Abstract
Background and Objectives
The role of the complement system in myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is not completely understood, and studies exploring its potential utility for diagnosis and prognosis are lacking. We aimed to investigate the value of complement factors (CFs) as diagnostic and prognostic biomarkers in patients with MOGAD.
Methods
Multicentric retrospective cohort study including patients with MOGAD, multiple sclerosis (MS) and aquaporin-4 seropositive neuromyelitis optica spectrum disorder (AQP4-NMOSD) with available paired serum and CSF samples. A panel of CFs were measured by multiplex ELISA, and the levels were compared between the 3 conditions. Univariable and multivariable analyses were performed to evaluate the association between levels of CFs and relapse and disability outcomes in MOGAD patients.
Results
Ninety-four patients (MOGAD, n = 60; MS, n = 18; AQP4-NMOSD, n = 16) were included. Mean (SD) age at sampling was 39.4 (16.7), 40.7 (7.0), and 43.3 (21.0), respectively. Female were predominant, especially in AQP4-NMOSD (88%). Combination of the serum levels of C3a, C4a, and C3a/C3 ratio showed excellent potential to discriminate MOGAD from patients with MS (area under the curve [AUC] [95% CI] 0.95 [0.90–0.99]) and from AQP4-NMOSD (AUC 0.88 [0.76–1.00]). In patients with MOGAD, CSF levels of CFs of the classical/lectin pathway influenced relapse-related outcomes, and lower C4 levels were associated with higher number of relapses during follow-up (incidence rate ratio [95% CI] 0.88 [0.78–0.99]; p = 0.04 in multivariable analysis), and a high C4a/C4 ratio was associated with increased risk of second relapse during the first year (hazard ratio [95% CI] 3.68 [1.26–10.78]; p = 0.02 in multivariable analysis). Time to second relapse was shorter in patients with MOGAD with a high CSF C4a/C4 ratio (log-rank p = 0.01). CSF levels of the membrane attack complex SC5b9 influenced disability-related outcomes, and baseline CSF SC5b9 levels were higher in patients who reached the final Expanded Disability Status Scale (EDSS) ≥ 3.0 (p = 0.002), and elevated SC5b9 levels were associated with increased risk of reaching EDSS ≥ 3.0 (odds ratio [95% CI] 1.79 [1.16–3.67]; p = 0.04 in multivariable analyses).
Discussion
Our results suggest that serum and CSF levels of CFs have diagnostic and prognostic value respectively in patients with MOGAD. These findings support the use of complement inhibitors as a therapeutic approach in these patients.
Introduction
Antibodies targeting the myelin oligodendrocyte glycoprotein (MOG-IgG) and aquaporin-4 protein (AQP4-IgG) define 2 distinct demyelinating diseases different from multiple sclerosis (MS).1,2 The complement, a key component of the innate immune system, has been shown to contribute to the pathogenesis of AQP4-IgG seropositive neuromyelitis optica spectrum disorder (AQP4-NMOSD), with anti-C5 therapies demonstrating remarkable efficacy in preventing relapses in patients with AQP4-NMOSD.3-7 However, the extent to which the complement system is involved in MOG-antibody-associated disease myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD) pathogenesis has been a subject of controversy in recent years.8-11
Previous studies comparing C3 and C4 complement factors (CFs) in blood between MOGAD and AQP4-NMOSD reported lower C4 levels in patients with AQP4-NMOSD.12-14 One of these studies failed to find significant differences in levels of CFs between patients with MOGAD and MS.12 By contrast, a more recent study reported for the first time systemic complement activation in MOGAD compared with both patients with MS and AQP4-NMOSD.15
Along with a better understanding of disease pathogenesis, the study of the complement system may lead to the identification of diagnostic and prognostic biomarkers playing a role in the disease. This is particularly relevant for MOGAD, a condition known to have clinical and radiologic features that may overlap with MS and AQP4-NMOSD, with an unpredictable disease course (i.e., approximately 50% of the cases are monophasic) and for which no prognostic biomarkers are available, yet.15-19
Bearing this in mind, in this study we aimed to explore the role of the complement system in MOGAD pathogenesis, diagnosis, and prognosis by measuring the levels of a panel of complement components (C3, C4, C5, C1q), complement activation products (C3a, C4a, C5a, Ba, Bb, and the membrane attack complex [MAC] or SC5b9), and complement regulators (Factor H, Factor I) in paired serum and CSF samples.
Methods
Study Design and Participants
This was a multicentric retrospective cohort study in 4 European centers (Vall d'Hebrón University Hospital, Barcelona, Spain; Clínic University Hospital, Barcelona, Spain; Dr. Josep Trueta University Hospital, Girona, Spain; Verona University Hospital, Verona, Italy) that included patients with MOGAD, relapsing-remitting MS, and AQP4-NMOSD fulfilling the most recent diagnostic criteria or recommendations for each disease at the moment of recruitment (December 2022).20-22 Selection of patients was based on availability of paired serum, and CSF samples never thawed or thawed at most once.
Serum and CSF Processing
CSF samples were collected by lumbar puncture for routine CSF diagnostics and centrifuged to remove cells. Peripheral blood was collected by standard venipuncture and allowed to clot spontaneously for 30 minutes, with serum collection by centrifugation. CSF and serum samples were aliquoted, frozen down at −80°C in 1–2 hours per protocol, and stored until used. The protocols for sample processing at each center contributing with samples are shown in eMethods.
Antibodies Assessment
Serum samples of patients with MOGAD and AQP4-NMOSD were tested for MOG-IgG and AQP4-IgG using live cell-based assays in their respective reference laboratories, as previously reported.23-25
Determination of CFs
CSF and serum samples were shipped to the University Hospital of Münster (Germany) on dry ice. For the quantification of serum and CSF levels of CFs (Ba, Bb, C3a, C4a, C5a, sC5b9, Factor H, Factor I, C1q, C3, C4, C5), multiplex enzyme-linked immunosorbent assays (ELISAs) based on chemiluminescence were used according to the manufacturer's recommendations (Quidel, San Diego, CA; cat. number: A900, A917) to systematically profile protein concentrations. Specifically, frozen specimens were thawed rapidly at 37°C using a water bath until just thawed, then immediately transferred to ice to prevent complement activation before dilution. Samples were analyzed within 45 minutes after thawing. Only one freeze/thaw cycle was performed for all samples. Each plate contained samples from all different groups to minimize interplate variation. Control samples provided by the manufacturer were included on each plate to ensure plate-to-plate consistency. For data points below lower limit or above upper limit of quantification, the respective threshold was used as value for analysis. eTable 1 summarizes the intra-assay and interassay coefficients of variation for each CF.
Clinical Information
The following information was recorded for all patients: sex; birth date; clinical information of the first event (date; topography; Expanded Disability Status Scale [EDSS] scores; visual acuity [VA] of the most affected eye; acute and chronic treatment before and at sampling; date of sampling). Samples collected within 3 months from the first or subsequent events were considered as acute onset or acute relapse, respectively. Otherwise, they were considered as remission.
Follow-up information was also recorded for patients with MOGAD and included dates of relapses; EDSS and VA at last follow-up; chronic treatment; and date of last follow-up.
Statistical Analysis
Levels of CFs were compared between disease groups using the Kruskal-Wallis test and a post hoc pairwise comparison analysis (Tukey test). To explore the association between serum and CSF levels of CFs and demographic and clinical characteristics, univariable linear regression models were fitted using clinical features as independent variables.
For the evaluation of clinical outcomes, different models were used depending on the outcome: (1) binary logistic regression model for EDSS ≥ 3.0 at last follow-up, expressed as odds ratios (ORs); (2) Poisson regression model for the total number of relapses, expressed as incidence rate ratio (IRR), using the follow-up duration as offset; (3) linear mixed regression models for EDSS and VA at last follow-up expressed as fixed-effects beta (β), using time (onset vs last follow-up) and measures (EDSS and VA) as fixed effects, and random intercept for the person; (4) Cox regression models for the time to second relapse expressed as hazard ratio (HR), and Kaplan-Meier curves with log-rank test for comparison of patients with values above or below the 75th-percentile of the significant CFs. Results were expressed with the corresponding 95% CIs.
Models were first fitted using each of the CFs as predictors (univariable models), and then, they were adjusted by sex, age, acute treatment, and follow-up duration (multivariable models).
Receiver operating characteristic (ROC) curves were built to evaluate model performance in discriminating MOGAD from MS and AQPNMOSD and for predicting EDSS ≥ 3 in patients with MOGAD. To determine 95% CIs for sensitivity (SE), specificity (SP) and area under the curve (AUC), bootstrapping with 1,000 resampling was done. The number needed to diagnose (NND) was calculated for the discrimination between diseases based on the ROC curves.
Statistical analyses were performed with R version 3.6.2. Values of p < 0.05 were considered significant. No adjustment for multiple comparisons was applied because our analyses were exploratory, and the objective was to generate hypothesis instead of validating previous data.
Standard Protocol Approvals, Registrations, and Patient Consents
This study received approval from the Clinical Research Ethics Committee at Vall d’Hebron University Hospital (EPA(AG)57/2013(3,834), PR(AG)400/2021). All patients signed written informed consents.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
Baseline Characteristics
The study included 94 patients (MOGAD, n = 60; MS, n = 18; AQP4-NMOSD, n = 16). Demographic and clinical information of the 3 cohorts is summarized in Table 1. Median interquartile range (IQR) age at sampling was 36 (28–49) years in MOGAD, 40 (35–46) in MS, and 43 (27–56) in AQP4-NMOSD. Female sex was predominant in the 3 cohorts, particularly in patients with AQP4-NMOSD (88%). The most common topography at first event was optic nerve (ON; 51%) in patients with MOGAD, spinal cord (SC; 44%) in patients with MS, and both locations (38%) in patients with AQP4-NMOSD. Median (IQR) EDSS at onset was lower in MS compared with patients with MOGAD and AQP4-NMOSD (2.0 [1.6–2.0] in MS vs 3.0 [2.3–4.0] and 3.0 [3.0–5.5] in MOGAD and AQP4-NMOSD, respectively; p = 0.001). Median time to sampling from disease onset was higher in MS (78.0 [42.8–133.0] days) vs patients with MOGAD and AQP4-NMOSD (15.0 [2.0–55.0] and 56.5 [26.2–357.0] days, respectively; p = 0.001). In 51/59 (86%) MOGAD, 11/18 (61%) MS and 9/16 (56%) patients with AQP4-NMOSD, samples were collected at acute onset. Sixteen (37%) MOGAD, 8 (67%) AQP4-NMOSD, and 4 (22%) MS received acute treatment within 1 month before sampling. Two (4%) patients with MOGAD and one (8%) patient with AQP4-NMOSD were under chronic treatment at sampling.
Table 1.
Demographic and Clinical Characteristics of Patients Included in the Study
| Characteristics | MOGAD (n = 60) | AQP4-NMOSD (n = 16) | MS (n = 18) | p Value |
| Baseline | ||||
| Sex female; no. (%) | 39 (65) | 14 (88) | 14 (78) | 0.18 |
| Age at sampling; median y (IQR) | 36 (28–49) | 43 (27–56) | 40 (35–46) | 0.68 |
| Index event topography; no. (%) | <0.001 | |||
| ON | 30/59 (51) | 6 (38) | 5 (28) | |
| SC | 7/59 (12) | 6 (38) | 8 (44) | |
| ON + SC | 4/59 (7) | 1 (6) | 0 (0) | |
| Infratentorial | 3/59 (5) | 3 (19) | 2 (11) | |
| Other | 15/59 (25) | 0 (0) | 3 (17) | |
| EDSS at onset; median (IQR) | 3.0 (2.3–4.0) | 3.0 (3.0–5.5) | 2.0 (1.6–2.0) | 0.001 |
| VA at onset; median (IQR) | 0.1 (0.0–0.5) | 0.2 (0.0–0.1) | — | 0.19 |
| Acute treatment within 1 month before samplinga; no. (%) | 16/43 (37) | 8/12 (67) | 4 (22) | 0.06 |
| Time to samplingb; median (IQR) d | 15.0 (2.0–55.0) | 56.5 (26.2–357.0) | 78.0 (42.8–133.0) | <0.001 |
| Sampling at acute onset; no. (%) | 51/59 (86) | 9 (56) | 11 (61) | 0.01 |
| Chronic treatment at samplingc; no. (%) | 2/47 (4) | 1/13 (8) | 0 (0) | 0.537 |
| Follow-up | ||||
| Chronic treatment during follow-upd; no. (%) | 27/47 (57) | — | — | |
| Presence of relapses; no. (%) | 16/48 (33) | — | — | |
| ARR; median (IQR) | 0.0 (0.0–0.3) | — | — | |
| EDSS at last follow-up; median (IQR) | 1.8 (0.0–3.0) | — | — | |
| Sustained EDSS 3.0; no. (%) | 11/52 (21) | — | — | |
| VA at last follow-up; median (IQR) | 0.8 (0.5–1.0) | — | — | |
| Follow-up duratione; median (IQR) | 1.3 (0.4–4.4) | — | — |
Abbreviations: AQP4-NMOSD = AQP4-IgG seropositive neuromyelitis optica spectrum disorder; ARR = annualized relapse rate; EDSS = Expanded Disability Status Scale; IQR = interquartile range; MOGAD = MOG-IgG antibody-associated disease; MS = multiple sclerosis; ON = optic nerve; SC = spinal cord; VA = visual acuity.
Additional missing values: age at sampling, n = 1 in MOGAD; EDSS score at onset, n = 14 in MOGAD, n = 2 in AQP4-NMOSD, n = 4 in MS; VA at onset, n = 22 in MOGAD, n = 4 in AQP4-NMOSD; ARR, n = 15; EDSS score at last follow-up, n = 8; VA at last follow-up, n = 25; follow-up duration, n = 8; time to sampling, n = 1.
Administration of corticosteroids, plasma exchange, IV immunoglobulin (IVIg), or combination of them within 1 month before sampling.
Calculated from disease onset.
Chronic therapy at sampling included 2 patients with MOGAD (one with IVIg and another with natalizumab) and one patient with AQP4-NMOSD (rituximab).
Chronic therapy during follow-up in patients with MOGAD included immunosuppressive treatments and disease-modifying therapies (rituximab, n = 11; azathioprine, n = 9; mycophenolate, n = 2; IVIg, n = 2; oral steroids, n = 1; IFN-beta, n = 2; fumarate, n = 1; natalizumab, n = 1).
Calculated from disease onset to last follow-up. Demographic and clinical features were compared between the 3 cohorts using the χ2 test (or Fisher exact test) for categorical variables and the t test (or nonparametric Wilcoxon-Mann-Whitney test) for continuous variables, as appropriate.
Associations Between CFs and Demographic and Clinical Characteristics
eTable 2 shows the univariable analysis for the association between serum and CSF levels of CFs and clinical features in the whole cohort. Age at sampling was positively associated with serum levels of Ba, C4, C5, and CSF levels of all factors except SC5b9, C3, C4, C5, and the C4a/C4 and C5a/C5 ratios. Male patients had higher CSF levels of all CFs except C3, C4, C5, and the C4a/C4 ratio as compared with female. Serum levels of only 3 CFs (Bb, C3a, and C4) were higher in samples collected at acute onset or relapse compared to remission. Regarding clinical presentation, CSF levels of C3a, C4a, and the C3a/C3 ratio were elevated in the SC topography compared with ON. Serum levels of C3, C4, and C5, as well as CSF levels of all CFs except C3, C5, and the C4a/C4 ratio were associated with EDSS at onset (all positively except C4 in CSF). Administration of acute treatment within 1 month before sampling influenced positively the serum levels of C3 and C4, and CSF levels of Ba, Bb, C3a, Factor H, C1q, and the C3/C3a ratio.
Serum Levels of Complement Activation Products Discriminate MOGAD From AQP4-NMOSD and MS
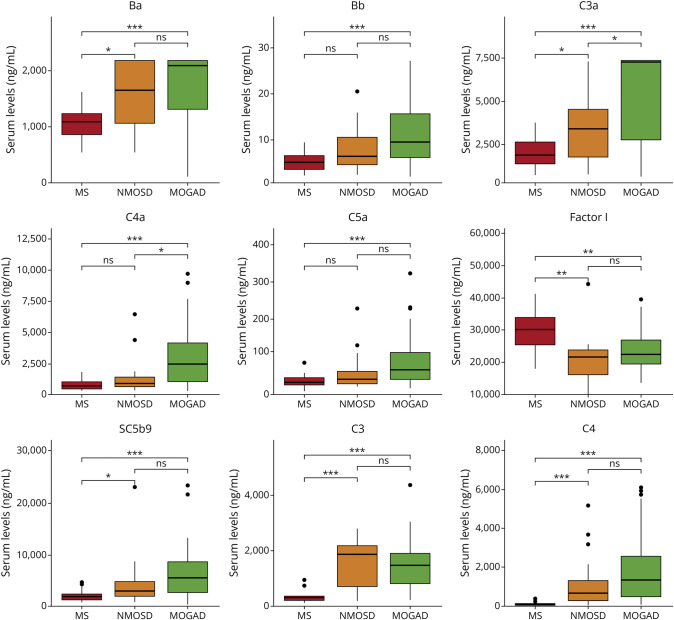
As shown in Figure 1, serum levels of CFs Ba, Bb, C3a, C4a, C5a, SC5b9, C3, and C4 were significantly increased in MOGAD compared with patients with MS. By contrast, Factor I and the C3a/C3 and C4a/C4 ratios were decreased in MOGAD compared with patients with MS (p < 0.01). Comparisons between patients with MOGAD and AQP4-NMOSD revealed significantly higher serum levels of C3a, C4a, and the C3a/C3 ratio in MOGAD compared with patients with AQP4-NMOSD (Figure 1). In CSF, levels of CFs Ba, C5a, Factor H, C3, and the C4a/C4 and C5a/C5 ratios were significantly elevated in MOGAD compared with patients with MS, whereas C4 levels were decreased in patients with MOGAD vs MS (eFigure 1). None of the CFs differed in their CSF levels between patients with MOGAD and AQP4-NMOSD. The remaining CFs not showing significant differences between the 3 conditions are shown in eFigure 2.
Figure 1. Comparison of Serum Levels of Complement Factors Between Patient With MOGAD, MS, and AQP4-NMOSD.
Boxplots depict the distribution of serum levels of complement factors showing statistically significant differences between the three different cohorts of patients. Median values are represented by the horizontal bar, IQR by hinges, and 1.5 × IQR by whiskers. Group comparisons were computed with the Kruskal-Wallis test and post hoc pairwise analysis. * <0.05, ** <0.01, *** <0.001. IQR = interquartile range; MOGAD = MOG-IgG antibody-associated disease; MS = multiple sclerosis.
Considering the significant differences observed in the disease phase at sampling (acute onset, acute relapse, or remission) between the 3 disease groups and the association found between levels of CFs and this variable, a sensitivity analysis including only patients with sampling at acute onset (MOGAD, n = 51; AQP4-NMOSD, n = 9; MS, n = 11) revealed very similar results (eFigure 3).
We next explored the diagnostic potential of CFs to differentiate patients with MOGAD from AQP4-NMOSD and MS. Analysis was focused only on the CFs whose levels significantly differed in MOGAD compared with both patients with MS and AQP4-NMOSD, i.e., C3a, C4a, and the C3a/C3 ratio in serum. eFigure 4A shows AUC and 95% CI resulting from the comparisons between patients with MOGAD and MS, which were 0.82 [0.72–0.91] for C3a, 0.81 [0.71–0.91] for C4a, and 0.76 [0.62–0.90] for the C3a/C3 ratio. eFigure 4A also shows the best cut-off values for each CF to discriminate between diseases with their corresponding sensitivities and specificities. Interestingly, the combination of serum C3a, C4a and C3a/C3 ratio showed very good potential to differentiate patients with MOGAD from patients with MS, with an AUC of 0.95 [0.90; 0.99], SE of 90% [70%–100%], and SP of 89% [78%–100%] (i.e., 54/60 patients with MOGAD and 16/18 patients with MS were correctly diagnosed in our cohort based on their assay results, yielding an NND of 1.12) (eFigure 4A).
The corresponding AUC [95%] in the comparisons between patients with MOGAD and AQP4-NMOSD were 0.73 [0.55; 0.90] for C3a, 0.64 [0.45; 0.82] for C4a, and 0.83 [0.69; 0.97] for the C3a/C3 ratio (eFigure 4B). The best cut-off values, sensitivities, and specificities for each CF are depicted in eFigure 4B. Similar to the comparison between patients with MOGAD and MS, the combination of the serum levels of the 3 CFs showed very good performance to discriminate patients with MOGAD from patients with AQP4-NMOSD with an AUC of 0.88 [0.76; 1.00], SE of 88% [50%–97%], and SP of 83% [50%–100%] (i.e., 53/60 patients with MOGAD and 13/16 patients with AQP4-NMOSD in our cohort received a correct diagnosis based on their assay results, yielding an NND of 1.15) (eFigure 4B).
Associations Between CFs and Demographic and Clinical Characteristics in Patients With MOGAD
eTable 3 shows the univariable analysis for the association between serum and CSF levels of CFs and clinical features of patients with MOGAD. Age at sampling was positively associated with serum levels of C4 and C5, and CSF levels of C4a, Factor I, C5, and the C3a/C3 ratio. Male patients had higher CSF levels of all CFs except C3, C4, and the C4a/C4 and C5a/C5 ratios as compared with female patients. In samples collected at acute onset, CSF levels of C3a and the C3a/C3 ratio were elevated in patients with SC topography compared with ON and, alongside with C5a, C5, SC5b9, and Factors H and I, were also associated with EDSS at onset. Acute treatment within 1 month before sampling influenced the CSF levels of Bb, C3a, C4a, and C1q. Only the CSF C5a/C5 ratio was influenced by disease phase at sampling (acute vs remission).
CSF Levels of CFs Influence Disease Prognosis in Patients With MOGAD
We finally investigated whether the complement system plays a prognostic role in patients with MOGAD by evaluating the association of serum and CSF levels of CFs with disease outcomes such as relapses, disability, and VA. Patients were followed for a median (IQR) time of 1.3 (0.4–4.4) years (Table 1). A total of 27 (57%) patients received chronic treatment during follow-up. Sixteen of 48 (33%) relapsed, with a median (IQR) annualized relapse rate of 0.0 (0.0–0.3) during the follow-up period. Of these, 13 (81%) patients relapsed during the first year of the disease. At last follow-up, median EDSS was 1.8 (0.0–3.0) and median VA was 0.8 (0.5–1.0) (Table 1). Eleven of 52 (21%) patients had a final EDSS ≥ 3.0. Baseline demographics, clinical features, and chronic treatment during follow-up had no effect on the different prognostic outcomes evaluated (eTable 4).
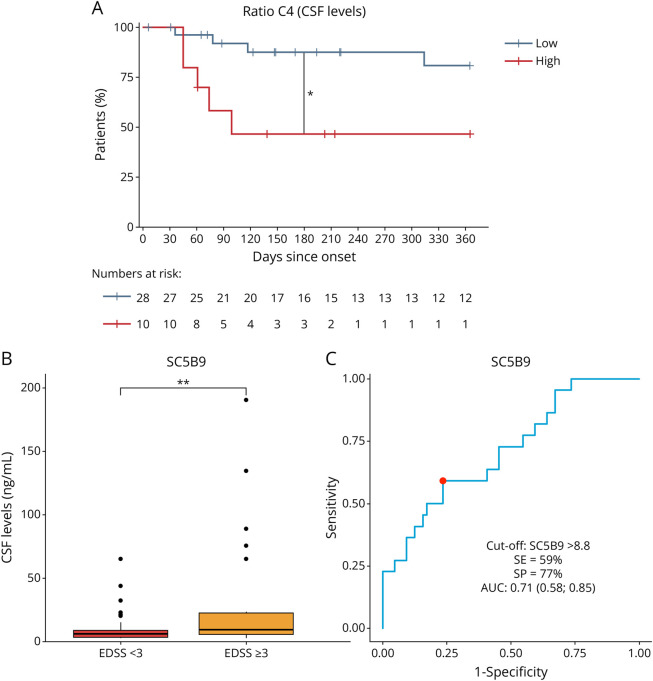
Among patients with MOGAD with sampling at acute onset, evaluation of relapse outcomes revealed an inverse association of CSF C4 levels with the total number of relapses at last follow-up in both univariable (IRR [95% CI] 0.91 [0.86–0.97]; p < 0.01) and multivariable (0.88 [0.78–0.99]; p = 0.04) analyses (Table 2). Associations were also observed for serum and CSF levels of C4a/C4 and C5a/C5 ratios, serum Bb, and C4a and CSF Ba levels at the univariable level, but significance disappeared after adjustment. A higher C4a/C4 ratio in the CSF was associated with an increased risk of having a second relapse during follow-up in both univariable (HR 3.89 [95% CI 1.66–9.13]; p < 0.01) and multivariable (3.68 [1.26–10.78]; p = 0.02) analyses (Table 2). An inverse association was also observed in univariable analysis for the CSF C4 levels (0.84 [0.72–0.97]; p = 0.02), remaining as a trend in multivariable analysis (0.84 [0.70–1.01]; p = 0.06). Of note, survival analysis using a cut-off value of 10.8 for the C4a/C4 ratio (corresponding to the 75th percentile in the MOGAD cohort) revealed that the time to relapse during the first year of disease was significantly shorter in patients with MOGAD with a C4a/C4 ratio above the cut-off compared with those below the cut-off (log-rank p = 0.01) (Figure 2A). At 1 year, 9/10 (90%) patients with MOGAD with a high C4a/C4 ratio relapsed compared with 16/28 (57%) patients with a lower ratio.
Table 2.
Univariable and Multivariable Regression Models to Test the Association Between Serum and CSF Levels of Complement Factors and Total Number of Recurrences and Time to Second Relapse in Patients With MOGAD
| Total number of recurrences | ||||
| Serum | CSF | |||
| Factors | Univariable IRR (95% CI)a; p Value | Multivariable IRR (95% CI)a; p Value | Univariable IRR (95% CI)a; p Value | Multivariable IRR (95% CI)a; p Value |
| Ba | 1.05 (1.00–1.12); 0.09 | 1.05 (0.96–1.14); 0.30 | 20.28 (2.36–154.55); <0.01 | 26.74 (0.45–1,116.11); 0.10 |
| Bb | 594.72 (1.71–1.24e+5); 0.02 | 11.14 (0.00–7.21e+4); 0.60 | 1.46e+166 (0.00); 0.32 | 9.88e+187 (0.00); 0.40 |
| C3a | 1.00 (0.98–1.01); 0.67 | 1.00 (0.98–1.02); 0.91 | 0.18 (0.00–15.32); 0.56 | 0.21 (0.00–49.62); 0.66 |
| C4a | 1.02 (1.00–1.03); <0.01 | 1.01 (0.98–1.04); 0.50 | 2.16 (0.79–4.96); 0.10 | 1.75 (0.18–15.34); 0.61 |
| C5a | 1.47 (0.87–2.31); 0.12 | 0.63 (0.14–1.73); 0.46 | 1.08e+35 (0.00–2.51e+78); 0.12 | 1.08e+50 (0.00–4.85e+117); 0.22 |
| Factor H | 1.02 (0.61–1.63); 0.94 | 0.83 (0.46–1.45); 0.52 | 125.28 (0.00–1.97e+09); 0.61 | 0.00 (0.00–1.10e+11); 0.37 |
| Factor I | 1.00 (0.99–1.00); 0.38 | 1.00 (0.99–1.00); 0.46 | 0.94 (0.59–1.23); 0.72 | 1.33 (0.56–2.82); 0.49 |
| SC5b9 | 1.01 (1.00–1.02); 0.07 | 1.00 (0.99–1.02); 0.80 | 0.67 (0.14–2.08); 0.56 | 1.76 (0.26–7.72); 0.50 |
| C1q | 0.93 (0.30–2.65); 0.89 | 1.00 (0.27–3.20); 0.99 | 0.00 (0.00–3.87e+67); 0.48 | 0.00 (0.00–4.64e+85); 0.52 |
| C3 | 1.00 (0.97–1.03); 0.76 | 1.05 (0.99–1.09); 0.06 | 1.58e+4 (0.22–3.33e+8); 0.07 | 4.49e+6(3.66–2.52e+12); 0.03 |
| C4 | 0.98 (0.95–1.01); 0.31 | 0.99 (0.94–1.03); 0.60 | 0.91 (0.86–0.97); <0.01 | 0.88 (0.78–0.99); 0.04 |
| C5 | 0.49 (0.21–1.12); 0.09 | 0.60 (0.18–1.91); 0.38 | 0.00 (0.00–4.23e+16); 0.17 | 0.00 (0.00–1.58e+69); 0.43 |
| C3a/C3 | 1.96 (0.92–3.84); 0.06 | 0.63 (0.04–6.50); 0.71 | 0.03 (0.00–1.71); 0.24 | 0.00 (0.00–3.13); 0.29 |
| C4a/C4 | 1.68 (1.03–2.58); 0.02 | 0.43 (0.03–2.99); 0.45 | 1.08 (1.02–1.13); <0.01 | 1.05 (0.97–1.12); 0.18 |
| C5a/C5 | 205.89 (1.30–1.77e+4); 0.03 | 0.06 (0.00–2.09e+6); 0.77 | 1.81 (1.15–2.68); <0.01 | 3.86 (0.36–19.74); 0.18 |
| Time to second relapse | ||||
| Serum | CSF | |||
| Factors | Univariable HR (95% CI); p Value | Multivariable HR (95% CI); p Value | Univariable HR (95% CI); p Value | Multivariable HR (95% CI); p Value |
| Ba | 1.00 (1.00–1.00); 0.10 | 1.00 (1.00–1.00); 0.26 | 1.04 (1.00–1.08); 0.06 | 1.04 (0.99–1.10); 0.16 |
| Bb | 1.06 (0.97–1.16); 0.19 | 1.09 (0.95–1.24); 0.23 | 1,574.70 (0.10–2.48e+7); 0.14 | 32,620.05 (0.06–1.85e+10); 0.12 |
| C3a | 1.00 (1.00–1.00); 0.59 | 1.00 (1.00–1.00); 0.69 | 1.00 (0.95–1.06); 0.87 | 0.99 (0.91–1.08); 0.83 |
| C4a | 1.00 (1.00–1.00); 0.24 | 1.00 (1.00–1.00); 0.25 | 1.02 (1.00–1.04); 0.05 | 1.02 (0.99–1.06); 0.14 |
| C5a | 1.00 (0.99–1.01); 0.98 | 1.00 (0.98–1.01); 0.69 | 1.80 (0.35–9.30); 0.48 | 1.43 (0.14–14.44); 0.76 |
| Factor H | 1.00 (1.00–1.01); 0.56 | 1.00 (0.99–1.01); 0.82 | 0.82 (0.39–1.75); 0.61 | 0.54 (0.24–1.22); 0.14 |
| Factor I | 1.00 (1.00–1.00); 0.79 | 1.00 (1.00–1.00); 0.41 | 1.00 (1.00–1.01); 0.19 | 1.00 (0.99–1.01); 0.72 |
| SC5b9 | 1.00 (1.00–1.00); 0.39 | 1.00 (1.00–1.00); 0.72 | 1.02 (0.99–1.06); 0.20 | 1.04 (1.00–1.09); 0.06 |
| C1q | 0.99 (0.97–1.02); 0.53 | 0.99 (0.97–1.01); 0.35 | 0.59 (0.01–23.54); 0.78 | 0.46 (0.00–53.16); 0.75 |
| C3 | 1.00 (1.00–1.00); 0.05 | 1.00 (1.00–1.00); 0.08 | 1.15 (0.99–1.33); 0.07 | 1.10 (0.92–1.32); 0.30 |
| C4 | 1.00 (1.00–1.00); 0.75 | 1.00 (1.00–1.00); 0.54 | 0.84 (0.72–0.97); 0.02 | 0.84 (0.70–1.01); 0.06 |
| C5 | 1.00 (0.98–1.01); 0.63 | 0.99 (0.98–1.01); 0.51 | 0.23 (0.00–18.67); 0.51 | 0.00 (0.00–24.94); 0.22 |
| C3a/C3 | 0.92 (0.66–1.27); 0.60 | 0.89 (0.61–1.30); 0.56 | 0.93 (0.59–1.46); 0.74 | 0.61 (0.14–2.76); 0.52 |
| C4a/C4 | 1.02 (0.83–1.26); 0.84 | 1.02 (0.80–1.30); 0.88 | 3.89 (1.66–9.13)a; <0.01 | 3.68 (1.26–10.78)a; 0.02 |
| C5a/C5 | 1.22 (0.28–5.28); 0.79 | 0.88 (0.11–7.05); 0.90 | 1.10 (0.92–1.31); 0.31 | 1.21 (0.97–1.52); 0.09 |
Abbreviations: HR = hazard ratio; IRR = incidence rate ratio.
Coefficients are transformed by multiplying complement values by 100. Association between serum and CSF levels of complement factors and recurrence outcomes were analyzed by a Poisson regression model for the total number of recurrences and by a Cox regression model for the time to second relapse. Detailed information on model adjustment is provided in the Methods section. Missing information: total number of recurrences, n = 15; time to second relapse, n = 13.
Figure 2. Potential of Complement Factors as Prognostic Biomarkers in Patients With MOGAD.
(A) Kaplan-Meier curves evaluating the time to second relapse in patients with high (>10.8) and low (<10.8) CSF values of the C4a/C4 ratio, revealing a higher risk of second relapse in patients with MOGAD with higher CSF values (log-rank test p value: 0.01). (B) Boxplots depicting the distribution of CSF SC5b9 levels in patients reaching and not reaching an EDSS of 3.0. Median values are represented by the horizontal bar, IQR by hinges, and 1.5 × IQR by whiskers. The comparison analysis (Mann-Whitney test) demonstrated higher CSF SC5b9 levels in patients reaching an EDSS of 3.0 (p = 0.002). (C) Performance of CSF SC5b9 levels (in ng/mL) to discriminate between patients with MOGAD who will and will not reach an EDSS of 3.0. AUC = area under the curve; EDSS = Expanded Disability Status Scale; IQR = interquartile range; MOGAD = MOG-IgG antibody-associated disease; SE = sensitivity; SP = specificity.
Regarding disability, CSF levels of several CFs including the complement activation products C3a, C5a, and the C3a/C3 ratio, the complement regulators Factors H and I, and the MAC SC5b9 were associated with EDSS at last follow-up in both univariable and multivariable analyses (Table 3). Among all these factors, CSF levels of SC5b9 influenced the risk of reaching a final EDSS ≥ 3.0 in univariable (OR [95% CI] 1.54 [1.16–2.51]; p = 0.02) and multivariable (1.79 [1.16–3.67]; p = 0.04) analyses (Table 4). Interestingly, CSF levels of SC5b9 were significantly higher in patients with MOGAD with final EDSS ≥ 3.0 compared with those with EDSS < 3.0 (median [IQR] CSF levels of 12.63 [9.40–82.47] ng/mL vs 6.77 [3.64–8.61], respectively; p < 0.01) (Figure 2B). Furthermore, as shown in Figure 2C, CSF levels of SC5b9 showed acceptable performance to predict a final EDSS ≥ 3.0, with an AUC [95% CI] of 0.71 [0.58–0.84]. A CSF SC5b9 value of 8.8 ng/mL resulted in the best cut-off to classify patients who will and will not reach this outcome, with SE of 59% and SP of 77% (Figure 2C).
Table 3.
Univariable and Multivariable Mixed Linear Models to Test the Association Between Serum and CSF Levels of Complement Factors and EDSS at Last Follow-Up in Patients With MOGAD
| EDSS at last follow-up | ||||
| Serum | CSF | |||
| Factors | Univariable β (95% CI)a; p Value | Multivariable β (95% CI)a; p Value | Univariable β (95% CI)a; p Value | Multivariable β (95% CI)a; p Value |
| Ba | 0.03 (0.0 to 0.10); 0.47 | 0.03 (−0.05 to 0.10); 0.48 | 3.17 (0.70 to 5.64); 0.01 | 0.98 (−1.76 to 3.71); 0.48 |
| Bb | 2.06 (−4.26 to 8.38); 0.52 | −0.63 (−7.28 to 6.03); 0.85 | 9.10 (2.10 to 16.02); 0.01 | 4.12 (−3.56 to 11.80); 0.29 |
| C3a | 0.01 (−0.01 to 0.02); 0.46 | 0.00 (−0.01 to 0.02); 0.59 | 3.69 (1.53 to 5.84); <0.01 | 2.69 (0.45 to 4.93); 0.02 |
| C4a | −0.01 (−0.03 to 0.01); 0.38 | −0.01 (−0.03 to 0.01); 0.23 | 1.76 (0.54 to 2.98); <0.01 | −0.03 (−1.65 to 1.58); 0.97 |
| C5a | −0.08 (−0.69 to 0.53); 0.80 | −0.33 (−0.95 to 0.29); 0.29 | 1.46 (0.66 to 2.26); <0.01 | 0.99 (0.12 to 1.87); 0.03 |
| Factor H | 0.08 (−0.37 to 0.53); 0.74 | 0.18 (−0.36 to 0.72); 0.51 | 4.03 (2.41 to 5.65); <0.01 | 2.68 (0.65 to 4.71); 0.01 |
| Factor I | 0.00 (0.00 to 0.01); 0.52 | 0.00 (0.00 to 0.01); 0.26 | 0.59 (0.33 to 0.84); <0.01 | 0.47 (0.11 to 0.83); 0.01 |
| SC5b9 | 0.00 (−0.01 to 0.01); 0.79 | −0.01 (−0.02 to 0.01); 0.31 | 2.27 (1.24 to 3.29); <0.01 | 1.82 (0.69 to 2.96); <0.01 |
| C1q | −0.36 (−1.82 to 1.10); 0.62 | −0.71 (−2.20 to 0.78); 0.35 | 1.53 (−0.67 to 3.73); 0.17 | 0.41 (−1.89 to 2.72); 0.72 |
| C3 | 0.01 (−0.04 to 0.06); 0.81 | 0.00 (−0.05 to 0.05); 0.98 | 1.76 (−10.45 to 13.98); 0.78 | 1.02 (−10.27 to 12.30); 0.86 |
| C4 | 0.00 (−0.02 to 0.03); 0.88 | 0.00 (−0.03 to 0.03); 0.98 | −0.98 (−9.29 to 7.34); 0.82 | 3.81 (−4.48 to 12.09); 0.36 |
| C5 | 0.36 (−0.78 to 1.50); 0.53 | 0.24 (−0.87 to 1.34); 0.67 | 2.74 (1.11 to 4.38); <0.01 | 1.77 (−0.24 to 3.77); 0.08 |
| C3a/C3 | 1.30 (−0.35 to 2.95); 0.12 | 0.92 (−0.96 to 2.79); 0.33 | 1.65 (0.77 to 2.54); <0.01 | 1.25 (0.37 to 2.14); <0.01 |
| C4a/C4 | 0.62 (−0.54 to 1.78); 0.29 | 0.04 (−1.43 to 1.51); 0.96 | −0.01 (−0.10 to to 0.08); 0.79 | −0.07 (−0.15 to 0.02); 0.12 |
| C5a/C5 | −0.71 (−9.08 to 7.65); 0.87 | −5.39 (−14.36 to 3.58); 0.24 | 0.72 (−0.34 to 1.78); 0.18 | 0.18 (−1.17 to 1.54); 0.79 |
Abbreviation: EDSS = Expanded Disability Status Scale.
Coefficients are transformed by multiplying complement values by 100, except for CSF factor H (multiplied by 10) and CSF Bb, C5a, C1q, and C5 (nontransformed). Missing information: n = 8.
Table 4.
Logistic Regression Models to Test the Association Between Serum and CSF Levels of Complement Factors and EDSS ≥ 3.0 at Last Follow-Up in Patients With MOGAD
| EDSS ≥3.0 at last follow-up | ||||
| Serum | CSF | |||
| Factors | Univariable OR (95% CI); p Value | Multivariable OR (95% CI); p Value | Univariable OR (95% CI); p Value | Multivariable OR (95% CI); p Value |
| Ba | 1.08 (0.95–1.26); 0.28 | 1.17 (0.96–1.57); 0.18 | 9.44 (0.15–608.49); 0.28 | 4.79 (0.01–1754.84); 0.60 |
| Bb | 74.79 (0.00–1.49e+6); 0.39 | 1,067.22 (0.00–1.56e+10); 0.38 | (0.00); 0.15 | (0.00); 0.23 |
| C3a | 1.01 (0.98–1.04); 0.65 | 1.02 (0.98–1.08); 0.31 | 19.87 (0.60–3,100.36); 0.14 | 68.48 (0.49–2.32e+5); 0.20 |
| C4a | 0.99 (0.96–1.02); 0.68 | 0.98 (0.92–1.03); 0.50 | 5.19 (0.74–40.39); 0.09 | 1.49 (0.04–47.85); 0.82 |
| C5a | 1.14 (0.40–2.87); 0.79 | 0.98 (0.14–4.05); 0.98 | 7.18e+62 (1,360.39–4.15e+141); 0.07 | 4.37e+64 (0.00–6.62e+168); 0.13 |
| Factor H | 1.23 (0.60–2.45); 0.54 | 1.25 (0.38–4.26); 0.71 | 1.48e+31 (2.57e+8–1.21e+69); 0.05 | 1.96e+24 (0.39–1.55e+74); 0.24 |
| Factor I | 1.00 (0.99–1.01); 0.54 | 1.01 (0.99–1.02); 0.46 | 2.14 (1.22–5.17); 0.04 | 3.18 (1.20–14.02); 0.06 |
| SC5b9 | 1.01 (0.99–1.02); 0.46 | 1.00 (0.97–1.03); 0.80 | 1.54 (1.16–2.51)a; 0.02 | 1.79 (1.16–3.67)a; 0.04 |
| C1q | 1.01 (0.99–1.02); 0.52 | 1.47 (0.07–24.10); 0.79 | 1.33e+34 (0.00–6.21e+173); 0.65 | 1.52e+70 (0.00–2.52e+278); 0.50 |
| C3 | 1.97 (0.19–17.97); 0.55 | 1.05 (0.96–1.17); 0.25 | 80.45 (0.00–6.09e+9); 0.64 | 2,345.17 (0.00–1.62e+13); 0.50 |
| C4 | 1.05 (0.97–1.14); 0.21 | 1.01 (0.95–1.06); 0.78 | 1.64 (0.00–1.58e+6); 0.94 | 3.66e+4 (0.00–3.15e+16); 0.37 |
| C5 | 1.00 (0.95–1.04); 0.87 | 0.81 (0.07–8.47); 0.86 | 2.05e+108 (0.00–1.52e+234); 0.06 | 4.36e+142 (0.00–); 0.13 |
| C3a/C3 | 0.98 (0.15–6.00); 0.98 | 1.92 (0.03–112.78); 0.75 | 4.52 (0.89–112.35); 0.19 | 5.55 (0.72–612.24); 0.29 |
| C4a/C4 | 2.70 (0.19–36.90); 0.44 | 0.01 (0.00–1.88); 0.18 | 0.92 (0.59–1.09); 0.53 | 0.52 (0.03–1.05); 0.56 |
| C5a/C5 | 1.25 (0.16–7.06); 0.81 | 2.00 (0.00–2.06e+08); 0.95 | 1.63 (0.28–7.51); 0.54 | 0.20 (0.00–5.32); 0.48 |
Abbreviations: EDSS = Expanded Disability Status Scale; OR = odds ratio.
Coefficients are transformed by multiplying complement values by 10. Missing values are indicated in Table 1.
Finally, CSF levels of CFs also influenced VA as prognostic outcome, and higher CSF levels of Ba, C5a, C3, and the C5a/C5 ratio predicted lower VA at last follow-up in both univariable and multivariable analyses (Table 5).
Table 5.
Mixed Linear Regression Models to Test the Association Between Serum and CSF Levels of Complement Factors and VA at Last Follow-Up in Patients With MOGAD
| Final VA | ||||
| Serum | CSF | |||
| Factors | Univariable estimate (95% CI); p Value | Multivariable estimate (95% CI); p Value | Univariable estimate (95% CI); p Value | Multivariable estimate (95% CI); p Value |
| Ba | −0.01 (−0.03 to 0.00); 0.11 | −0.01 (−0.02 to 0.01); 0.22 | −0.67 (−1.19 to −0.16); 0.01 | −0.62 (−1.13 to −0.12); 0.02 |
| Bb | −0.31 (−1.62 to 1.00); 0.64 | −0.11 (−1.67 to 1.44); 0.88 | −74.35 (−369.46 to 220.77); 0.62 | −78.17 (−354.80 to 198.46); 0.57 |
| C3a | 0.00 (0.00–0.00); 0.56 | 0.00 (−0.01 to 0.00); 0.43 | −0.15 (−1.16 to 0.87); 0.77 | −0.21 −1.17 to 0.76); 0.67 |
| C4a | 0.00 (−0.01 to 0.00); 0.20 | 0.00 (−0.01 to 0.00); 0.14 | −0.24 (−0.64 to 0.16); 0.24 | 0.00 (−0.46 to 0.46); >0.99 |
| C5a | 0.04 (−0.09 to 0.16); 0.55 | 0.07 (−0.07 to 0.21); 0.33 | −31.45 (−56.96 to −5.95); 0.02 | −29.26 (−54.69 to −3.83); 0.03 |
| Factor H | −0.02 (−0.12 to 0.07); 0.64 | −0.02 (−0.15 to 0.12); 0.81 | −2.33 (−11.31 to 6.65); 0.61 | 2.84 (−6.74 to 12.43); 0.55 |
| Factor I | 0.00 (0.00 to 0.00); 0.66 | 0.00 (0.00 to 0.00); 0.82 | −0.10 (−0.22 to 0.02); 0.11 | −0.08 (−0.19 to 0.04); 0.20 |
| SC5b9 | 0.00 (0.00 to 0.00); 0.49 | 0.00 (0.00 to 0.00); 0.54 | −0.14 (−0.74 to 0.46); 0.65 | −0.23 (−0.81 to 0.34); 0.41 |
| C1q | 0.02 (−0.29 to 0.34); 0.89 | 0.11 (−0.23 to 0.46); 0.51 | 12.72 (−43.71 to 69.14); 0.65 | 7.49 (−45.34 to 60.32); 0.78 |
| C3 | −0.01 (−0.02 to 0.00); 0.03 | −0.01 (−0.02 to 0.00); 0.05 | −3.25 (−5.43 to −1.08); <0.01 | −3.34 (−5.44 to −1.23); <0.01 |
| C4 | 0.00 (−0.01 to 0.01); 0.85 | 0.00 (−0.01 to 0.01); 0.54 | 2.02 (0.31 to 3.73); 0.02 | 1.91 (−0.12 to 3.94); 0.06 |
| C5 | −0.23 (−0.49 to 0.03); 0.09 | −0.23 (−0.49 to 0.04); 0.10 | 6.83 (−43.82 to 57.48); 0.79 | 13.38 (−36.02 to 62.78); 0.59 |
| C3a/C3 | 0.05 (−0.27 to 0.37); 0.77 | 0.18 (−0.19 to 0.56); 0.33 | 0.16 (−0.49 to 0.80); 0.63 | 0.13 (−0.50 to 0.77); 0.68 |
| C4a/C4 | −0.16 (−0.38 to 0.07); 0.17 | −0.17 (−0.49 to 0.14); 0.28 | −0.01 (−0.03 to 0.01); 0.24 | −0.01 (−0.03 to 0.01); 0.31 |
| C5a/C5 | 0.66 (−0.89 to 2.21); 0.40 | 1.39 (−0.36 to 3.13); 0.12 | −0.25 (−0.42 to −0.08); <0.01 | −0.37 (−0.58 to −0.16); <0.01 |
Abbreviation: VA = visual acuity.
Missing values are indicated in Table 1.
Discussion
In this study, we found that serum levels of CFs played an important role as diagnostic biomarkers, whereas CSF complement levels were more associated with prognostic outcomes in patients with MOGAD. The combination of the serum levels of 3 complement activation products showed excellent discriminatory potential to differentiate MOGAD from both patients with MS and AQP4-NMOSD, with very low NND (i.e., the number of patients needed to determine these proteins to avoid a misdiagnosis), highlighting the possible utility of these biomarkers in clinical practice. This might be especially useful in patients with overlapping presentations and uncertain antibody results (e.g., low titer).
A recent study reported increased serum complement activation in MOGAD compared with MS and AQP4-NMOSD.15 Previous studies found lower blood levels of C3 and C4 in AQP4-NMOSD compared with MOGAD,12-14 but, to our knowledge, no other groups measured complement activation products in patients with MOGAD. Our findings indicate that, similar to AQP4-NMOSD, the complement classical pathway is also playing an important role in the pathogenesis of MOGAD. This is in line with previous studies reporting the demyelinating potential of the MOG-IgG in the presence of complement in in vivo26 and in vitro models27-29 and the presence of complement deposition in active white matter lesions in histopathologic studies.9
Despite the contribution of body fluid biomarkers to diagnosis and knowledge about the pathogenesis of MOGAD, studies demonstrating their value for predicting relapses and long-term disability are lacking.15,17-19 This is especially relevant because MOGAD has an unpredictable course in comparison with MS and AQP4-NMOSD.23 Again, we found that CFs of the classical/lectin pathway influenced relapses in patients with MOGAD, based on the findings that lower CSF C4 levels (probably reflecting compound consumption in the context of an inflammatory environment) were associated with a higher number of relapses during follow-up, and a higher CSF C4a/C4 ratio increased the risk of a second event. Regarding disability, although several CFs were associated with EDSS at last follow-up, only the CSF SC5b9 levels influenced the risk of reaching EDSS ≥ 3.0. SC5b9 or MAC is the final stage of the 3 complement pathways, resulting in direct injury by the formation of a permeability pore in cell membranes.30 Interestingly, CSF levels of SC5b9 above 8.8 ng/mL showed moderate accuracy for predicting EDSS ≥ 3.0. Altogether, these results point toward a possible relationship between the intrathecal activation of the complement system and a more relapsing and disabling disease course in patients with MOGAD. Similarly, these findings could explain the reported worse prognosis in patients with MOG-IgG in CSF.31
The inhibition of the complement cascade by IV immunoglobulins as a possible effector mechanism30,32,33 could explain its high efficacy preventing relapses in patients with MOGAD.34,35 However, there are no approved preventive therapies for MOGAD, and the evidences come from observational studies. Eculizumab and ravulizumab, 2 humanized monoclonal antibodies that prevent MAC formation by inhibiting C5, were approved for AQP4-NMOSD treatment because they demonstrated high efficacy in preventing relapses.6 In our cohort, of 11 patients with MOGAD reaching final EDSS ≥ 3.0, 8 patients had CSF levels of SC5b9 above 8.8 ng/mL based on the best cut-off predicting this outcome. Therefore, these 8 patients with MOGAD could be considered for complement-targeting therapies based on their ELISA results. Overall, our data suggest that inhibition of the classical or alternative pathway proximal to C5 could provide additional benefit in preventing relapses and disability in patients with MOGAD.
Study limitations included the retrospective design, the absence of longitudinal assessment of CFs, and the small sample size of the MS and AQP4-NMOSD cohorts. However, the aim of this study was to profile complement activation and its association with prognosis in patients with MOGAD, and this cohort was well represented despite the low disease prevalence. Albeit samples were obtained out of acute onset in almost half of the patients with AQP4-NMOSD and MS, a sensitivity analysis including only patients at acute onset showed similar results. The presence of infection close to sampling could also have affected the levels of CFs. Although our study was not specifically designed for this purpose, we believe the influence of a prior infection on levels of CFs should be explored in future studies. Another limitation is the short follow-up (median 1.3 years) of the MOGAD cohort. Nevertheless, relapses within the first year have the highest impact in long-term prognosis of MOGAD.36 Finally, no correction for multiple comparisons was performed because our analyses were exploratory. Further studies are needed to confirm our findings.
Our study contributes to the amount of evidence of the relevant implication of the complement system in MOGAD, providing insights into future therapeutic options and supporting the use of CFs as useful biomarkers for aiding the diagnosis and predicting prognosis.
Glossary
- AUC
area under the curve
- CF
complement factor
- EDSS
Expanded Disability Status Scale
- HR
hazard ratio
- IQR
interquartile range
- IRR
incidence rate ratio
- MAC
membrane attack complex
- MOGAD
myelin oligodendrocyte glycoprotein antibody–associated disease
- MOG-Ig
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- NND
number needed to diagnose
- OR
odds ratio
- ROC
receiver operating characteristic
- SC
spinal cord
- SE
sensitivity
- SP
specificity
- VA
visual acuity
Appendix. Authors
| Name | Location | Contribution |
| Javier Villacieros-Álvarez, MD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jan D. Lunemann, MD, PhD | Department of Neurology with Institute of Translational Neurology, University Hospital Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Maria Sepulveda, MD | Neuroimmunology and Multiple Sclerosis Unit, Hospital Clinic de Barcelona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Adrián Valls-Carbó, MD | Fundación INCE (Iniciativa para las Neurociencias), Madrid, Spain | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Alessandro Dinoto, MD | Neurology Unit, Department of Neurosciences, Biomedicine, and Movement Sciences, University of Verona, Italy | Major role in the acquisition of data; Analysis or interpretation of data |
| Victoria Fernández, MD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Andreu Vilaseca, MD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Mireia Castillo | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Spain | Major role in the acquisition of data; analysis or interpretation of data |
| Georgina Arrambide | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Analysis or interpretation of data |
| Luca Bollo | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Carmen Espejo | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Analysis or interpretation of data |
| Sara Llufriu, MD, PhD | Neuroimmunology and Multiple Sclerosis Unit, Hospital Clinic de Barcelona, Neuroimmunology Program, Neurology Service, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic de Barcelona, University of Barcelona, Spain | Analysis or interpretation of data |
| Yolanda Blanco, MD, PhD | Neuroimmunology and Multiple Sclerosis Unit, Hospital Clinic de Barcelona, Neuroimmunology Program, Neurology Service, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic de Barcelona, University of Barcelona, Spain | Analysis or interpretation of data |
| Thais Armangue, PhD | Neuroimmunology Program, Neurology Service, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Hospital Clínic de Barcelona, Pediatric Neuroimmunology Unit, Neurology Department, Sant Joan de Déu Children's Hospital, University of Barcelona, Spain | Analysis or interpretation of data |
| Gary Álvarez Bravo, MD, MSc | Neurodegeneration and Neuroinflammation research group, IDIBGI, Department of Medical Sciences, Faculty of Medicine, University of Girona, Girona Neuroimmunology and Multiple Sclerosis Unit, Neurology Department, Dr. Josep Trueta University Hospital and Santa Caterina Hospital, Spain | Analysis or interpretation of data |
| Ana Quiroga-Varela | Neurodegeneration and Neuroinflammation research group, IDIBGI, Girona-Salt, Redes de Investigación Cooperativa Orientada a Resultados en Salud (RICORS), Red de Enfermedades inflamatorias (RD21/0002/0063), Instituto de Salud Carlos III, Madrid, Spain | Analysis or interpretation of data |
| Lluís Ramió Torrentà, PhD | Neurodegeneration and Neuroinflammation research group, IDIBGI, Department of Medical Sciences, Faculty of Medicine, University of Girona, Redes de Investigación Cooperativa Orientada a Resultados en Salud (RICORS), Red de Enfermedades inflamatorias (RD21/0002/0063), Instituto de Salud Carlos III, Madrid, Girona Neuroimmunology and Multiple Sclerosis Unit, Neurology Department, Dr. Josep Trueta University Hospital and Santa Caterina Hospital, Girona-Salt, Spain | Analysis or interpretation of data |
| Alvaro Cobo-Calvo, MD, PhD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Mar Tintore, MD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Sara Mariotto | Neurology Unit, Department of Neurosciences, Biomedicine, and Movement Sciences, University of Verona, Italy | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Xavier Montalban, MD, PhD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Manuel Comabella, MD, PhD | Neurology-Neuroimmunology Department, Multiple Sclerosis Center of Catalonia, Vall d'Hebron Barcelona Hospital Campus, Vall d'Hebron Research Institute, Autonomous University of Barcelona, Spain | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The study was funded thanks to a legacy from Mrs. Adela Arbó. J.V.-A. received grant from Instituto de Salud Carlos III, Spain; FI21/00282.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Wingerchuk DM, Lucchinetti CF. Neuromyelitis optica spectrum disorder. N Engl J Med. 2022;387(7):631-639. doi: 10.1056/NEJMra1904655 [DOI] [PubMed] [Google Scholar]
- 2.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762-772. doi: 10.1016/S1474-4422(21)00218-0 [DOI] [PubMed] [Google Scholar]
- 3.Takai Y, Misu T, Suzuki H, et al. Staging of astrocytopathy and complement activation in neuromyelitis optica spectrum disorders. Brain. 2021;144(8):2401-2415. doi: 10.1093/brain/awab102 [DOI] [PubMed] [Google Scholar]
- 4.Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133(pt 2):349-361. doi: 10.1093/brain/awp309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221-2231. doi: 10.1212/01.WNL.0000289761.64862.ce [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med. 2019;381(7):614-625. doi: 10.1056/NEJMoa1900866 [DOI] [PubMed] [Google Scholar]
- 7.Pittock SJ, Barnett M, Bennett JL, et al. Ravulizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. Ann Neurol. 2023;93(6):1053-1068. doi: 10.1002/ana.26626 [DOI] [PubMed] [Google Scholar]
- 8.Takai Y, Misu T, Kaneko K, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain. 2020;143(5):1431-1446. doi: 10.1093/brain/awaa102 [DOI] [PubMed] [Google Scholar]
- 9.Höftberger R, Guo Y, Flanagan EP, et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020;139(5):875-892. doi: 10.1007/s00401-020-02132-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.More Efficient Complement Activation by Anti-Aquaporin-4 Compared With Anti-Myelin Oligodendrocyte Glycoprotein Antibodies—PubMed. Accessed November 7, 2023. pubmed.ncbi.nlm.nih.gov/36414427/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCombe JA, Flanagan EP, Chen JJ, Zekeridou A, Lucchinetti CF, Pittock SJ. Investigating the immunopathogenic mechanisms underlying MOGAD. Ann Neurol. 2022;91(2):299-300. doi: 10.1002/ana.26279 [DOI] [PubMed] [Google Scholar]
- 12.Pache F, Ringelstein M, Aktas O, et al. C3 and C4 complement levels in AQP4-IgG-positive NMOSD and in MOGAD. J Neuroimmunol. 2021;360:577699. doi: 10.1016/j.jneuroim.2021.577699 [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Wu Y, Hang H, Lu J, Ding Y. Plasma complement 3 and complement 4 are promising biomarkers for distinguishing NMOSD from MOGAD and are associated with the blood-brain-barrier disruption in NMOSD. Front Immunol. 2022;13:853891. doi: 10.3389/fimmu.2022.853891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C, Chen B, Tao R, et al. The clinical value of complement proteins in differentiating AQP4-IgG-positive from MOG-IgG-positive neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2019;35:1-4. doi: 10.1016/j.msard.2019.06.035 [DOI] [PubMed] [Google Scholar]
- 15.Keller CW, Lopez JA, Wendel EM, et al. Complement activation is a prominent feature of MOGAD. Ann Neurol. 2021;90(6):976-982. doi: 10.1002/ana.26226 [DOI] [PubMed] [Google Scholar]
- 16.Sechi E, Cacciaguerra L, Chen JJ, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a review of clinical and MRI features, diagnosis, and management. Front Neurol. 2022;13:885218. doi: 10.3389/fneur.2022.885218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariotto S, Ferrari S, Gastaldi M, et al. Neurofilament light chain serum levels reflect disease severity in MOG-Ab associated disorders. J Neurol Neurosurg Psychiatry. 2019;90(11):1293-1296. doi: 10.1136/jnnp-2018-320287 [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Lee EJ, Kim S, et al. Serum biomarkers in myelin oligodendrocyte glycoprotein antibody–associated disease. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e708. doi: 10.1212/NXI.0000000000000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang X, Huang W, Wang L, et al. Serum neurofilament light and GFAP are associated with disease severity in inflammatory disorders with aquaporin-4 or myelin oligodendrocyte glycoprotein antibodies. Front Immunol. 2021;12:647618. doi: 10.3389/fimmu.2021.647618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 22.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi: 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: international MOGAD Panel proposed criteria. Lancet Neurol. 2023;22(3):268-282. doi: 10.1016/S1474-4422(22)00431-8 [DOI] [PubMed] [Google Scholar]
- 24.Höftberger R, Sepulveda M, Armangue T, et al. Antibodies to MOG and AQP4 in adults with neuromyelitis optica and suspected limited forms of the disease. Mult Scler. 2015;21(7):866-874. doi: 10.1177/1352458514555785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariotto S, Ferrari S, Monaco S, et al. Clinical spectrum and IgG subclass analysis of anti-myelin oligodendrocyte glycoprotein antibody-associated syndromes: a multicenter study. J Neurol. 2017;264(12):2420-2430. doi: 10.1007/s00415-017-8635-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mader S, Ho S, Wong HK, et al. Dissection of complement and Fc-receptor-mediated pathomechanisms of autoantibodies to myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci U S A. 2023;120(13):e2300648120. doi: 10.1073/pnas.2300648120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohyama K, Nishida H, Kaneko K, Misu T, Nakashima I, Sakuma H. Complement-dependent cytotoxicity of human autoantibodies against myelin oligodendrocyte glycoprotein. Front Neurosci. 2023;17:1014071. doi: 10.3389/fnins.2023.1014071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yandamuri SS, Filipek B, Obaid AH, et al. MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity. JCI Insight. 2023;8(11):e165373. doi: 10.1172/jci.insight.165373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerch M, Schanda K, Lafon E, et al. More efficient complement activation by anti–aquaporin-4 compared with anti–myelin oligodendrocyte glycoprotein antibodies. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200059. doi: 10.1212/NXI.0000000000200059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalakas MC, Alexopoulos H, Spaeth PJ. Complement in neurological disorders and emerging complement-targeted therapeutics. Nat Rev Neurol. 2020;16(11):601-617. doi: 10.1038/s41582-020-0400-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carta S, Cobo Calvo Á, Armangué T, et al. Significance of myelin oligodendrocyte glycoprotein antibodies in CSF: a retrospective multicenter study. Neurology. 2023;100(11):e1095-e1108. doi: 10.1212/WNL.0000000000201662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter M, Baksmeier C, Steckel J, et al. Dose-dependent inhibition of demyelination and microglia activation by IVIG. Ann Clin Transl Neurol. 2016;3(11):828-843. doi: 10.1002/acn3.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lünemann JD, Nimmerjahn F, Dalakas MC. Intravenous immunoglobulin in neurology--mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80-89. doi: 10.1038/nrneurol.2014.253 [DOI] [PubMed] [Google Scholar]
- 34.Chen JJ, Huda S, Hacohen Y, et al. Association of maintenance intravenous immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2022;79(5):518-525. doi: 10.1001/jamaneurol.2022.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Kong L, Zhao Z, et al. Effectiveness and tolerability of different therapies in preventive treatment of MOG-IgG-associated disorder: a network meta-analysis. Front Immunol. 2022;13:953993. doi: 10.3389/fimmu.2022.953993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen B, Gomez-Figueroa E, Redenbaugh V, et al. Do early relapses predict the risk of long-term relapsing disease in an adult and paediatric cohort with MOGAD? Ann Neurol. 2023;94(3):508-517. doi: 10.1002/ana.26731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.