Abstract
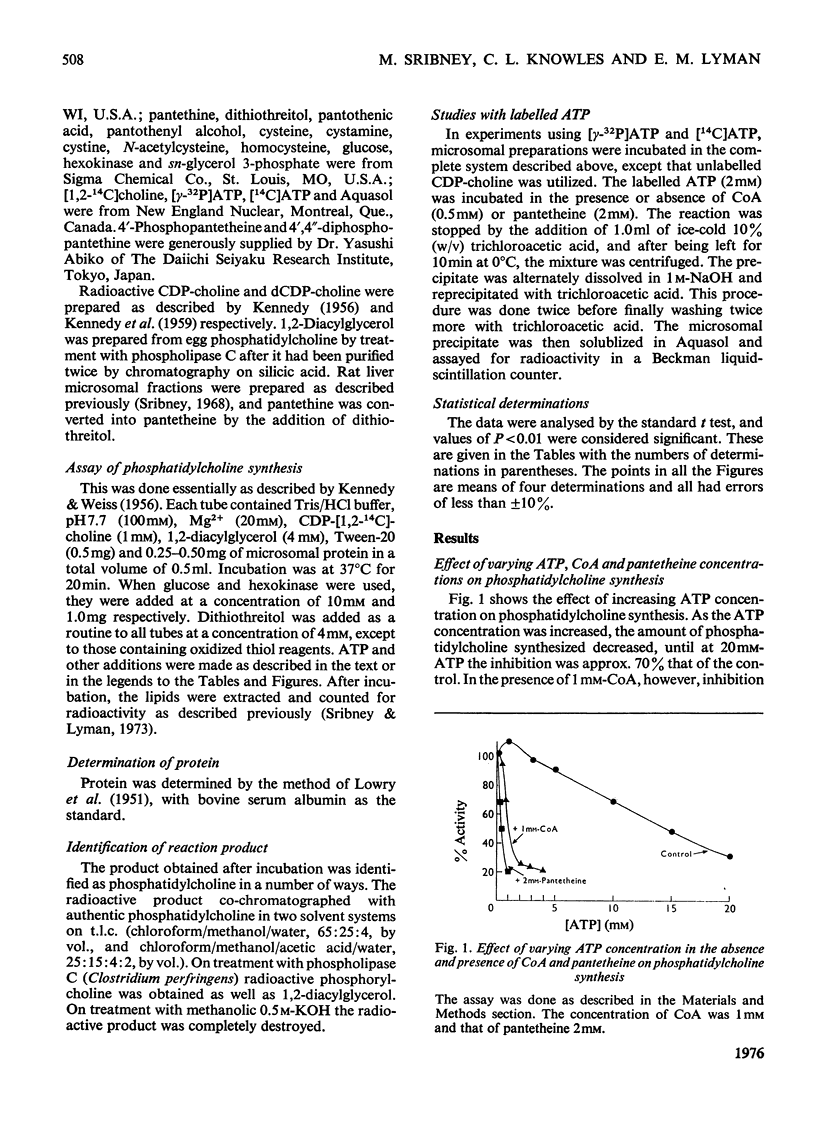
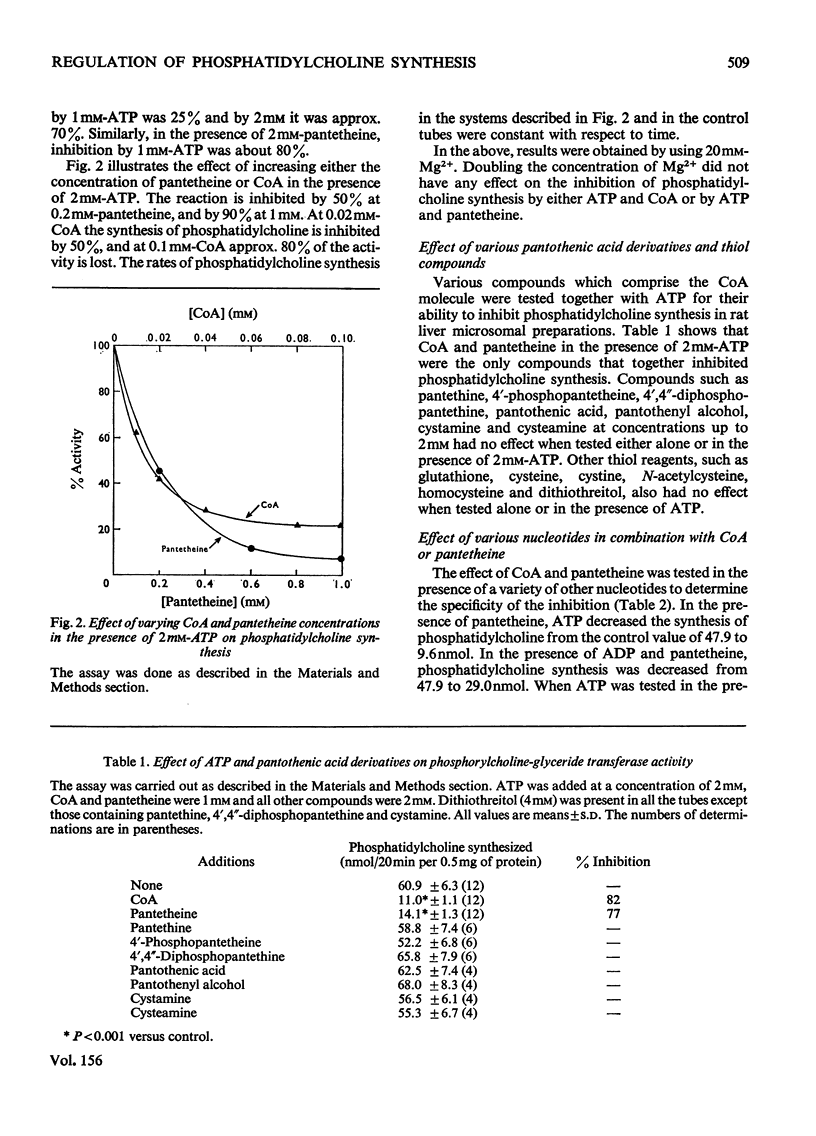
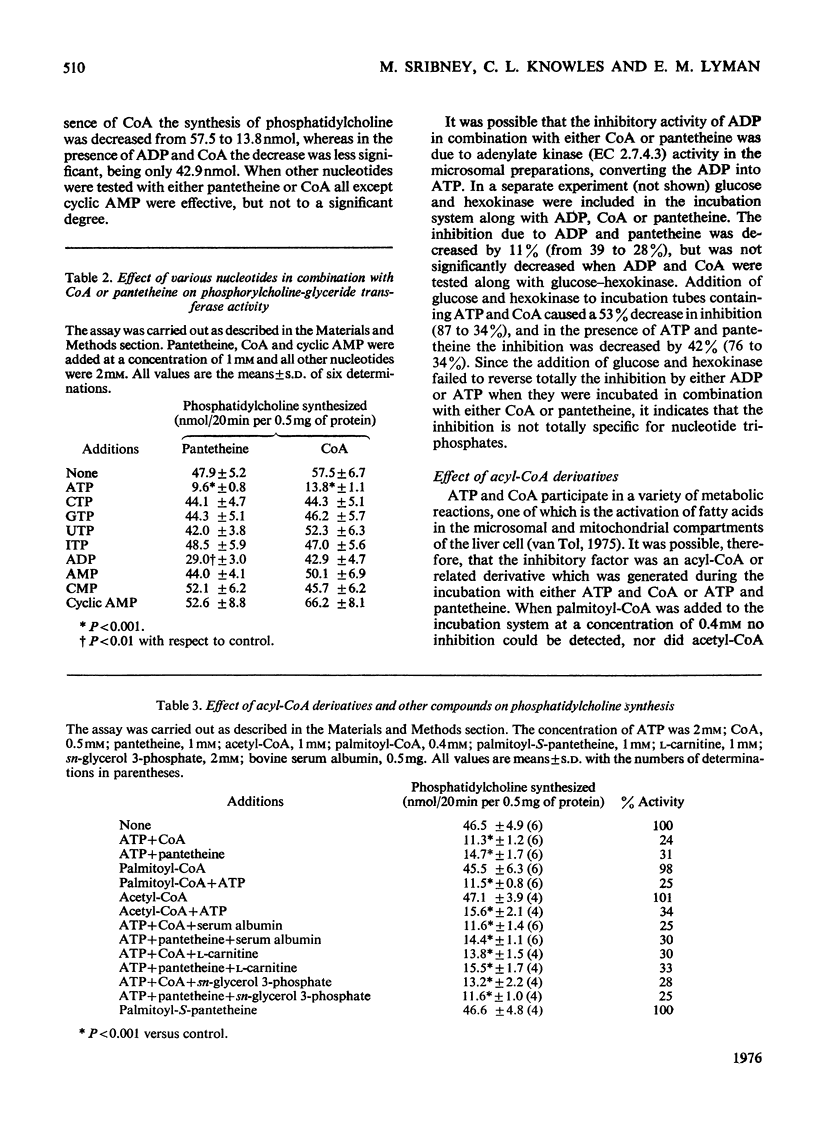
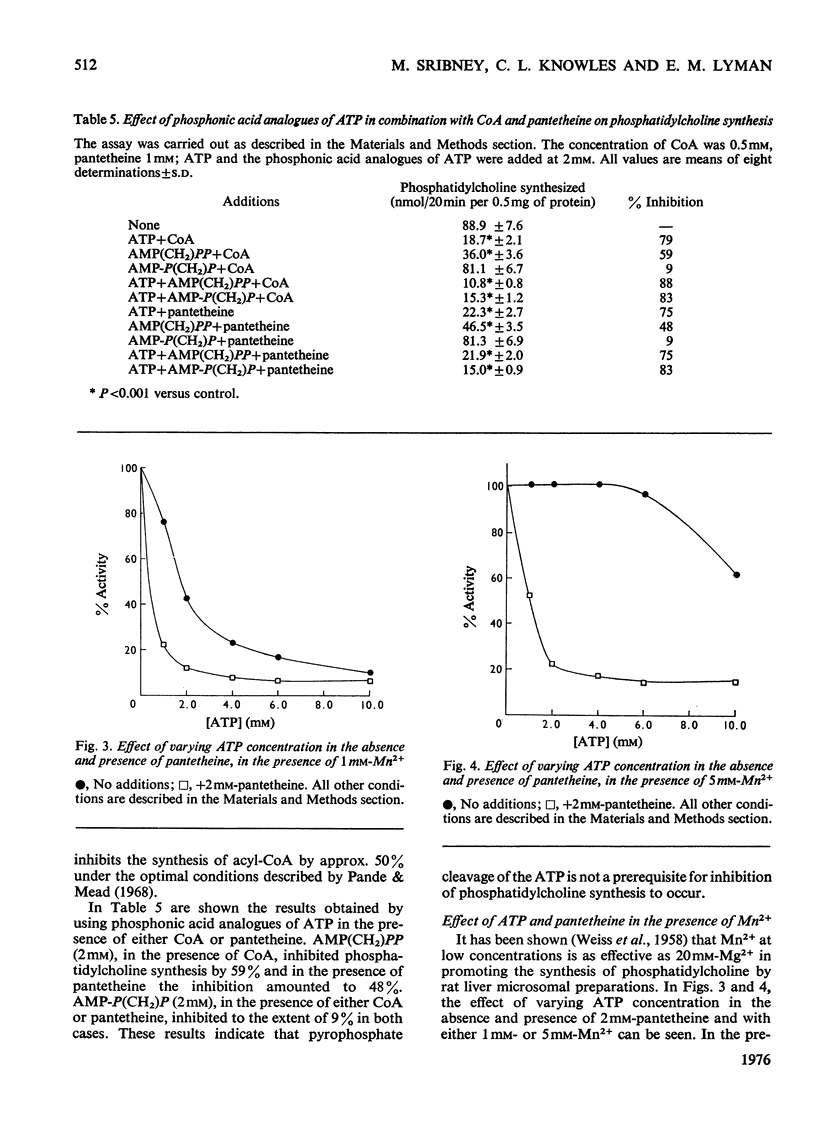
The biosynthesis of phosphatidylcholine in rat liver microsomal preparations catalysed by CDP-choline-1,2-diacylglycerol cholinephosphotransferase (EC 2.7.8.2) was inhibited by a combination of ATP and CoA or ATP and pantetheine. ATP alone at high concentrations (20 mM) inhibits phosphatidylcholine formation to the extent of 70%. In the presence of 0.1 mM-CoA, ATP (2 mM) inhibits to the extent of 80% and in the presence of 1 mM-pantetheine to the extent of 90%. ADP and other nucleotide triphosphates in combination with either CoA or pantetheine are only 10-30% as effective in inhibiting phosphatidylcholine synthesis. AMP(CH2)PP [adenosine 5'-(alphabeta-methylene)triphosphate] together with CoA inhibits to the extent of 59% and with pantetheine by 48%. AMP-P(CH2)P [adenosine 5'-(betagamma-methylene)triphosphate] together with either CoA or pantetheine had no significant effect on phosphatidylcholine formation. Other closely related derivatives of pantothenic acid were without effect either alone or in the presence of ATP, as were thiol compounds such as cysteine, homocysteine, cysteamine, dithiothreitol and glutathione. Several mechanisms by which this inhibition might take place were ruled out and it is concluded that ATP together with either CoA or pantetheine interacts reversibly with phosphatidylcholine synthetase to cause temporarily the inhibition of phosphatidylcholine formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Tana J., Rose G., Shapiro B. The purification and properties of microsomal palmitoyl-coenzyme A synthetase. Biochem J. 1971 Apr;122(3):353–362. doi: 10.1042/bj1220353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barden R. E., Cleland W. W. 1-Acylglycerol 3-phosphate acyltransferase from rat liver. J Biol Chem. 1969 Jul 10;244(13):3677–3684. [PubMed] [Google Scholar]
- DILS R. R., HUBSCHER G. Metabolism of phospholipids. III. The effect of calcium ions on the incorporation of labelled choline into rat-liver microsomes. Biochim Biophys Acta. 1961 Jan 29;46:505–513. doi: 10.1016/0006-3002(61)90581-9. [DOI] [PubMed] [Google Scholar]
- De Kruyff B., Van Golde L. M., Van Deenen L. L. Utilization of diacylglycerol species by cholinephosphotransferase, ethanolaminephosphotransferase and diacylglycerol acyltransferase in rat liver microsomes. Biochim Biophys Acta. 1970 Sep 8;210(3):425–435. doi: 10.1016/0005-2760(70)90038-x. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B. CARNITINE AND ITS ROLE IN FATTY ACID METABOLISM. Adv Lipid Res. 1963;1:285–334. [PubMed] [Google Scholar]
- Fiscus W. G., Schneider W. C. The role of phospholipids in stimulating phosphorylcholine cytidyltransferase activity. J Biol Chem. 1966 Jul 25;241(14):3324–3330. [PubMed] [Google Scholar]
- Gatt S., Barenholz Y. Enzymes of complex lipid metabolism. Annu Rev Biochem. 1973;42(0):61–90. doi: 10.1146/annurev.bi.42.070173.000425. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., BROCKERHOFF H., BARRON E. J. The site of attack of phospholipase (lecithinase) A on lecithin: a re-evaluation. Position of fatty acids on lecithins and triglycerides. J Biol Chem. 1960 Jul;235:1917–1923. [PubMed] [Google Scholar]
- KENNEDY E. P., BORKENHAGEN L. F., SMITH S. W. Possible metabolic functions of deoxycytidine diphosphate choline and deoxycytidine diphosphate ethanolamine. J Biol Chem. 1959 Aug;234(8):1998–2000. [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- KENNEDY E. P. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem. 1956 Sep;222(1):185–191. [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J Biol Chem. 1960 Aug;235:2233–2237. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOVELLI G. D., SCHMETZ F. J., Jr, KAPLAN N. O. Enzymatic degradation and resynthesis of coenzyme A. J Biol Chem. 1954 Feb;206(2):533–545. [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Long chain fatty acid activation in subcellular preparations from rat liver. J Biol Chem. 1968 Jan 25;243(2):352–361. [PubMed] [Google Scholar]
- Pande S. V. Reversal by CoA of palmityl-CoA inhibition of long chain acyl-CoA synthetase activity. Biochim Biophys Acta. 1973 Apr 13;306(1):15–20. doi: 10.1016/0005-2760(73)90202-6. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Meiners B., Mudd J. B. Regulation by cytidine nucleotides of the acylation of sn-(14C)glycerol 3-phosphate. Regional and subcellular distribution of the enzymes responsible for phosphatidic acid synthesis de novo in the central nervous system of the rat. Biochem J. 1973 Mar;132(3):381–394. doi: 10.1042/bj1320381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. R., Alexandre A., Galzigna L., Sartorelli L., Gibson D. M. 4'-phosphopantetheine, a cofactor bound to guanosine triphosphate-dependent acyl coenzyme A synthetase. J Biol Chem. 1970 Jun;245(12):3110–3114. [PubMed] [Google Scholar]
- SPITZER H. L., KYRIAKIDES E. C., BALINT J. A. BILIARY PHOSPHOLIPIDS IN VARIOUS SPECIES. Nature. 1964 Oct 17;204:288–288. doi: 10.1038/204288a0. [DOI] [PubMed] [Google Scholar]
- Segal H. L. Enzymatic interconversion of active and inactive forms of enzymes. Science. 1973 Apr 6;180(4081):25–32. doi: 10.1126/science.180.4081.25. [DOI] [PubMed] [Google Scholar]
- Shribney M. Stimulation and inhibition of sphingomyelin synthetase. Arch Biochem Biophys. 1968 Sep 10;126(3):954–955. doi: 10.1016/0003-9861(68)90489-x. [DOI] [PubMed] [Google Scholar]
- Sribney M., Lyman E. M. Stimulation of phosphorylcholine-glyceride transferase activity by unsaturated fatty acids. Can J Biochem. 1973 Nov;51(11):1479–1486. doi: 10.1139/o73-196. [DOI] [PubMed] [Google Scholar]
- Taketa K., Pogell B. M. The effect of palmityl coenzyme A on glucose 6-phosphate dehydrogenase and other enzymes. J Biol Chem. 1966 Feb 10;241(3):720–726. [PubMed] [Google Scholar]
- Tol V. A. Aspects of long-chain acyl-COA metabolism. Mol Cell Biochem. 1975 Apr 30;7(1):19–31. doi: 10.1007/BF01732160. [DOI] [PubMed] [Google Scholar]
- Trams E. G., Stahl W. L., Robinson J. Formation of S-acyl pantetheine from acyl-coenzyme A by plasma membranes. Biochim Biophys Acta. 1968 Dec 10;163(4):472–482. doi: 10.1016/0005-2736(68)90076-x. [DOI] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Saturated fatty acid biosynthesis and its regulation. Annu Rev Biochem. 1973;42:21–60. doi: 10.1146/annurev.bi.42.070173.000321. [DOI] [PubMed] [Google Scholar]
- WEISS S. B., KENNEDY E. P., KIYASU J. Y. The enzymatic synthesis of triglycerides. J Biol Chem. 1960 Jan;235:40–44. [PubMed] [Google Scholar]
- WEISS S. B., SMITH S. W., KENNEDY E. P. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958 Mar;231(1):53–64. [PubMed] [Google Scholar]


