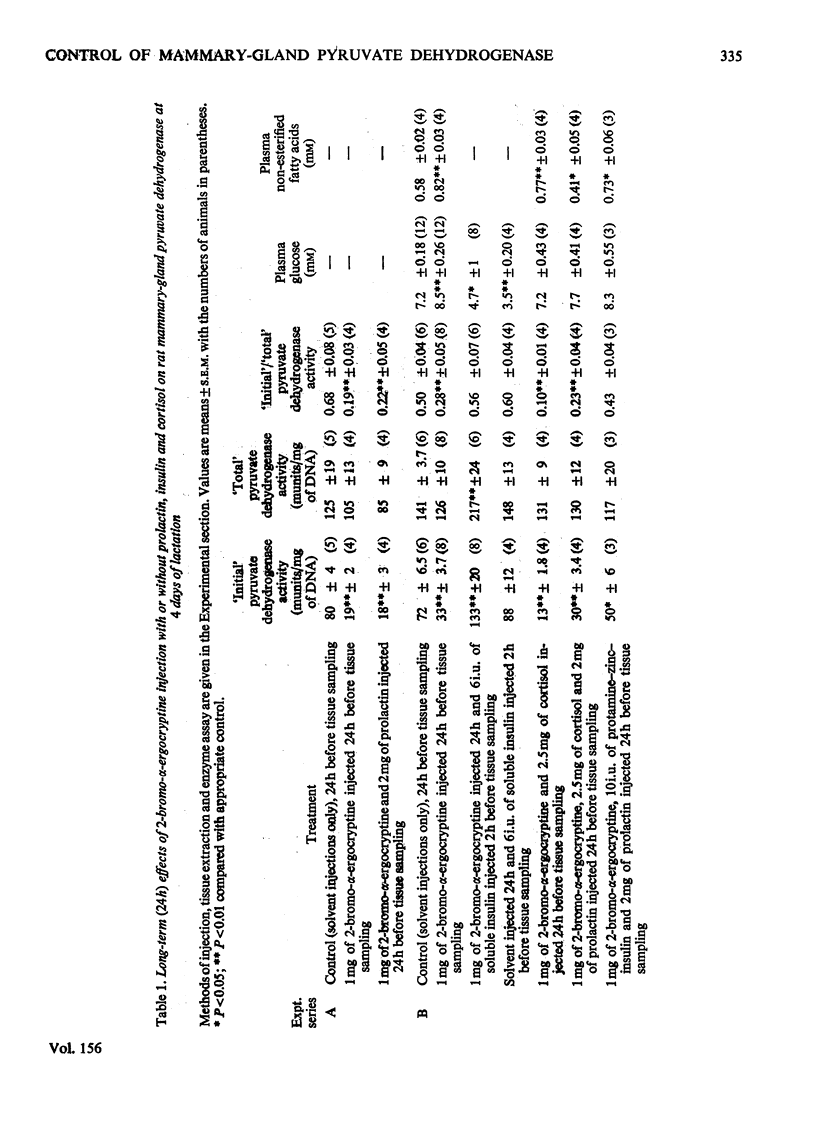
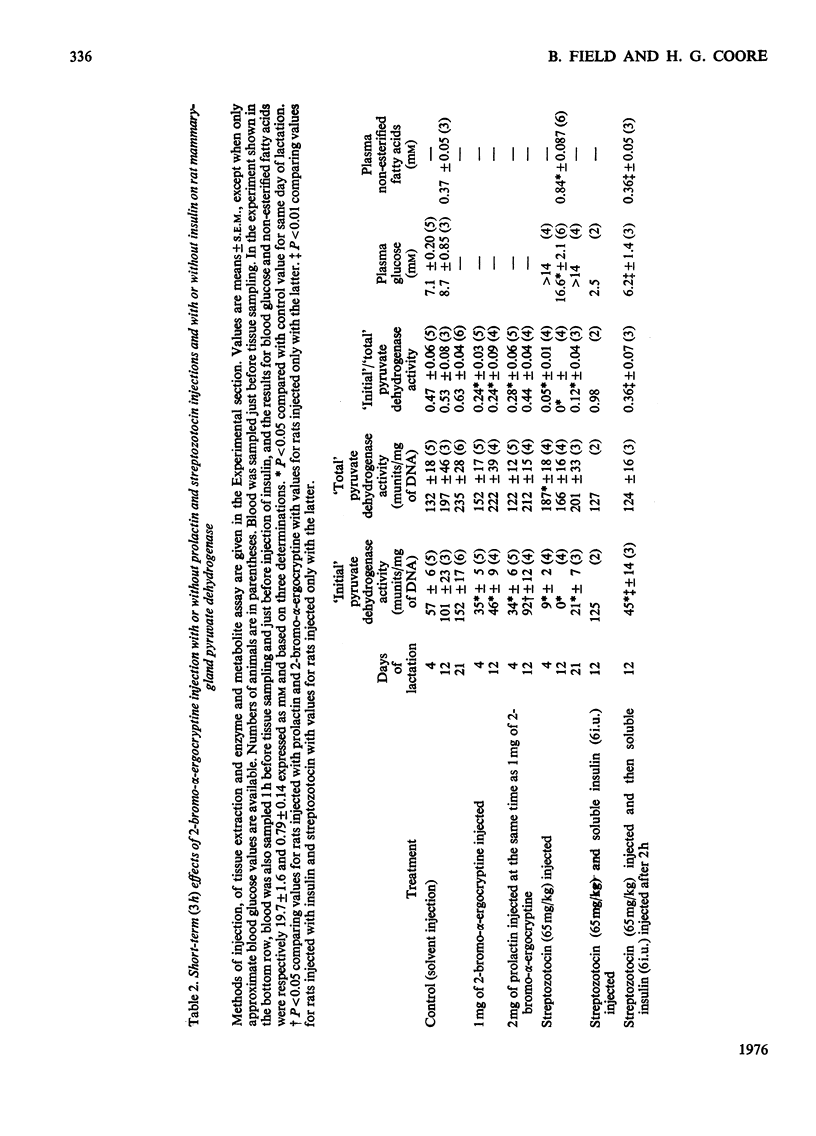
Abstract
Withdrawal of prolactin or of insulin from the circulation of lactating rats leads, within 3h, to increased inactivation by phosphorylation of mammary-gland pyruvate dehydrogenase. Prolactin may act by priming the tissue to respond directly to normal concentrations of circulating insulin and by this means be responsible for the increased activation of the enzyme during the course of normal lactation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenomori Y., Chen C. L., Meites J. Serum prolactin levels in rats during different reproductive states. Endocrinology. 1970 Mar;86(3):506–510. doi: 10.1210/endo-86-3-506. [DOI] [PubMed] [Google Scholar]
- Coore H. G., Denton R. M., Martin B. R., Randle P. J. Regulation of adipose tissue pyruvate dehydrogenase by insulin and other hormones. Biochem J. 1971 Nov;125(1):115–127. doi: 10.1042/bj1250115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coore H. G., Field B. Properties of pyruvate dehydrogenase of rat mammary tissue and its changes during pregnancy, lactation and weaning. Biochem J. 1974 Jul;142(1):87–95. doi: 10.1042/bj1420087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field B., Coore H. G. Effects of prolactin withdrawal on activity of pyruvate dehydrogenase of rat mammary gland. Biochem Soc Trans. 1975;3(2):258–261. doi: 10.1042/bst0030258. [DOI] [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hallowes R. C., Wang D. Y., Lewis D. J., Strong C. R., Dils R. The stimulation by prolactin and growth hormone of fatty acid synthesis in explants from rat mammary glands. J Endocrinol. 1973 May;57(2):265–276. doi: 10.1677/joe.0.0570265. [DOI] [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of insulin on isolated rat mammary cell metabolism: glucose utilization and metabolite patterns. Endocrinology. 1971 Nov;89(5):1263–1269. doi: 10.1210/endo-89-5-1263. [DOI] [PubMed] [Google Scholar]
- Mendelson C. R., Scow R. O. Uptake of chylomicron-triglyceride by perfused mammary tissue of lactating rats. Am J Physiol. 1972 Dec;223(6):1418–1423. doi: 10.1152/ajplegacy.1972.223.6.1418. [DOI] [PubMed] [Google Scholar]
- Simpson A. A., Simpson M. H., Sinha Y. N., Schmidt G. H. Changes in concentration of prolactin and adrenal corticosteroids in rat plasma during pregnancy and lactation. J Endocrinol. 1973 Sep;58(3):675–676. doi: 10.1677/joe.0.0580675. [DOI] [PubMed] [Google Scholar]
- Sutter-Dub M. T., Leclercq R., Sutter B. C., Jacquot R. Plasma glucose, progesterone and immunoreactive insulin levels in the lactating rat. Horm Metab Res. 1974 Jul;6(4):297–300. doi: 10.1055/s-0028-1093852. [DOI] [PubMed] [Google Scholar]


