, Longson D. Dementia: assessment, management and support: summary of updated nice guidance. BMJ. 2018;361:k2438. [DOI] [PubMed] [Google Scholar]
- 7. Shaji KS, Sivakumar PT, Rao GP, Paul N. Clinical practice guidelines for management of dementia. Indian J Psychiatry. 2018;60:S312‐S328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsunaga S, Fujishiro H, Takechi H. Efficacy and safety of cholinesterase inhibitors for mild cognitive impairment: a systematic review and meta‐analysis. J Alzheimers Dis. 2019;71:513‐523. [DOI] [PubMed] [Google Scholar]
- 9. Kelley BJ. Treatment of mild cognitive impairment. Curr Treat Options Neurol. 2015;17:372. [DOI] [PubMed] [Google Scholar]
- 10. Weinstein AM, Barton C, Ross L, Kramer JH, Yaffe K. Treatment practices of mild cognitive impairment in California Alzheimer's Disease Centers. J Am Geriatr Soc. 2009;57:686‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ban CX, Xiao SF, Lin X, et al. Clinicians' prescription preferences for treating patients with Alzheimer's disease in Shanghai. Transl Neurodegener. 2016;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vohra N, Hadi MA, Khanal S, Kurmi OP, Paudyal V. Impact of deprivation, dementia prevalence and regional demography on prescribing of antidementia drugs in England: a time trend analysis. Br J Clin Pharmacol. 2021;87:3747‐3755. [DOI] [PubMed] [Google Scholar]
- 13. Dementia in Australia. 2023. Australian Institute of Health and Welfare. Accessed November 16, 2023. https://www.aihw.gov.au/reports/dementia/dementia-in-aus
- 14. May B, Feng M. Classical Chinese medicine literature. In: Xue CC, Lu C, eds. Evidence‐based Clinical Chinese Medicine—Volume 8: Alzheimer's Disease. 1st ed. World Scientific Publishing; 2018:41‐74. [Google Scholar]
- 15. May B, Feng M. Classical Chinese medicine literature. In: Xue CC, Lu C, eds. Evidence‐based Clinical Chinese Medicine—Volume 9: Vascular Dementia. 1st ed. World Scientific Publishing; 2020:41‐69. [Google Scholar]
- 16. Liu L, Zhang CS, Zhang AL, Cai Y, Xue CC. The efficacy and safety of Chinese herbal medicine for mild cognitive impairment: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Front Pharmacol. 2024;15:1341074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane. 2022. Accessed May 08, 2023. http://www.training.cochrane.org/handbook
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeFeudis F. A brief history of egb 761® and its therapeutic uses. Pharmacopsychiatry. 2003;36:2‐7. [DOI] [PubMed] [Google Scholar]
- 20. Grade Handbook for Grading Quality of Evidence and Strength of Recommendations (updated October 2013). 2013. Accessed May 08, 2023. https://gdt.gradepro.org/app/handbook/handbook.html
- 21. Gan P, Wu D, Ji K, Huang L, Liu L. Treatment of type 2 diabetes mellitus with mild cognitive impairment by bu shen qing nao ultrafine granular powder. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2020;40:422‐426. [Google Scholar]
- 22. Gao F, Ji Y, Cao H, Wang H. Effects of Chinese medicine for nourishing kidney, eliminating phlegm and damp on secondary mild vascular cognitive dysfunction in elderly patients with diabetes and the effects on oxidative stress index and acetylcholinesterase. Mod J Integr Tradit Chin West Med. 2017;26:3324‐3327. [Google Scholar]
- 23. Gao H. Observation on the therapeutic effect of modified shu yu pill in the early treatment of non‐dementia vascular cognitive impairment. Mod J Integr Tradit Chin West Med. 2017;26:2336‐2339. [Google Scholar]
- 24. Gu L. Analysis on the effectiveness and safety of yi zhi wen dan decoction in the treatment of senile mild cognitive impairment. Pract J Clin Med. 2018;15:55‐58. [Google Scholar]
- 25. Guo D, Bai Y. Effects of donepezil combined with yang xue qing nao granule on hs‐CRP, Hcy, cerebral blood perfusion and electrophysiology in patients with vascular cognitive impairment caused cerebral small vessel disease. Mod J Integr Tradit Chin West Med. 2019;28:352‐356. +368. [Google Scholar]
- 26. Han L. Clinical Observation of Bu Shen Yi Zhi Decoction for MCI after Stroke (Kidney Deficiency Syndrome) [Master]. Shanxi University of Chinese Medicine; 2020. [Google Scholar]
- 27. He J, Jiang X, Wu Z, Zhang J. Effect of huan nao yi cong decoction combined with conventional medicine in the treatment of senile mild cognitive impairment and its influence on hemorheology. Mod J Integr Tradit Chin West Med. 2017;26:1989‐1991. [Google Scholar]
- 28. Li Q, Jia S, Guan H. Clinical effect observation of di huang yin zi in adjuvant treatment of deficiency of kidney and marrow syndrome type 2 diabetes mellitus with mild cognitive impairment. Lishizhen Med Mater Med Res. 2022;33:410‐412. [Google Scholar]
- 29. Li Q, Zhou L. Clinical observation of di huang yi zi for the treatment of mild cognitive impairment. Zhejiang J Integr Tradit Chin West Med. 2014;24:625‐626. [Google Scholar]
- 30. Li X, Wang H. Clinical observation on therapeutic effect of spleen and removing phlegm method on patients with Alzheimer's disease MCI. Clini J Tradit Chin Med. 2020;32:1099‐1102. [Google Scholar]
- 31. Liu X. Clinical observation of bu yang huan wu decoction combined with donepezil in the treatment of mild cognitive impairment. J Pract Tradit Chin Med. 2020;36:359‐360. [Google Scholar]
- 32. Ma C, Liu X, Wang X, et al. Clinical effect observation of jian pi bu shen huo xue prescription on MCI of white matter lesion in cerebral small vessel disease. Lishizhen Med Mater Med Res. 2022;33:400‐403. [Google Scholar]
- 33. Qian D. The Clinical Study on the Treatment of Mild Cognitive Impairment (MCI) from Deficiency of Fluid [Master]. Shandong University of Traditional Chinese Medicine; 2019. [Google Scholar]
- 34. Shen L. Therapeutic effect and pharmacological analysis of huang lian wen dan decoction in senile patients with mild cognitive impairment. Health Prot Promot. 2014;2:202‐203. [Google Scholar]
- 35. Shou F, Wu C, Fu D, Mao Y. Clinical study on modified sheng hui decoction combined with donepezil for senile mild cognitive impairment with kidney essence depletion syndrome. J New Chin Med. 2022;54:58‐60. [Google Scholar]
- 36. Wang L, Wang L, Han S. Clinical effect observation of yang xue qing nao granule combined with donepezil in patients with mild cognitive impairment. J Epileptol Electroneurophysiol. 2018;27:176‐177. [Google Scholar]
- 37. Wang X, Piao M, Wang Z, et al. Clinical observation of san bu recipe in treating amnestic mild cognitive impairment with deficiency of spleen and kidney. Guid J Tradit Chin Med Pharm. 2021;27:80‐84. [Google Scholar]
- 38. Xie L, Chen C. Bu yang huan wu decoction combined with donepezil in the treatment of 30 cases of mild cognitive impairment. Jiangxi J Tradit Chin Med. 2015;46:26‐28. [Google Scholar]
- 39. Xie S, Liu X, Wang X. Therapeutic effect analysis of yang xue qing nao pill combined with acetylcholinase inhibitors on patients with mild cognitive impairment with leukoaraiosis. J Med Forum. 2020;41:149‐151. [Google Scholar]
- 40. Yang J, Tian Q, Sun X. Effect of aricept combined with wu ling capsule for the treatment of mild cognitive impairment. Chin J Gerontol. 2011;31:3886‐3888. [Google Scholar]
- 41. Interpretation of the Moca. Is There a Cut‐off Score Between Mild Cognitive Impairment (MCI) and Alzheimer's Disease (AD)? 2023. MoCA Test. Accessed August 11, 2023. https://mocacognition.com/faq
- 42. Lu J, Li D, Li F, et al. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: a population‐based study. J Geriatr Psychiatry Neurol. 2011;24:184‐190. [DOI] [PubMed] [Google Scholar]
- 43. Cao C, Xiao J, Liu M, et al. Active components, derived from kai‐xin‐san, a herbal formula, increase the expressions of neurotrophic factor NGF and BDNF on mouse astrocyte primary cultures via camp‐dependent signaling pathway. J Ethnopharmacol. 2018;224:554‐562. [DOI] [PubMed] [Google Scholar]
- 44. Yi P, Zhang Z, Huang S, Huang J, Peng W, Yang J. Integrated meta‐analysis, network pharmacology, and molecular docking to investigate the efficacy and potential pharmacological mechanism of kai‐xin‐san on Alzheimer's disease. Pharm Biol. 2020;58:932‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiao Y, Zhang J, Qiao W, et al. Kai‐xin‐san inhibits tau pathology and neuronal apoptosis in aged samp8 mice. Mol Neurobiol. 2022;59:3294‐3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Su S, Chen G, Gao M, et al. Kai‐xin‐san protects against mitochondrial dysfunction in Alzheimer's disease through SIRT3/NLRP3 pathway. Chin Med. 2023;18:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu Y, Lu F, Xu H, et al. Kai‐xin‐san improves cognitive impairment via Wnt/β‐catenin and IRE1/XBP1s signalings in APP/PS1 mice. Rejuvenation Res. 2023;26:105‐115. [DOI] [PubMed] [Google Scholar]
- 48. Zuo H, Zhang Q, Chen C, Yang F, Yu H, Hu YJ. Molecular evidence of herbal formula: a network‐based analysis of si‐wu decoction. Phytochem Anal. 2021;32:198‐205. [DOI] [PubMed] [Google Scholar]
- 49. Liu Y, Yang G, Cui W, Zhang Y, Liang X. Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: a review. Front Pharmacol. 2022;13:948600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bian Z, Zhang R, Zhang X, et al. Extraction, structure and bioactivities of polysaccharides from rehmannia glutinosa: a review. J Ethnopharmacol. 2023;305:116132. [DOI] [PubMed] [Google Scholar]
- 51. Zhang R, Li M, Jia Z. Rehmannia glutinosa: review of botany, chemistry and pharmacology. J Ethnopharmacol. 2008;117:199‐214. [DOI] [PubMed] [Google Scholar]
- 52. Fu C, Wu Y, Liu S, et al. Rehmannioside a improves cognitive impairment and alleviates ferroptosis via activating pi3k/akt/nrf2 and slc7a11/gpx4 signaling pathway after ischemia. J Ethnopharmacol. 2022;289:115021. [DOI] [PubMed] [Google Scholar]
- 53. Long Y, Li D, Yu S, et al. Medicine‐food herb: Angelica sinensis, a potential therapeutic hope for Alzheimer's disease and related complications. Food Funct. 2022;13:8783‐8803. [DOI] [PubMed] [Google Scholar]
- 54. Duan M, Wang L, Jiang Y, Pei Y, Guan D, Qiu Z. Angelica sinensis reduced Aβ‐induced memory impairment in rats. J Drug Target. 2016;24:340‐347. [DOI] [PubMed] [Google Scholar]
- 55. May BH, Lu C, Bennett L, Hügel HM, Xue CC. Evaluating the traditional Chinese literature for herbal formulae and individual herbs used for age‐related dementia and memory impairment. Biogerontology. 2012;13:299‐312. [DOI] [PubMed] [Google Scholar]
- 56. Manayi A, Omidpanah S, Barreca D, et al. Neuroprotective effects of paeoniflorin in neurodegenerative diseases of the central nervous system. Phytother Res. 2017;16:1173‐1181. [Google Scholar]
- 57. Hong H, Lu X, Wu C, et al. A review for the pharmacological effects of paeoniflorin in the nervous system. Front Pharmacol. 2022;13:898955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Smith L, Shin JI, Jacob L, et al. Sleep problems and mild cognitive impairment among adults aged ≥50 years from low‐ and middle‐income countries. Exp Gerontol. 2021;154:111513. [DOI] [PubMed] [Google Scholar]
- 59. Rozzini L, Conti MZ, Riva M, et al. Non‐amnestic mild cognitive impairment and sleep complaints: a bidirectional relationship? Aging Clin Exp Res. 2018;30:661‐668. [DOI] [PubMed] [Google Scholar]
- 60. Guarnieri B, Sorbi S. Sleep and cognitive decline: a strong bidirectional relationship. It is time for specific recommendations on routine assessment and the management of sleep disorders in patients with mild cognitive impairment and dementia. Eur Neurol. 2015;74:43‐48. [DOI] [PubMed] [Google Scholar]
- 61. Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017‐1028. [DOI] [PubMed] [Google Scholar]
- 62. Hsieh C, Tseng P, Chen T, et al. The association of changes of sleep architecture related to donepezil: a systematic review and meta‐analysis. J Formos Med Assoc. 2022;121:1466‐1477. [DOI] [PubMed] [Google Scholar]
- 63. Blackman J, Swirski M, Clynes J, Harding S, Leng Y, Coulthard E. Pharmacological and non‐pharmacological interventions to enhance sleep in mild cognitive impairment and mild Alzheimer's disease: a systematic review. J Sleep Res. 2021;30:e13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders (5th ed., text rev.). 2022. doi: 10.1176/appi.books.9780890425787.x17_Neurocognitive_Disorders [DOI]
- 65. Zhang X, Lian S, Zhang Y, Zhao Q. Efficacy and safety of donepezil for mild cognitive impairment: a systematic review and meta‐analysis. Clin Neurol Neurosurg. 2022;213:107134. [DOI] [PubMed] [Google Scholar]
- 66. Donepezil. 2023. Accessed June 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK513257/
- 67. Kasper S, Bancher C, Eckert A, et al. Management of mild cognitive impairment (MCI): the need for national and international guidelines. World J Biol Psychiatry. 2020;21:579‐594. [DOI] [PubMed] [Google Scholar]
- 68. Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). 2016;22:404‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mufson EJ, Binder L, Counts SE, et al. Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol. 2012;123:13‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gonzalez‐Escamilla G, Atienza M, Garcia‐Solis D, Cantero JL. Cerebral and blood correlates of reduced functional connectivity in mild cognitive impairment. Brain Struct Funct. 2016;221:631‐645. [DOI] [PubMed] [Google Scholar]
- 71. Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry. 2006;77:429‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA 2020 checklist.
Table S2. Search strategy.
Table S3. Characteristics of included studies.
Table S4. Participants characteristics of included studies.
Table S5. Ingredients of Chinese herbal medicine preparations of included studies.
Table S6. Most frequently used herbs in included studies.
Table S7. MMSE and MoCA: Baseline, end‐of‐treatment, and change scores.
Table S8. Grade assessment for MMSE and MoCA.
Table S9. Summary of adverse events.
Table S10. Frequency distribution of different adverse events.
Table S11. Pharmacological mechanisms of Kai xin san and Si wu decoction.
Figure S1. Risk of bias in included studies.
Figure S2. Overall meta‐analysis on change scores of MMSE.
Figure S3. Subgroup analysis on MMSE at the end of treatment (based on the MCI subtypes).
Figure S4. Subgroup analysis on MMSE at the end of treatment (based on the treatment duration).
Figure S5. Univariate meta‐regression analyses for the end‐of‐treatment effects on MMSE.
Figure S6. Overall meta‐analysis on change scores of MoCA.
Figure S7. Subgroup analysis on MoCA at the end of treatment (based on the treatment duration).
Figure S8. Univariate meta‐regression analysis for the end‐of‐treatment effects on MoCA.
Figure S9. Sensitivity analysis based on MMSE (one‐by‐one exclusion method).
Figure S10. Sensitivity analysis based on MoCA (one‐by‐one exclusion method).
Figure S11. Funnel plot of studies reporting MMSE at the end of treatment.
Figure S12. Funnel plot of studies reporting MoCA at the end of treatment.
Figure S13. Meta‐analysis on adverse events at the end of treatment.


