Abstract
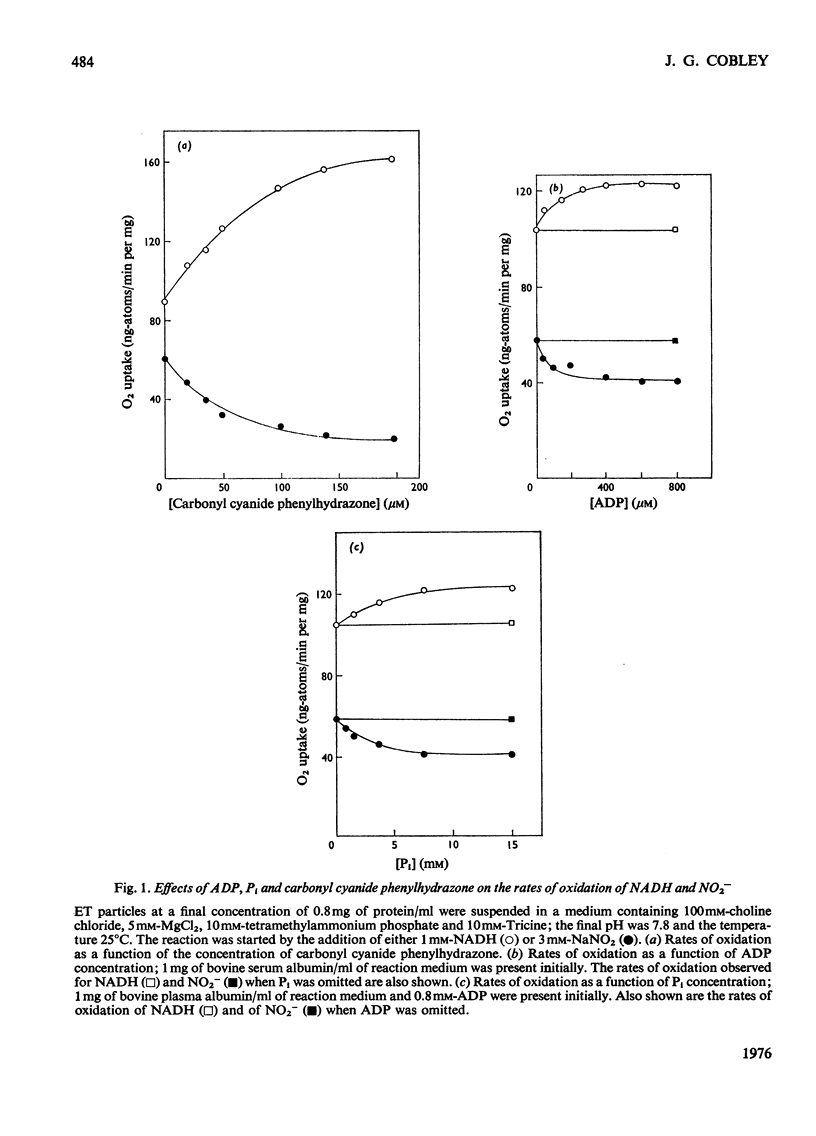
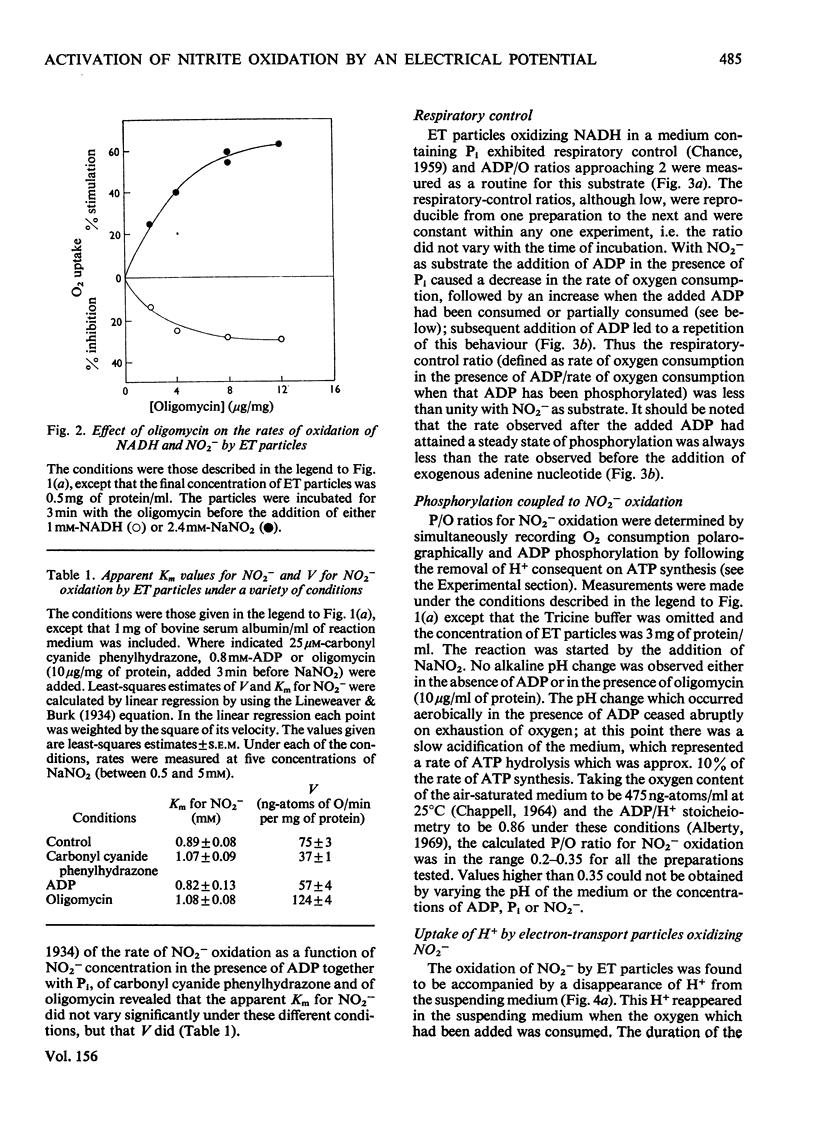
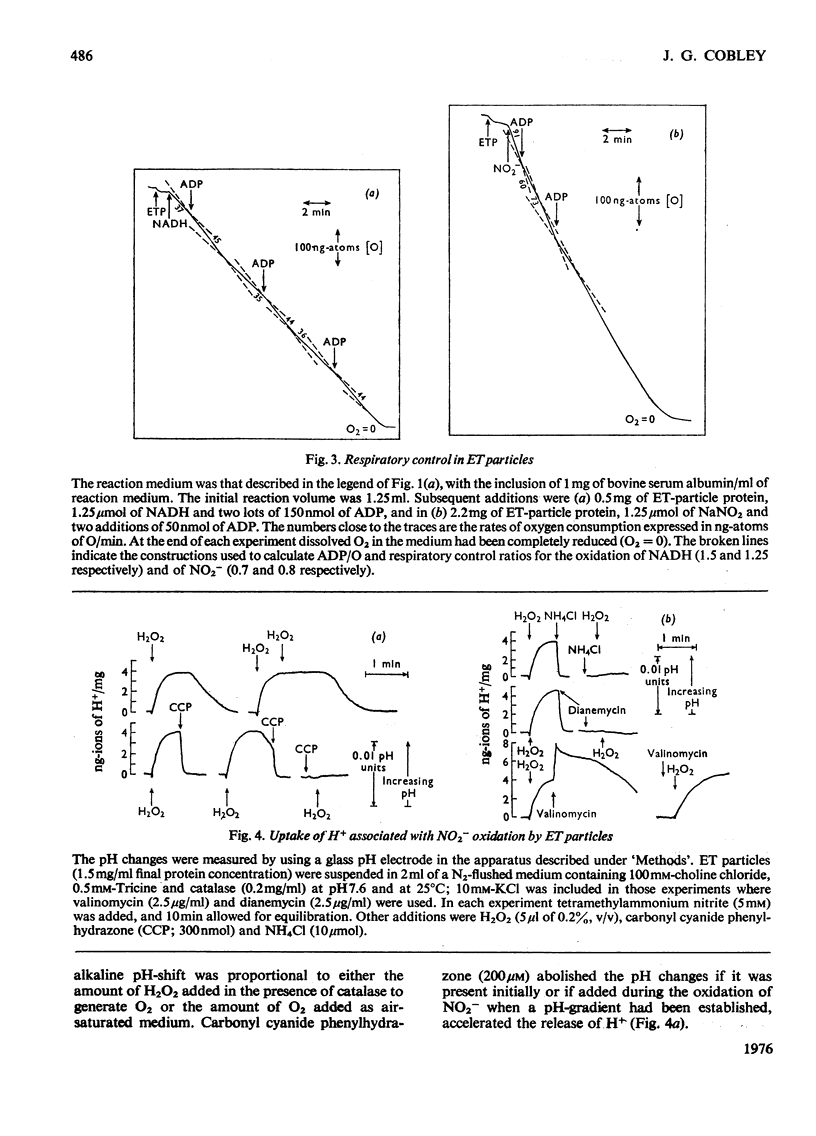
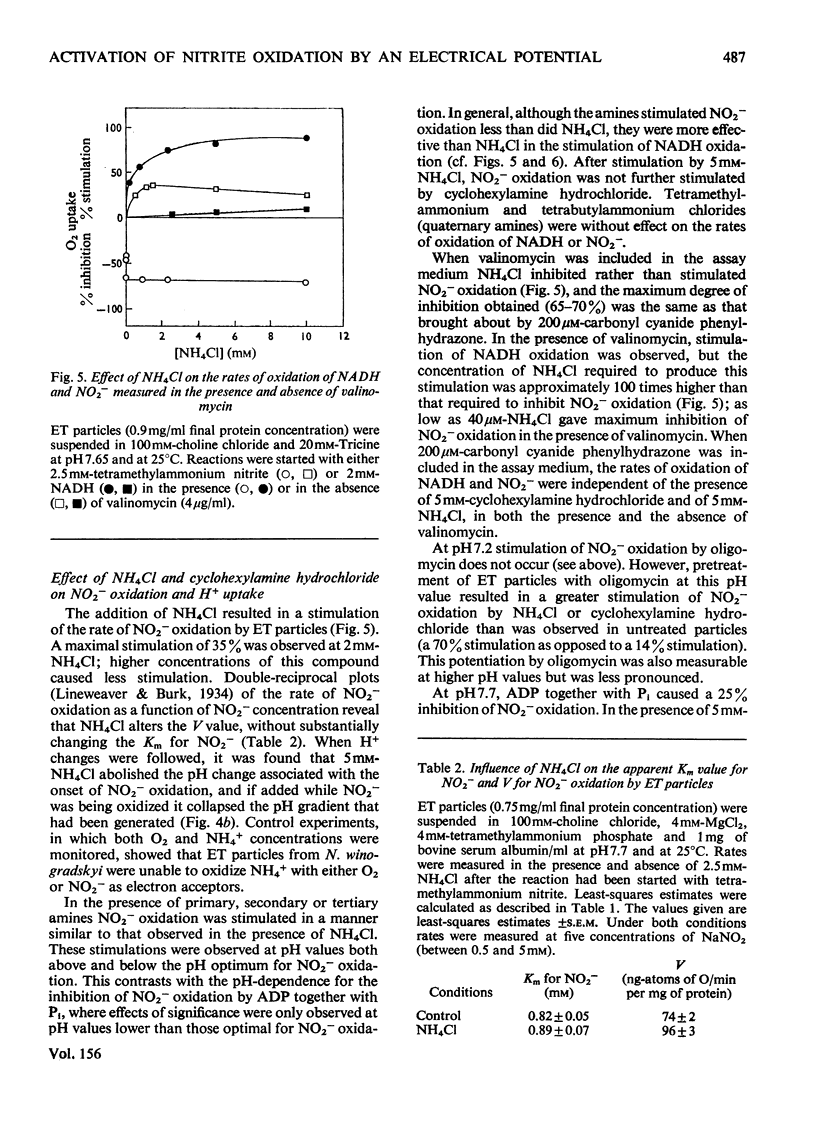
1. In electron-transport particles (ET particles) prepared from Nitrobacter winogradskyi, the uncoupling agent carbonyl cyanide phenylhydrazone increased the rate of NADH oxidation but decreased the rate of oxidation of NO2-. Its effectiveness in stimulating NADH oxidation closely paralleled its effectiveness in inhibiting NO2- oxidation. 2. In the presence of ADP and phosphate the oxidation of NADH was stimulated, whereas the oxidation of NO2- was inhibited. In the presence of excess of Pi the concentration dependence with respect to ADP was the same for acceleration of NADH oxidation and inhibition of NO2- oxidation. 3. Oligomycin inhibited NADH oxidation and stimulated the oxidation of NO2-. The concentration of oligomycin required to produce half-maximal effect in both systems was the same. 4. The apparent Km for NO2- was not affected by ADP together with Pi, by uncoupling agent or by oligomycin. 5. With NADH as substrate, classical respiratory control was observed. With NO2- as substrate the respiratory-control ratio was less than unity. 6. A reversible uptake of H+ accompanied the oxidation of NO2- by ET particles. 7. In the presence of NH4Cl or cyclohexylamine hydrochloride, H+ uptake was abolished and increased rates of NO2- oxidation were observed. When valinomycin was present in the reaction medium, low concentrations of NH4Cl inhibited NO2- oxidation. 8. Pretreatment of ET particles with oligomycin enhanced the stimulation of NO2- oxidation induced by NH4Cl or by cyclohexylamine hydrochloride. Pretreatment with the uncoupler carbonyl cyanide phenylhydrazone prevented these stimulations. 9. In the presence of dianemycin together with K+, the uptake of H+ was abolished and the rate of NO2- oxidation was increased. In contrast, in the presence of valinomycin together with K+, the uptake of H+ was increased, and the rate of NO2- oxidation decreased. 10. Sodium tetraphenylboron was found to be an inhibitor of NO2- oxidation, but caused a stimulation of NADH oxidation which was dependent on the presence of NH4Cl or cyclohexylamine hydrochloride. 11. It is concluded that the enhanced rate of NO2- oxidation observed in the absence of energy-dissipating processes clearly relates to some state before the involvement of adenine nucleotides, and it is suggested that the oxidation of NO2- generates a protonmotive force, the electrical component of which controls the rate of NO2- oxidation.
Full text
PDF


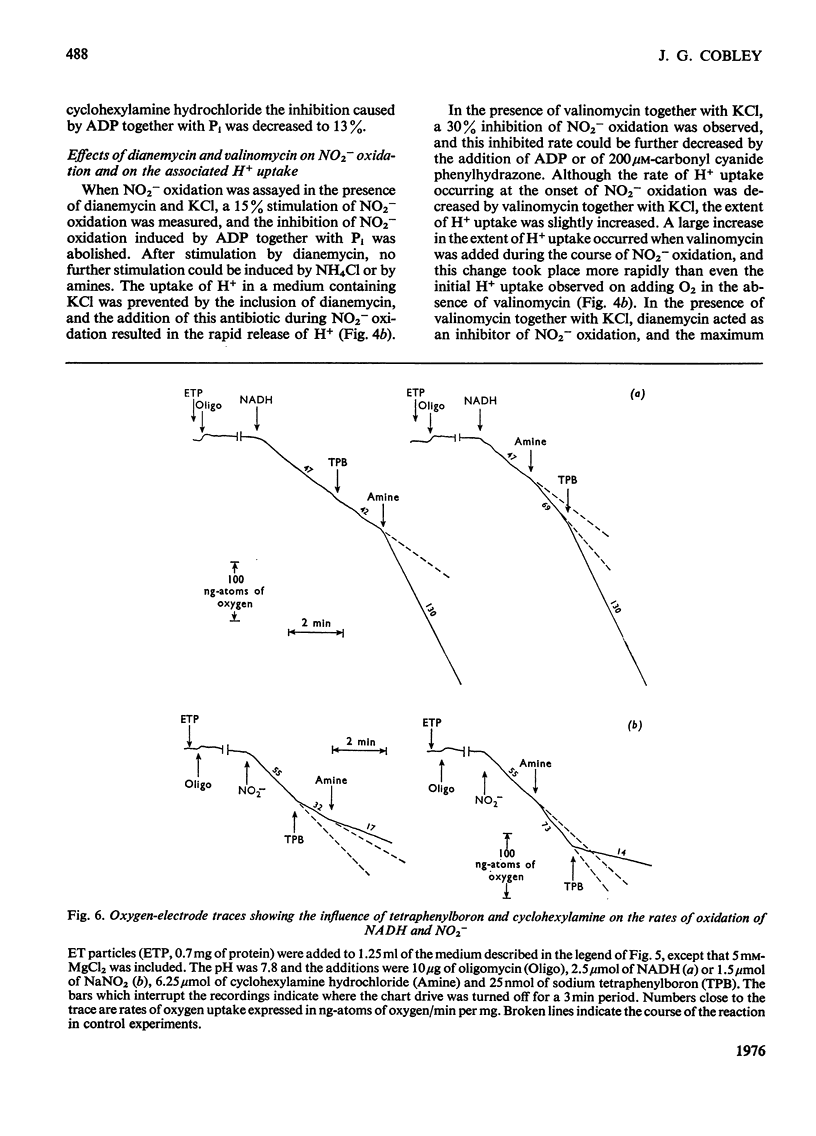
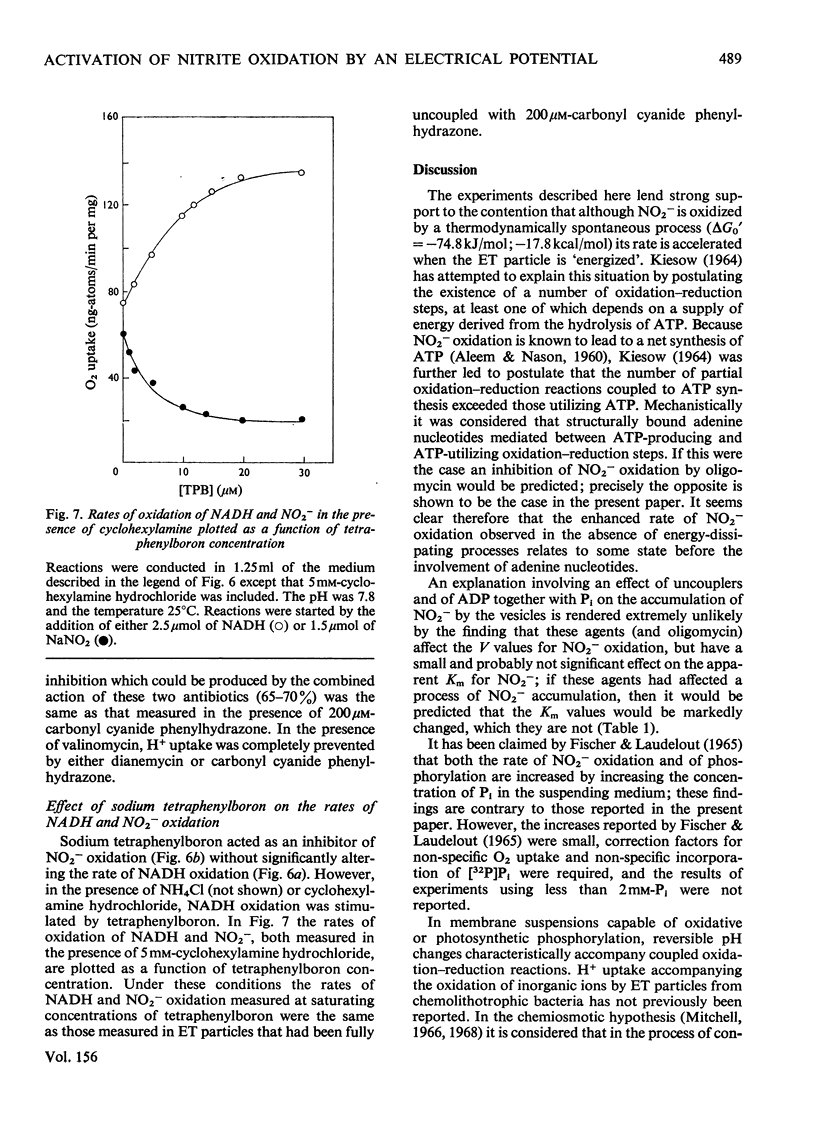


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEEM M. I., ALEXANDER M. Cell-free nitrification by Nitrobacter. J Bacteriol. 1958 Nov;76(5):510–514. doi: 10.1128/jb.76.5.510-514.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Aleem M. I., Nason A. PHOSPHORYLATION COUPLED TO NITRITE OXIDATION BY PARTICLES FROM THE CHEMOAUTOTROPH, NITROBACTER AGILIS. Proc Natl Acad Sci U S A. 1960 Jun;46(6):763–769. doi: 10.1073/pnas.46.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem M. I. Path of carbon and assimilatory power in chemosynthetic bacteria. I. Nitrobacter agilis. Biochim Biophys Acta. 1965 Aug 24;107(1):14–28. doi: 10.1016/0304-4165(65)90384-3. [DOI] [PubMed] [Google Scholar]
- BECK J. V., SHAFIA F. M. EFFECT OF PHOSPHATE ION AND 2,4-DINITROPEHENOL ON THE ACTIVITY OF INTACT CELLS OF THIOBACILLUS FERROOXIDANS. J Bacteriol. 1964 Oct;88:850–857. doi: 10.1128/jb.88.4.850-857.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT W. D., LEES H. Nitrite oxidation by Nitrobacter in the presence of certain nitrophenols. Nature. 1960 Oct 8;188:147–148. doi: 10.1038/188147b0. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOOD N. E. Activation of the Hill reaction by amines. Biochim Biophys Acta. 1960 Jun 3;40:502–517. doi: 10.1016/0006-3002(60)91391-3. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- KIESOW L. ON THE ASSIMILATION OF ENERGY FROM INORGANIC SOURCES IN AUTOTROPHIC FORMS OF LIFE. Proc Natl Acad Sci U S A. 1964 Oct;52:980–988. doi: 10.1073/pnas.52.4.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIESOW L. UBER DIE REDUKTION VON DIPHOSPHOPYRIDINNUCLEOTID BEI DER CHEMOSYNTHESE. Biochem Z. 1963;338:400–406. [PubMed] [Google Scholar]
- Krogmann D. W., Jagendorf A. T., Avron M. Uncouplers of Spinach Chloroplast Photosynthetic Phosphorylation. Plant Physiol. 1959 May;34(3):272–277. doi: 10.1104/pp.34.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudelout H., Simonart P. C., van Droogenbroeck R. Calorimetric measurement of free energy utilization by Nitrosomonas and Nitrobacter. Arch Mikrobiol. 1968;63(3):256–277. doi: 10.1007/BF00412842. [DOI] [PubMed] [Google Scholar]
- Liberman E. A., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. IV. General discussion. Biochim Biophys Acta. 1970 Aug 4;216(1):30–42. doi: 10.1016/0005-2728(70)90156-8. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Tsien H. C., Laudelout H. Minimal size of Nitrobacter membrane fragments retaining nitrite oxidizing activity. Arch Mikrobiol. 1968 May 8;61(3):280–291. doi: 10.1007/BF00446613. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Crofts A. R. Photosynthesis. Annu Rev Biochem. 1970;39:389–428. doi: 10.1146/annurev.bi.39.070170.002133. [DOI] [PubMed] [Google Scholar]


