Abstract
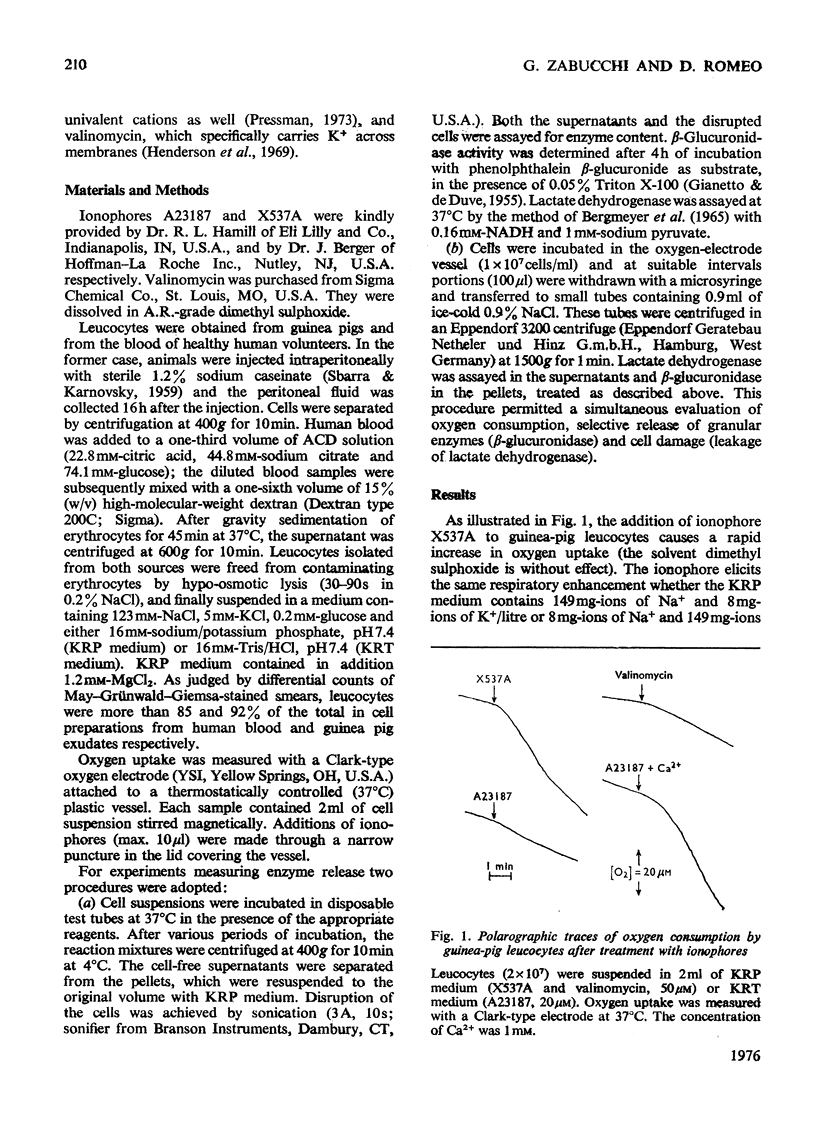
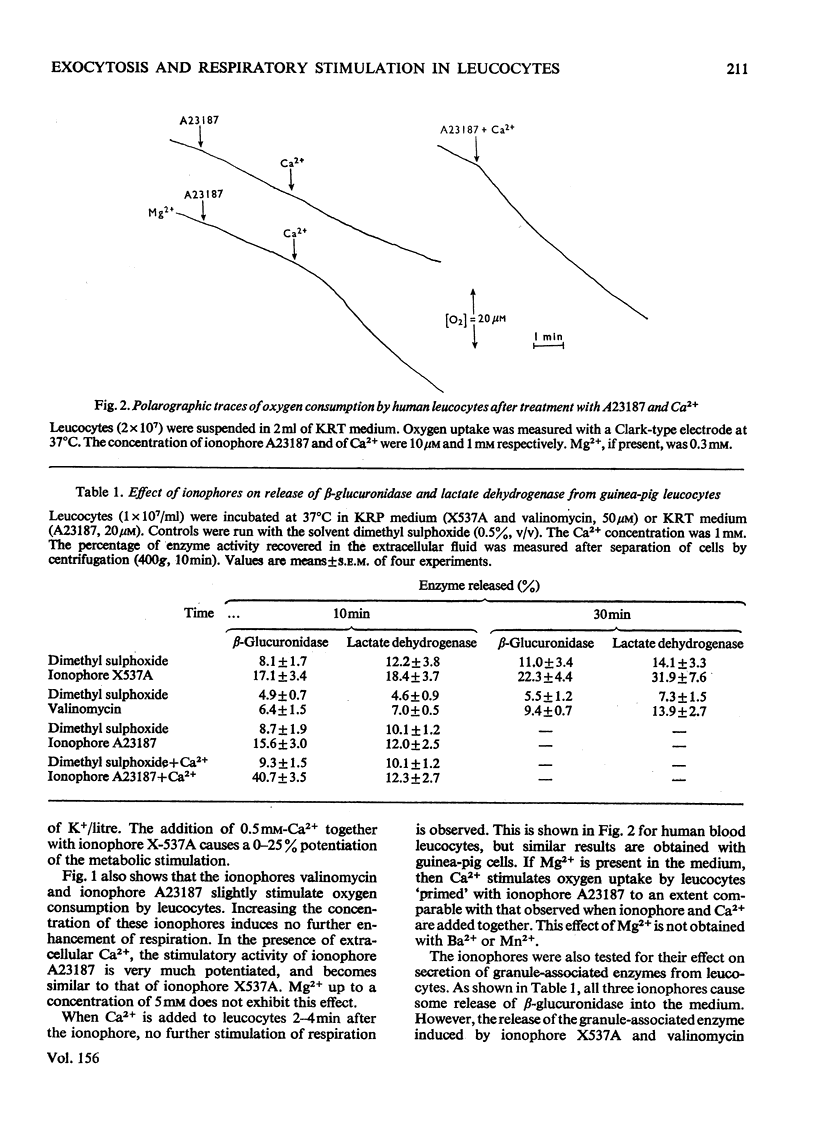
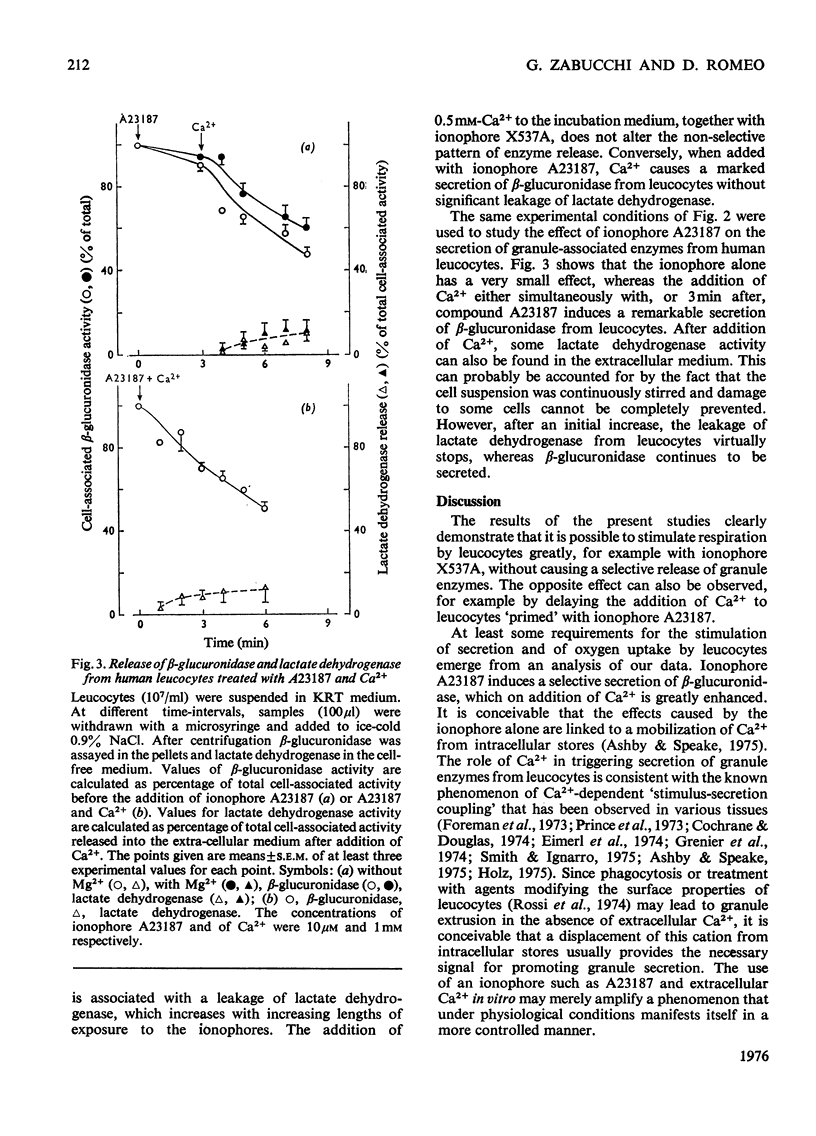
By exploiting the unique characteristics of three ionophores, experimental conditions were found which permit the dissociation of respiratory stimulation from secretion in polymorphonuclear leucocytes. A marked stimulation of respiration was produced by ionophore X537A, which binds and transports both alkali-earth and alkali cations. The stimulatory activity of this ionophore was the same at either high or low Na+/K+ ratios in the medium and was virtually unaffected by extracellular Ca2+. A slight stimulation of oxygen consumption was also caused by the K+-selective ionophore valinomycin and by ionophore A23187, which complexes and transfers bivalent cations. Ionophore X537A and valinomycin were unable to stimulate selective release of granuleassociated beta-glucuronidase and gradually increased cell fragility, as monitored by increased leakage of lactate dehydrogenase. Ionophore A23187 slightly increased exocytosis of beta-glucuronidase. In a Mg2+-free medium, Ca2+, added simultaneously with ionophore A23187, greatly enhanced respiration and secretion of the granule enzyme. If Ca2+ was added a few minutes after the ionophore, exocytosis occurred, but no respiratory burst was observed. If the latter experiment was repeated in the presence of extracellular Mg2+, both secretion and respiration were stimulated. This effect was not produced by Mn2+ or Ba2+. It is proposed that Ca2+ is required for triggering selective secretion of granule enzymes from leucocytes is caused by an intracellular redistribution of cations, which may invovle Mg2+-dependent mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J. P., Speake R. N. Insulin and glucagon secretion from isolated islets of Langerhans. The effects of calcium ionophores. Biochem J. 1975 Jul;150(1):89–96. [PMC free article] [PubMed] [Google Scholar]
- Cochrane D. E., Douglas W. W. Calcium-induced extrusion of secretory granules (exocytosis) in mast cells exposed to 48-80 or the ionophores A-23187 and X-537A. Proc Natl Acad Sci U S A. 1974 Feb;71(2):408–412. doi: 10.1073/pnas.71.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimerl S., Savion N., Heichal O., Selinger Z. Induction of enzyme secretion in rat pancreatic slices using the ionophore A-23187 and calcium. An experimental bypass of the hormone receptor pathway. J Biol Chem. 1974 Jun 25;249(12):3991–3993. [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier G., Van Sande J., Glick D., Dumont J. E. Effect of ionophore A23187 on thyroid secretion. FEBS Lett. 1974 Dec 1;49(1):96–99. doi: 10.1016/0014-5793(74)80640-x. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., COHN Z. A. Degranulation of polymorphonuclear leucocytes following phagocytosis of microorganisms. J Exp Med. 1960 Dec 1;112:1005–1014. doi: 10.1084/jem.112.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn D. C., Lehrer R. I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J Clin Invest. 1975 Apr;55(4):707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz R. W. The release of dopamine from synaptosomes from rat striatum by the ionophores X 537A and A 23187. Biochim Biophys Acta. 1975 Jan 14;375(1):138–152. doi: 10.1016/0005-2736(75)90079-6. [DOI] [PubMed] [Google Scholar]
- Patriarca P., Cramer R., Moncalvo S., Rossi F., Romeo D. Enzymatic basis of metabolic stimulation in leucocytes during phagocytosis: the role of activated NADPH oxidase. Arch Biochem Biophys. 1971 Jul;145(1):255–262. doi: 10.1016/0003-9861(71)90034-8. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Prince W. T., Rasmussen H., Berridge M. J. The role of calcium in fly salivary gland secretion analyzed with the ionophore A-23187. Biochim Biophys Acta. 1973 Nov 2;329(1):98–107. doi: 10.1016/0304-4165(73)90012-3. [DOI] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Romeo D., Zabucchi G., Miani N., Rossi F. Ion movement across leukocyte plasma membrane and excitation of their metabolism. Nature. 1975 Feb 13;253(5492):542–544. doi: 10.1038/253542a0. [DOI] [PubMed] [Google Scholar]
- Rossi F., Romeo D., Patriarca P. Mechanism of phagocytosis-associated oxidative metabolism in polymorphonuclear leucocytes and macrophages. J Reticuloendothel Soc. 1972 Aug;12(2):127–149. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Schell-Frederick E. Stimulation of the oxidative metabolism of polymorphonuclear leucocytes by the calcium ionophore A23187. FEBS Lett. 1974 Nov 1;48(1):37–40. doi: 10.1016/0014-5793(74)81056-2. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Ignarro L. J. Bioregulation of lysosomal enzyme secretion from human neutrophils: roles of guanosine 3':5'-monophosphate and calcium in stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1975 Jan;72(1):108–112. doi: 10.1073/pnas.72.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Henson P., Holmes B., Good R. A. An in vitro study of exocytosis of neutrophil granule enzymes in chronic granulomatous disease neutrophils. J Immunol. 1974 Apr;112(4):1383–1386. [PubMed] [Google Scholar]
- Weissmann G. The role of lysosomes in inflammation and disease. Annu Rev Med. 1967;18:97–112. doi: 10.1146/annurev.me.18.020167.000525. [DOI] [PubMed] [Google Scholar]
- Zabucchi G., Soranzo M. R., Rossi F. Exocytosis in human polymorphonuclear leukocytes induced by A 23187 and calcium. FEBS Lett. 1975 Jun 1;54(1):44–48. doi: 10.1016/0014-5793(75)81064-7. [DOI] [PubMed] [Google Scholar]


