Abstract
Domestication process effects are manifold, affecting genotype and phenotype, and assumed to be universal in animals by part of the scientific community. While mammals and birds have been thoroughly investigated, from taming to intensive selective breeding, fish domestication remains comparatively unstudied. The most widely bred and traded ornamental fish species worldwide, the goldfish, underwent the effect of long-term artificial selection on differing skeletal and soft tissue modules through ornamental domestication. Here, we provide a global morphological analysis in this emblematic ornamental domesticated fish. We demonstrate that goldfish exhibit unique morphological innovations in whole-body, cranial, and sensory (Weberian ossicles and brain) anatomy compared to their evolutionary clade, highlighting a remarkable morphological disparity within a single species comparable to that of a macroevolutionary radiation. In goldfish, as in the case of dogs and pigeons in their respective evolutionary contexts, the most ornamented varieties are extremes in the occupied morphological space, emphasizing the power of artificial selection for nonadaptive traits. Using 21st century tools on a dataset comprising the 16 main goldfish breeds, 23 wild close relatives, and 39 cypriniform species, we show that Charles Darwin’s expressed wonder at the goldfish is justified. There is a commonality of overall pattern in the morphological differentiation of domesticated forms selected for ornamental purposes, but the singularity of goldfish occupation and extension within (phylo)morphospaces, speaks against a universality in the domestication process.
Keywords: artificial selection, fish domestication, phenomics
Introduction
Goldfish (Carassius auratus) is the most morphologically diverse domestic fish species (Darwin, 1868) and the most widely bred and traded ornamental fish species worldwide. The domestic form arose from different populations of carp from ancient China and has been strongly selected for ornamental purposes over a millennium (Ota & Abe, 2016; Smartt, 2008). Whereas the genomic (Chen et al., 2020) and developmental genetics (Kon et al., 2020; Ota, 2021) of goldfish diversification are increasingly better understood, morphological research on these animals has remained at the level of traditional breed descriptions (Smartt, 2008) and the intense study of few specific traits (Li et al., 2019), leaving unexamined what Darwin (1868) called “the most extraordinary modifications of structure” among domestic animals. Goldfish belong to the Cypriniformes (Figure 1), the world’s largest clade of freshwater fishes (~4200 species; Betancur-R et al., 2017), and has been superseded as a preferred “model organism” by the zebrafish among Cypriniformes given the genome duplications that occurred in the lineages of Carassius and Cyprinus (Glasauer & Neuhauss, 2014), leading to increased difficulty in studying their genome. The genome duplication resulting in 50 chromosomes, i.e., twice that of most other Cyprinidae (Ohno et al., 1967), could have been a major driver for the morphological disparity of goldfish (Ota & Abe, 2016), disparity being defined here as the measure of morphological variation among species and higher taxa (Hopkins & Gerber, 2017).
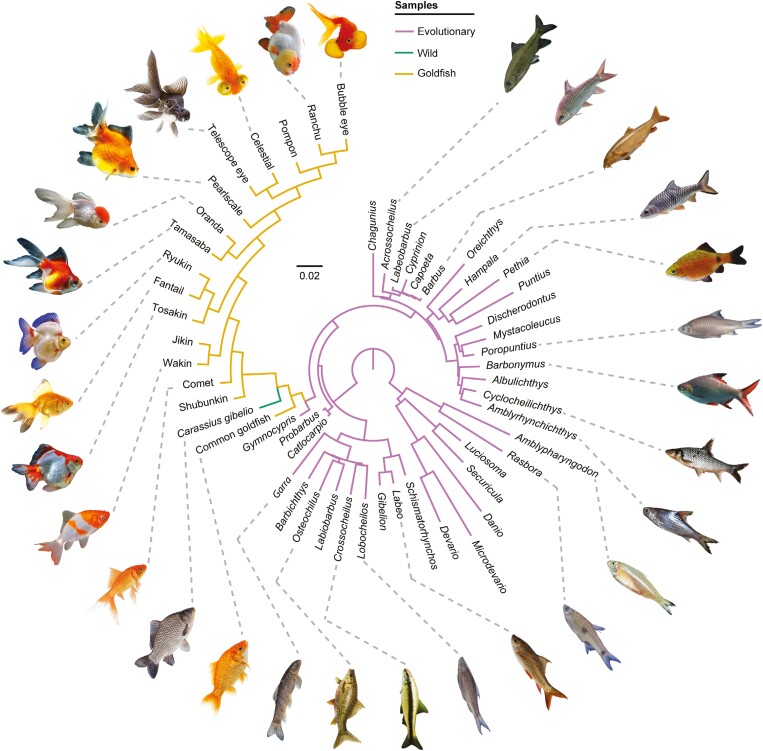
Figure 1.
Fully dichotomous phylogeny used in PCoA. We crafted the phylogeny based on the genera in our sample present in the robust molecular phylogeny of Stout et al. (2016), conserving branch lengths. We added goldfish and some Cyprininae to the tree based on the phylogenies of Tang et al. (2011) and Podlesnykh et al. (2015), duplicating the branch lengths of Gymnocypris from the phylogeny of Tang et al. (2011). We forced the dichotomy of incorporated taxa by following the phylogeny of Chen et al. (2020) and the diagrammatic genealogy of Smartt (2008). The production of this tree in addition to the tree shown in Supplementary Figure S2 is justified by the need to incorporate a fully resolved tree for PCoA. As a control test, we provide additional analyses without the incorporation of phylogeny (Supplementary Figure S3). Fish illustrations are not scaled, and references are available in Supplementary Table S7.
Goldfish belongs to the megadiverse Ostariophysi, a clade notably characterized by the Weberian apparatus (Figure 2), a key innovation in the auditory system that enables a broader range of detectable frequencies and sound sensitivity (Bird & Hernandez, 2007). It consists of the structurally modified first four cervical vertebrae as a paired set of four ossicles, connecting the anterior swim bladder to the inner ear (Diogo, 2009), two fundamental organs for hydrostatic functions and hearing. Selection in goldfish has been largely for ornamental traits, also characteristic in companion dogs and “fancy pigeon” breeds. In the wild, fish ornamentation can serve for visual attraction and is associated with sexual selection (Amundsen & Forsgren, 2001). Experimental studies in fishes suggest that ornamentation can conflict with natural selection, ornamented specimens being more prone to predation (Kemp et al., 2018). The domestication process can lead to functional relaxation (e.g., no predation), allowing the selection of morphological traits that are nonadaptive and potentially deleterious in a natural environment (Van Valen, 1960). Domestication can also lead to changes in the skull, the brain, and sensory organs (Sánchez-Villagra, 2022). The neurocranium, encapsulating sensory organs, has been a central marker of change of the domestication process (Sánchez-Villagra, 2022), but is only poorly documented in this context for domestic fishes because its extraction is complicated due to bone thinness and the composite nature of fish anatomy.
Figure 2.
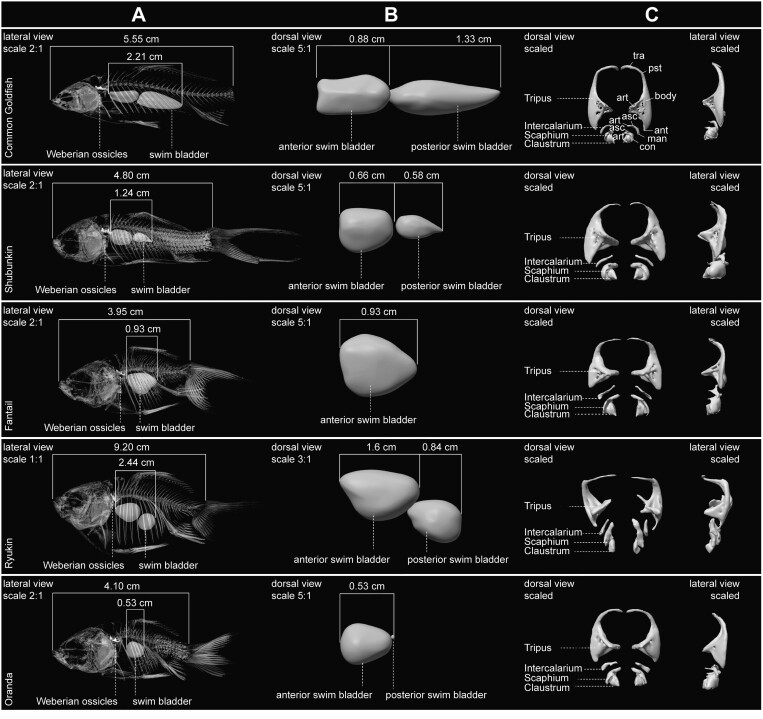
Anatomical plate of virtual skeleton, swim bladder and Weberian ossicle models. (A) Lateral view of scaled skeleton with accentuated swim bladder and Weberian ossicles. (B) Dorsal view of scaled anterior and posterior swim bladder. (C) Dorsal (left) and lateral (right) view of scaled Weberian ossicles. Anatomical abbreviations following Bird and Hernandez (2007): Ant, anterior process; art, articular process; asc, ascending process; con, concha; man, manubrium; pst, posterior process; tra, transformator process.
In the present study, our aim is to compare for the first time the phenomics of goldfish with their wild and evolutionary representatives along different phenotypic axes in (phylo)morphospaces (Mitteroecker & Huttegger, 2009). In addition to identifying phenotypic variation resulting from the domestication process of goldfish, we wonder which is the major axis of differentiation between domesticated and wild forms across several anatomical units. Ultimately, our goal is to identify how artificial selection for ornamentation drives the distribution of domesticated forms in morphospaces. Thus, we question whether goldfish evolution is a special case of domestication or not, and if the patterns and trajectories of morphological differentiation have parallels in the domestication processes of other species.
We use state-of-the-art of phenomics to investigate goldfish diversity through their 16 main breeds/varieties (N = 30) in relation to their close wild relative, the Prussian carp (C. gibelio; N = 24), and several other closely related cypriniform species (N = 39), and thus explore what fascinated Darwin. To compare the phenotypic diversity of goldfish within its evolutionary clade, we focused our sampling mainly on Cyprinidae (N = 33) and Danionidae (N = 7), thus covering a case of phenotypic evolution starting in the early Cenozoic (Tao et al., 2019). We explore the issue of whether goldfish diversity mirrors its evolutionary group’s morphological disparity by quantifying and comparing overall phenotype within the clade, leading to also examine how the selection for ornamental traits has produced morphological innovations in diverse anatomical modules. This issue has been explored in studies of the skulls of pigeons (Young et al., 2017) and dogs (Drake & Klingenberg, 2010), providing a comparative context for assessing the impact of the domestication process.
Materials and methods
Sampling
To investigate phenomics in a domestication context, comparative sampling should include several domesticated breeds, individuals of a wild close relative, and related-clade species (Sánchez-Villagra, 2022). For goldfish, we initially selected at least 2 individuals of the 16 main breeds/varieties, following Smartt’s (2008) diagram to cover the breadth of goldfish morphological traits. From the 37 specimens stored at the Natural History Museum of Bern (NMBE), 7 were not included in the analyses because of a premature ontogenetic stage or the presence of morphological pathologies. These are nevertheless included in Supplementary Table S1 to refer to the collection with the other 30 specimens at the NMBE. For their wild close relatives, we built a sampling focused on C. gibelio, the second-best candidate after wild C. auratus (Podlesnykh et al., 2015), reaching 24 specimens. For the other cypriniforms, in line with the phylogeny of Stout et al. (2016), we sampled 39 specimens available on the MorphoSource platform, covering 8 of 11 subfamilies of Cyprinidae (N = 32) and the three subfamilies of Danionidae (N = 7), their sister group. Due to acquisition constraints, such as indiscernible Weberian ossicles in some individuals, sampling varies slightly among the analyses. In addition, for the other cypriniforms, the cladistic matrix was constructed using the same genera from Stout et al. (2016), but not always the same species, as for the other analyses, due to the literature available (see below). All specimens and whether/how they were included in each analysis are listed in Supplementary Table S1. Another limiting factor in our sampling is the absence of data on the sex of each individual. However, to our knowledge, no sexual dimorphism is known in goldfish, with the exception of a slight variation in the morphology of the wild type goldfish associated with the presence/absence of breeding tubercles and the shape of the cloaca (Ota, 2021), anatomical parts that we do not quantify here. We therefore assume that sexual dimorphism is negligible for our analyses of goldfish phenomics.
Cladistic matrix construction
To investigate the external morphological traits of the whole body, the 19 discretely coded morphological traits from qualitative and quantitative data were selected and completed in agreement with Smartt (2008) to build the cladistic matrix (illustrated in Supplementary Figure S1). In addition to our observations, for the other cypriniforms, our coding was completed using descriptions available in the literature. Due to missing descriptions, 16 taxa were coded using different species than from Stout et al. (2016) and should therefore be considered here at the generic level. All characters were treated as unordered. The completed cladistic matrix contains only eight missing data points (i.e., ~0.7%) and is available in Supplementary Table S2, including all related references.
Phylogeny
As a fundamental prerequisite, phylogeny should be incorporated into analyses including more than two species. Although several studies have improved our conception of the Cypriniformes phylogeny (Betancur-R et al., 2017), intrarelationships remain nonconsensual in the Cyprinoidei phylogeny. We reconstructed our phylogeny based on the robust molecular phylogeny of Stout et al. (2016), conserving branch lengths. To match our sampling, we removed the set of species from Stout et al. (2016) unavailable for our study. Due to the lack of Cyprininae, in addition to the set of goldfish, we added other Carassius species (i.e., C. cuvieri and C. langsdorfii), and the genus Gymnocypris (i.e., reconstructing the position of G. dobula from G. przewalskii), based on the phylogeny of Tang et al. (2011) and Podlesnykh et al. (2015). Thus, we extracted the branch length between the node defining Cyprininae and Gymnocypris in the phylogeny of Tang et al. (2011). Then, we incorporated this branch length into the tree of Stout et al. (2016) by duplicating the branch length for all Cyprininae in our sampling. Due to their polyphyletic origin (Podlesnykh et al., 2015), goldfish are split into two groups: the common goldfish, as a sister group to other Carassius, and the remaining goldfish in an apical position. For the neurocranial phylomorphospace construction, to limit assumptions, we preserved a polytomy for both goldfish groups and for C. gibelio. However, as principal coordinate analysis (PCoA) for whole-body external morphology requires a resolved, i.e., fully dichotomous, tree, we constrained the dichotomy within these three groups, following the phylogeny of Chen et al. (2020) and complementing with the diagrammatic genealogy of Smartt (2008). It is noteworthy here that our tree-reconstruction does not correspond to a new phylogeny but only to reduce the impact of phylogeny in our morphological analyses. The fully resolved tree is illustrated in Figure 1 and the tree conserving polytomies is available in Supplementary Figure S2. As a control test, we provide the same analyses without the incorporation of phylogeny in Supplementary Figure S3.
Imagery, staining, and virtual reconstruction
All goldfish, C. gibelio and two other Carassius species were imaged using X-ray microcomputed tomography (μCT) at the Irchel campus of the University of Zurich with a Nikon XT H 225 ST. To optimize scan parameters, each specimen was scanned at least twice: for the whole body (+anterior and posterior swim bladder) and with a focus on the head (neurocranium—orbital ring + Weberian ossicles). For brain volume extraction, the five selected specimens along the first PC of the neurocranial morphospace were stained (see Results), then scanned a third time with a focus on the brain. The staining procedure followed the slightly modified protocol of Camilieri-Asch et al. (2020), specifically defined for goldfish brain extraction. The modifications occurred in the preparation of the specimens, as our goldfish were not fixed and the lenses were not extracted, and in the staining time, as our specimens showed different sizes. Our preparation involved placing the specimens on a stirrer plate at room temperature (~20 °C) in an aqueous solution of Lugol’s Iodine (I2KI), including 1% wt/vol of iodine (I2) and 2% wt/vol of potassium iodide (KI) in distilled water (1g I2 + 2g KI in 100 mm of distilled water). The solution was changed every 24 hr. Due to the size of each specimen, the amount of solution and staining time varied: Common goldfish NMBE 1105140 (100 ml; 96 hr); Tosakin NMBE 1105164 (200 ml; 120 hr); Oranda NMBE 1105157 (250 ml; 120 hr); Bubble eye NMBE 1105163 (250 ml; 120 ml); Celestial NMBE 1105153 (200 ml; 120 hr). As a result, the entire endocranial cavity is filled by the organ, assuming little or no shrinkage, as expected with the protocol of Camilieri-Asch et al. (2020). Three-dimensional reconstructions of the neurocrania associated with the orbital ring, Weberian ossicles, swim bladders, and brains were performed using stacks of digital μCT images with AVIZO v. 8.0.0 software (Visualization Sciences Group, Burlington, MA, USA). The composite and locally extremely fine anatomy of fish makes the segmentation stage highly time consuming and difficult, partly explaining the lack of studies on 3D teleost reconstructions. When the anatomy was not too fine, we used the online software Biomedisa (Lösel et al., 2020) to support the segmentation work completed by hand. Each 3D model of the targeted anatomical structures was then exported in PLY format for landmarking. The volume of the swim bladder and brain (and its regions) was extracted directly from AVIZO and is available in Supplementary Tables S3 and S4.
Three-dimensional geometric morphometrics
To quantify the neurocranial and Weberian ossicles shape, we opted for 3D geometric morphometrics. Regarding the neurocranium, we used 68 anatomical landmarks covering the structure composed of the neurocranium, but also the orbital rings, in order to capture maximal anatomical associated variation (see Supplementary Figure S4 for an illustration and Supplementary Table S5 for their description). To remove the asymmetrical component, we symmetrized the neurocranial complex along the bilateral axis. Some specimens (N = 16/86) displayed segmentation difficulties (alteration, fine region, etc.) leading to the systematic definition of missing landmarks, which we estimated using their symmetric (0.30% of the whole landmark set) or regression (0.09% of the whole landmark set). The estimate of missing landmarks for a single specimen never exceeds 3 landmarks, making missing data highly negligible. Finally, a generalized Procrustes analysis was applied to the whole dataset to extract shape variation only. For Weberian ossicles, quantification is complicated by the composite nature of the ossicle set. We followed the approach of Thomas et al. (2023), quantifying by block (i.e., in this case, ossicle) and then combining these same blocks, including a total of 16 anatomical landmarks and 96 sliding semi-landmarks distributed over 13 curves (see Supplementary Figure S5 for an illustration and Supplementary Table S6 for a description). For this object type, the asymmetrical component has not yet been implemented. Rather than considering eight blocks that are biologically dependent, we preferred to quantify only one side (i.e., the left), confirming qualitatively the absence of strong asymmetry. We favored complete specimens rather than the estimation of missing landmarks, leading to our dataset to be free of them. A generalized Procrustes analysis was performed on each block independently, then all blocks were recombined, enabling us to analyze shape variation per block and in a superblock (Thomas et al., 2023). Further details of the preprocessing, functions and packages used with R software v. 4.3.249 (R Core Team, 2021) are available in the provided code. Landmarks were placed manually using Stratovan Checkpoint v2022.07.21.1321 (Stratovan Corporation).
Statistics
Many different multivariate statistical tools exist to analyze the distribution of variance, here through (phylo)morphospaces. Among them, due to the composition of each dataset, we have compiled different ordinations to investigate various parts of the phenotype separately. Because the cladistic matrix corresponds to qualitative data and discretized continuous data, we computed a distance matrix based on the cladistic matrix and performed a PCoA, including phylogeny. To measure the dissimilarity between each individual on the whole distance matrix, we computed a neighbor-joining network. In addition, to confirm visual inspection of the phylomorphospace, we grouped distances within each cluster (i.e., domesticated vs. wild) and performed a permutational multivariate ANOVA (i.e., PERMANOVA; Anderson, 2001) to test the difference in variance between clusters using permutation (iteration = 10,000). For analyses based on 3D geometric morphometrics, we used: a phylogenetically aligned component analysis (PaCA; Collyer & Adams, 2021) to integrate phylogeny into the analysis of the neurocranial complex shape including more than two species; a classical principal component analysis (PCA) to analyze the shape of the neurocranial complex between goldfish and C. gibelio; and a regularized consensus PCA (RCPCA) to investigate multipart objects such as Weberian ossicles (Thomas et al., 2023). Different levels of analysis are possible, notably through various considerations such as phylogeny and allometry (Drake & Klingenberg, 2010). Here, we have not considered allometry because this phenomenon is practically unsupported in our study (Supplementary Table S8). For each analysis incorporating phylogeny, we conducted similar analyses without its incorporation for comparative purposes (Supplementary Figure S3), all confirming the main signal detected. With regard to morphological disparity, we assessed the amount of morphological variation by comparing the Procrustes variance of each group. Finally, to measure the correlation between Weberian ossicles shape and swim bladder volume, we performed a multivariate regression of Weberian ossicles Procrustes coordinates on the log swim bladder volume. Further details of the functions and packages used with R software (R Core Team, 2021) are available in the provided code.
Results
External morphology
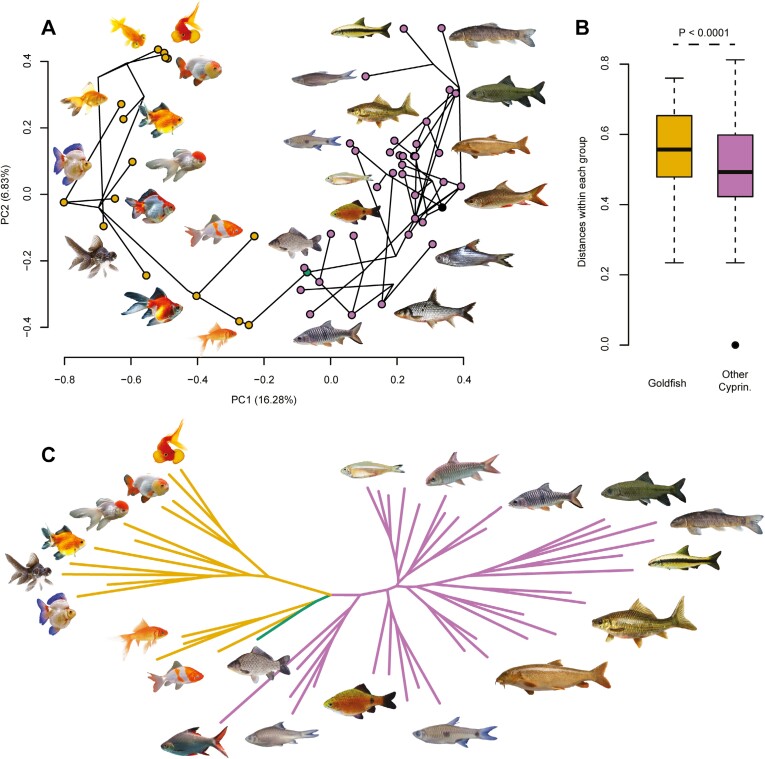
The phylomorphospace defined by the first two principal components (PCs = 22.46%) shows a clear demarcation between the occupation of goldfish and other cypriniforms (Figure 3A). The distinction between the goldfish and the evolutionary cluster is contained at the PC1, mainly influenced by body shape and fin configuration. Ornamental domestication led goldfish to occupy a new space in the phylomorphospace, mainly through the selection for globular body shape and duplicated caudal and anal fins, in opposition to fusiform, elongated, and single-tail fish (Figure 3A). PC2 reveals the enlargement of each cluster mainly based on fin length and shape. For goldfish, this second axis distinguishes breeds with atrophied fins from others (Figure 3A). Overall summarized morphological distances among specimens support the strong demarcation detected by the first two PCs (Figure 3C), confirming the major morphological disparities between goldfish and the cypriniforms sampling (Figure 3B).
Figure 3.
The new occupation of goldfish in the cypriniform phylomorphospace is mainly driven by the shape of the body and fins (N = 53). (A) Phylomorphospace (PCs 1–2 = 23.11%) from the PCoA performed on a distance matrix extracted from our cladistic matrix comprising 19 discretely coded morphological traits (Supplementary Table S2). The black dot in the phylomorphospace corresponds to the root of the tree. (B) Boxplot of the distance between each individual of each group (goldfish vs. other cypriniforms), resulting from the distance matrix. PERMANOVA rejected an approximately equal multivariate dispersion between goldfish and other cypriniforms (F = 16.665; P < 0.0001). (C) Dissimilarity neighbor-joining network covering all the variance from the distance matrix. Abbreviation: Cyprin., Cypriniformes. The color code follows that of Figure 1. Fish illustrations are not scaled, and references are available in Supplementary Table S7.
Neurocranial shape
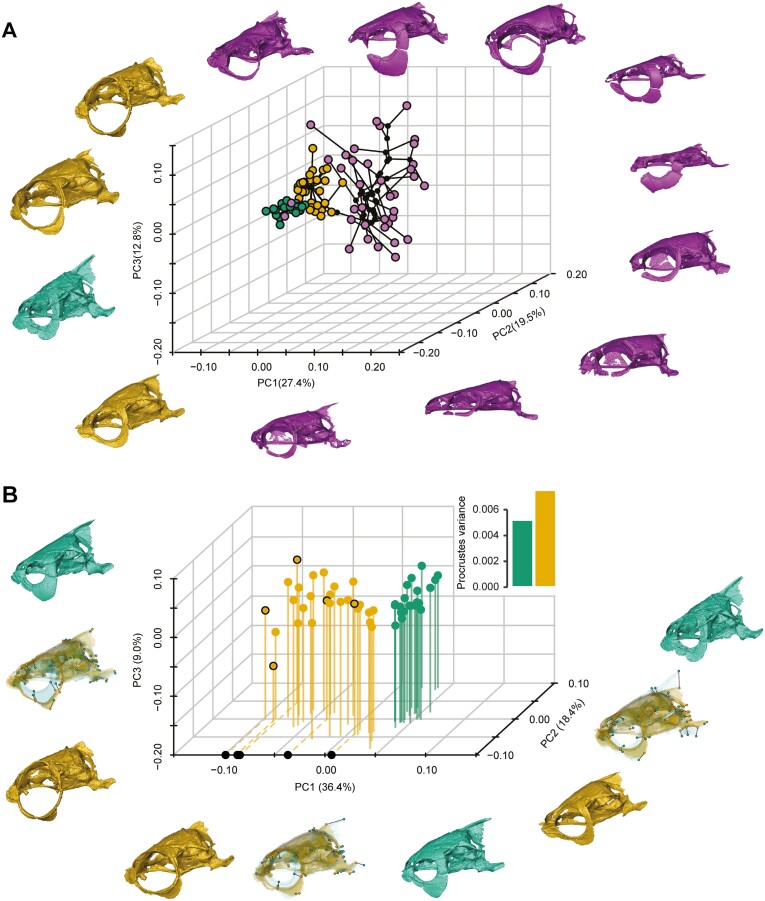
We conducted two analyses: (1) The first three PCs of the PaCA account for 59.7% of the total variance and reveal that goldfish occupy a different place in the phylomorphospace than their wild counterparts (Figure 4A); (2) a PCA including only the C. gibelio and the goldfish, as in the previous analysis other cyprinids mask the morphological diversification of goldfish versus their closest wild relatives. The first three PCs of the new morphospace account for 63.8% of the total variance and confirm the clear separation between goldfish and C. gibelio, with morphospace occupancy and morphological disparity higher for goldfish (Figure 4B). Goldfish breeds show extreme shape variation in the orbital ring, with a drastic variation in the orbital size and a reduction in the dimensions of the ring bones (Figure 4B). This major shape variation is accompanied by a face shortening, a cranial roof flattening, a strong reduction of the supraoccipital crest and the relative proportions of the basioccipital (Figure 4B).
Figure 4.
Domestication impacts all neurocranial shape components, especially the orbit and supraoccipital crest. (A) 3D neurocranial phylomorphospace (PCs 1–3 = 59.6%; N = 84) from a PaCA, including goldfish, C. gibelio, and other cypriniforms (32 cyprinids + 7 danionids). Unscaled 3D meshes in lateral view were added to highlight the shape diversity expressed in the 3D phylomorphospace. The black dot corresponds to the nodes of the tree. (B) 3D neurocranial morphospace (PCs 1–3 = 63.8%; N = 49) from a PCA, focusing on goldfish and C. gibelio and including the morphological disparity analysis computed and compared using Procrustes variance. Neurocranial shape changes in lateral view between the most distant goldfish and C. gibelio on each PC of the morphospace are provided. The 3D meshes were superimposed and rendered transparent to highlight the vectors and the associated shape deformations. The five breeds projected on the PC1 correspond to the selected breeds for the brain volume comparisons. The color code follows that of Figure 1.
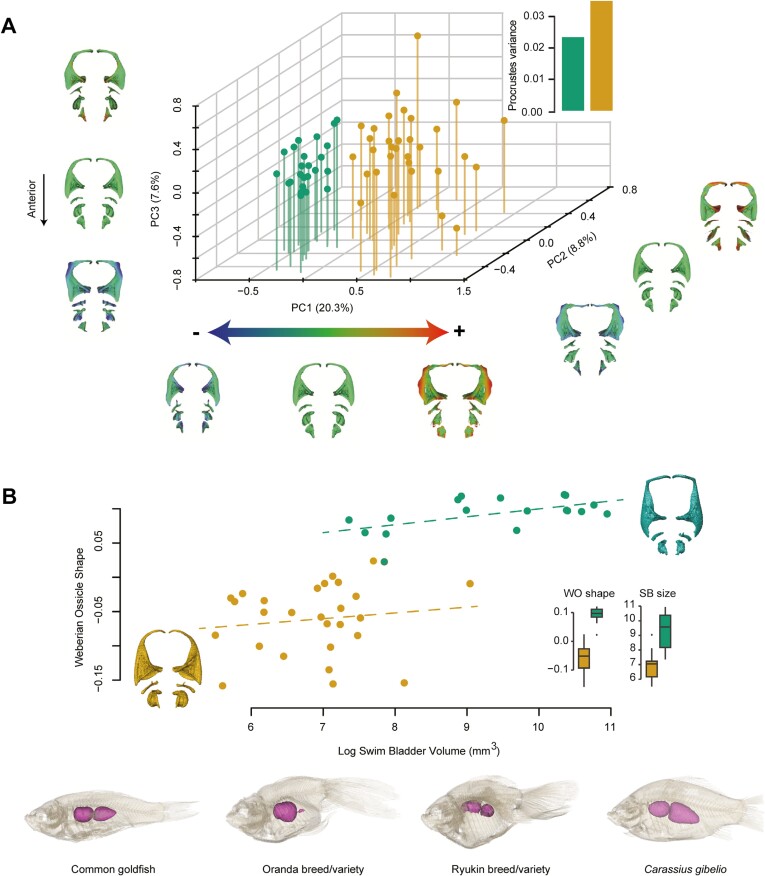
Weberian ossicles—swim bladder complex
The first three PCs of the RCPCA account for 36.7% of the total variance, and like the neurocranium (Figure 4B), the morphospace contains two well-differentiated clusters with wider occupancy and higher morphological disparity in goldfish (Figure 5A). In the morphospace, all four ossicle processes show variation, except for the anterior process of the Tripus (Figure 2). Only PC1 clearly distinguishes goldfish from the wild strain, while PC2 and PC3 show strong morphological variations carried by these ossicles within goldfish (Figure 5A). Ornamental domestication has led goldfish to undergo relative enlargement and/or elongation of the processes of each ossicle, particularly for the transformator, posterior, and articular processes of the Tripus (Figure 5A). Our results reveal that Weberian ossicle shape and swim bladder size are correlated (Figure 5B), with similar slopes coefficient for goldfish and C. gibelio (Figure 5B). However, we detected that swim bladder size varies much more in C. gibelio than in goldfish (Figure 5B), and inversely for Weberian ossicle shape, implying that ornamental domestication of goldfish has led to smaller fish but with higher variation in Weberian ossicle shape as swim bladder size varies.
Figure 5.
Goldfish show a highly disparate shape for the Weberian ossicles—swim bladder complex compared to C. gibelio. (A) 3D Weberian ossicle morphospace (PCs 1–3 = 36.7%; N = 56) from a RCPCA associated with the morphological disparity expressed through Procrustes variances of goldfish and C. gibelio. Heat maps were used to illustrate the shape deformations of each Weberian ossicle from the consensual shape to every extreme of each PC. 3D meshes are unscaled and in dorsal view. The right side was duplicated from the left for visualization purposes. (B) Multivariate regression of the Weberian ossicle shape with the log swim bladder volume (N = 45). The dotted lines show the slopes of each group. Boxplots have been added to highlight the amount of shape and size variation in goldfish and C. gibelio. An example of the high diversity of swim bladders in goldfish compared to C. gibelio is provided. Unscaled specimens are shown in transparency and lateral view to reveal their swim bladder (3D models). The color code follows that of Figure 1. Abbreviations: SB, swim bladder; WO, Weberian ossicles.
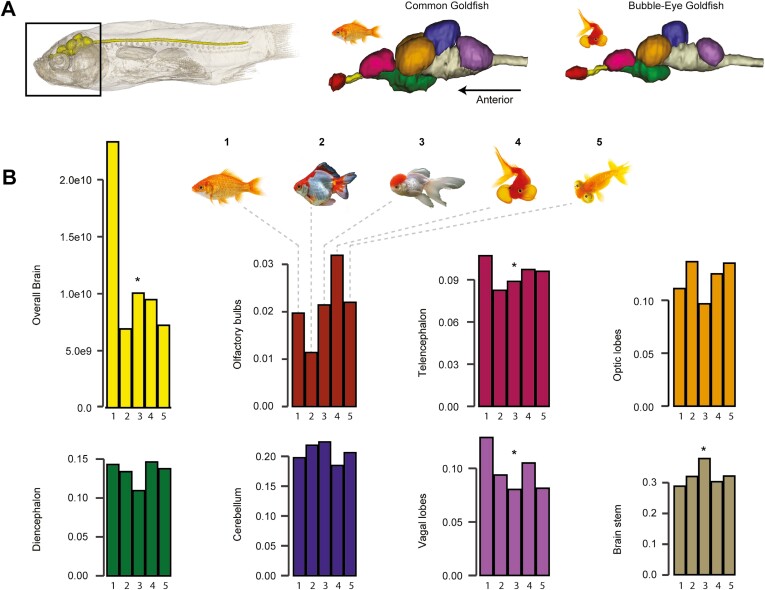
Brain size
We discovered large differences in total relative brain volume between the common goldfish, i.e., the strain recognized as a phenotypic equivalent of wild goldfish with different coloration (Omori & Kon, 2019), and the most extreme breeds, the latter with a brain proportionally more than twice smaller than in the common goldfish (~64% smaller, Figure 6). If the volume of each brain region is standardized by the total brain volume for each specimen, we detect no trend toward reduction or increase for the olfactory bulbs, optic lobes, diencephalon, and cerebellum (Figure 6). However, our study reveals that the most distantly related goldfish breeds tend to have a relatively smaller telencephalon (average ~15%) and vagal lobe (average ~30%), and a relatively larger brainstem (pons + medulla oblongata—average ~15%), suggesting differential reductions of brain portions (Figure 6).
Figure 6.
Domestication leads to a drastic reduction of the brain size in its entirety and regionally. (A) Overall brain anatomy at the same scale illustrated in lateral view for the goldfish closest to its wild counterparts, i.e., common goldfish, and one of the most distant, i.e., Bubble-eye breed. Brains show parcellated coloration to illustrate the different brain regions. (B) Histogram of relative brain volume and related relative regions volumes, with one being the closest relative to wild counterparts, i.e., common goldfish, and five being the most distant, i.e., Celestial breed (see Figure 4 for the five-breed selection). Total brain volume was standardized using neurocranial centroid size, while each brain region was standardized by total brain volume. Asterisks indicate brain region subject to distinct volume trends between common goldfish and other breeds. The color code in (B) follows the color code from (A). Fish illustrations are not scaled, and references are available in Supplementary Table S7.
Discussion
The millennia-old ornamental domestication of goldfish has generated much morphological novelty and variation across the phenotype, as exemplified by our extensive study of the whole body, neurocranium, Weberian ossicles, and the brain. The lack of overlap between the goldfish cluster and that of 40 other cypriniform species for whole-body and neurocranium (phylo)morphospaces is notable and different in its totality from the reported partial overlap in skull morphospace between wild and domesticated dogs and pigeon. For pigeons (Young et al., 2017) and domestic dogs (Drake & Klingenberg, 2010), the outliers in the domestic morphospace in contrast to the wild ones are the “fancy pigeons” and the companion breeds, respectively. In goldfish as well, the intensity of breeding selection for ornamental purposes led to major morphological innovation. Some traits have never been recorded in wild specimens, such as the caudal bifurcation of the axial skeleton in twin-tail breeds (Abe et al., 2014). Goldfish do not include as many breeds (Ota, 2021; Smartt, 2008) as other domestic animals, but there is considerable morphological diversity in them. In contrast, there are hundreds of recognized breeds of dogs and pigeons (Sánchez-Villagra, 2022). Concerning the time and mode of origin of such diversity, historical sources document how already the Song (960–1279) and Ming (1368–1598) Chinese dynasties record the major kinds of goldfish, including most of the breeds present in our sample from the 15th and 16th centuries (Smartt, 2008). There must have been an early occupation of morphospace in goldfish, a short fuse model analogous to that of carp domestication and to mammalian diversification patterns before or around the Cretaceous-Paleogene extinction event (Sánchez-Villagra, 2022). The intensification of goldfish breeding in the 20th century led to the recognition of many named varieties (Smartt, 2008), but the goldfish morphospace had already evolved centuries ago (Ota & Abe, 2016).
Husbandry has led to goldfish with extreme, nonadaptive features to be viable and reproductive. The shortening of the bodies of many goldfish breeds is correlated to stronger spinal curvature and a reduction of the abdominal and caudal vertebra count (Ota & Abe, 2016), causing a misshaped coelomic cavity with more limited space to fit organs (Brown et al., 2018), impairments in the undulation of the body and caudal fin for the typical cyprinid propulsion (Friedman et al., 2021), and shortened lifespan (Brown et al., 2018). Besides parasitic, viral, bacterial, or fungal infections, buoyancy disorders observed in globular goldfish are also often related to organ malformation and compaction caused by inbreeding and rounding of the body cavity (Brown et al., 2018). The fin configurations that differentiate the morphologically most distant goldfish in our phylomorphospace study includes the nonindependent twin-tail (Abe et al., 2014), longer-tail, and dorsal-finless phenotypes. Twin-tail phenotypes could be associated with more long-tail phenotypes, showing an elongation of caudal fin-lobes accompanied by a higher tendency of elongation in all other fins (Kon et al., 2020). While the biomechanical advantages and/or disadvantages of twin-tail and long-tail phenotypes are still unknown, dorsal-finless breeds exhibit greatly impaired locomotion, reflected by reduced directional stability, balance, swimming speed, and acceleration (Blake et al., 2009). The neurocranium has been a useful marker in investigations of the effect of the domestication process (Sánchez-Villagra, 2022) as it is composed of several bones derived either from the neural crest or mesoderm (Teng et al., 2019) and houses the main sensory organs. Here, we demonstrated that the domestication process had strongly affected not only the shape of the orbit, but also all the components of the neurocranium. Orbital variation in goldfish ranges from the relatively common position and shape of the eyes in common goldfish to the large and fluid-filled vesicles seen in Bubble-eye varieties, leading to a skyward displacement of the iris. Such orbital variations have no equivalent in other domestic animals or natural occurring species. The protuberant head and the high orbital shape changes are among the most extreme external morphological variations within goldfish. Ornamental head features present in “fancy goldfish” breeds are sensitive to mechanical damage and vision impairment since most of them encompass or surround the eyes (Matsumura et al., 1982). As reported for cypriniforms in general (Bird & Hernandez, 2007), the diversity in Weberian ossicle shape in goldfish is mostly related to changes in length and shape of ossicle processes. We detected strong shape variation for the Tripus and Intercalarium in goldfish, supporting the suggestion that the shape of the posteriorly located ossicles is less conserved (Boyle & Herrel, 2018). While the Tripus and the Intercalarium might play a greater role in specialized sound detection, the Scaphium acts mostly as a reliable transductor and the Claustrum as a stabilizing and protective structure (Bird & Hernandez, 2007; Diogo, 2009). The goldfish swim bladder is relatively smaller than that of C. gibelio, with no overlap with the extensive sample of the latter. The swim bladder is a hydrostatic gas-filled organ acting as a stabilizing agent, enabling active control over buoyancy and depth maintenance (Pelster, 2017). Impinging sound pressure waves can cause oscillations of the elastic swim bladder walls, and through the connection over the Weberian ossicles, sound waves are transferred to the inner ear (Watson, 1939), explaining the fundamental role of this complex for hearing. In catfish, piranhas and pacus, a reduced swim bladder could negatively affect hearing abilities, in particular at higher frequencies (Boyle & Herrel, 2018; Lechner & Ladich, 2008). These size reductions could either assist in retaining high-frequency hearing in loud environments by reducing the inertia of the complex or show a reduced investment into specialized hearing (Boyle & Herrel, 2018; Lechner & Ladich, 2008). Resulting from ornamental domestication, goldfish of differing breeds thus possibly display greater differences in individualistic high-frequency detection abilities than wild counterparts, from potentially highly sound-sensitive to almost deaf goldfish. As generally the case for mammals and birds (Balcarcel et al., 2022), most domestic fishes exhibit a reduction in brain size compared to wild forms (Sánchez-Villagra, 2022), especially the telencephalon, optic tectum, olfactory bulbs, and cerebellum. A comparative study including goldfish and crucian carp as a wild strain showed lower brain mass in domestic fish, with the vagal lobe as a region particularly impacted by the reduction (Masai et al., 1983). In our study, we detected a drastic reduction in goldfish brain size compared with the common and less specialized goldfish, and a relative cerebral regionalized reduction only in the telencephalon and vagal lobe. The telencephalon is a key region for cognitive abilities, including sensorimotor and cerebellar processing (Barton, 2012). Although the correlation between brain size and cognitive ability has long been debated, the absolute number of neurons in the telencephalon has been shown to be a good proxy for cognitive abilities (Herculano-Houzel, 2017), this number being correlated with brain mass (Marhounová et al., 2019). It is likely then that the reduction in brain size detected in our study suggests that goldfish possess reduced cognitive capacities in contrast to their wild counterparts. The vagal lobe contains the sensorimotor function of the palatal organ, and thus corresponds to the gustatory center, with cypriniforms showing many morphological novelties related to the masticatory apparatus (Hernandez & Cohen, 2019). Our results confirm that the vagal lobe in goldfish is relatively smaller than the vagal lobe of C. gibelio, also suggesting a reduction in gustatory senses (Masai et al., 1983).
Much is discussed in the search for general patterns of transformation in the domestication process (Lord et al., 2020; Wright et al., 2020), agreeing on the lack of universality of features of a potential “syndrome” and other aspects such as brain size reduction (Balcarcel et al., 2022) or skull changes (Sánchez-Villagra, 2022) and the heritability of immune responses among fishes (Milla et al., 2021). The pattern of morphological diversification of goldfish in comparison to their evolutionary clade is different from that reported for other species and as such an example of the lack of universals in the domestication process (Sánchez-Villagra, 2022), also valid for the developmental process in general (Richardson, 2022). Consequently, we question any model species to understand domestication. In evolution in the wild, as in domestication, the combination of selection regimes and phylogenetic baggage can result in a myriad of conditions for evolution, which together with contingency can produce different patterns of change. The domestication process has the added aspect of the influence of human culture, introducing another level of contingency and of selection, the latter perhaps analogous to the evolution of ornaments in the wild. In contrast to the uniqueness of the goldfish morphological transformations, there is commonality with pigeons and dogs in the fact that the morphological innovation as reflected in new morphospace occupation concerns breeds that are ornamental. Phenotypic variation is often modeled in microevolutionary studies as continuous and unbiased (isotropic) in relation to the adaptive needs of organisms (Charlesworth et al., 1982). The pattern of morphological diversification we report on goldfish would seem to follow this, as many phenotypic peculiarities responding to the peculiar fancy of breeders exist and do not overlap with the evolutionary pattern of evolution of the large, inclusive clade.
Supplementary material
Supplementary material is available online at Evolution Letters.
Acknowledgments
We would like to thank Dominic Pascal Stalder for participation in the preliminary analyses that gave rise to the present study; Gabriel Aguirre Fernandez (Zurich) for methodological help in using cladistic matrix; Jorge D. Carrillo Briceño (Zurich) for μCTs acquisitions; Olivia Plateau (Cambridge) for her discussion and help with R script; Carolin Sommer-Trembo (Zurich) for her discussion and proofreading; Philippe Keith (Paris), Zora Gabsi (Paris), and Ralf Britz (Dresden) for loans of Carassius specimens; Gilson Rivas (Maracaibo) and Tito Barros (Maracaibo) for access to specimens; Niklaus Heeb (Zurich), Simon Tschachtli (Zurich), Alexandra Kaufamnn (Zurich), Elena Kaeser (Zurich), and Evelyne Pfeffer (Zurich) for some illustrative contents. Our special thank goes to Lukas Rüber (Bern) for his advice on cypriniform systematics and his help with the goldfish collection at NMBE. We also acknowledge the suggestions from two anonymous reviewers and by editors Judith Mank and Andy Gardner. Finally, we thank all MorphoSource contributors (Supplementary Table S1) and platform members.
Contributor Information
Kévin Le Verger, Department of Paleontology, University of Zurich, Zurich, Switzerland.
Laurelle C Küng, Department of Paleontology, University of Zurich, Zurich, Switzerland.
Anne-Claire Fabre, Institute of Ecology and Evolution, Universität Bern, Bern, Switzerland; Naturhistorisches Museum der Burgergemeinde Bern, Bern, Switzerland; Department of Life Sciences, Natural History Museum, London, United Kingdom.
Thomas Schmelzle, Department of Paleontology, University of Zurich, Zurich, Switzerland.
Alexandra Wegmann, Department of Paleontology, University of Zurich, Zurich, Switzerland.
Marcelo R Sánchez-Villagra, Department of Paleontology, University of Zurich, Zurich, Switzerland.
Data and code availability
All data are available in the main text, or the supplementary data. All original code and documents have been deposited at Zenodo and are publicly available as of the date of publication (https://doi.org/10.5281/zenodo.10403067). (DICE-)CT-scans, together with acquisition parameters, and the 3D models produced will be made available progressively on MorphoSource following publication of the present work in accordance with institutional policies (https://www.morphosource.org/projects/000602063/about?locale=en).
Author contributions
Conceptualization: M.R.S.V.; Methodology: K.L.V., L.C.K., A.F.; Investigation: K.L.V., L.C.K., A.F.; Visualization: K.L.V., L.C.K., ACF., T.S., A.W.; Funding acquisition: M.R.S.V.; Project administration: M.R.S.V.; Supervision: K.L.V., M.R.S.V.; Writing—original draft: K.L.V., M.R.S.V.; Writing—review & editing: K.L.V., L.C.K., A.F., T.S., A.W., M.R.S.V.
Funding
This work was funded by Swiss National Science Foundation project SNF 310030_212395 to M.R.S.V. Our source of funding supports Open Access publishing.
Conflict of interest: The authors declare no conflicts of interest.
References
- Abe, G., Lee, S. H., Chang, M., Liu, S. C., Tsai, H. Y., & Ota, K. G. (2014). The origin of the bifurcated axial skeletal system in the twin-tail goldfish. Nature Communications, 5(1), 3360. https://doi.org/ 10.1038/ncomms4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundsen, T., & Forsgren, E. (2001). Male mate choice selects for female coloration in a fish. Proceedings of the National Academy of Sciences of the United States of America, 98(23), 13155–13160. https://doi.org/ 10.1073/pnas.211439298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, M. J. (2001). A new method for non‐parametric multivariate analysis of variance. Austral Ecology, 26(1), 32–46. https://doi.org/ 10.1111/j.1442-9993.2001.01070.pp.x [DOI] [Google Scholar]
- Balcarcel, A. M., Geiger, M., Clauss, M., & Sánchez‐Villagra, M. R. (2022). The mammalian brain under domestication: Discovering patterns after a century of old and new analyses. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 338(8), 460–483. https://doi.org/ 10.1002/jez.b.23105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton, R. A. (2012). Embodied cognitive evolution and the cerebellum. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 367(1599), 2097–2107. https://doi.org/ 10.1098/rstb.2012.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur-R, R., Wiley, E. O., Arratia, G., Acero, A., Bailly, N., Miya, M., Lecointre, G., & Orti, G. (2017). Phylogenetic classification of bony fishes. BMC Evolutionary Biology, 17(162), 1–40. https://doi.org/ 10.1186/s12862-017-0958-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, N. C., & Hernandez, L. P. (2007). Morphological variation in the Weberian apparatus of Cypriniformes. Journal of Morphology, 268(9), 739–757. https://doi.org/ 10.1002/jmor.10550 [DOI] [PubMed] [Google Scholar]
- Blake, R. W., Li, J., & Chan, K. H. S. (2009). Swimming in four goldfish Carassius auratus morphotypes: Understanding functional design and performance employing artificially selected forms. Journal of Fish Biology, 75(3), 591–617. https://doi.org/ 10.1111/j.1095-8649.2009.02309.x [DOI] [PubMed] [Google Scholar]
- Boyle, K. S., & Herrel, A. (2018). Relative size variation of the otoliths, swim bladder, and Weberian apparatus structures in piranhas and pacus (Characiformes: Serrasalmidae) with different ecologies and its implications for the detection of sound stimuli. Journal of Morphology, 279(12), 1849–1871. https://doi.org/ 10.1002/jmor.20908 [DOI] [PubMed] [Google Scholar]
- Brown, C., Wolfenden, D., & Sneddon, L. (2018). Goldfish (Carassius auratus). In Yeates J. (Ed.), Companion animal care and welfare: The UFAW companion animal handbook. Wiley Blackwell. https://doi.org/ 10.1002/9781119333708.ch23 [DOI] [Google Scholar]
- Camilieri-Asch, V., Shaw, J. A., Mehnert, A., Yopak, K. E., Partridge, J. C., & Collin, S. P. (2020). diceCT: A valuable technique to study the nervous system of fish. Eneuro, 7(4), ENEURO.0076-20.2020. https://doi.org/ 10.1523/ENEURO.0076-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, B., Lande, R., & Slatkin, M. (1982). A neo-Darwinian commentary on macroevolution. Evolution, 36(3), 474–498. https://doi.org/ 10.1111/j.1558-5646.1982.tb05068.x [DOI] [PubMed] [Google Scholar]
- Chen, D., Zhang, Q., Tang, W., Huang, Z., Wang, G., Wang, Y., Shi, J., Xu, H., Lin, L., Li, Z., Chi, W., Huang, L., Xia, J., Zhang, X., Guo, L., Wang, Y., Ma, P., Tang, J., Zhou, G., … Zhang, J. (2020). The evolutionary origin and domestication history of goldfish (Carassius auratus). Proceedings of the National Academy of Sciences of the United States of America, 117(47), 29775–29785. https://doi.org/ 10.1073/pnas.2005545117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collyer, M. L., & Adams, D. C. (2021). Phylogenetically aligned component analysis. Methods in Ecology and Evolution, 12(2), 359–372. https://doi.org/ 10.1111/2041-210x.13515 [DOI] [Google Scholar]
- Darwin, C. (1868). The variation of animals and plants under domestication. John Murray. [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. (2009). Origin, evolution and homologies of the Weberian apparatus: A new insight. International Journal of Morphology, 27(2), 333– 354. http://www.scielo.cl/scielo.php?script=sci_arttext&pid=S0717-95022009000200008 [Google Scholar]
- Drake, A. G., & Klingenberg, C. P. (2010). Large-scale diversification of skull shape in domestic dogs: Disparity and modularity. The American Naturalist, 175(3), 289–301. https://doi.org/ 10.1086/650372 [DOI] [PubMed] [Google Scholar]
- Friedman, S. T., Price, S. A., & Wainwright, P. C. (2021). The effect of locomotion mode on body shape evolution in teleost fishes. Integrative Organismal Biology (Oxford, England), 3(1), obab016. https://doi.org/ 10.1093/iob/obab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasauer, S. M., & Neuhauss, S. C. (2014). Whole-genome duplication in teleost fishes and its evolutionary consequences. Molecular Genetics and Genomics : MGG, 289(6), 1045–1060. https://doi.org/ 10.1007/s00438-014-0889-2 [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel, S. (2017). Numbers of neurons as biological correlates of cognitive capability. Current Opinion in Behavioral Sciences, 16, 1–7. https://doi.org/ 10.1016/j.cobeha.2017.02.004 [DOI] [Google Scholar]
- Hernandez, L. P., & Cohen, K. E. (2019). The role of developmental integration and historical contingency in the origin and evolution of cypriniform trophic novelties. Integrative and Comparative Biology, 59(2), 473–488. https://doi.org/ 10.1093/icb/icz056 [DOI] [PubMed] [Google Scholar]
- Hopkins, M. J., & Gerber, S. (2017). Morphological disparity. In Nuno de la Rosa L., & Müller G. (Eds.), Evolutionary developmental biology. Springer. https://doi.org/ 10.1007/978-3-319-33038-9_132-1 [DOI] [Google Scholar]
- Kemp, D. J., Batistic, F. K., & Reznick, D. N. (2018). Predictable adaptive trajectories of sexual coloration in the wild: Evidence from replicate experimental guppy populations. Evolution, 72(11), 2462–2477. https://doi.org/ 10.1111/evo.13564 [DOI] [PubMed] [Google Scholar]
- Kon, T., Omori, Y., Fukuta, K., Wada, H., Watanabe, M., Chen, Z., Iwasaki, M., Mishina, T., Matsuzaki, S. -I. S., Yoshihara, D., Arakawa, J., Kawakami, K., Toyoda, A., Burgess, S. M., Noguchi, H., & Furukawa, T. (2020). The genetic basis of morphological diversity in domesticated goldfish. Current Biology: CB, 30(12), 2260–2274.e6. https://doi.org/ 10.1016/j.cub.2020.04.034 [DOI] [PubMed] [Google Scholar]
- Lechner, W., & Ladich, F. (2008). Size matters: Diversity in swimbladders and Weberian ossicles affects hearing in catfishes. The Journal of Experimental Biology, 211(Pt 10), 1681–1689. https://doi.org/ 10.1242/jeb.016436 [DOI] [PubMed] [Google Scholar]
- Li, I. J., Lee, S. H., Abe, G., & Ota, K. G. (2019). Embryonic and postembryonic development of the ornamental twin‐tail goldfish. Developmental Dynamics, 248(4), 251–283. https://doi.org/ 10.1002/dvdy.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, K. A., Larson, G., Coppinger, R. P., & Karlsson, E. K. (2020). The history of farm foxes undermines the animal domestication syndrome. Trends in Ecology & Evolution, 35(2), 125–136. https://doi.org/ 10.1016/j.tree.2019.10.011 [DOI] [PubMed] [Google Scholar]
- Lösel, P. D., van de Kamp, T., Jayme, A., Ershov, A., Faragó, T., Pichler, O., Tan Jerome, N., Aadepu, N., Bremer, S., Chilingaryan, S. A., Heethoff, M., Kopmann, A., Odar, J., Schmelzle, S., Zuber, M., Wittbrodt, J., Baumbach, T., & Heuveline, V. (2020). Introducing Biomedisa as an open-source online platform for biomedical image segmentation. Nature Communications, 11(1), 5577. https://doi.org/ 10.1038/s41467-020-19303-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhounová, L., Kotrschal, A., Kverková, K., Kolm, N., & Němec, P. (2019). Artificial selection on brain size leads to matching changes in overall number of neurons. Evolution, 73(9), 2003–2012. https://doi.org/ 10.1111/evo.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai, H., Takatsuji, K., & Sato, Y. (1983). Morphological variability of the brains under domestication from the crucian carp to the goldfish. Journal of Zoological Systematics and Evolutionary Research, 20(2), 112–118. https://doi.org/ 10.1111/j.1439-0469.1983.tb00256.x [DOI] [Google Scholar]
- Matsumura, M., Ohkuma, M., & Honda, Y. (1982). Retinal degeneration in celestial goldfish: developmental study. Ophthalmic Research, 14(5), 344–353. https://doi.org/ 10.1159/000265212 [DOI] [PubMed] [Google Scholar]
- Milla, S., Pasquet, A., El Mohajer, L., & Fontaine, P. (2021). How domestication alters fish phenotypes. Reviews in Aquaculture, 13(1), 388–405. https://doi.org/ 10.1111/raq.12480 [DOI] [Google Scholar]
- Mitteroecker, P., & Huttegger, S. M. (2009). The concept of morphospaces in evolutionary and developmental biology: Mathematics and metaphors. Biological Theory, 4(1), 54–67. https://doi.org/ 10.1162/biot.2009.4.1.54 [DOI] [Google Scholar]
- Ohno, S., Muramoto, J., Christian, L., & Atkin, N. B. (1967). Diploid-tetraploid relationship among old-world members of the fish family Cyprinidae. Chromosoma, 23(1), 1–9. https://doi.org/ 10.1007/bf00293307 [DOI] [Google Scholar]
- Omori, Y., & Kon, T. (2019). Goldfish: An old and new model system to study vertebrate development, evolution and human disease. Journal of Biochemistry, 165(3), 209–218. https://doi.org/ 10.1093/jb/mvy076 [DOI] [PubMed] [Google Scholar]
- Ota, K. G. (2021). Goldfish development and evolution. Springer. https://doi.org/ 10.1007/978-981-16-0850-6 [DOI] [Google Scholar]
- Ota, K. G., & Abe, G. (2016). Goldfish morphology as a model for evolutionary developmental biology. Wiley Interdisciplinary Reviews: Developmental Biology, 5(3), 272–295. https://doi.org/ 10.1002/wdev.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelster, B. (2017). Swimbladder function and buoyancy control in fishes. Reference Module in Life Science, Elsevier. https://doi.org/ 10.1016/B978-0-12-809633-8.03063-6 [DOI] [Google Scholar]
- Podlesnykh, A. V., Brykov, V. A., & Skurikhina, L. A. (2015). Polyphyletic origin of ornamental goldfish. Food and Nutrition Sciences, 06(11), 1005–1013. https://doi.org/ 10.4236/fns.2015.611104 [DOI] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org [Google Scholar]
- Richardson, M. K. (2022). Theories, laws, and models in evo‐devo. Journal of Experimental Zoology. Part B. Molecular and Developmental Evolution, 338(1-2), 36–61. https://doi.org/ 10.1002/jez.b.23096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villagra, M. (2022). The process of animal domestication. Princeton University Press. [Google Scholar]
- Smartt, J. (2008). Goldfish varieties and genetics: A handbook for breeders. John Wiley & Sons. [Google Scholar]
- Stout, C. C., Tan, M., Lemmon, A. R., Lemmon, E. M., & Armbruster, J. W. (2016). Resolving Cypriniformes relationships using an anchored enrichment approach. BMC Evolutionary Biology, 16(1), 244–213. https://doi.org/ 10.1186/s12862-016-0819-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, K. L., Agnew, M. K., Chen, W. J., Hirt, M. V., Raley, M. E., Sado, T., Schneider, L. M., Yang, L., Bart, H. L., He, S., Liu, H., Miya, M., Saitoh, K., Simons, A. M., Wood, R. M., & Mayden, R. L. (2011). Phylogeny of the gudgeons (Teleostei: Cyprinidae: Gobioninae). Molecular Phylogenetics and Evolution, 61(1), 103–124. https://doi.org/ 10.1016/j.ympev.2011.05.022 [DOI] [PubMed] [Google Scholar]
- Tao, W., Yang, L., Mayden, R. L., & He, S. (2019). Phylogenetic relationships of Cypriniformes and plasticity of pharyngeal teeth in the adaptive radiation of cyprinids. Science China Life Sciences, 62(4), 553–565. https://doi.org/ 10.1007/s11427-019-9480-3 [DOI] [PubMed] [Google Scholar]
- Teng, C. S., Cavin, L., Maxson, R. E., Sánchez-Villagra, M. R., & Crump, J. G. (2019). Resolving homology in the face of shifting germ layer origins: Lessons from a major skull vault boundary. Elife, 8, e52814. https://doi.org/ 10.7554/eLife.52814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, D. B., Harmer, A. M., Giovanardi, S., Holvast, E. J., McGoverin, C. M., & Tenenhaus, A. (2023). Constructing a multiple‐part morphospace using a multiblock method. Methods in Ecology and Evolution, 14(1), 65–76. https://doi.org/ 10.1111/2041-210X.13781 [DOI] [Google Scholar]
- Van Valen, L. (1960). Nonadaptive aspects of evolution. The American Naturalist, 94(877), 305–308. https://doi.org/ 10.1086/282132 [DOI] [Google Scholar]
- Watson, J. M. (1939). The development of the Weberian ossicles and anterior vertebrae in the goldfish. Proceedings of the Royal Society of London. Series B-Biological Sciences, 127(849), 452–472. https://doi.org/ 10.1098/rspb.1939.0034 [DOI] [Google Scholar]
- Wright, D., Henriksen, R., & Johnsson, M. (2020). Defining the domestication syndrome: Comment on Lord et al. 2020. Trends in Ecology & Evolution, 35(12), 1059–1060. https://doi.org/ 10.1016/j.tree.2020.08.009 [DOI] [PubMed] [Google Scholar]
- Young, N. M., Linde-Medina, M., Fondon, J. W., Hallgrímsson, B., & Marcucio, R. S. (2017). Craniofacial diversification in the domestic pigeon and the evolution of the avian skull. Nature Ecology & Evolution, 1(4), 0095. https://doi.org/ 10.1038/s41559-017-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text, or the supplementary data. All original code and documents have been deposited at Zenodo and are publicly available as of the date of publication (https://doi.org/10.5281/zenodo.10403067). (DICE-)CT-scans, together with acquisition parameters, and the 3D models produced will be made available progressively on MorphoSource following publication of the present work in accordance with institutional policies (https://www.morphosource.org/projects/000602063/about?locale=en).