Abstract
Liver fibrosis is a pathological change characterized by excessive deposition of extracellular matrix caused by chronic liver injury, and the mechanisms underlying its development are associated with endothelial cell injury, inflammatory immune cell activation, and HSC activation. Furthermore, hepatic macrophages exhibit remarkable heterogeneity and hold central functions in the evolution of liver fibrosis, with different subgroups exerting dual effects of promotion and regression. Currently, targeted macrophage therapy for reversing hepatic fibrosis has been extensively studied and has shown promising prospects. In this review, we will discuss the dual role of macrophages in liver fibrosis and provide new insights into reversing liver fibrosis based on macrophages.
Keywords: liver fibrosis, macrophages, therapeutics
INTRODUCTION
Liver fibrosis is a pathological alteration that occurs in the reparative and healing processes as a result of the response to chronic liver injury, mainly including viral hepatitis and cholestatic injury. It is characterized by excessive deposition of extracellular matrix (ECM) within the liver and serves as a critical step in the progression of liver cirrhosis and hepatocellular carcinoma.1,2,3 Additionally, liver fibrosis can lead to a series of complications, such as portal hypertension, liver failure, and HE. Moreover, liver cirrhosis and its complications cause approximately 1 million deaths worldwide each year, posing a significant public health concern.4 However, the mechanisms underlying the development of liver fibrosis are complex and not fully elucidated. It is likely associated with the injury of endothelial cells, the recruitment of inflammatory immune cells, and the activation of HSCs, as the interactions between these cells contribute to the promotion of liver fibrosis.5 Recently, the main approaches for treating hepatic fibrosis include removing the injurious factors causing chronic liver damage, eliminating or inactivating myofibroblasts, suppressing inflammatory responses, and degrading ECM.6 However, significant breakthroughs in treatment have yet to be achieved.
Hepatic macrophages are a crucial component of the liver’s innate immune system. In addition to their robust phagocytic ability and antigen-presenting function, they can recognize and eliminate pathogens, cellular debris, or apoptotic cells, and can also generate reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS), reactive nitrogen species, cytokines, chemokines, and growth factors to trigger cascades of inflammatory reactions and other biological responses, playing a vital role in tissue repair, inflammatory responses, lipid metabolism, and tumor development.7,8 Besides, hepatic macrophages, as key regulatory cells, play a crucial role in the progression of liver fibrosis, which is a dynamic and reversible wound-healing process that involves both progression and regression. Importantly, single-cell sequencing has revealed that distinct subpopulations of hepatic macrophages have dual effects, both promoting and resolving liver fibrosis.9 This article provides a comprehensive review of the dual role of macrophages in liver fibrosis and explores new perspectives on how to reverse liver fibrosis based on macrophage-mediated mechanisms.
The origin of liver macrophages
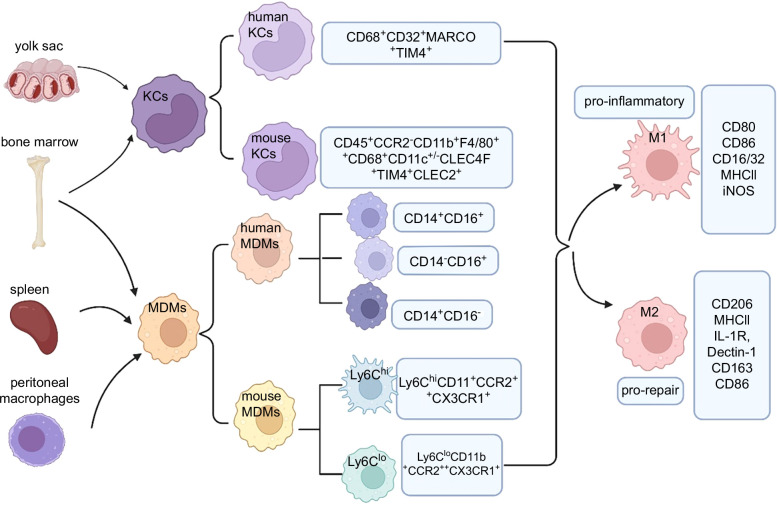
Hepatic macrophages are composed of different subpopulations, primarily including resident hepatic macrophages, monocyte-derived macrophages (MDMs), and peritoneal macrophages. Using single-cell sequencing, hepatic macrophages can now be more accurately classified based on relevant selection markers10 (Figure 1).
FIGURE 1.
The origin of hepatic macrophage subsets in mice and humans. Hepatic macrophages are mainly comprised of KCs and MDMs. KCs are located in the sinusoid of the liver with a yolk sac or bone marrow origin. Infiltrating monocytes are categorized into 2 subsets: Ly6Chi and Ly6Clo monocytes, mainly derived from the bone marrow as well as the spleen. The peritoneal macrophages originating from the peritoneum also contribute to the infiltrating monocytes. Activated macrophages undergo differentiation or polarization into 2 distinct subtypes, namely M1 (proinflammatory) and M2 (prorepair). Abbreviations: MDMs, monocyte-derived macrophages; MHC, major histocompatibility complex.
First, hepatic resident macrophages, also known as KCs, originate from the yolk sac erythro-myeloid progenitors and play a dominant role among hepatic macrophages, thereby, serving as the first line of defense in immune responses within the liver. In addition, KCs are activated by inflammatory factors, lipid mediators, and dysbiosis of the gut microbiota, and recruit circulating bone marrow-derived macrophages to the liver for differentiation and replenishment.11,12 Current studies have found that mouse KCs can specifically express CLEC4F,13 TIM4,14 CLEC2,15 etc., to distinguish them from monocyte-derived macrophages (MDMs); for example, the specific expression markers of mouse KCs include CD45+ chemokine receptor 2 (CCR2)−CD11b+F4/80++CD68+CD11c+/−CLEC4F+TIM4+CLEC2+. Blériot et al16,17 have classified mouse KCs into 2 subpopulations, KC1 (CD206loESAM−) and KC2 (CD206hiESAM+), based on the expression of CD206 and ESAM. These 2 subpopulations exhibit distinct functional characteristics, with KC1 showing stronger immunological features, while KC2 specifically expresses genes involved in cell adhesion and hepatic lipid metabolism pathways. In humans, KCs exhibit high expression of TIM4 and MARCO, therefore, the surface markers for human KCs are CD68+CD32+MARCO+TIM4+. Furthermore, a study18 using spatial transcriptomics has revealed that KCs (CD68+MARCO+) are localized in the portal area, while recruited MDMs (CD68+MARCO-) are located near the central vein.
Second, MDMs primarily consist of those originating from the bone marrow and the spleen, possessing immunogenicity and acquiring different phenotypes and functions under the influence of the local microenvironment.19 Interestingly, in mice, studies have shown that MDMs can differentiate into macrophages with different phenotypes in response to the distinct microenvironment within the liver, known as Ly-6Chi and Ly-6Clo. The Ly-6Chi subset primarily originates from the bone marrow, while the Ly-6Clo subset is derived from the spleen.20 Furthermore, studies in mice have found that MDMs with high expression of Ly6C (Ly6ChiCD11b+CCR2++CX3CR1+) exhibit a proinflammatory and profibrotic phenotype, while MDMs with low expression of Ly6C (Ly6CloCD11b+CCR2++CX3CR1+) display a prorepair and antifibrotic phenotype.21,22 In humans, based on the expression of CD14 and CD16, macrophages can be classified into 3 subtypes: CD14+CD16−, CD14+CD16+ and CD14−CD16+ subsets. Moreover, in humans, there is no antigen equivalent to mouse Ly-6C. It is generally believed that CD14+CD16− monocyte-derived macrophages are similar to mouse Ly6Chi macrophages, while CD14+CD16+ monocyte-derived macrophages are similar to Ly6Clo macrophages.23 However, CD14+CD16+monocyte-derived macrophages accumulate and release inflammatory factors in the damaged liver, contrary to the role of mouse Ly-6Clo macrophages. Consistently, both cell types can promote ECM degradation or fibrinolysis.
Finally, peritoneal macrophages are located in the subcapsular region of the liver and have been confirmed to exist in both humans and mice24 (Figure 1). They express common macrophage markers, such as F4/80 and CD64, but do not express markers specific to KCs, such as VSIG4, CLEC4F, FOLR2, or CLEC2.25 In addition to bone marrow–derived macrophages, peritoneal macrophages also undergo self-renewal and promote liver regeneration through their migration via the mesothelial cells when the liver injury occurs.
In general, activated macrophages undergo differentiation or polarization into two distinct subtypes, namely M1 (proinflammatory) and M2(prorepair)26 (Figure 1). Lipopolysaccharide, TNF-α, and colony-stimulating factors for granulocytes and macrophages can induce polarization of macrophages toward M1 phenotype.27 Current researches indicate that M1 macrophages can be polarized via the toll-like receptor-4/NF-κB, JAK/STAT1, and Notch signaling pathway,28,29,30,31 characterized by specific markers such as CD80, CD86, CD16/32, major histocompatibility complex II, and iNOS, producing a large amount of proinflammatory cytokines and chemokines such as IL-1b, IL-6, IL-12, IL-23, TNF-a, CXCL1~3, CXCL8~10, chemokine ligand (CCL2)~5, and CCL11,32 and mainly exerting antigen-presenting function, as well as proinflammatory and pathogenic microorganism scavenging capabilities. On the other hand, M2 macrophages, known as anti-inflammatory macrophages, are primarily activated by IL-4 and IL-13, secreting anti-inflammatory factors such as IL-10, IL-4, IL-13, TGFβ, etc.; therefore, have the ability to suppress inflammation, promote tissue remodeling and prevent parasitic infections. In addition, common mechanisms for M2 macrophage polarization include the JAK/STAT6 and TGFβ/Smads signaling pathways.33,34 In accordance with the expression of activation markers and various functions, M2 macrophages are typically categorized into 4 subpopulations: M2a, M2b, M2c, and M2d subtypes.35 First, induced M2a macrophages are characterized by specific markers, such as CD206, major histocompatibility complex II, IL-1R, and Dectin-1, having various functions, including anti-inflammation, wound healing, and Th2 immune response;36 second, M2b macrophages are characterized by specific markers such as CD206, major histocompatibility complex II, and CD86, which have functions of immune regulation, protumor and pro-infection;37 third, M2c macrophages are characterized by specific markers, such as CD206 and CD163, that have functions of phagocytosis, immunosuppression, and tissue remodeling.37 Finally, M2d macrophages are characterized by specific marker CD206, and have functions of promoting tumor growth and angiogenesis.38 Furthermore, transcriptomic studies have revealed that the phenotypes of Ly-6Chi and Ly-6Clo cells do not strictly correspond to M1 and M2 macrophage types.39 However, it is now recognized that the M1/M2 dichotomy is too simplistic and limited to describe the many distinct polarization phenotypes unraveled by single-cell RNA sequencing. Nevertheless, the M1/M2 phenotyping of liver macrophages can still reflect various dynamic pathological changes in the liver.
The dual role of macrophages in the formation of liver fibrosis
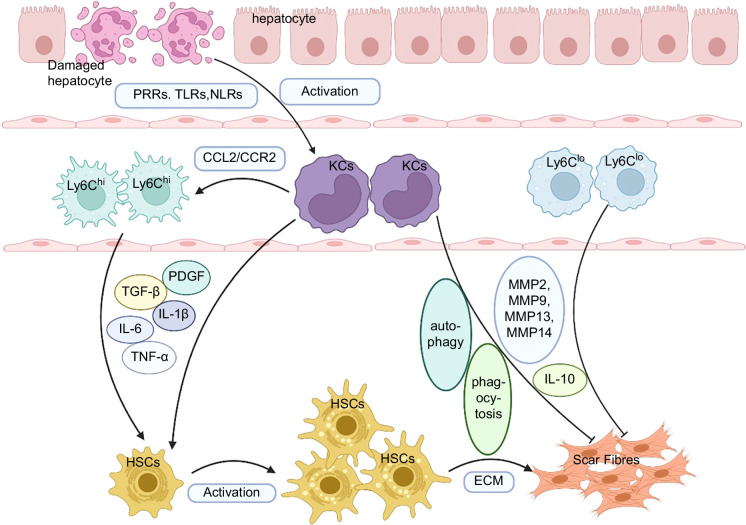
ECM is a complex structure composed of various molecules, including type I and III collagen fibers, fibronectin, laminin, and glycosaminoglycans.40 Liver fibrosis formation is a process characterized by excessive deposition of ECM components produced by myofibroblasts. In fibrotic liver, the primary sources of fibrogenic myofibroblasts have been identified as liver-resident–activated HSCs and activated portal fibroblasts.1 The activation of HSCs and their transformation into myofibroblasts are key steps in the formation of liver fibrosis, while liver macrophages play a crucial role in regulating HSC activation.41 Different subsets of macrophages exhibit distinct polarization states, as changes in the tissue microenvironment can induce macrophages of different origins to adopt different phenotypes, hence crucial in promoting or resolving fibrosis at different stages of liver fibrosis.42 During the initiation and progression of liver fibrosis, activated hepatic macrophages upregulate profibrotic factors and inflammatory cytokines, such as TGF-β, PDGF, CCL2/CCR2), TNF-α, IL-1β, and so on. These factors activate HSCs and contribute to the deposition of ECM and liver fibrosis.43 However, in the late stage of liver fibrosis, under various exogenous stimuli, hepatic macrophages can also degrade ECM by producing matrix metalloproteinases (MMPs), and produce large amounts of IL-4, IL-5, and IL-10, working together with IL-13 to exert antifibrotic effects44,45 (Figure 2). Therefore, hepatic macrophages play a dual role in liver fibrosis progression and its resolution.
FIGURE 2.
The dual role of hepatic macrophages in the progression and regression of liver fibrosis. KCs are activated by damaged hepatocytes and upregulate TGF-β, PDGF, CCL2/ CCR2, TNF-α, IL-1β, and so on. The increased levels of CCL2/CCR2 promote the recruitment of Ly-6Chi monocytes to the liver injury. The activated KCs and Ly6Chi monocytes both exert profibrotic effects by promoting the activation of HSCs, leading to the excessive deposition of ECM and scar formation. On the contrary, under various exogenous stimuli, KCs and Ly6Clo monocytes can degrade ECM by producing MMPs and IL-10 to exert antifibrotic effects. In addition, autophagy and phagocytosis of KCs can promote the regression of liver fibrosis. Abbreviations: CCL2/CCR2, chemokine ligand 2/chemokine receptor 2; ECM, extracellular matrix; MMPs, matrix metalloproteinases; NLRs, nod-like receptors; PRRs, pattern recognition receptors; TLRs, toll-like receptors.
Macrophages promote liver fibrosis progression
Activated KCs can upregulate proinflammatory and profibrotic factors through pattern recognition receptors,46 classical TGF-β,47 and PDGF48 pathway, thereby promoting liver fibrosis. When liver injury occurs, pathogen-associated molecular patterns and damage-associated molecular patterns can be recognized by pattern recognition receptors on the cell surface or within the cells, such as toll-like receptors and nod-like receptors. This recognition triggers the production of inflammatory mediators, such as TNF-α, IL-6, IL-12, IL-1β, and CCL2, promoting the activation and proliferation of HSCs, leading to the deposition of ECM and the development of liver fibrosis49 (Figure 2). Moreover, various cytokines secreted by macrophages can activate and enhance T-cell functions.50 For example, IL-6 can promote the differentiation of Th17 cells and IL-4 promotes the differentiation of Th2 cells. Th17 cells can secrete pro-inflammatory factors, such as IL-17, IL-22, and IL-23, to activate HSCs and promote liver fibrosis.51,52 Current studies suggest that when there is an imbalance between Th17 cells and regulatory T cells, HSCs become activated and stimulate liver fibrosis.52,53 Th2 cells produce cytokines IL-4 and IL-13 to activate HSCs, leading to ECM proliferation and promoting liver fibrosis.54,55 Indeed, the most potent inducers of liver fibrogenesis are TGF-β and PDGF, which are primarily secreted by KCs. During liver injury, KCs produce a significant amount of TGF-β, which binds to the highly affinitive TGF-β receptor on the surface of HSCs, then induces sustained phosphorylation of downstream membrane receptor-regulated Smad, downregulates inhibitory Smad expression, facilitates the translocation of the signal into the cell nucleus and triggers the activation of HSCs as well as the development of fibrosis.56 Whereas, PDGF secreted by hepatic macrophages during liver injury binds to its receptors on HSCs, leading to the dimerization and autophosphorylation of its subunits, thereby inducing sustained activation of HSCs.57
Furthermore, during liver injury, recruited MDMs in the liver can activate HSCs and promote liver fibrosis by releasing inflammatory mediators, such as ROS,58 iNOS,59 and reactive nitrogen species.60 Nicotinamide adenine dinucleotide phosphate oxidase 2 is an inflammatory mediator that promotes apoptosis in hepatocytes. Also, it has been reported that ROS generated by nicotinamide adenine dinucleotide phosphate oxidase 2 released from MDMs can exacerbate liver fibrosis induced by CCl4.61 MDMs can catalyze the production of a large amount of proinflammatory macrophage factor, nitric oxide, by inducing the expression of iNOS from L-arginine, which can increase the production of prostaglandin E2 through the activation of cyclooxygenase, thereby promoting the occurrence of liver fibrosis.62 Additionally, the reduced form of nitric oxide can react with ROS to form reactive nitrogen species, such as the highly reactive and toxic peroxynitrite anion (ONOO-), which can activate HSCs.60 In the early stages of liver injury, Ly6Chi monocyte-derived macrophages are recruited to the injured liver through the action of the CCL2/CCR2 axis. Then, these macrophages release various inflammatory and profibrotic factors that act on HSCs, promoting their proliferation and activation, thereby driving the development of liver fibrosis1 (Figure 2). In the human body, CD14+ CD16+ monocyte-derived macrophages in MDMs are the main cell types involved in the formation of liver fibrosis, accumulating in the damaged liver and releasing inflammatory and fibrotic factors to promote the development of liver fibrosis22 (Figure 2).
Macrophages are involved in the regression of liver fibrosis
Several studies36,37,38 have demonstrated that liver fibrosis is reversible. The regression of liver fibrosis may be associated with a decrease in proinflammatory or profibrotic cytokine production, an increase in collagen degradation activity,63 the elimination of hepatic myofibroblasts,59 the inhibition of ECM production, and the resolution of fibrous scar tissue. Moreover, KCs can promote the regression of liver fibrosis through various mechanisms, such as producing anti-inflammatory factors like IL-10, recruiting natural killer cells to induce apoptosis of activated HSCs, phagocytosing damaged liver cells, and producing MMPs that degrade the ECM, including MMPs-2, 9, 13, and 1464,65 (Figure 2). Furthermore, IL-10 and IL-12 secreted by macrophages promote the differentiation and proliferation of regulatory T cells and Th1 cells, respectively. Regulatory T cells not only directly inhibit the activation and proliferation of HSCs, thereby reducing the deposition of ECM, but they also suppress the activation of Ly6Chi macrophages, by reducing chronic inflammation and alleviating liver fibrosis.50,66,67 Th1 cells can produce interferon-γ to decrease the activation of HSCs and the accumulation of ECM, as well as enhance natural killer cell activity and promote apoptosis of HSCs.68 Additionally, hepaticmacrophages in the liver synthesize and secrete TRAIL, which can induce apoptosis in HSCs via decreasing the expression of TIMPs in HSCs, thereby promoting extracellular matrix degradation and exerting an antifibrotic effect.69
During the regression of liver fibrosis, the population of Ly6Clo subset is enriched in the mouse liver, which exhibits reduced secretion of proinflammatory factors and can secrete multiple proteins, including MMP9 and MMP2, that degrade the ECM70 (Figure 2).
New ideas for macrophage-based treatment of liver fibrosis
Due to the significant role of hepatic macrophages in the development and regression of liver fibrosis, there is currently a growing number of researches focused on macrophage-based therapies to improve liver fibrosis, which holds promising prospects.20 So far, the main therapeutic strategies for reversing fibrosis based on macrophages include antifibrotic treatments targeting macrophage immune metabolism,71 anti-liver fibrosis treatments targeting macrophage-related signaling pathways,72 and the use of autologous macrophages for treating liver fibrosis73 (Table 1).
TABLE 1.
Targeting hepatic macrophages as a therapeutic strategy for liver fibrosis
| Categories | Therapeutics | Mechanism | Drugs | References |
|---|---|---|---|---|
| Targeting macrophage immune metabolism | ACC inhibitor | Prevent macrophage activation and infiltration | WZ66 | 68,73 |
| PPAR | Promoting macrophage differentiation toward M2 | Elafibranor | 69,70,71 | |
| FXR | Increasing cholesterol transport in macrophages | Obeticholic acid | 72 | |
| Targeting macrophage-related signaling pathways | Antibiotics | Removes intestinal bacteria and inhibits macrophage activity | Rifaximin | 74 |
| IL-1β antagonists | Inhibit the activation of inflammasomes | IL-1Ra | 75 | |
| CCR2/5 antagonists | Inhibition of monocyte recruitment | Cenicriviroc | 76,77 | |
| Gal-3 antagonists | Inhibition of inflammatory macrophage function | GR-MD-02 | 78,79 | |
| Targeting autologous macrophages | CD45+CD14+25F9hi cells | Proreparative macrophages reverse liver fibrosis | — | 64 |
Abbreviations: ACC-inhibitor, acetyl-CoA carboxylase-inhibitor; CCR2/5, chemokine receptor 2/5; FXR, farnesoid X receptor; Gal-3 antagonists, galectin-3 antagonists; PPAR, peroxisome proliferator-activated receptors.
The metabolic reprogramming of macrophages in response to changes in the local microenvironment of the liver after injury can influence the polarization of macrophage subsets toward proinflammatory or anti-inflammatory phenotypes, thereby affecting the activation of HSCs.80 In addition, M1 and M2 macrophages exhibit distinct metabolic characteristics: M1 macrophages are primarily involved in the enhancement of glycolysis and the pentose phosphate pathway, and the activation of tricarboxylic acid cycle, whereas, M2 macrophages are primarily involved in the enhancement of fatty acid oxidation and arginase pathway, and the activation of the tricarboxylic acid cycle.74 Previous studies have demonstrated that the glycolytic pathway promotes the polarization of macrophages toward the M1 phenotype, activating inflammation pathways and releasing inflammatory cytokines such as TNF-α, IL-1β, IL-6, etc.75 Targeting macrophage metabolism has a unique advantage in improving liver fibrosis and reducing drug side effects. Recently, relevant studies have shown that targets based on macrophage immune metabolism include the inhibitor WZ66 of acetyl-CoA carboxylase (ACC), which is involved in lipid metabolism,76 peroxisome proliferator-activated receptors (PPAR),77,78,79and farnesol X receptor, which induces the expression of genes related to lipoprotein metabolism.81 Gao et al76 demonstrated that by using the acetyl-CoA carboxylase inhibitor WZ66, acetyl-CoA carboxylase could be inhibited and, therefore, prevent macrophage activation and infiltration and reduce HSC activation so as to alleviate hepatic steatosis. Notably, the peroxisome PPARs are a family of nuclear transcription factors, including four subtypes: α, β, δ, and γ, which are involved in the regulation of lipid metabolism and glucose metabolism. Previous studies have found that during liver inflammation, PPARα is redistributed from hepatocytes to KCs, and activation of PPARα can induce a phenotypic transformation of macrophages into the M2 phenotype.83 On the other hand, farnesoid X receptor is a bile acid receptor that can inhibit gene expression related to hepatic triglyceride synthesis and regulate lipid metabolism. Currently, clinical trials are underway for the farnesol X receptor agonist obeticholic acid in patients with liver fibrosis.81 However, metabolic targets are nonspecific, and it is necessary to accurately describe the spatiotemporal characteristics of macrophage metabolism to make targeted macrophage metabolism therapy more precise.
Currently, the development of drugs targeting macrophage-related signaling pathways is also a hot topic in the context of anti-liver fibrosis. Antibiotics such as rifaximin, vancomycin, gentamicin, and meropenem have been shown to clear the gut microbiota to inhibit macrophage activation, thereby reducing inflammatory responses and alleviating liver fibrosis.84 In addition, IL-1β signaling pathway antagonists,85 chemokine receptor 2/5 (CCR2/5) antagonists,86,87and galectin-3 antagonists88 have been shown to inhibit the activation of inflammasomes produced by KCs and suppress the recruitment of monocytes. Additionally, IL-1Ra is an antagonist of IL-1β that binds to IL-1R to regulate inflammation. Current clinical trial is underway to monitor the levels of endotoxins, IL-1, TNF-α, and other markers in patients’ serum to evaluate the efficacy of IL-1Ra (anakinra) in treating liver diseases.85 Mulder et al87 found that CCR2 plays a crucial role in recruiting immune cells to white adipose tissue and the liver, but the CCR2 inhibitor (cenicriviroc) can alleviate liver inflammation and the progression of liver fibrosis. Also, galectin-3, a lectin protein, is a required factor for TGF-β–mediated myofibroblast activation and ECM generation. Notably, in the model of schistosome-induced liver fibrosis, KCs are recruited to the fibrotic tissue and exhibit high expression of Galectin-3.89 Currently, clinical studies are underway for testing the efficacy of these agents.
A large recruitment of bone marrow-derived proreparative macrophages in the liver has been shown to reverse liver fibrosis by secreting MMPs and degrading ECM. Previous research has proposed the use of autologous macrophage therapy for the treatment of liver fibrosis. For example, Moroni and colleagues isolated CD45+CD14+25F9hi cells from peripheral blood monocytes of patients with liver cirrhosis using macrophage colony-stimulating factor and then infused these cells back into the patients. Follow-up assessments of liver fibrosis indicators such as transient elastography, pro-collagen type III, and type III collagen protein degradation products showed a decrease in their levels.73 This study demonstrated the potential application of autologous macrophages CD14+25F9+ in the treatment of liver fibrosis. However, further clinical research data is needed to supplement and confirm these findings. Additionally, it remains to be elucidated whether other cell subpopulations have similar functions in the context of liver fibrosis treatment.
CONCLUSIONS
With the application of single-cell sequencing, subpopulations of liver macrophages can be accurately classified based on relevant selection markers. These subpopulations of liver macrophages play a dual role in the process of liver fibrosis. In the early stages of liver injury, Ly6Chi monocyte-derived macrophages are recruited to the liver and release various inflammatory and profibrotic factors, which activate and proliferate HSCs. While, during the resolution phase of fibrosis, Ly6Clo monocyte-derived macrophages can degrade ECM by secreting various MMPs such as MMP-12 and MMP-13. Therefore, macrophage-based therapies have become a promising approach for improving liver fibrosis, with extensive research being conducted in this area. These include antifibrotic treatments targeting macrophage immune metabolism, anti-liver fibrosis treatments targeting macrophage-related signaling pathways, and the use of autologous macrophages for liver fibrosis treatment, all of which are currently in clinical trial stages. Further identification of reparative macrophage subpopulations and clarification of their differentiation pathways and regulatory mechanisms will provide new strategies and hope for the treatment of liver fibrosis in the future.
AUTHOR CONTRIBUTIONS
Drafting of the article: Jinqiu Ran, Shengxia Yin, and Rahma Iss. Study review and/or revision of the manuscript: all authors. Study concept and study supervision: Jie Li, Chao Wu, Qun Zhang.
FUNDING INFORMATION
This work was supported by the National Natural Science Fund of China(No.82170609,81970545), NSFCRGC Forum for Young Scholars(No.82411560273),the Natural Science Foundation of Jiangsu Province(No.BK20231118), National Science and Technology Innovation 2030 Major Program(No.2023ZD0508802).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Jinqiu Ran, Shengxia Yin, and Rahma Issa contributed equally.
Abbreviations: CCL2, chemokine ligand; CCR2, chemokine receptor 2; ECM, extracellular matrix; iNOS, inducible nitric oxide synthase; MDM, monocyte-derived macrophage; MMP, matrix metalloproteinase; ROS, reactive oxygen species.
Contributor Information
Jinqiu Ran, Email: 18324179627@163.com.
Shengxia Yin, Email: att717@163.com.
Rahma Issa, Email: Maghawri53@yahoo.com.
Qianwen Zhao, Email: cola@njmu.edu.cn.
Guangqi Zhu, Email: zhuguangqidoctor@163.com.
Huan Zhang, Email: zh2811080706@163.com.
Qun Zhang, Email: slim888@163.com.
Chao Wu, Email: dr.wu@nju.edu.cn.
Jie Li, Email: lijier@nju.edu.cn.
REFERENCES
- 1.Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18:151–166. [DOI] [PubMed] [Google Scholar]
- 2.Schwabe RF, Tabas I, Pajvani UB. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Baumgartner K, Bositis C. Cirrhosis: Diagnosis and management. Am Fam Physician. 2019;100:759–770. [PubMed] [Google Scholar]
- 6.Caligiuri A, Gentilini A, Pastore M, Gitto S, Marra F. Cellular and molecular mechanisms underlying liver fibrosis regression. Cells. 2021;10:2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao T, Wu W, Sui L, Huang Q, Nan Y, Liu J, et al. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact Mater. 2022;7:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heide D, Weiskirchen R, Bansal R. Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol. 2019;10:2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guillot A, Tacke F. Liver macrophages: Old dogmas and new insights. Hepatol Commun. 2019;3:730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nati M, Chung KJ, Chavakis T. The role of innate immune cells in nonalcoholic fatty liver disease. J Innate Immun. 2022;14:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran S, Baba I, Poupel L, Dussaud S, Moreau M, Gélineau A, et al. Impaired Kupffer cell self-renewal alters the liver response to lipid overload during non-alcoholic steatohepatitis. Immunity. 2020;53:627–640.e5. [DOI] [PubMed] [Google Scholar]
- 13.Sakai M, Troutman TD, Seidman JS, Ouyang Z, Spann NJ, Abe Y, et al. Liver-derived signals sequentially reprogram myeloid enhancers to initiate and maintain Kupffer cell identity. Immunity. 2019;51:655–670.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott CL, Zheng F, De Baetselier P, Martens L, Saeys Y, De Prijck S, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remmerie A, Martens L, Thoné T, Castoldi A, Seurinck R, Pavie B, et al. Osteopontin expression identifies a subset of recruited macrophages distinct from Kupffer cells in the fatty liver. Immunity. 2020;53:641–657.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blériot C, Barreby E, Dunsmore G, Ballaire R, Chakarov S, Ficht X, et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity. 2021;54:2101–2116.e6. [DOI] [PubMed] [Google Scholar]
- 17.De Simone G, Andreata F, Bleriot C, Fumagalli V, Laura C, Garcia-Manteiga JM, et al. Identification of a Kupffer cell subset capable of reverting the T cell dysfunction induced by hepatocellular priming. Immunity. 2021;54:2089–2100.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews TS, Atif J, Liu JC, Perciani CT, Ma XZ, Thoeni C, et al. Single-cell, single-nucleus, and spatial RNA sequencing of the human liver identifies cholangiocyte and mesenchymal heterogeneity. Hepatol Commun. 2022;6:821–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beattie L, Sawtell A, Mann J, Frame TCM, Teal B, de Labastida Rivera F, et al. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. [DOI] [PubMed] [Google Scholar]
- 21.Weston CJ, Zimmermann HW, Adams DH. The role of myeloid-derived cells in the progression of liver disease. Front Immunol. 2019;10:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sierro F, Evrard M, Rizzetto S, Melino M, Mitchell AJ, Florido M, et al. A liver capsular network of monocyte-derived macrophages restricts hepatic dissemination of intraperitoneal bacteria by neutrophil recruitment. Immunity. 2017;47:374–388. [DOI] [PubMed] [Google Scholar]
- 25.Guilliams M, Bonnardel J, Haest B, Vanderborght B, Wagner C, Remmerie A, et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell. 2022;185:379–396.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22:429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Investig. 2012;122:787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu K, Ma J, Zhan Y, Liu K, Ye Z, Chen J, et al. Down-regulation of microRNA-214 contributed to the enhanced mitochondrial transcription factor A and inhibited proliferation of colorectal cancer cells. Cell Physiol Biochem. 2018;49:545–554. [DOI] [PubMed] [Google Scholar]
- 29.Gong J, Li J, Dong H, Chen G, Qin X, Hu M, et al. Inhibitory effects of berberine on proinflammatory M1 macrophage polarization through interfering with the interaction between TLR4 and MyD88. BMC Complement Altern Med. 2019;19:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Zhang S, Jeon R, Vuckovic I, Jiang X, Lerman A, et al. Interferon gamma induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine. 2018;30:303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei W, Li ZP, Bian ZX, Han QB. Astragalus polysaccharide RAP induces macrophage phenotype polarization to M1 via the notch signaling pathway. Molecules. 2019;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. [DOI] [PubMed] [Google Scholar]
- 33.Gao S, Zhou J, Liu N, Wang L, Gao Q, Wu Y, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J Mol Cell Cardiol. 2015;85:131–139. [DOI] [PubMed] [Google Scholar]
- 34.Lu H, Wu L, Liu L, Ruan Q, Zhang X, Hong W, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem Pharmacol. 2018;154:203–212. [DOI] [PubMed] [Google Scholar]
- 35.Shouhed D, Steggerda J, Burch M, Noureddin M. The role of bariatric surgery in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2017;11:797–811. [DOI] [PubMed] [Google Scholar]
- 36.Funes SC, Rios M, Escobar-Vera J, Kalergis AM. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Zhang S, Wu H, Rong X, Guo J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol. 2019;106:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grinberg S, Hasko G, Wu D, Leibovich SJ. Suppression of PLCbeta2 by endotoxin plays a role in the adenosine A(2A) receptor-mediated switch of macrophages from an inflammatory to an angiogenic phenotype. Am J Pathol. 2009;175:2439–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–E3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, et al. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep. 2016;36:e00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells. 2020;9:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen Y, Lambrecht J, Ju C, Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell Mol Immunol. 2021;18:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233–242. [DOI] [PubMed] [Google Scholar]
- 44.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. [DOI] [PubMed] [Google Scholar]
- 45.Du P, Ma Q, Zhu ZD, Li G, Wang Y, Li QQ, et al. Mechanism of Corilagin interference with IL-13/STAT6 signaling pathways in hepatic alternative activation macrophages in schistosomiasis-induced liver fibrosis in mouse model. Eur J Pharmacol. 2016;793:119–126. [DOI] [PubMed] [Google Scholar]
- 46.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao XA, Chen GM, Liu Y, Chen YX, Wu HY, Chen J, et al. Inhibitory effect of silymarin on CCl(4)-induced liver fibrosis by reducing Ly6C(hi) monocytes infiltration. Int J Clin Exp Pathol. 2017;10:11941–11951. [PMC free article] [PubMed] [Google Scholar]
- 48.Wang YH, Twu YC, Wang CK, Lin FZ, Lee CY, Liao YJ. Niemann-pick type C2 protein regulates free cholesterol accumulation and influences hepatic stellate cell proliferation and mitochondrial respiration function. Int J Mol Sci. 2018;19:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saeed AFUH, Ruan X, Guan H, Su J, Ouyang S. Regulation of cGAS-mediated immune responses and immunotherapy. Adv Sci (Weinh). 2020;7:1902599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun R, Xiang Z, Wu B. T cells and liver fibrosis. PH&C. 2022;1:125–132. [Google Scholar]
- 51.Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396–403. [DOI] [PubMed] [Google Scholar]
- 52.Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, et al. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835–1844. [DOI] [PubMed] [Google Scholar]
- 53.Sun XF, Gu L, Deng WS, Xu Q. Impaired balance of T helper 17/T regulatory cells in carbon tetrachloride-induced liver fibrosis in mice. World J Gastroenterol. 2014;20:2062–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 Axis in Organ Fibrosis. Front Immunol. 2018;9:2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wynn TA, Hesse M, Sandler NG, Kaviratne M, Hoffmann KF, Chiaramonte MG, et al. P-selectin suppresses hepatic inflammation and fibrosis in mice by regulating interferon gamma and the IL-13 decoy receptor. Hepatology. 2004;39:676–687. [DOI] [PubMed] [Google Scholar]
- 56.Cai X, Li Z, Zhang Q, Qu Y, Xu M, Wan X, et al. CXCL6-EGFR-induced Kupffer cells secrete TGF-β1 promoting hepatic stellate cell activation via the SMAD2/BRD4/C-MYC/EZH2 pathway in liver fibrosis. J Cell Mol Med. 2018;22:5050–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bansal R, Prakash J, De Ruiter M, Poelstra K. Interferon gamma peptidomimetic targeted to hepatic stellate cells ameliorates acute and chronic liver fibrosis in vivo. J Control Release. 2014;179:18–24. [DOI] [PubMed] [Google Scholar]
- 58.Iwakiri Y. Nitric oxide in liver fibrosis: The role of inducible nitric oxide synthase. Clin Mol Hepatol. 2015;21:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. [DOI] [PubMed] [Google Scholar]
- 60.Li TH, Lee PC, Lee KC, Hsieh YC, Tsai CY, Yang YY, et al. Down-regulation of common NFκB-iNOS pathway by chronic Thalidomide treatment improves hepatopulmonary syndrome and muscle wasting in rats with biliary cirrhosis. Sci Rep. 2016;6:39405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah RA, Alkhouri N, Kowdley KV. Emerging drugs for the treatment of non-alcoholic steatohepatitis: A focused review of farnesoid X receptor agonists. Expert Opin Emerg Drugs. 2020;25:251–260. [DOI] [PubMed] [Google Scholar]
- 62.de Oliveira da Silva B, Ramos LF, Moraes KCM. Molecular interplays in hepatic stellate cells: Apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol Int. 2017;41:946–959. [DOI] [PubMed] [Google Scholar]
- 63.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61:416–426. [DOI] [PubMed] [Google Scholar]
- 65.Feng M, Ding J, Wang M, Zhang J, Zhu X, Guan W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int J Biol Sci. 2018;14:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu K, Qian Q, Zhou J, Sun D, Duan Y, Zhu X, et al. Regulatory T cells (Tregs) in liver fibrosis. Cell Death Discov. 2023;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ikeno Y, Ohara D, Takeuchi Y, Watanabe H, Kondoh G, Taura K, et al. Foxp3+ Regulatory T cells inhibit CCl(4)-induced liver inflammation and fibrosis by regulating tissue cellular immunity. Front Immunol. 2020;11:584048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roderfeld M, Rath T, Pasupuleti S, Zimmermann M, Neumann C, Churin Y, et al. Bone marrow transplantation improves hepatic fibrosis in Abcb4-/- mice via Th1 response and matrix metalloproteinase activity. Gut. 2012;61:907–916. [DOI] [PubMed] [Google Scholar]
- 69.Pellicoro A, Ramachandran P, Iredale JP. Reversibility of liver fibrosis. Fibrogenesis & Tissue Repair. 2012;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. [DOI] [PubMed] [Google Scholar]
- 71.Xu F, Guo M, Huang W, Feng L, Zhu J, Luo K, et al. Annexin A5 regulates hepatic macrophage polarization via directly targeting PKM2 and ameliorates NASH. Redox Biol. 2020;36:101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan BW, Liu YJ, Li XN, Han MM, Yu HY, Hong HY, et al. An autologous macrophage-based phenotypic transformation-collagen degradation system treating advanced liver fibrosis. Adv Sci (Weinh). 2024;11:2306899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moroni F, Dwyer BJ, Graham C, Pass C, Bailey L, Ritchie L, et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat Med. 2019;25:1560–1565. [DOI] [PubMed] [Google Scholar]
- 74.O’Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol. 2016;16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Gao M, Yang Z, Zhao Y, Guo K, Sun B, et al. Macrophages and metabolic reprograming in the tumor microenvironment. Front Oncol. 2022;12:795159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gao Y, Qian M, Wei Q, Duan X, Wang S, Hu H, et al. WZ66, a novel acetyl-CoA carboxylase inhibitor, alleviates nonalcoholic steatohepatitis (NASH) in mice. Acta Pharmacol Sin. 2020;41:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Francque S, Szabo G, Abdelmalek MF, Byrne CD, Cusi K, Dufour JF, et al. Nonalcoholic steatohepatitis: the role of peroxisome proliferator-activated receptors. Nat Rev Gastroenterol Hepatol. 2021;18:24–39. [DOI] [PubMed] [Google Scholar]
- 78.Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. [DOI] [PubMed] [Google Scholar]
- 79.Gilgenkrantz H, Mallat A, Moreau R, Lotersztajn S. Targeting cell-intrinsic metabolism for antifibrotic therapy. J Hepatol. 2021;74:1442–1454. [DOI] [PubMed] [Google Scholar]
- 80.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: Where are we (going)? Trends Immunol. 2017;38:395–406. [DOI] [PubMed] [Google Scholar]
- 81.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 82. Weng SY, Schuppan D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J Hepatol. 2013;58:619–621. [DOI] [PubMed] [Google Scholar]
- 83.Orfila C, Lepert JC, Alric L, Carrera G, Béraud M, Pipy B. Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem Cell Biol. 2005;123:585–593. [DOI] [PubMed] [Google Scholar]
- 84.Madsen BS, Trebicka J, Thiele M, Israelsen M, Arumugan M, Havelund T, et al. Antifibrotic and molecular aspects of rifaximin in alcoholic liver disease: Study protocol for a randomized controlled trial. Trials. 2018;19:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mathews S, Gao B. Therapeutic potential of interleukin 1 inhibitors in the treatment of alcoholic liver disease. Hepatology. 2013;57:2078–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulder P, van den Hoek AM, Kleemann R. The CCR2 inhibitor propagermanium attenuates diet-iInduced insulin resistance, adipose tissue inflammation and non-alcoholic steatohepatitis. PLoS One. 2017;12:e0169740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, et al. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44:1183–1198. [DOI] [PubMed] [Google Scholar]
- 89.de Oliveira FL, Carneiro K, Brito JM, Cabanel M, Pereira JX, Paiva LA, et al. Galectin-3, histone deacetylases, and Hedgehog signaling: Possible convergent targets in schistosomiasis-induced liver fibrosis. PLoS Negl Trop Dis. 2017;11:e0005137. [DOI] [PMC free article] [PubMed] [Google Scholar]