Abstract
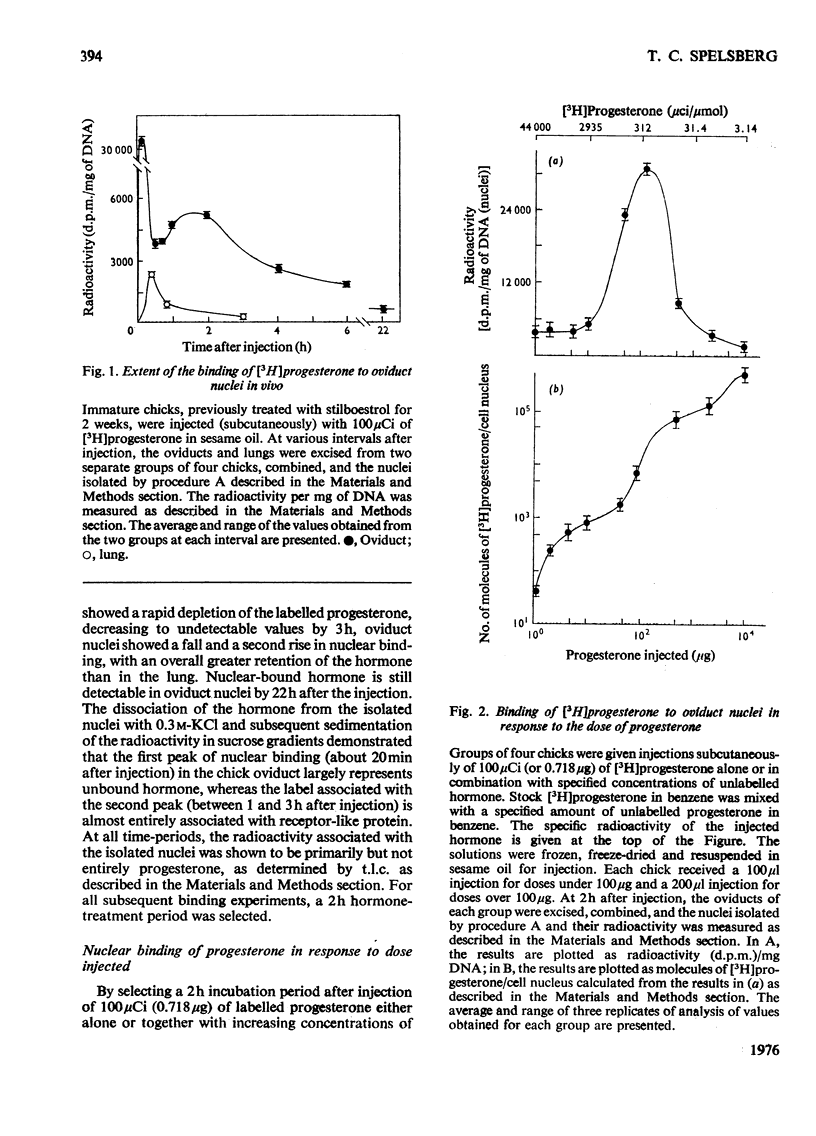
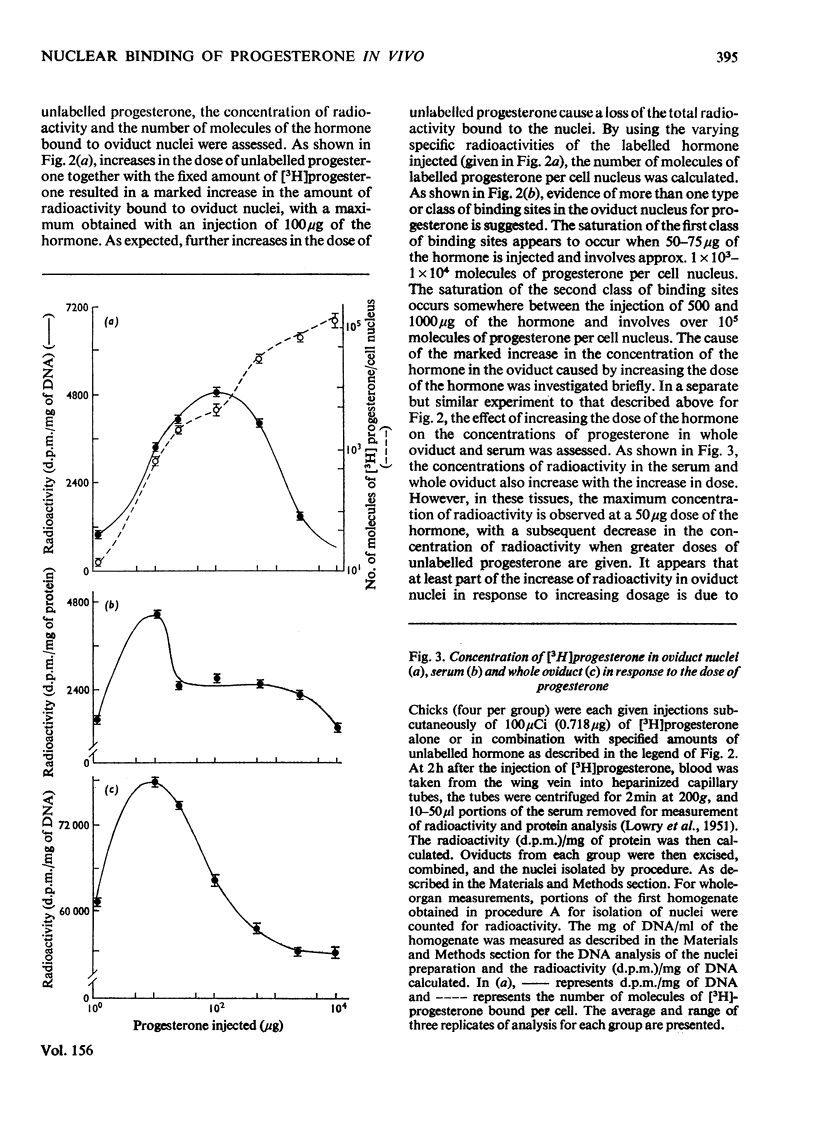
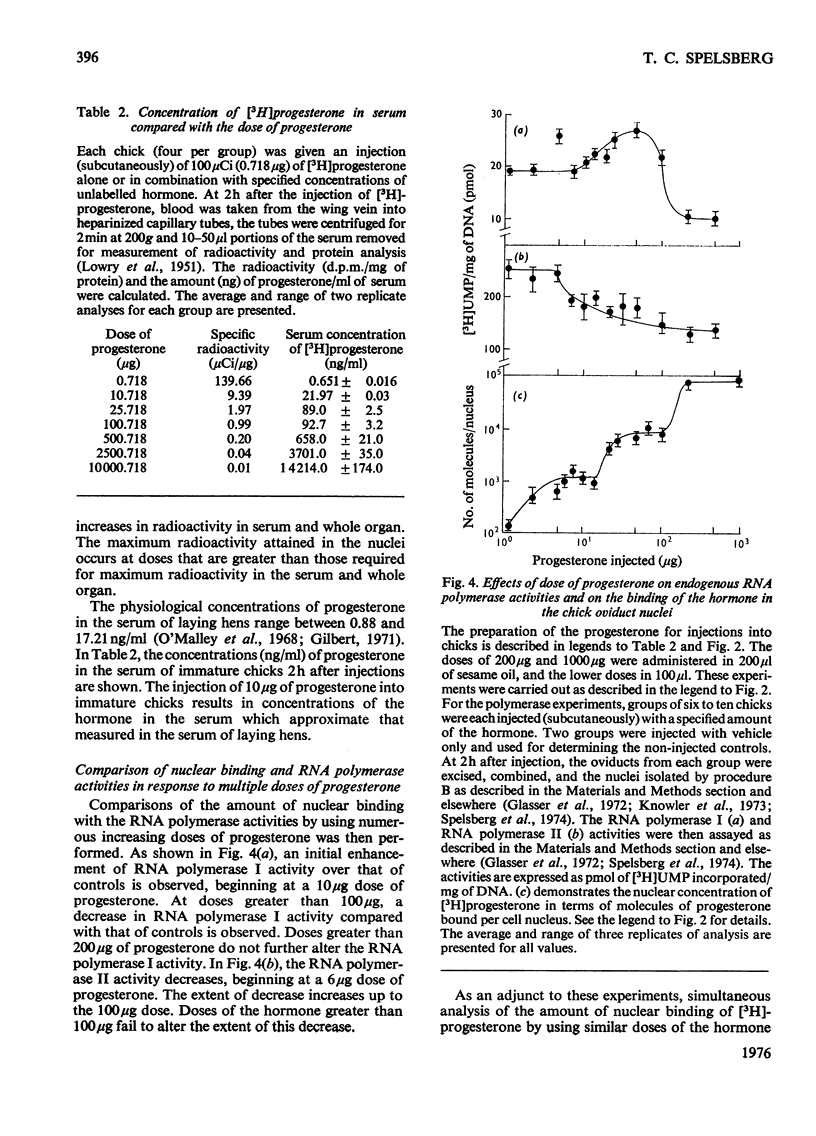
1. Varied doses of labelled or unlabelled progesterone were injected into immature chicks which had previously been stimulated with oestrogen. The concentrations of nuclear bound [3H]progesterone were correlated with the effects of the hormone on endogenous RNA polymerase I and II activities in isolated oviduct nuclei. 2. The extent of nuclear localization of [3H]progesterone in oviduct (a progesterone target tissue) was shown to be much greater than in lung (non-target tissue). The conccentration of bivalent cations in solvents used in the nuclei isolations has a marked effect on the amount of bound hormone in the nuclei. 3. Evidence for the existence of several classes of binding sites for progesterone in the oviduct nuclei is given. These classes represent about 1000) 10000 and 100000 molecules of the hormone per cell nucleus and are saturated by injecting approx. 10, 100 and 1000 mug of progesterone respectively. 4. When saturation of the first (highest affinity) class of nuclear sites occurs, a marked inhibition in RNA polymerase II (but not RNA polymerase I) activity was observed. When the second class of sites was saturated, alterations in both RNA polymerase I and II activities were observed. Binding to the third class of nuclear binding sites was not accompained by further changes in polymerase activity. It is suggested that the first two classes of nuclear binding sites may represent functional sites for progesterone action in the chick oviduct.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing R. J., Barbiroli B., Smellie R. M. The early effects of oestradiol-17-beta on ribonucleic acid synthesis in rat uterus. Biochem J. 1968 Oct;109(4):705–706. doi: 10.1042/bj1090705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick N. M., Smellie R. M. The effects of oestradiol-17beta on the ribonucleic acid polymerases of immature rabbit uterus. Biochem J. 1975 Apr;147(1):91–101. doi: 10.1042/bj1470091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. F., Sauerwein H. Studies on the mode of action of progesterone on chicken oviduct epithelium. I. Morphological changes associated with early differentiation of the tissue. Exp Cell Res. 1970 Jul;61(1):79–90. doi: 10.1016/0014-4827(70)90260-0. [DOI] [PubMed] [Google Scholar]
- Glasser S. R., Chytil F., Spelsberg T. C. Early effects of oestradiol-17 on the chromatin and activity of the deoxyribonucleic acid-dependent ribonucleic acid polymerases (I and II) of the rat uterus. Biochem J. 1972 Dec;130(4):947–957. doi: 10.1042/bj1300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser S. R., Spelsberg T. C. Mammalian RNA polymerases I and II: independent diurnal variations in activity. Biochem Biophys Res Commun. 1972 May 26;47(4):951–958. doi: 10.1016/0006-291x(72)90585-2. [DOI] [PubMed] [Google Scholar]
- Hamilton T. H., Widnell C. C., Tata J. R. Synthesis of ribonucleic acid during early estrogen action. J Biol Chem. 1968 Jan 25;243(2):408–417. [PubMed] [Google Scholar]
- Knowler J. T., Moses H. L., Spelsberg T. C. Comparison and characterization of nuclear isolation procedures as applied to chick oviduct. J Cell Biol. 1973 Dec;59(3):685–695. doi: 10.1083/jcb.59.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler J. T., Smellie R. M. The synthesis of ribonucleic acid in immature rat uterus responding to oestradiol-17 beta. Biochem J. 1971 Nov;125(2):605–614. doi: 10.1042/bj1250605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. O., Grimley P. M., O'Malley B. W. Estrogen-induced cytodifferentiation of the ovalbumin-secreting glands of the chick oviduct. J Cell Biol. 1969 Jan;40(1):8–27. doi: 10.1083/jcb.40.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASON R. C. Synergistic and antagonistic effects of progesterone in combination with estrogens on oviduct weight. Endocrinology. 1952 Dec;51(6):570–572. doi: 10.1210/endo-51-6-570. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Kirschner M. A., Bardin C. W. Estimation of plasma androgenic and progestational steroids in the laying hen. Proc Soc Exp Biol Med. 1968 Feb;127(2):521–523. doi: 10.3181/00379727-127-32730. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., McGuire W. L., Kohler P. O., Korenman S. G. Studies on the mechanism of steroid hormone regulation of synthesis of specific proteins. Recent Prog Horm Res. 1969;25:105–160. doi: 10.1016/b978-0-12-571125-8.50006-5. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Rosenfeld G. C., Comstock J. P., Means A. R. Steroid hormone induction of a specific translatable messenger RNA. Nat New Biol. 1972 Nov 8;240(97):45–48. doi: 10.1038/newbio240045a0. [DOI] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. I. Antagonistic effect of progesterone on estrogen-induced proliferation and differentiation of tubular gland cells. J Cell Biol. 1969 Jun;41(3):816–831. doi: 10.1083/jcb.41.3.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Schimke R. T. Interaction of estrogen and progesterone in chick oviduct development. II. Effects of estrogen and progesterone on tubular gland cell function. J Cell Biol. 1969 Oct;43(1):123–137. doi: 10.1083/jcb.43.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Palmiter R. D., Smith L. T. Synergistic effects of oesterogen and progesterone on ovomucoid and conalbumin mRNA synthesis in chick oviduct. Nat New Biol. 1973 Nov 21;246(151):74–76. doi: 10.1038/newbio246074a0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Wrenn J. T. Interaction of estrogen and progesterone in chick oviduct development. 3. Tubular gland cell cytodifferentiation. J Cell Biol. 1971 Sep;50(3):598–615. doi: 10.1083/jcb.50.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple ribonucleic acid polymerases and ribonucleic acid synthesis during sea urchin development. Biochemistry. 1970 Jun 9;9(12):2543–2553. doi: 10.1021/bi00814a023. [DOI] [PubMed] [Google Scholar]
- Rosenfeld G. C., Comstock J. P., Means A. R., O'Malley B. W. Estrogen-induced synthesis of ovalbumin messenger RNA and its translation in a cell-free system. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1695–1703. doi: 10.1016/0006-291x(72)90805-4. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Knowler J. T., Moses H. L. Specific methods for the isolation of nuclei from chick oviduct. Methods Enzymol. 1974;31:263–279. doi: 10.1016/0076-6879(74)31028-2. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Steggles A. W., O'Malley B. W. Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem. 1971 Jul 10;246(13):4188–4197. [PubMed] [Google Scholar]
- Teng C. S., Hamilton T. H. The role of chromatin in estrogen action in the uterus, I. The control of template capacity and chemical composition and the binding of H3-estradiol-17 beta. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1410–1417. doi: 10.1073/pnas.60.4.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachewsky D., Segal S. J. Differential synthesis of ribonucleic acid in uterine nuclei: evidence for selective gene transcription induced by estrogens. Eur J Biochem. 1968 Apr;4(3):279–285. doi: 10.1111/j.1432-1033.1968.tb00206.x. [DOI] [PubMed] [Google Scholar]
- WILSON J. D. The nature of the RNA response to estradiol administration by the uterus of the rat. Proc Natl Acad Sci U S A. 1963 Jul;50:93–100. doi: 10.1073/pnas.50.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]


