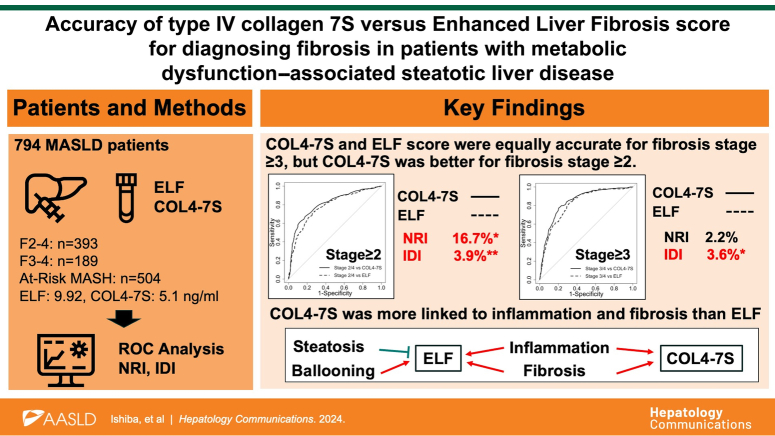
Abstract
Background:
Various noninvasive tests can be used to identify high-risk groups of patients with metabolic dysfunction–associated steatotic liver disease/steatohepatitis (MASLD). In this study, we compared the diagnostic performance of serum type 4 collagen 7S (COL4-7S) and the Enhanced Liver Fibrosis (ELF) score for detecting fibrosis in patients with MASLD.
Methods:
Among 1368 patients with MASLD who underwent liver biopsy, 794 with values for both serum COL4-7S and the ELF score were enrolled in this multicenter study. The diagnostic performance of COL4-7S and ELF for detecting fibrosis stage ≥2, fibrosis stage ≥3, and at-risk metabolic dysfunction–associated steatohepatitis were evaluated using ROC curve, continuous net reclassification improvement, and integrated discrimination improvement analyses.
Results:
Both COL4-7S and ELF scores increased significantly with increasing fibrosis. The AUROC for each outcome was higher for COL4-7S than ELF, but not significantly. The diagnostic performance for detecting fibrosis stage ≥2 was significantly better for COL4-7S than for the ELF score (s net reclassification improvement=16.7%, p=0.018; integrated discrimination improvement=3.9%, p<0.01). In patients without diabetes, the diagnostic performance for each outcome did not differ significantly between COL4-7S and ELF score, but in patients with diabetes, the diagnostic performance for fibrosis stage ≥2 was higher for COL4-7S than for the ELF score (AUROC=0.817 vs. 0.773, p=0.04; s net reclassification improvement=32.7%, p<0.01; integrated discrimination improvement=5.6%, p<0.01).
Conclusions:
The diagnostic performance of serum COL4-7S (a single marker) for identifying more advanced disease in patients with MASLD was at least equivalent to that of the ELF score (a combined marker).
Keywords: at-risk MASH, ELF test, liver fibrosis, metabolic dysfunction–associated steatotic liver disease/steatohepatitis, type IV collagen 7S
INTRODUCTION
The prognosis of metabolic dysfunction–associated steatotic liver disease (MASLD) worsens as liver fibrosis progresses.1 Although liver biopsy is the gold standard for quantifying hepatic fibrosis, the clinical efficacy of various noninvasive tests, such as the fibrosis-4 index, has been studied.2 Current evidence suggests that fibrosis-4 index has good performance for not only diagnosing advanced liver fibrosis but also for predicting the future occurrence of liver-related events and HCC.3
Other surrogate fibrosis markers, such as the Enhanced Liver Fibrosis (ELF) score and type IV collagen 7S (COL4-7S), can also accurately diagnose liver fibrosis.4,5 These markers focus on products of extracellular matrix turnover. Extensive dynamic extracellular matrix remodeling involving both the interstitial and basement membrane matrices occurs during fibrogenesis. Proteolytic fragments of different collagen subtypes are released during fibrogenesis and/or fibrinolysis and can be used as noninvasive biomarkers.6
The ELF score is based on 3 serum fibrosis markers: hyaluronic acid (HA), TIMP-1, and N-terminal peptide of procollagen III.7 Sharma et al8 reported that the ELF score showed good diagnostic accuracy for advanced fibrosis and cirrhosis in several cohorts of patients with chronic liver disease. The AUROCs were >0.80. Furthermore, the ELF score is recommended as a noninvasive test in guidelines published by the European Association for the Study of the Liver9 and in the practice guidance for NAFLD published by the American Association for the Study of Liver Diseases.10
COL4-7S is a fragment of type IV collagen, which is the most abundant structural component of the basement membrane. COL4-7S is a well-established biochemical marker of liver fibrosis11,12 and is stable in blood because of its relative resistance to proteolytic enzymes. Serum COL4-7S levels are increased during the progression of liver fibrosis in various types of chronic hepatitis, including metabolic dysfunction–associated steatohepatitis (MASH, previously known as NASH).13 COL4-7S has been used to study fibrogenic activity in both animal models11 and humans.3 It has been covered by insurance in Japan since 1989 and has become one of the most useful liver fibrosis markers at our institution.14 We previously reported the effectiveness of COL4-7S for diagnosing fibrosis in MASLD.5 Importantly, the effectiveness of CO4-7S for diagnosing advanced liver fibrosis was not affected by the presence of type 2 diabetes mellitus (T2DM) in patients with MASLD (the AUROC was 0.883 in patients without T2DM and 0.872 in those with T2DM). The diagnostic accuracy of the ELF score for liver fibrosis is also unaffected by the presence or absence of T2DM.15
The ELF score is widely used as a fibrosis marker for MASLD in the West, while the effectiveness of COL4-7S has been rarely studied outside Japan despite it being one of the oldest fibrosis markers.16 This study aimed to compare the diagnostic performance of COL4-7S and ELF for liver fibrosis and at-risk MASH (MASH at increased risk of disease progression).
METHODS
Patients
Among 1368 patients with biopsy-confirmed MASLD, 794 with values for both serum COL4-7S and the ELF score were enrolled in this multicenter study. This is a retrospective, observational, cross-sectional study comparing the diagnostic accuracy for fibrosis between ELF and COL4-7S. The patients were enrolled from January 1990 to February 2020 at 6 centers in Japan: Saga University, Kyoto Prefectural University of Medicine, Yokohama City University, Saiseikai Suita Hospital, Ogaki Municipal Hospital, and Gifu Municipal Hospital. The exclusion criteria were daily consumption of alcohol-associated beverages; the presence of other liver diseases (including viral hepatitis, autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, alpha-1-antitrypsin deficiency–associated liver disease, Wilson disease, and drug-induced liver disease); and evidence of HCC, biliary tract cancer, or pancreatic cancer. This study was conducted in accordance with the Declaration of Helsinki and Istanbul approved by the institutional review board at each participating institution. Participants were enrolled using an opt-out consent process.
Laboratory and clinical parameters
Physical characteristics, medical history, lifestyle habits, and clinical laboratory data were collected for all patients. Blood samples were analyzed using standard techniques in the clinical laboratory at each institution. T2DM, hypertension, and dyslipidemia were diagnosed according to standard criteria.17,18,19,20 T2DM was diagnosed in accordance with the following: physician diagnosis of T2DM, documented use of oral hypoglycemic medication and a random glucose level ≥200 mg/dL, or a fasting plasma glucose level ≥126 mg/dL and hemoglobin A1c ≥6.5%.17 Blood samples were collected 1 day before and after the liver biopsy was performed, and then the blood tests were performed. ELF was measured using serum stored at that time.
The serum COL4-7S level and the ELF score were determined in all patients. COL4-7S levels were measured using double-antibody radioimmunoassay. The ELF score was calculated using values from serum samples obtained with the patient’s consent when the liver biopsy was performed. After measuring serum levels of HA, PIIP, and TIMP-1 (Siemens Health Care Diagnostics Inc., Tokyo, Japan), the ELF score was calculated as follows: 2.278 + 0.851 ln [HA] + 0.751 ln [peptide of procollagen III] + 0.394 ln [TIMP-1].
Liver histology
All patients underwent percutaneous liver biopsy with ultrasonic guidance. Liver specimens were read and scored at a central location (Kyushu University) by a single, experienced pathologist (Shinichi Aishima) who was blinded to the clinical and laboratory data. A 16G needle was used for liver biopsy; specimens measuring 1.5–2 cm with enough evaluable portal tracts were considered eligible. The Kleiner scoring system was used for histologic assessment of MASLD, including the determination of the amount of steatosis (grades 0–3), lobular inflammation (grades 0–3), and hepatocellular ballooning (grades 0–2), as well as the stage of fibrosis (stages 0–4).21,22 MASH was diagnosed according to the fatty liver inhibition of progression algorithm.23 At-risk MASH was defined as MASH with an NAFLD activity score ≥4 or a fibrosis stage ≥2.
Statistical analysis
ROC analysis, continuous net reclassification improvement (cNRI) analysis, and IDI analysis were used to assess the prognosis accuracy of COL4-7S and ELF for fibrosis stage ≥3, fibrosis stage ≥2, and at-risk MASH.24 These analyses were repeated in subgroups of patients with or without T2DM to determine whether the presence of T2DM affected the diagnostic accuracy of COL4-7S or the ELF score for fibrosis stage ≥3, fibrosis stage ≥2, or at-risk MASH. The Jonckheere-Terpstra trend test was used to assess the association between each histologic feature of MASLD (steatosis, inflammation, ballooning, and fibrosis) and COL4-7S or ELF.
We standardized the values for COL4-7S, the ELF score, HA, peptide of procollagen III, and TIMP-1 (mean=0 and SDs=±1) to assess the relative impact of each histologic features at the same level when performing the regression analysis. We then performed multiple regression analysis to calculate regression coefficients and ordinal logistic regression analysis to estimate ORs for each histologic feature. All statistical analyses were performed using R, version 4.2.2. Nominal two-sided p-values <0.05 were considered statistically significant.
RESULTS
Patient characteristics
Characteristics of the enrolled patients are shown in Table 1. A total of 794 patients were included in the study, 348 (43.8%) of whom were male. The median patient age was 59 years. A total of 393 patients (49.5%) had fibrosis stage ≥2, 189 patients (23.8%) had fibrosis stage ≥3, and 504 patients (63.5%) met the criteria for at-risk MASH. Twenty-five cases of grade 0 included liver fibrosis, and 4 cases were burned-out MASH. The median serum COL4-7S was 5.1 ng/mL, and the median ELF score was 9.92.
TABLE 1.
Patient characteristics
| Characteristic | Total (n=794) | Non-DM (n=396) | DM (n=398) | p |
|---|---|---|---|---|
| Age (y) | 59 [17, 85] | 57 [17, 85] | 61 [19, 84] | <0.001 |
| Male sex | 348 (43.8) | 182 (46.0) | 166 (41.7) | 0.256 |
| BMI (kg/m2) | 27.9 [15.4, 62.8] | 27.4 [15.4, 62.8] | 28.6 [16.8, 53.2] | <0.001 |
| Total protein (g/dL) | 7.2 [4.4, 9.6] | 7.2 [4.4, 8.4] | 7.2 [4.9, 9.6] | 0.7 |
| Albumin (g/dL) | 4.2 [2.5, 6.0] | 4.3 [2.8, 6.0] | 4.2 [2.5, 5.2] | 0.003 |
| Platelet counts (108/μL) | 19.8 [4.4, 63.7] | 20.2 [6.0, 44.5] | 19.4 [4.4, 63.7] | 0.026 |
| AST (IU/L) | 46 [11, 608] | 46 [11, 251] | 47 [13, 608] | 0.824 |
| ALT (IU/L) | 60 [2, 401] | 66 [2, 401] | 57 [10, 323] | 0.005 |
| GGT (IU/L) | 59 [11, 1070] | 55 [11, 1070] | 62 [11, 566] | 0.96 |
| Total cholesterol (mg/dL) | 192 [68, 423] | 196 [68, 347] | 187 [77, 423] | 0.139 |
| LDL-C (mg/dL) | 121 [17, 259] | 125 [34, 259] | 116 [17, 243] | <0.001 |
| HDL-C (mg/dL) | 47 [16, 115] | 47 [16, 113] | 47 [22, 115] | <0.001 |
| Triglycerides (mg/dL) | 134 [39, 751] | 132 [40, 751] | 138 [39, 614] | 0.482 |
| COL4-7S (ng/mL) | 5.1 [0.9, 19.8] | 4.8 [0.9, 19.8] | 5.5 [2.7, 14.0] | <0.001 |
| HA (ng/mL) | 69.11 [4.33, 1632.40] | 57.81 [4.33, 1086.14] | 91.03 [7.59, 1632.40] | <0.001 |
| P3P (U/mL) | 13.3 [1.4, 148.3] | 13.6 [1.4, 62.4] | 13.2 [2.7, 148.3] | 0.264 |
| TIMP-1 (ng/mL) | 232.0 [26.2, 935.6] | 222.5 [26.2, 806.0] | 243.1 [92.5, 935.6] | <0.001 |
| ELF score | 9.92 [5.07, 14.06] | 9.70 [5.07, 13.37] | 10.20 [7.49, 14.06] | <0.001 |
| Fibrosis stages 2–4 | 393 (49.5) | 168 (42.4) | 225 (56.5) | <0.001 |
| Fibrosis stages 3–4 | 189 (23.8) | 67 (16.9) | 122 (30.7) | <0.001 |
| At-risk MASH | 504 (63.5) | 234 (59.1) | 270 (67.8) | 0.013 |
| Steatosis grades 0 : 1 : 2 : 3 | 40 (5.0) : 546 (68.8) : 138 (17.4) : 70 (8.8) | 17 (4.3) : 269 (67.9) : 69 (17.4) : 41 (10.4) | 23 (5.8) : 277 (69.6) : 69 (17.3) : 29 (7.3) | 0.381 |
| Inflammation grades 0 : 1 : 2 : 3 | 33 (4.2) : 515 (64.9) : 197 (24.8) : 49 (6.2) | 22 (5.6) : 258 (65.2) : 98 (24.7) : 18 (4.5) | 11 (2.8) : 257 (64.6) : 99 (24.9) : 31 (7.8) | 0.068 |
| Ballooning grades 0 : 1 : 2 | 263 (33.1) : 366 (46.1) : 165 (20.8) | 143 (36.1) : 180 (45.5) : 73 (18.4) | 120 (30.2) : 186 (46.7) : 92 (23.1) | 0.117 |
Note: Results are presented as number (%) for qualitative data or as median [range] for quantitative data.
p-values were obtained using the Mann-Whitney U test or χ2 test.
Abbreviations: BMI, body mass index; COL4-7S, type IV collagen 7S; DM, diabetes mellitus; ELF, Enhanced Liver Fibrosis; HA, hyaluronic acid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MASH, metabolic dysfunction–associated steatohepatitis; P3P, procollagen III protein.
A total of 398 patients had T2DM. Compared to patients without T2DM, those with T2DM were significantly older (61 vs. 57 y, p<0.01), had a higher median COL4-7S level (5.5 vs. 4.8 ng/mL, p<0.01), and ELF score (10.2 vs. 9.7, p<0.01); and had significantly higher rates of fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH. However, there were no significant differences in rates of steatosis, inflammation, or ballooning between patients with or without T2DM.
Comparison of the diagnostic accuracy of COL4-7S versus ELF score for fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH
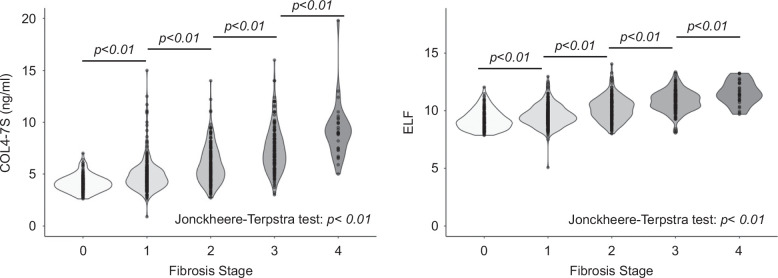
Violin plots of the distribution of COL4-7S levels and ELF scores at each stage are shown in Figure 1. The distribution of both markers increased significantly with increasing stage. However, the distribution was wider for COL4-7S than for the ELF score at each stage.
FIGURE 1.
Violin plots of COL4-7S levels and ELF scores for each fibrosis stage. Abbreviations: COL4-7S, type IV collagen 7S; ELF, Enhanced Liver Fibrosis.
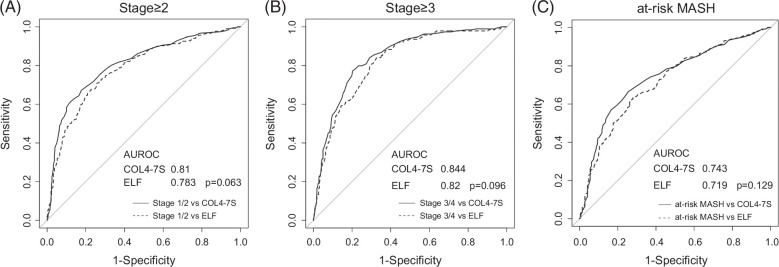
ROC analysis was performed, and the Delong test was used to compare the diagnostic accuracy of COL4-7S versus ELF score for fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH. The AUROC of COL4-7S was higher than that of ELF for all 3 outcomes, although the differences were not statistically significant (Figure 2). Specifically, the AUROCs of COL4-7S and ELF were 0.81and 0.783, respectively, for fibrosis stage ≥2 (p=0.063), 0.844 and 0.820 for fibrosis stage ≥3 (p=0.096); and 0.743 and 0.719 for at-risk MASH (p=0.129).
FIGURE 2.
Area under the receiver operating characteristics curve of COL4-7S and ELF score for diagnosing fibrosis stage ≥2 (A), fibrosis stage ≥3 (B), and at-risk MASH (C). Abbreviations: COL4-7S, type IV collagen 7S; ELF, Enhanced Liver Fibrosis; MASH, metabolic dysfunction–associated steatohepatitis.
The cutoff values of ELF and COL4-7S for each outcome on sensitivity 90% and specificity 90% were the following: the cutoff values of COL4-7S were 4.2 and 6.0 for stage≥2, respectively; 5.1 and 7.2 for stage≥3; and 4.2 and 6.0 for at-risk MASH. On the other hand, the values of ELF were 9.2 and 10.7 for stage≥2, respectively; 9.8 and 11.0 for stage≥3; and 8.9 and 10.8 for at-risk MASH (Supplemental Table S1, http://links.lww.com/HC9/B70).
cNRI and IDI analyses were also performed to compare the diagnostic accuracy of COL4-7S and ELF for detecting fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH (Table 2). cNRI was 0.167 (p=0.018) and IDI was 0.039 (p=0.003) for fibrosis stage ≥2. The accuracy of COL4-7S was significantly higher than that of ELF for detecting fibrosis stage ≥2 according to both types of analysis. cNRI was 0.022 (p=0.796) and IDI was 0.036 (p=0.021) for diagnosing fibrosis stage ≥3. Thus, there was no significant difference in the accuracy of COL4-7S and ELF scores for detecting fibrosis stage ≥3 according to cNRI analysis, but the diagnostic accuracy of COL4-7S was significantly higher than that of ELF according to IDI analysis. In terms of detecting at-risk MASH, cNRI was 0.098 (p=0.183), and IDI was 0.011 (p=0.261), indicating that there was no significant difference in the accuracy of COL4-7S and ELF for diagnosing at-risk MASH according to either type of analysis.
TABLE 2.
Diagnostic performance of COL4-7S and the ELF score according to ROC curve, net reclassification improvement, and integrated discrimination improvement analyses
| Method of analysis | Fibrosis stage ≥3 | p | Fibrosis stage ≥2 | p | At-risk MASH | p |
|---|---|---|---|---|---|---|
| AUROC | — | 0.0961 | — | 0.0632 | — | 0.129 |
| COL4-7S | 0.844 | — | 0.81 | — | 0.743 | — |
| ELF score | 0.82 | — | 0.783 | — | 0.719 | — |
| ELF score → COL4-7S | ||||||
| NRI | 0.0215 | 0.796 | 0.167 | 0.018 | 0.098 | 0.183 |
| NRI for events | 0.0794 | 0.274 | 0.139 | 0.005 | 0.064 | 0.153 |
| NRI for nonevents | −0.0579 | 0.154 | 0.0274 | 0.583 | 0.035 | 0.557 |
| IDI | 0.0361 | 0.021 | 0.0389 | 0.003 | 0.011 | 0.261 |
Abbreviations: COL4-7S, type IV collagen 7S; ELF, Enhanced Liver Fibrosis; IDI, integrated discrimination improvement; MASH, metabolic dysfunction–associated steatohepatitis; NRI, net reclassification improvement.
Comparison of the diagnostic accuracy of COL4-7S and ELF score for fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH in patients with or without T2DM
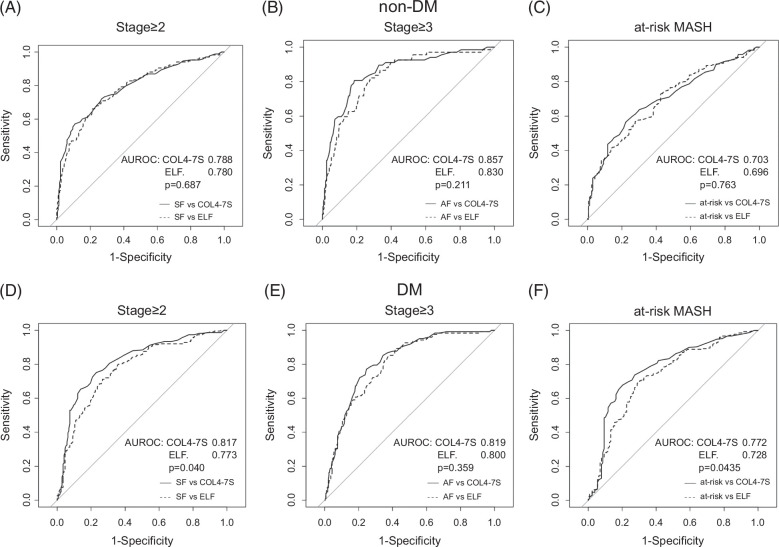
ROC, cNRI, and IDI analyses were also performed to assess the performance of COL4-7S and ELF for detecting fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH in patients with or without T2DM. In patients without T2DM, the diagnostic accuracy was not significantly different between COL4-7S and the ELF score for all three outcomes, using any of the 3 methods of analysis (AUROC, cNRI, and IDI) (Figure 3, Table 3A). In patients with T2DM, the diagnostic accuracy for fibrosis stage ≥2 was significantly better for COL4-7S than for the ELF score during AUROC analysis (AUROC of COL4-7S vs. ELF: 0.817 vs. 0.773 of ELF, p=0.040), cNRI analysis (cNRI=0.327, p<0.01), and IDI analysis (IDI=0.056, p<0.01). Conversely, the diagnostic accuracy for fibrosis stage ≥3 did not differ significantly between COL4-7S and ELF score during AUROC analysis, cNRI analysis (cNRI=−0.094, p=0.386), or IDI analysis (IDI=0.0152, p=0.444). For diagnosing at-risk MASH, the AUROC was significantly higher for COL4-7S than for the ELF score (0.772 vs. 0.728, p=0.044), but no significant difference between markers was observed during cNRI analysis (cNRI=0.20, p=0.061) or IDI analysis (IDI=0.024, p=0.093) (Figure 3, Table 3B).
FIGURE 3.
AUROC of COL4-7S and ELF score for diagnosing fibrosis stage ≥2, fibrosis stage ≥3, and at-risk MASH in patients without type 2 DM (A–C) and patients with type 2 DM (D–F). Abbreviations: COL4-7S, type IV collagen 7S; DM, diabetes mellitus; ELF, Enhanced Liver Fibrosis; MASH, metabolic dysfunction–associated steatohepatitis.
TABLE 3.
Diagnostic performance of COL4-7S and the ELF score according to ROC curve, net reclassification improvement, and integrated discrimination improvement analyses in patients with or without type 2 diabetes mellitus
| Method of analysis | Fibrosis stage ≥3 | p | Fibrosis stage ≥2 | p | At-risk MASH | p |
|---|---|---|---|---|---|---|
| Patients without T2DM | ||||||
| AUROC | — | 0.830 | — | 0.687 | — | 0.763 |
| COL4 | 0.857 | — | 0.788 | — | 0.703 | — |
| ELF score | 0.830 | — | 0.780 | — | 0.696 | — |
| ELF score → COL4-7S | ||||||
| NRI | 0.221 | 0.0924 | 0.045 | 0.657 | 0.046 | 0.649 |
| NRI for events | 0.224 | 0.0601 | 0.071 | 0.353 | 0.034 | 0.601 |
| NRI for nonevents | −0.003 | 0.956 | −0.026 | 0.691 | 0.012 | 0.875 |
| IDI | 0.062 | 0.011 | 0.018 | 0.317 | 4.47×10−5 | 0.997 |
| Patients with T2DM | ||||||
| AUROC | — | 0.359 | — | 0.040 | — | 0.044 |
| COL4 | 0.819 | — | 0.817 | — | 0.772 | — |
| ELF score | 0.800 | — | 0.773 | — | 0.728 | — |
| ELF → COL4-7S | ||||||
| NRI | −0.094 | 0.386 | 0.327 | 0.001 | 0.2 | 0.061 |
| NRI for events | 0 | 0.999 | 0.182 | 0.005 | 0.0593 | 0.329 |
| NRI for nonevents | −0.094 | 0.116 | 0.145 | 0.055 | 0.141 | 0.108 |
| IDI | 0.0152 | 0.444 | 0.056 | 0.002 | 0.024 | 0.093 |
Abbreviations: COL4-7S, type IV collagen 7S; ELF, Enhanced Liver Fibrosis; IDI, integrated discrimination improvement; MASH, metabolic dysfunction–associated steatohepatitis; NRI, net reclassification improvement; T2DM, type 2 diabetes mellitus.
Association of COL4-7S and ELF score with specific histologic features of MASLD
Relationships between both fibrosis makers (COL4-7S and ELF) and histologic features of MASLD were assessed using the Jonckheere-Terpstra trend test. The COL4-7S level tended to decrease as steatosis progressed, although the trend was not statistically significant (p=0.07), whereas the ELF score decreased significantly as steatosis increased (p<0.01). Regarding other histologic features, COL4-7S levels and ELF scores increased significantly as inflammation, ballooning, and fibrosis progressed (p<0.01) (Supplemental Figure S1A–C, http://links.lww.com/HC9/B71).
The results of multiple regression analysis after standardization of COL4-7S levels and ELF scores are shown in Supplemental Table S2, http://links.lww.com/HC9/B70. The coefficients for COL-7S were −0.022 (p=0.591) for steatosis, 0.206 (p<0.01) for inflammation, 0.092 (p=0.06) for ballooning, and 0.434 (p<0.01) for fibrosis. The coefficients for ELF score were −0.091 (p=0.032) for steatosis, 0.132 (p=0.013) for inflammation, 0.163 (p<0.01) for ballooning, and 0.394 (p<0.01) for fibrosis.
Ordinal logistic regression analysis results are shown in Supplemental Table S3, http://links.lww.com/HC9/B70. Higher COL4-7S levels and ELF scores were significantly associated with a higher fibrosis stage, with an OR of 2.65 (95% CI: 2.16–3.25, p<0.01) for COL4-7S and 1.86 (95% CI: 1.53–2.24, p<0.01) for ELF score.
The rate of steatosis was higher in patients with fibrosis stage ≥2 than in those with fibrosis stage ≥3 (Supplemental Table S4, http://links.lww.com/HC9/B70).
DISCUSSION
In this study, we compared the diagnostic performance of serum COL4-7S level versus the ELF score for detecting liver fibrosis. We noted several key findings. First, there was no significant difference in diagnostic accuracy between COL4-7S and ELF score for fibrosis stage ≥3 or at-risk MASH. Second, the diagnostic ability of both markers for liver fibrosis was not significantly different between patients with or without T2DM. Third, COL4-7S was superior to the ELF score for diagnosing fibrosis stage ≥2. COL4-7S was also more closely associated with the grade of inflammation and stage of fibrosis than ELF. Thus, COL4-7S appears to be an effective noninvasive marker for detecting higher stages of fibrosis in patients with MASLD. As such, it may not only have an important role in the risk-stratification of these patients, but it may also be useful for evaluating treatment efficacy.
We previously reported the effectiveness of COL4-7S for diagnosing MASH and advanced fibrosis in patients with MASLD.25 Sumida et al26 reported that the NAFIC score, which consists of serum ferritin, insulin, and COL4-7S levels, predicted MASH. Okanoue et al27 reported that the CA index, which consists of COL4-7S and aspartate aminotransferase, predicted MASH-related and MASH-related fibrosis. Furthermore, the good diagnostic accuracy of COL4-7S for detecting fibrosis stage ≥3 was maintained in patients with T2DM, with an AUROC of 0.883 in patients with MASLD who do not have DM and 0.872 in those with MASLD plus DM.5
The ELF score, as a noninvasive marker of fibrosis, has demonstrated excellent performance in detecting fibrosis stage ≥3, with an AUROC of 0.90.28 However, Vali and colleagues reported that the ELF score had an AUROC of 0.83 for detecting advanced fibrosis, with high sensitivity but limited specificity for excluding advanced fibrosis at low cutoff values. Furthermore, the diagnostic performance of the ELF score at higher thresholds was limited in low-prevalence settings.29 Furthermore, Seko et al30 reported that the AUROC for fibrosis stage ≥3 was 0.802, which was not superior to that of fibrosis-4 index. As with COL4-7S, the diagnostic accuracy of the ELF score was maintained whether or not T2DM was present.15 ELF scores using a low cutoff value were reported to have a high sensitivity (≥91.4%) and high negative predictive value (≥96.8%), regardless of the presence or absence of T2DM.
As in previous investigations, both COL4-7S and ELF scores exhibited high diagnostic performance in detecting fibrosis stage ≥3 in the current study. However, the diagnostic accuracy tended to be lower for the fibrosing stage ≥2 or at-risk MASH compared to the fibrosis stage ≥3. Interestingly, the diagnostic performance for diagnosing fibrosis stage ≥2 was significantly higher for COL4-7S than for the ELF score in patients with T2DM. ELF scores were associated with each of the assessed histologic features of MASLD (steatosis, inflammation, ballooning, and fibrosis), whereas COL4-7S levels were associated with only inflammation and fibrosis. While most of these associations were positive, the ELF score was negatively associated with steatosis grade. This negative association can be explained by the higher rate of steatosis in patients with fibrosis stage ≥2 than in those with fibrosis stage ≥3. Therefore, the ELF score appears to be influenced by this negative relationship, which may lower its value and reduce its diagnostic performance. In contrast, COL4-7S was not associated with steatosis, likely contributing to its superior performance as an indicator of hepatic fibrosis. In the violin plot of this study, the ELF score and COL4-7S statistically significantly distinguish fibrosis stages, but in practice, at lower stages (stages 0–1), their values overlap and cannot be clearly distinguished. In this study, the ELF is ~8–12 for stages 0–1, but similar ranges have been reported in another article.31 Because of the heterogeneous effects of fat deposition, inflammation, and ballooning at low stages, ELF may be less stable in values at low stages than COL4-7S.
COL4-7S and the ELF score appeared to be overall less useful for detecting earlier-stage MASLD than the later-stage disease (ie, fibrosis stage ≥3). As MASLD progresses very slowly, this drawback might be improved by periodic measurements of these markers, with more emphasis on changes over time rather than just absolute values. Future studies are necessary to confirm our observations and to determine appropriate monitoring intervals.
This study has some limitations. For example, COL4-7S was measured by radioimmunoassay, whereas the currently preferred method is the chemiluminescent enzyme immunoassay. However, Shima et al32 reported a close correlation between COL4-7S-radioimmunoassay and COL4-7S-chemiluminescent enzyme immunoassay values in patients with NAFLD (r=0.888, p<0.01), suggesting that our results are likely also applicable to COL4-7S measured by chemiluminescent enzyme immunoassay. Another limitation was that this study was conducted in Japanese patients. Further validation studies in other populations should be considered. If measurements of COL4-7S become available outside of Japan in the future, we believe it will be possible to conduct validation studies and evaluate the diagnosis performance of COL4-7S in the future.
In conclusion, serum COL4 levels had comparable diagnostic power for fibrosis stage ≥3 and at-risk MASLD, compared to the ELF score, but were superior to ELF for diagnosing fibrosis stage ≥2. The ELF score, which is calculated as a composite of 3 serum protein levels, was associated with steatosis, inflammation, ballooning, and fibrosis, whereas COL4-7S was associated with only inflammation and fibrosis. Our results suggest that diagnosing fibrosis stage ≥2 using noninvasive markers may be influenced by differences in histologic background, resulting in the ELF score being less useful for diagnosing earlier-stage fibrosis than COL4-7S.
Supplementary Material
AUTHOR CONTRIBUTIONS
Hiroshi Ishiba, Hideki Fujii, Yoshio Sumida, and Yoshihiro Kamada: manuscript drafting and analysis. Hideki Fujii, Yoshihiro Kamada, Hirokazu Takahashi, Yuya Seko, and Atsushi Nakajima: study concept and design. Data acquisition: Yoshio Sumida, Hidenori Toyoda, Hideki Hayashi, Kanji Yamaguchi, Michihiro Iwaki, Masato Yoneda, Taeang Arai, Toshihide Shima, Asahiro Morishita, Kazuhito Kawata, Kengo Tomita, Miwa Kawanaka, Yuichi Yoshida, Tadashi Ikegami, Kazuo Notsumata, Satoshi Oeda, Hideaki Fukushima, Eiji Miyoshi, and Shinichi Aishima. Statistical analysis: Hiroshi Ishiba. Material support: Hideki Fujii. Study supervision: Yoshito Itoh, Takeshi Okanoue, and Atsushi Nakajima.
CONFLICTS OF INTEREST
Yoshio Sumida is on the speaker’s bureau for MSD, Novo Nordisk, Taisho, Kowa, Mochida, and Siemens Healthcare. Hirokazu Takahashi is on the speaker’s bureau for Kowa, Taisho Pharma, and Novo Nordisk. He received grants from Sysmex and Abbvie GK. Hidenoru Toyoda is on the speaker’s bureau for Gilead Sciences, Abbvie, Eisai, Fujifilm WAKO, Terumo, Takeda, Chugai, Kowa, and Bayer. Masato Yoneda received grants from Gilead Sciences Incorporated. Hideaki Fukushima is employed by Siemens Healthcare Diagnostics K.K. The remaining authors report no conflict of interest.
Footnotes
Abbreviations: cNRI, continuous net reclassification improvement; COL4-7S, type 4 collagen 7S; ELF, Enhanced Liver Fibrosis; HA, hyaluronic acid; IDI, integrated discrimination improvement; MASLD, metabolic dysfunction–associated steatotic liver disease; T2DM, type 2 diabetes mellitus.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Hiroshi Ishiba, Email: chiroinu@koto.kpu-m.ac.jp.
Hideki Fujii, Email: rolahdieki@med.osaka-cu.ac.jp.
Yoshihiro Kamada, Email: ykamada@sahs.med.osaka-u.ac.jp.
Yoshio Sumida, Email: sumida19701106@yahoo.co.jp.
Hirokazu Takahashi, Email: takahas2@cc.saga-u.ac.jp.
Yuya Seko, Email: yuyaseko@koto.kpu-m.ac.jp.
Hidenori Toyoda, Email: hmtoyoda@spice.ocn.ne.jp.
Hideki Hayashi, Email: hidekihaya884@gmail.com.
Kanji Yamaguchi, Email: ykanji@koto.kpu-m.ac.jp.
Michihiro Iwaki, Email: michihirokeidai@yahoo.co.jp.
Masato Yoneda, Email: dryoneda@yahoo.co.jp.
Taeang Arai, Email: taeangpark@yahoo.co.jp.
Toshihide Shima, Email: shima0301d@suita.saiseikai.or.jp.
Asahiro Morishita, Email: asahiro@med.kagawa-u.ac.jp.
Kazuhito Kawata, Email: kawata@hama-med.ac.jp.
Kengo Tomita, Email: kengo@boreas.dti.ne.jp.
Miwa Kawanaka, Email: m.kawanaka@med.kawasaki-m.ac.jp.
Yuichi Yoshida, Email: yu1yoshida@gmail.com.
Tadashi Ikegami, Email: ikegamit@tokyo-med.ac.jp.
Kazuo Notsumata, Email: kazuo.notsumata@gmail.com.
Satoshi Oeda, Email: ooedasa@cc.saga-u.ac.jp.
Hideaki Fukushima, Email: hideaki.fukushima@siemens-healthineers.com.
Eiji Miyoshi, Email: emiyoshi@sahs.med.osaka-u.ac.jp.
Shinichi Aishima, Email: aishima.shinichi.476@m.kyushu-u.ac.jp.
Yoshito Itoh, Email: yitoh@koto.kpu-m.ac.jp.
Takeshi Okanoue, Email: okanoue@suita.saiseikai.or.jp.
Atsushi Nakajima, Email: nakajima-tky@umin.ac.jp.
REFERENCES
- 1.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut. 2021;70:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagström H, Nasr P, Ekstedt M, Stål P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring systems in assessing risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:1148–56.e4. [DOI] [PubMed] [Google Scholar]
- 4.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–60. [DOI] [PubMed] [Google Scholar]
- 5.Ishiba H, Sumida Y, Seko Y, Tanaka S, Yoneda M, Hyogo H, et al. Type IV collagen 7S is the most accurate test for identifying advanced fibrosis in NAFLD with type 2 diabetes. Hepatol Commun. 2020;5:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan W, Torok NJ. Editorial: Noninvasive fibrosis biomarkers in patients with NASH with diabetes. Hepatol Commun. 2021;5:553–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg WMC, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology. 2004;127:1704–13. [DOI] [PubMed] [Google Scholar]
- 8.Sharma C, Cococcia S, Ellis N, Parkes J, Rosenberg W. Systematic review: Accuracy of the enhanced liver fibrosis test for diagnosing advanced liver fibrosis and cirrhosis. J Gastroenterol Hepatol. 2021;36:1788–802. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) . EASL-EASDEASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–40. [DOI] [PubMed] [Google Scholar]
- 10.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 11.Leeming DJ, Nielsen MJ, Dai Y, Veidal SS, Vassiliadis E, Zhang C, et al. Enzyme-linked immunosorbent serum assay specific for the 7S domain of Collagen Type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol Res. 2012;42:482–493. [DOI] [PubMed] [Google Scholar]
- 12.Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2005;11:255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefano JT, Guedes LV, de Souza AAA, Vanni DS, Alves VAF, Carrilho FJ, et al. Usefulness of collagen type IV in the detection of significant liver fibrosis in nonalcoholic fatty liver disease. Ann Hepatol. 2021;20:100253. [DOI] [PubMed] [Google Scholar]
- 14.Murawaki Y, Ikuta Y, Koda M, Koda M, Kawasaki H. Serum type III procollagen peptide, type IV collagen 7S domain, central triple-helix of type IV collagen and tissue inhibitor of metalloproteinases in patients with chronic viral liver disease: Relationship to liver histology. Hepatology. 1994;20(4 Pt 1):780–787. [DOI] [PubMed] [Google Scholar]
- 15.Arai T, Takahashi H, Seko Y, Toyoda H, Hayashi H, Yamaguchi K, et al. Accuracy of the enhanced liver fibrosis test in patients with type 2 diabetes mellitus and its clinical implications. Clin Gastroenterol Hepatol. 2024;22:789–797.e8. [DOI] [PubMed] [Google Scholar]
- 16.Kamada Y, Nakamura T, Isobe S, Hosono K, Suama Y, Ohtakaki Y, et al. SWOT analysis of noninvasive tests for diagnosing NAFLD with severe fibrosis: An expert review by the JANIT Forum. J Gastroenterol. 2023;58:79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1(suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481. [DOI] [PubMed] [Google Scholar]
- 19.Teramoto T, Sasaki J, Ueshima H, Egusa G, Kinoshita M, Shimamoto K, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:155–8. [DOI] [PubMed] [Google Scholar]
- 20.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. [DOI] [PubMed] [Google Scholar]
- 23.Bedossa P, FLIP Pathology Consortium . Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology. 2014;60:565–75. [DOI] [PubMed] [Google Scholar]
- 24.Jin J. Risk assessment for cardiovascular disease with nontraditional risk factors. JAMA. 2018;320:316. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda M, Mawatari H, Fujita K, Yonemitsu K, Kato S, Takahashi H, et al. Type IV collagen 7s domain is an independent clinical marker of the severity of fibrosis in patients with nonalcoholic steatohepatitis before the cirrhotic stage. J Gastroenterol. 2007;42:375–81. [DOI] [PubMed] [Google Scholar]
- 26.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in non-alcoholic fatty liver disease. J Gastroenterol. 2011;46:257–68. [DOI] [PubMed] [Google Scholar]
- 27.Okanoue T, Ebise H, Kai T, Mizuno M, Shima T, Ichihara J, et al. A simple scoring system using type IV collagen 7S and aspartate aminotransferase for diagnosing nonalcoholic steatohepatitis and related fibrosis. J Gastroenterol. 2018;53:129–39. [DOI] [PubMed] [Google Scholar]
- 28.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–60. [DOI] [PubMed] [Google Scholar]
- 29.Vali Y, Lee J, Boursier J, Spijker R, Löffler J, Verheij J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J Hepatol. 2020;73:252–62. [DOI] [PubMed] [Google Scholar]
- 30.Seko Y, Takahashi H, Toyoda H, Hayashi H, Yamaguchi K, Iwaki M, et al. Diagnostic accuracy of enhanced liver fibrosis test for nonalcoholic steatohepatitis-related fibrosis: Multicenter study. Hepatol Res. 2023;53:312–21. [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Felix S, Jeffers T, Younossi E, Nader F, Pham H, et al. Performance of the enhanced liver fibrosis test to estimate advanced fibrosis among patients with nonalcoholic fatty liver disease. JAMA Netw Open. 2021;4:e2123923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shima T, Ohtakaki Y, Kikuchi H, Uchino H, Isomura M, Aoyagi K, et al. A novel rapid immunoassay of serum type IV collagen 7S for the diagnosis of fibrosis stage of nonalcoholic fatty liver diseases. Hepatol Res. 2021;51:263–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.