Abstract
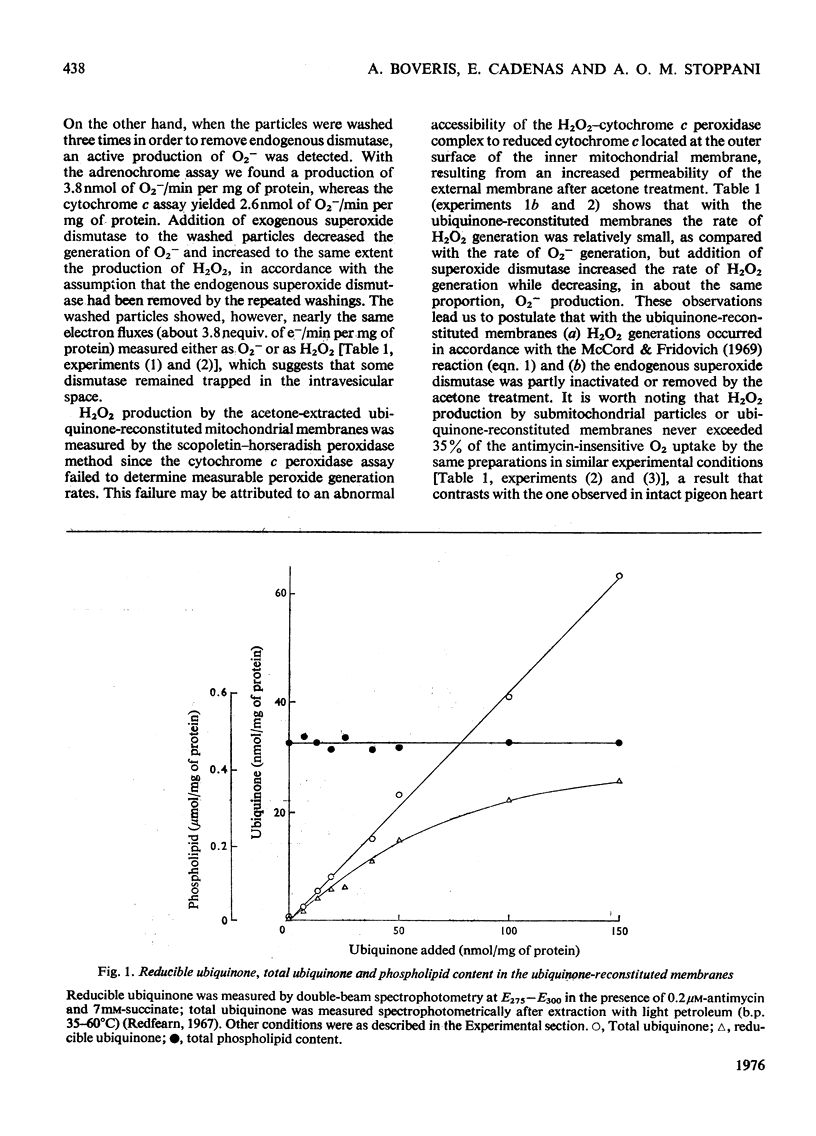
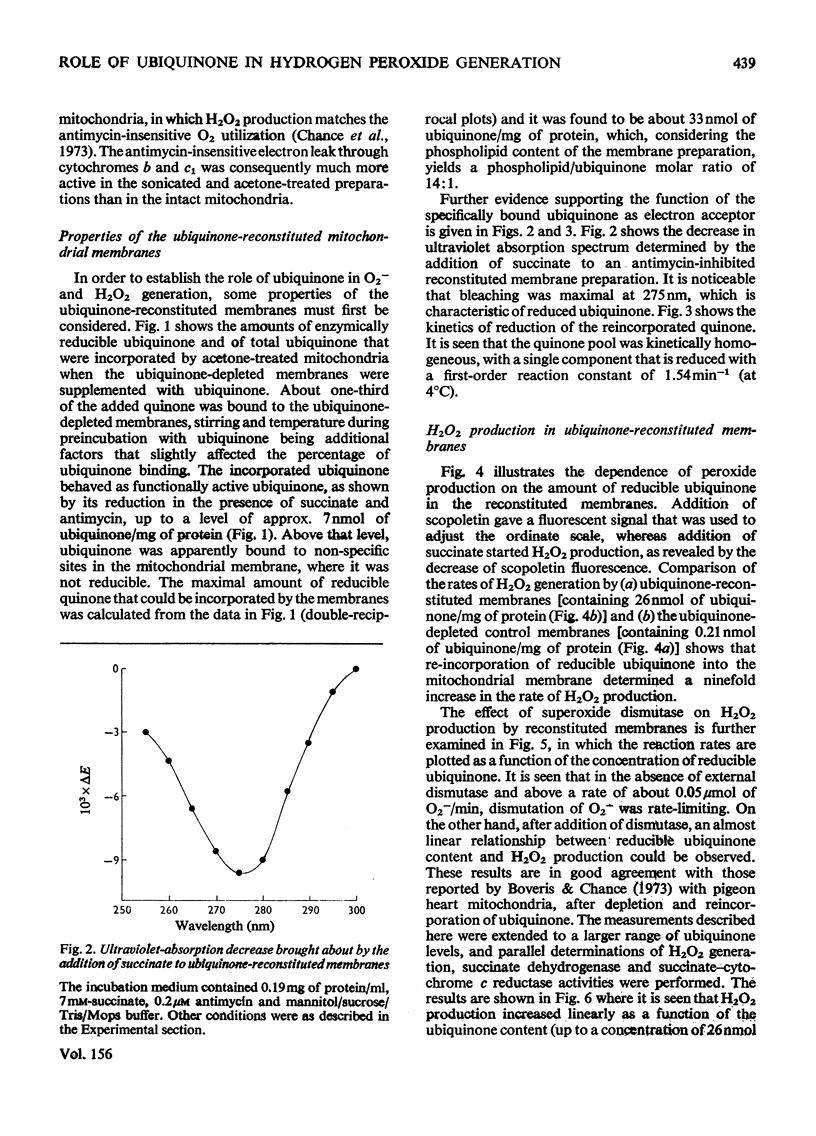
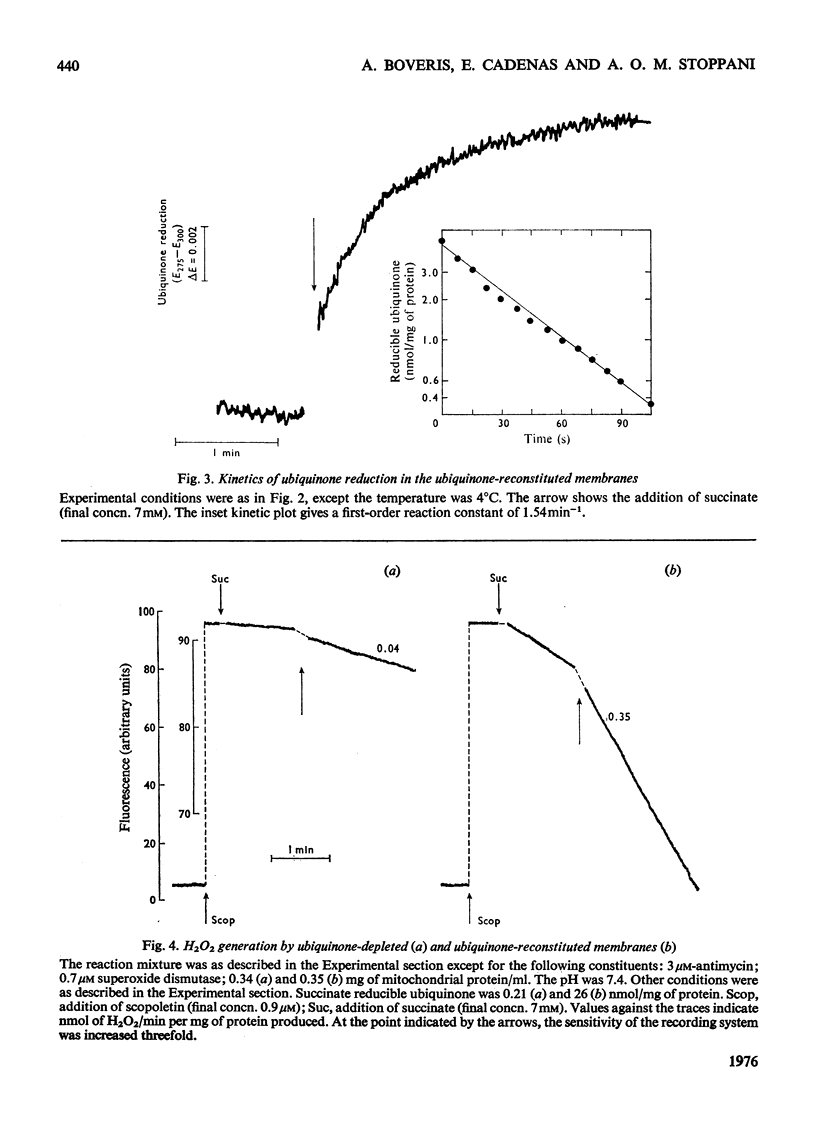
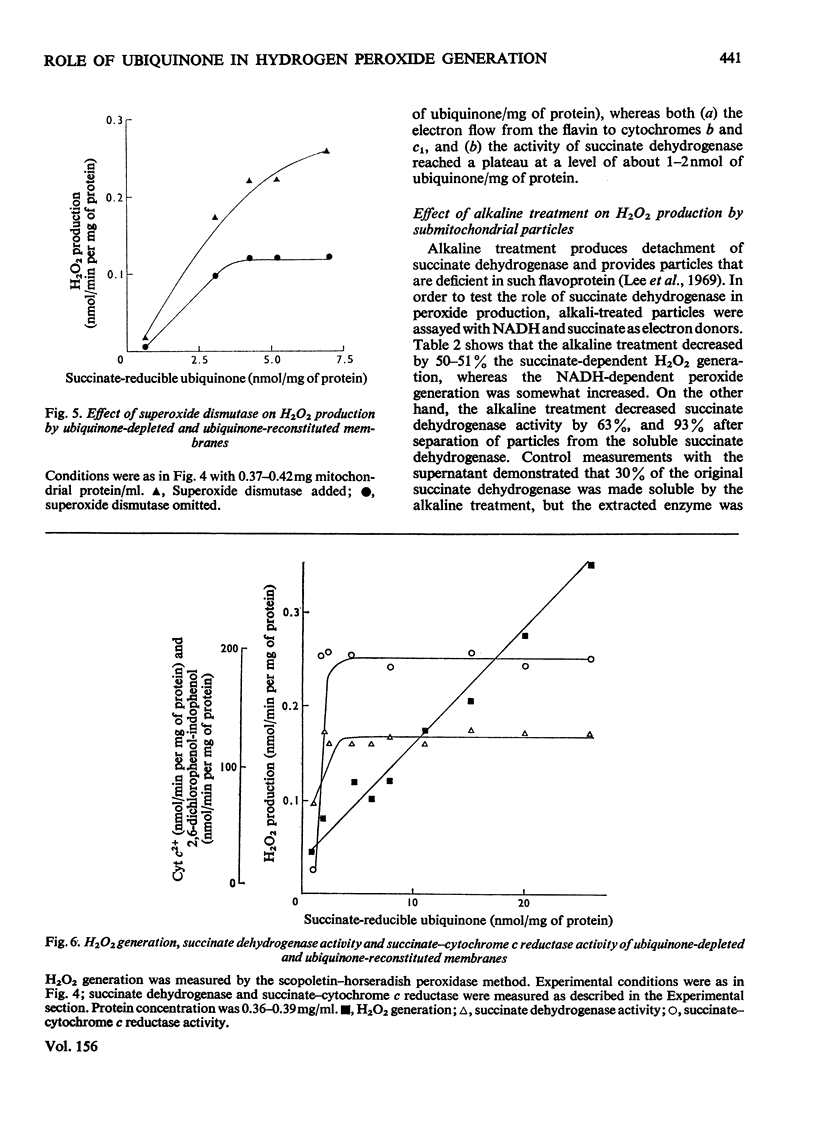
Antimycin-inhibited bovine heart submitochondrial particles generate O2- and H2O2 with succinate as electron donor. H2O2 generation involves the action of the mitochondrial superoxide dismutase, in accordance with the McCord & Fridovich [(1969) j. biol. Chem. 244, 6049-6055] reaction mechanism. Removal of ubiquinone by acetone treatment decreases the ability of mitochondrial preparations to generate O2- and H2O2, whereas supplementation of the depleted membranes with ubiquinone enhances the peroxide-generating activity in the reconstituted membranes. Addition of superoxide dismutase to ubiquinone-reconstituted membranes is essential in order to obtain maximal rates of H2O2 generation since the acetone treatment of the membranes apparently inactivates (or removes) the mitochondrial superoxide dismutase. Parallel measurements of H2O2 production, succinate dehydrogenase and succinate-cytochrome c reductase activities show that peroxide generation by ubiquinone-supplemented membranes is a monotonous function of the reducible ubiquinone content, whereas the other two measured activities reach saturation at relatively low concentrations of reducible quinone. Alkaline treatment of submitochondrial particles causes a significant decrease in succinate dehydrogenase activity and succinate-dependent H2O2 production, which contrasts with the increase of peroxide production by the same particles with NADH as electron donor. Solubilized succinate dehydrogenase generates H2O2 at a much lower rate than the parent submitochondrial particles. It is postulated that ubisemiquinone (and ubiquinol) are chiefly responsible for the succinate-dependent peroxide production by the mitochondrial inner membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREAE W. A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature. 1955 May 14;175(4463):859–860. doi: 10.1038/175859a0. [DOI] [PubMed] [Google Scholar]
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Boveris A., Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975 Jul 1;54(3):311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973 Jul;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Erecińska M., Wagner M. Reduction kinetics of cytochromes b. Biochim Biophys Acta. 1972 Feb 28;256(2):223–242. doi: 10.1016/0005-2728(72)90055-2. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- DOIZAKI W. M., ZIEVE L. Quantiative estimation of some phosphatides and their hydrolysis products by thin layer chromatography. Proc Soc Exp Biol Med. 1963 May;113:91–94. doi: 10.3181/00379727-113-28285. [DOI] [PubMed] [Google Scholar]
- Dionisi O., Galeotti T., Terranova T., Azzi A. Kinetics of redox changes of nicotinamide-adenine dinucleotides in Ehrlich ascites tumour cells. FEBS Lett. 1973 Feb 15;30(1):84–88. doi: 10.1016/0014-5793(73)80624-6. [DOI] [PubMed] [Google Scholar]
- Ernster L., Lee I. Y., Norling B., Persson B. Studies with ubiquinone-depleted submitochondrial particles. Essentiality of ubiquinone for the interaction of succinate dehydrogenase, NADH dehydrogenase, and cytochrome b. Eur J Biochem. 1969 Jun;9(3):299–310. doi: 10.1111/j.1432-1033.1969.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Kennedy J. A. Role of superoxide radical in mitochondrial dehydrogenase reactions. Biochem Biophys Res Commun. 1974 Oct 8;60(3):1044–1050. doi: 10.1016/0006-291x(74)90418-5. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Kennedy J. Superoxide production and electron transport in mitochondrial oxidation of dihydroorotic acid. J Biol Chem. 1975 Jun 10;250(11):4322–4326. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Gutman M., Kearney E. B., Singer T. P. Control of succinate dehydrogenase in mitochondria. Biochemistry. 1971 Dec 7;10(25):4763–4770. doi: 10.1021/bi00801a025. [DOI] [PubMed] [Google Scholar]
- LESTER R. L., FLEISCHER S. Studies on the electron-transport system. 27. The respiratory activity of acetoneextracted beef-heart mitochondria: role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Feb 18;47:358–377. doi: 10.1016/0006-3002(61)90297-9. [DOI] [PubMed] [Google Scholar]
- Lee C., Johansson B., King T. E. Reconstitution of respiratory control of succinate oxidation in submitochondrial particles. Biochem Biophys Res Commun. 1969 Apr 29;35(2):243–248. doi: 10.1016/0006-291x(69)90274-5. [DOI] [PubMed] [Google Scholar]
- Loschen G., Azzi A., Richter C., Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974 May 15;42(1):68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The generation of superoixide radical during the autoxidation of ferredoxins. J Biol Chem. 1971 Nov 25;246(22):6886–6890. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972 May 25;247(10):3170–3175. [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Peeters-Joris C., Vandevoorde A. M., Baudhuin P. Subcellular localization of superoxide dismutase in rat liver. Biochem J. 1975 Jul;150(1):31–39. doi: 10.1042/bj1500031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E., Norling B., Persson B., Ernster L. Studies with ubiquinone-depleted submitochondrial particles. Effects of extraction and reincorporation of ubiquinone on the kinetics of succinate dehydrogenase. Eur J Biochem. 1970 Nov;16(3):508–513. doi: 10.1111/j.1432-1033.1970.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Iyanagi T., Yamazaki I. Relation between redox potentials and rate constants in reactions coupled with the system oxygen-superoxide. Biochemistry. 1975 Aug 26;14(17):3761–3764. doi: 10.1021/bi00688a007. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J. 1975 Jun;147(3):493–504. doi: 10.1042/bj1470493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973 Jul 10;248(13):4793–4796. [PubMed] [Google Scholar]
- Weisiger R. A., Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973 May 25;248(10):3582–3592. [PubMed] [Google Scholar]


