Abstract
Jasminum subtriplinerve Blume tea is a traditional Vietnamese medicine used to treat impetigo, menstruation issues, and painful menstrual hematometra. Previous studies have shown that extracts and isolated compounds from J. subtriplinerve possess diverse pharmacological properties, such as antioxidant, antibacterial, and antidiabetic effects. However, their potential anticancer effects and underlying mechanisms of action have not been clear. Here, we examined the effects of J. subtriplinerve extracts against three human cancer cell lines. We also conducted in vivo analyses using a mouse model of 7,12-dimethylbenz[a]anthracene-induced breast cancer, including an investigation of changes in histological sections. The effect of the J. subtriplinerve ethyl acetate fraction on cytokine levels (IL-2, PGE2, TNF-α) in serum was determined using ELISA kits. Results showed that the ethyl acetate (EtOAc) fraction had the highest anti-proliferative activity (IC50 = 13.7 mg/ml) against the breast cancer (MCF-7) cell line, while the butanol (BuOH) and water fractions did not show any anticancer effects. Additionally, the EtOAc fraction at a dose of 14.4 mg/kg was able to elevate IL-2 levels and suppress the expression of PGE2 in the serum of mice. A remarkable decrease in the percentage of death and tumor incidence in mice was achieved following treatment with the EtOAc fraction at a dose of 14.4mg/kg. No abnormal parameters in blood were observed in the J. subtriplinerve treatment groups. These results suggest that J. subtriplinerve, when used as tea or a functional food, is nontoxic and has clear chemopreventive effects against breast cancer.
Keywords: Jasminium subtriplinerve Blume, breast cancer, DMBA, IL-2
Introduction
Natural compounds play a crucial role in the development of functional foods and in the early stages of screening for, and the evaluation and development of, new drugs [1]. Such compounds tend to be cost-effective, safe, and efficient, making them highly valuable for use in health supplements and in efforts to identify novel drugs. By leveraging the benefits of natural substances, researchers aim to innovate and improve treatments while ensuring accessibility and benefits for a wide range of individuals.
For example, green tea and coffee contain bioactive compounds that help treat various conditions, including cardiovascular diseases and certain types of cancer [2]. The antioxidants present in these natural products help reduce inflammation and oxidative stress, thereby promoting overall health. Ongoing research into such natural compounds continues to reveal their potential in preventive and therapeutic applications, demonstrating that the integration of natural substances into modern medicine and functional foods can significantly improve public health. This approach enhances treatment efficacy while ensuring safety and affordability.
Cancer remains the primary cause of death worldwide [3]. According to 2019 data from the World Health Organization (WHO), cancer is either the primary or secondary cause of premature death (before the age of 70) in 112 of 183 nations worldwide; it is also ranked third or fourth in an additional 23 countries [4]. Consequently, pharmaceutical companies and independent research institutions around the world prioritize the development of novel cancer drugs. Surgical, chemotherapeutic, radiotherapeutic, targeted, and immunological therapies are currently used to treat cancer [5]. The main goal of chemotherapy is to either completely destroy malignant cells or convert them into benign cells without concurrently damaging healthy cells. Thus, there is an urgent need for the creation of innovative, safe, and effective chemopreventive drugs [6]. Accordingly, the use of medicinal plants as a natural drug source is important.
Jasminum subtriplinerve Blume belongs to the jasmine family (Oleaceae) and is widely distributed, particularly in southern Asia [7] (including the southern provinces of China and Hainan Island). Several studies have investigated the chemical composition of J. subtriplinerve, which includes the following main components: terpene glycosides, flavonoid glycosides, phenylethanoid glycosides, phenylpropanoid glycosides, steroids, triterpenoids, and phenolics [7-9]. In traditional Vietnamese medicine, J. subtriplinerve is believed to have various effects such as clearing heat, relieving rheumatism, activating blood circulation, regulating menstruation, and reducing inflammation. It is used to treat conditions such as amenorrhea, metritis, mastitis, rheumatism, and jaundice. In Vietnam, J. subtriplinerve is widely utilized for multiple purposes including improving liver function, increasing bile secretion, stimulating digestion, enhancing appetite, and promoting good sleep. Moreover, it is used to treat rheumatism, bone and joint pain, and skin diseases, as well as snake bites. Its leaves are primarily used to address irregular menstruation, high postpartum fever in women, lymphadenitis, metritis, mastitis, breast abscess, vaginal discharge, rheumatism-induced bone and joint pain, scabies, sores, impetigo, and itchy skin [8-10]. Numerous studies regarding the pharmacological effects of green tea have demonstrated that it has antibacterial, anti-inflammatory, antioxidant, and cell stimulation effects [7]. J. subtriplinerve, like green tea, is used daily by local people in Asian countries for similar traditional medicinal purposes. However, their potential anticancer effects and underlying mechanisms of action are unclear.
Here, we explored the effects of extracts of J. subtriplinerve leaves on three human cancer cell lines using the MTT assay, as reported in previous studies [11, 12]. We also conducted in vivo analyses using a mouse model of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer, including an investigation of changes in histological sections.
Material and Methods
Materials
Leaves of J. subtriplinerve were collected from Nghe An province, Vietnam, in August 2022 and taxonomically identified by Dr. Ngo Duc Phuong. The voucher specimen (accession 2301/TVN-DV) is stored at the Herbarium of the Institute of Traditional Medicine Science in Hanoi, Vietnam.
Sample Preparation
Ethanol extract of Jasminum subtriplinerve. Dried leaves of J. subtriplinerve (7.5 kg) were extracted by reflux with 70% ethanol (three times, 3 h each) at 80°C. The extracts were filtered, combined, and evaporated under reduced pressure, resulting in a green residue (1125.0 g), which was then suspended in water and sequentially partitioned with n-hexane, ethyl acetate (EtOAc), and n-butanol (BuOH). The fractionated extracts were pooled and evaporated under reduced pressure to yield the respective fractions: an n-hexane extract weighing 127.5 g, an EtOAc extract weighing 183 g, an n-BuOH extract weighing 155 g, and a water layer obtained after solvent removal.
Isolation major compounds. Based on liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) screening and thin layer chromatography analysis combined with bioassay guidance (Fig. 1), the EtOAc extract was selected for further separation of bioactive compounds. Using combined chromatographic separation techniques, major compounds were isolated from the extract. Briefly, the EtOAc fraction (36.6 g) was subjected to silica gel column chromatography (CC) and eluted with CHCl3/MeOH (100:1, 60:1, 40:1, 10:1, and 2:1) to produce six fractions (E1–E6). Compound 1 (500.8 mg) was obtained after fraction E5 had been subjected to silica gel CC and elution with CHCl3/MeOH/H2O (4:1:0.1, v/v/v), followed by purification on the Sephadex LH-20 CC system using MeOH/H2O (4:1, v/v). The structure was identified via NMR analysis, including 1D and 2D NMR, and HR-ESI-MS data.
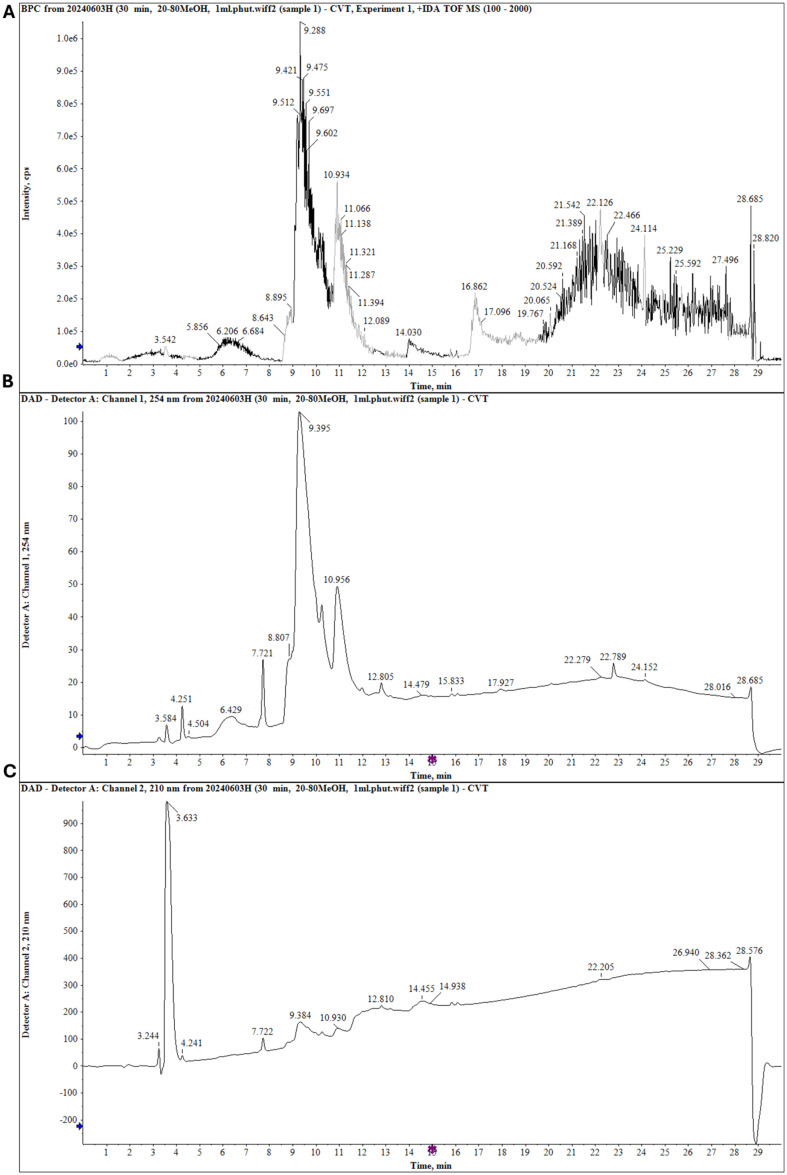
Fig. 1. Screening secondary metabolites from an MeOH extract of J. subtriplinerve using LC-QTOF MS/MS in positive mode.
(A) Positive mass (MS) chromatogram. (B) Ultraviolet (UV; 254 nm) chromatogram. (C) Ultraviolet (UV; 210 nm) chromatogram of the MeOH extract from J. subtriplinerve.
Acteoside: yellow powder; 1H-NMR (600 MHz, CD3OD) δH: 4.40 (d, J = 8.0 Hz, H-1), 3.41 (dd, J = 9.0, 8.0 Hz, H-2), 3.85 (1H, t, J = 9.0 Hz, H-3), 4.95 (t, J = 9.5 Hz, H-4), 3.55 (m, H-5), 3.56 (1H, m, H-6α), 3.64 (d, J =10.0 Hz, H-6β), 5.20 (d, J = 1.5 Hz, H-1'), 3.95 (dd, J = 3.1, 1.8 Hz, H-2'), 3.60 (m, H-3'), 3.32 (m, H-4'), 3.60 (m, H-5'), 1.11 (3H, d, J = 6.2 Hz, H-6'), 7.08 (d, J = 2.0 Hz, H-2''), 6.80 (d, J = 8.2 Hz, H-5''), 6.97 (dd, J = 8.0, 2.0 Hz, H-6''), 7.62 (d, J = 16.0 Hz, H-7''), 6.30 (d, J = 16.0 Hz, H-8''), 6.72 (d, J = 2.0 Hz, H-2'''), 6.70 (d, J = 8.0 Hz, H-5'''), 6.55 (dd, J = 8.0, 2.0 Hz, H-6'''), 2.81 (2H, dd, J = 13.0, 6.7 Hz, H-7'''), 3.74 (m, H-8α'''), 4.05 (ddd, J = 9.5, 8.5, 7.0 Hz, H-8β'''); 13C-NMR (150 MHz, CD3OD) δC: 104.2 (C-1), 76.0 (C-2), 81.6 (C-3), 70.6 (C-4), 76.2 (C-5), 62.3 (C-6), 103.0 (C-1'), 72.3 (C-2'), 70.4 (C-3'), 73.8 (C-4'), 72.0 (C-5'), 18.4 (C-6'), 127.6 (C-1''), 115.2 (C-2''), 146.8 (C-3''), 149.8 (C-4''), 116.5 (C-5''), 123.2 (C-6''), 148.0 (C-7''), 114.7 (C-8''), 168.3 (C-9''), 131.5 (C-1'''), 117.1 (C-2'''), 146.1 (C-3'''), 144.7 (C-4'''), 116.3 (C-5'''), 121.3 (C-6'''), 36.5 (C-7'''), 72.2 (C-8'''); HR-ESI-MS m/z 623.1980 [M-H]- (Calcd. 623.1981).
Extract preparation for oral gavage. For in vivo experiments, dried extracts of J. subtriplinerve were ground into powder, which was diluted with distilled water to achieve final concentrations of 4.8 g/kg and 14.4 g/kg.
HPLC Analysis via LC-Q-TOF MS/MS
The major secondary metabolites from J. subtriplinerve were screened in accordance with previously reported methods [13, 14]. Briefly, HR-QTOF-MS/MS was performed on an X500 QTOF mass spectrometer (High Performance Benchtop Instruments). The extract was separated using a Capcell Pak C18 analysis column. Solvent A (0.1% v/v formic acid in H2O) and solvent B (0.1% v/v formic acid in MeOH) were chosen for the mobile phase. The gradient protocol consisted of increasing MeOH from 20% to 80% over 30 min, with a flow rate of 0.6 ml/min and injection volume of 10.0 μl.
Cytotoxicity Measurement
The cytotoxic effects of the extract on cancer cell lines were assessed using an MTT assay [15-17]. Briefly, three cancer cell lines, including MCF-7 (human breast cancer), SK-LU-1 (human lung adenocarcinoma), and HepG2 (human hepatoblastoma), were seeded in 96-well plates (Corning, USA) with RPMI medium. After treatment with several amounts of ethanol extract and its fractions (EtOAc, BuOH, and water fractions), the cells were incubated for 48 h at 37°C with 5% CO2. Independent experiments were carried out at least three times. Ellipticine was used as a positive control. Data are presented as means ± standard errors (SEs).
The degree of cell inhibition was computed as follows:
where OD (blank) is the optical density of the well containing cancer cells without any reagent. IC50 values were calculated to evaluate the inhibitory effects of the samples.
Growth Rate Assay
MCF7 cells were cultured in DMEM supplemented with penicillin G sodium (100 units/ml), streptomycin sulfate (100 μg/ml), amphotericin B (0.25 μg/ml), and 10% fetal bovine serum (FBS). MCF-7 cells were seeded into 12-well plates at a density of 1 × 105 cells per well and then treated with the IC50 of the EtOAc fraction or the IC50 of ellipticine. Vehicle wells were treated with an equal volume of culture medium. At 2, 6, 24, and 48 h after treatment, live cells were collected and counted using 0.4% trypan blue staining. The number of cells at each time point was recorded.
Mouse Model of DMBA-Induced Cancer
Animals. Female Swiss albino mice aged 7–8 weeks were purchased from the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, and maintained in the animal center of the Department of Pharmacology, University of Medicine and Pharmacy, Vietnam National University, Hanoi, Vietnam, under pathogen-free conditions. All mice were provided adequate food and water daily. The experimental protocol was approved by the Institutional Animal Ethics Committee of the University of Medicine and Pharmacy, Vietnam National University (Approval number: QG.22.69).
Experimental design. Breast tumors were induced using multiple doses of DMBA, which was purchased from Sigma-Aldrich (USA), diluted in olive oil, and administered by gavage. Each animal received 50 mg/kg DMBA weekly for 4 weeks [18].
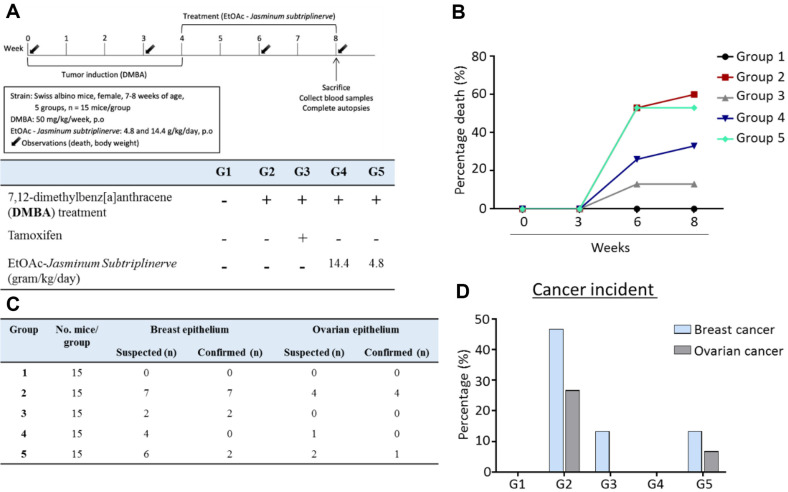
Briefly, the mice were divided into five groups (n = 15 mice each). After acclimatization, the mice were randomly assigned to groups. Group 1 (control group) received only sesame oil vehicle by oral gavage. Groups 2, 3, 4, and 5 were treated with 50 mg/kg DMBA dissolved in sesame oil by oral gavage once weekly for the first 4 weeks of the experimental period; however, group 2 did not receive any additional treatments. During the subsequent 4 weeks, mice in group 3 were treated daily with tamoxifen, whereas mice in groups 4 and 5 were treated with the EtOAc extract of J. subtriplinerve. Mice were sacrificed 1 day after the last dose. Blood samples were collected for assays of cytokines and hematological parameters. Tumors in breast and ovarian tissues were collected for morphological analysis (Fig. 2).
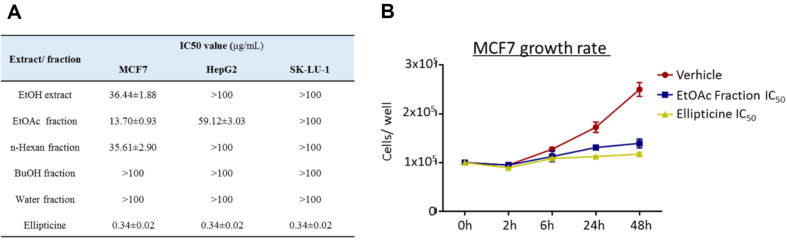
Fig. 2. Anti- cancer effects of J. Subtriplinerve extract (EtOH) and EtOH fractions in-vitro experiment.
EtOH extract and its fractions was treated on three human cancer cell lines in 48 h. The cytotoxicity of J. Subtriplinerve on cancer cell lines was assessed using the MTT assay. IC50 values was calculated (A). Population doubling times are shown (B). Data are presented as the means ± SD of three independent experiments.
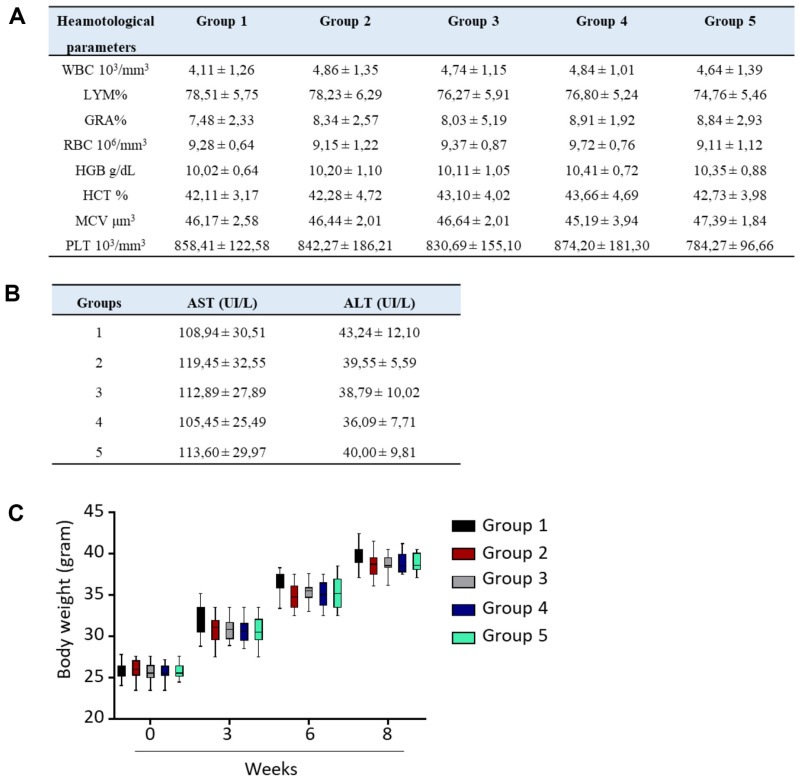
Clinical symptoms. All mice were monitored daily throughout the experimental period for signs of distress and mortality. Their body weights were measured periodically throughout the experimental period. Hematological parameters were assessed using blood collected from the carotid arteries. Samples (0.3 ml) in ethylenediaminetetraacetic acid (EDTA) were used to measure hemoglobin (Hb) and hematocrit levels; red blood cell (RBC) and white blood cell (WBC) counts; and the percentages of neutrophils, lymphocytes, and platelets. We also estimated liver marker enzymes. For these measurements, blood was collected in non-heparinized tubes and then centrifuged at 3,000 rpm for 10 min. The separated serum was analyzed using the Erba Chem 5v3 Clinical Chemistry Analyzer (Erba Mannheim, India) to measure liver function marker enzymes such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Histological analysis. Mice were sacrificed at the end of the eighth week of the experimental period to collect breast and ovarian samples for histological analysis. Additionally, breast and ovarian samples were collected from any mice that died before the specified time point. All tissues were washed with phosphate-buffered saline (PBS), fixed with 10% formalin, processed, and embedded in paraffin blocks. Sections (5 μm) were cut using a microtome (Leica Microsystems, Germany) and stained with hematoxylin and eosin (H&E) for general analysis. Stained sections were observed under an autofocus microscope (Motic, USA) at high power field (HPF) magnification of 200× to confirm cancer status.
Cytokine measurement. Blood was collected from each mouse, and serum was isolated by centrifugation. Then, serum samples were prepared for cytokine analyses. Levels of IL-2 and PGE2 were quantified using commercial enzyme-linked immunosorbent assay (ELISA) kits (Bioassay Technology Laboratory, China), in accordance with the manufacturer’s instructions.
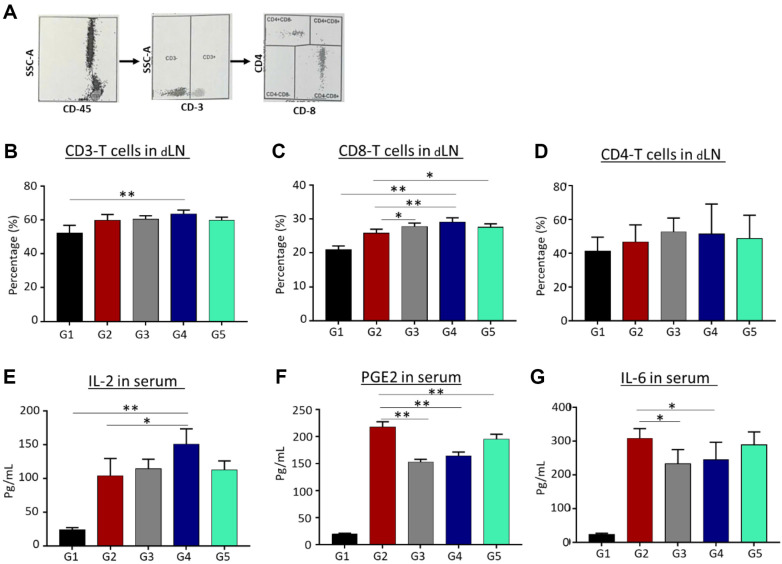
CD3-CD4-CD8 T cell count test. CD3, CD4, and CD8 T cell populations were detected in mouse draining lymph node cells (dLNs). The dLNs were harvested from mice at the end of the experiment, and single-cell suspensions were prepared. The dLNs (1 × 106 cells) in 100 μl PBS buffer were plated in 24-well culture plates and stained with FITC-labeled anti-CD3, PE-labeled anti-CD4, and PE-labeled anti-CD8 monoclonal antibodies for 20 min at room temperature. These samples were analyzed via flow cytometry.
Statistical analysis. Comparisons between the negative control group and treatment groups were performed using nonparametric one-way analysis of variance (ANOVA; i.e., Kruskal-Wallis test) and confirmed with post hoc Bonferroni correction. P-values < 0.05 were considered statistically significant, and p-values < 0.01 were considered highly significant.
Results
Screening of Chemical Constituents via LC-QTOF-MS
The major components of the ethanol extract from the leaves of J. subtriplinerve were assessed via liquid chromatography–mass spectrometry (LC-MS). Several major compounds were identified, including phenylethanoid glycosides, flavonoid glycosides, terpen glycosides, triterpenoids, and secoiridoid glycoside, by comparing their retention times, MS/MS fragments, maximum ultraviolet (UV) absorptions (UV max), and molecular weights to those of reference compounds (Fig. 1). Indeed, several secondary metabolites were identified, including astragalin, rutin, isoquercetin, isoquercitrin, kaempferol, rutinoside, nicotiflorin, chevangin A, and jasnervosid A. Next, to identify specific chemicals, we utilized LC-QTOF MS/MS.
Anticancer Effects in In Vitro Assays
Ethanol extracts and fractions of J. subtriplinerve were examined for cytotoxic effects on human cancer cell lines. MCF-7, SK-LU-1, and HepG2 cells were seeded in 96-well plates and treated with various concentrations of these extracts and fractions for 48 h. Cytotoxicity was measured using the MTT assay. Both the full extract and each of its three fractions (EtOAc, n-hexane, and n-BuOH) significantly inhibited MCF-7 breast cancer cells, with IC50 values lower than 50 μg/ml (Fig. 2A). Ellipticine, a well-known anticancer agent, showed IC50 values of approximately 1 μg/ml. For MCF-7 cells, the EtOAc fraction exhibited the highest cytotoxicity with an IC50 value of 13.70 ± 0.93 μg/ml; other fractions were less effective. In addition, the EtOAc fraction of J. subtriplinerve significantly attenuated the proliferation of MCF-7 at IC50 in 48 h culture (Fig. 2B).
Effects of Extract Fractions on Cancer Mortality
DMBA was orally administered for 4 weeks before the samples were treated. Throughout the experiment, all mice were monitored daily for signs of tumors and mortality. As shown in Fig. 3, approximately 60% of mice in group 2 (DMBA administration only) died. Treatment with a dose of 4.8 mg/kg fractionated extract did not significantly reduce mortality: 53.3% of the mice died. However, a high dose of 14.4 mg/kg fractionated extract or tamoxifen strongly reduced mortality to 33% and 13%, respectively.
Fig. 3. Effects of J. subtriplinerve fractionated extract (EtOAc) on clinical symptoms in a mouse model of DMBA-induced cancer.
Clinical symptoms were observed during the experimental period. (A) Experimental scheme. (B) Survival rate. (C) Number of mice suspected to have tumors and confirmed via H&E analysis. (D) Cancer incidence. Data are presented as means ± standard deviations of 15 mice per group. Statistical significance was assessed via one-way ANOVA followed by Bonferroni’s post hoc test; *p < 0.05, **p < 0.01, vs. group 2.
The numbers of mice that died with suspected tumors were recorded; tissue samples were collected for histological analysis to confirm the cause of death in each group. In group 2, all mice with suspected breast or ovarian tumors were subsequently confirmed to have cancer (46.67% breast cancer and 26.7% ovarian cancer)(Fig. 3). In Group 3 (tamoxifen treatment), two mice were confirmed to have breast cancer; none had ovarian cancer. Similarly, in group 5, two of six and one of two mice were suspected to have breast cancer and ovarian cancer, respectively. No cases of cancer were histologically detected after 4 weeks of treatment with high doses of the extract fraction in group 4, although four mice had previously been suspected to have breast tumors.
Effects of J. subtriplinerve on DMBA-Induced Histopathological Changes in Mammary and Ovarian Tissues
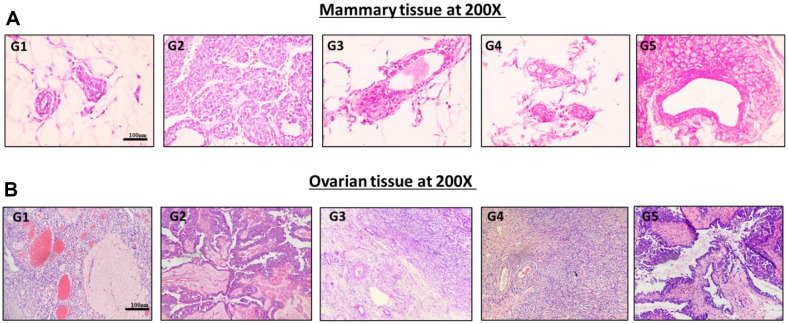
Histological sections of mammary tissues from the control group showed a normal mammary gland structure, with small mammary ducts surrounded by a small amount of fibrous connective tissue (Fig. 4). In contrast, mice in the DMBA group exhibited various histopathological changes. Typical findings included hyperplasia in some ducts, mild ductal proliferations, and focal epithelial hyperplasia with hyperchromatic enlarged nuclei. Tissues in the extract treatment groups showed a normal structure, although group 5 displayed dysplastic mammary glands with increased duct number and irregular cell division. Ovarian tissues in controls and group 4 mice (treated with J. subtriplinerve) showed normal ovarian structure. In contrast, group 2 displayed diffuse tumors that disrupted the normal ovarian histological structure. The cells were large and had irregular nuclei, coarse chromatin, and a high nucleus-to-cytoplasm ratio. The rate of division was high.
Fig. 4. Changes in histological sections.
Histological sections of mouse mammary tissues and ovarian tissues were viewed under 200 × magnification after H&E staining. (A) Groups 1 and 4 showed normal mammary gland structures, with small mammary ducts surrounded by a small amount of fibrous connective tissue. Group 2 showed various histopathological changes such as hyperplasia in some ducts, mild ductal proliferations, and focal epithelial hyperplasia with enlarged, hyperchromatic nuclei. Group 5 exhibited dysplastic mammary glands, an increase in channel number, and irregular cell division. (B) Normal ovarian structure in groups 1 (controls) and 4. Group 2 displayed diffuse tumors that disrupted the normal ovarian histological structure. Cells were large and had irregular nuclei, coarse chromatin, and a high nucleus-tocytoplasm ratio. They also showed a high rate of division.
Lymphocytosis in Tissue
Flow cytometry of lymphocytes using the CD3-CD4-CD8 count test showed that compared with the vehicle group, DMBA treatment significantly increased the percentages of CD3 and CD8 T cells Fig. 5A and 5B to Fig. 5B and 5C, respectively no differences was observed in CD4 T cells compared to group 2, even hough it’s percentage was higher than group 1 (Fig. 5D). Furthermore, treatment with the fractionated extract in groups 3 and 4 resulted in higher percentages of CD8 T cells in the blood compared with the untreated group.
Fig. 5. Effects of extracts on cytokines and lymphocytes.
Treated mice were dissected 1 day after the last treatment. Blood and draining lymph nodes were collected for tests. (A) The gating of CD3, CD8, CD4 T cells by flow cytometer analysis, (B) percentage CD3 T cells, (C) percentage CD8 T cells and (D) percentage CD4 T cells were counted by CD3-CD4-CD8 test. The level of (E) IL-2 and (F) PGE2, (G) IL-6 expression in serum were measured by ELISA. Data are presented as means ± standard deviations of three mice per group. Statistical significance was assessed via one-way ANOVA followed by Bonferroni’s post hoc test; *p < 0.05, **p < 0.01, vs. group 2.
Effects of Extracts on Th1 Cytokine Levels in Serum
IL-2 levels, determined using an ELISA kit, rapidly increased after DMBA treatment compared with the levels in controls (group 1). After 4 weeks of treatment with a high dose of the extract fraction (14.4 mg/kg; group 4), the levels increased even more noticeably. PGE2 and IL-6 levels in serum also increased in response to DMBA; tamoxifen and the higher dose of extract fraction significantly inhibited this increase.
Dose Toxicity Effects
Treatment with different doses of the extract fraction had no effect on hematological parameters, and there were no changes in serum levels of ALT or AST compared with controls (Fig. 6). Additionally, no abnormal changes in body weight were noted.
Fig. 6. Dose toxicity effects.
Female mice were administered J. subtriplinerve daily for 4 weeks and clinical observations were made. (A) Changes in hematological results. (B) Levels of liver marker enzymes. (C) Changes in body weight.
Identification of the Active Compound with Anticancer Activity against MCF-7
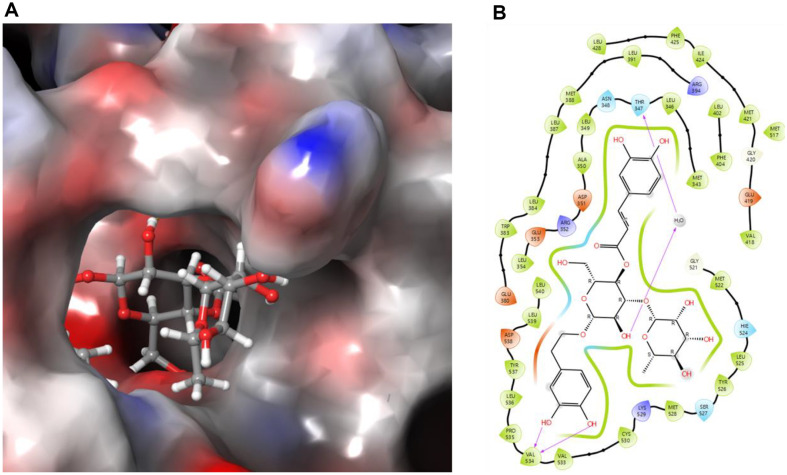
To investigate the underlying mechanisms of the anticancer effects of J. subtriplinerve extract, we performed molecular docking simulations using Maestro v. 13.4.134 (Schrödinger software, MMshare v. 6.0.134, released 2022-4, Platform Windows-x64) [19]. The crystal structure of the breast cancer target was downloaded from the Protein Data Bank (PDB ID: 3ERT). Then, the compound detected in the highest amount, acteoside, was identified by LC-QTOF-MS and TLC. Acteoside was docked into the active site of mechanistic target 3ERT; the results indicated that it bound to the active site of 3ERT with a docking score of −11.949 kcal/mol, which was significant compared to ellipticine. Notably, THR347 and VAL534 induced and stabilized the active conformation of 3ERT (Fig. 7). Based on its high binding affinity energies, hydrogen bond interactions, and ability to generate the ligand-binding pocket, acteoside from J. subtriplinerve shows inhibitory potential in breast cancer treatment.
Fig. 7. Molecular docking simulation of acteoside with 3ERT.
(A) Three-dimensional binding interactions and (B) two-dimensional diagram of observed ligand–receptor interactions between acteoside and breast cancer protein (3ERT).
Discussion
The MTT assay is an uncomplicated method for evaluating the inhibitory potential of natural extracts or pure compounds with potential anticancer properties [20]. Thus, it was used to assess the degree of inhibition of J. subtriplinerve crude extracts and fractions (in EtOAc, n-hexane, and n-BuOH) against three cancer cell lines. All preparations significantly inhibited MCF-7 breast cancer cells with IC50 values lower than 50 μg/ml. Ellipticine, a well-known anticancer agent, was used as a reference for comparison [21].
The IC50 value of ellipticine is approximately 1 μg/ml, whereas the EtOAc fraction of J. subtriplinerve had a value of 13.70 ± 0.93 μg/ml. This difference highlights the need for further comprehensive chemical studies to isolate pure compounds that may possess enhanced anticancer properties. To elucidate the anticancer effects of the EtOAc fraction, we administered this fraction to mice, daily by oral gavage for 4 weeks. At high doses (14.4 mg/kg), the mortality rate and cancer incidence both were significantly reduced. T cells play a crucial role in cancer pathogenesis and are categorized into two main types: TH1 and TH2. In immune responses, CD4 cells (i.e., helper T cells) stimulate B cells to produce antibodies and activate cytotoxic T cells (CD8 cells). CD8 cells then directly participate in tumor destruction; indeed, these cells are under consideration for use in tumor vaccines [22]. In the present study, mice with DMBA-induced cancer exhibited a significant increase in the percentage of CD3 T cells compared with controls. We also evaluated the percentages of CD4 and CD8 T cells, the latter of which showed a significant increase in the cancer groups. Group 4, treated with a high dose of the EtOAc extract, showed the highest percentage of CD8 cells; this was significantly higher than the percentage in the negative control group. CD8 T cells function by directly attacking virus-infected cells and cancer cells [23, 24]. Therefore, exploration of the impacts of extract fractions on the percentages of CD4 and CD8 T cells can help elucidate their effects on cell-mediated immune responses, as well as the underlying anticancer mechanism.
In addition to immune organs and competent immune cells, the immune system involves extensive cytokine activity. Cytokines are important substances secreted by antigen-activated immune cells. In particular, IL-2, TNF-α, and IL-6 play crucial roles in the immune response to inflammation as well as cancer [23].
IL-2 is secreted by activated Th lymphocytes and stimulates both CD4 and CD8 T cells; it also promotes the development and differentiation of B lymphocytes by enhancing natural killer (NK) cells and lymphokine-activated killer (LAK) cells. IL-2 is secreted when an antigen binds to the T cell receptor (TCR) and induces the expression of IL-2 receptors (IL-2R). The subsequent interaction between IL-2 and IL-2R stimulates the growth, differentiation, and survival of Tc cells. IL-2/S4B6 immune complexes exhibit high stimulatory activity toward NK cells and CD8 T cells; they could potentially replace conventional IL-2 in cancer immunotherapy. This hypothesis is supported by the proportional increase in IL-2 secretion as CD8 T cell percentage increases during tumor-targeting therapy. Moreover, preclinical and clinical studies have demonstrated that IL-2 can augment the development of immune cells, enhancing their abilities to eliminate cancer cells, particularly in renal cell carcinoma and malignant melanoma. With appropriate IL-2 therapy, the cure rates for these two types of cancer can reach 18% for malignant melanoma and 37% for renal cell carcinoma [25, 26]. The EtOAc extract led to the highest levels of IL-2 and CD8 T cells, highlighting its anticancer potential. The inflammatory environment and immune response are closely associated with cancer progression and recurrence. In various types of tumors, development and metastasis are characterized by the epithelial–mesenchymal transition (EMT), initiation of tumor formation, and angiogenesis, processes that are increasingly linked to intrinsic or extrinsic inflammation. Among known inflammatory mediators, PGE2 increases the invasiveness and progression of epithelial tumors by promoting their growth, helping cells to evade apoptosis, activating the transcription of tyrosine kinase growth factor receptors, and inducing angiogenesis. Furthermore, it plays a critical role in the tumor microenvironment by suppressing antitumor immunity and regulating tumor immune evasion, thus supporting tumor progression [27].
IL-6 is a major pro-inflammatory cytokine involved in the inflammatory response and cancer progression. It is secreted by various types of immune cells, including T cells, macrophages, and tumor cells [1-3]. Its overexpression has been reported in almost all types of tumors. Normally, high levels of IL-6 in the tumor microenvironment are thought to reflect the relationship between inflammation and cancer. In this study, DMBA induced an increase in IL-6 levels in mice, but with the higher dose of EtOAc fraction treatment, a significant decrease in IL-6 was found. This also suggests this fraction’s ability to reduce inflammation or prevent cancer in future studies.
The development of drugs or functional foods for cancer prevention or treatment requires assessing their effectiveness in terms of inhibiting tumor growth while ensuring user safety. Our results indicate that after 4 weeks of continuous treatment with extracts of J. subtriplinerve leaves, there were no significant differences in blood and biochemical parameters between the treated and control groups (i.e., the treatment was safe).
J. subtriplinerve belongs to the Oleaceae family; thus far, no study has revealed any toxicities associated with its use. For example, a study of J. sambac L. extracts demonstrated no acute oral toxicity at a dose of 5,000 mg/kg [28]. A study of Litsea elliptica demonstrated no acute oral toxicity at doses ranging from 400 to 5,000 mg/kg; moreover, doses of 125, 250, and 500 mg/kg administered daily for 28 consecutive days did not lead to changes in body weight, food intake, or water consumption [29]. Additionally, a 50% ethanol extract of J. subtriplinerve did not induce any signs of toxicity in mice at a maximum dose of 20 g/kg. Finally, a study showed that acteoside, a key compound in J. subtriplinerve, did not cause toxicity when administered at sub-chronic doses [30]. These findings, together with our results, support the safety and efficacy of this medicinal plant.
Conclusion
Cancer is a leading cause of death worldwide, with millions of new cases diagnosed each year. Accordingly, there is a critical need to discover safe, effective, and natural anticancer agents that ensure safe, affordable, and accessible treatment. By focusing on natural compounds, researchers aim to develop therapies that effectively combat cancer while minimizing adverse side effects; this approach will make long-term treatment more tolerable and sustainable for patients.
Breast cancer is the third most common cancer among women, after lung and bronchial cancers. Despite significant diagnostic and therapeutic advancements in both industrialized and developing nations, breast cancer remains a leading cause of mortality among women [31]. This disease significantly threatens health and places substantial burdens on patients and society. Breast cancer typically originates in the milk ducts or lobules of breast tissue. Multiple internal and external factors can contribute to its development, including hormones, immune system factors, genetic mutations, chemicals, and radiation [32].
J. subtriplinerve belongs to the jasmine family (Oleaceae) and is widely distributed, particularly in southern Asia. In traditional Vietnamese medicine, this plant is renowned for its therapeutic effects in terms of clearing heat, relieving rheumatism, promoting blood circulation, regulating menstruation, and reducing inflammation [9]. It is used to treat conditions such as amenorrhea, metritis, mastitis, rheumatism, and jaundice. It also supports liver function, increases bile secretion, improves digestion, stimulates appetite, and promotes restful sleep [8]. It has antibacterial and anti-inflammatory properties and is used to treat rheumatism, bone and joint pain, skin ailments, and snake bites. Its leaves are used in the management of irregular menstruation, high postpartum fever in women, lymphadenitis, metritis, mastitis, breast abscess, vaginal discharge, rheumatism with bone and joint pain, scabies, sores, impetigo, and various itchy skin conditions.
We comprehensively assessed the use of J. subtriplinerve as treatment for cancer and found that a 70% ethanol extract showed chemopreventive efficacy in a mouse model of breast cancer. Our results suggest that J. subtriplinerve extract can help to prevent the development of breast cancer without causing toxicity.
Supplemental Materials
Acknowledgments
Hong Minh Phan was funded by the Master's and PhD Scholarship Programme of the Vingroup Innovation Foundation (VINIF), code: VINIF. 2023.TS.068.
Footnotes
Funding
This research received a grant from the Vietnam National University Project, 'Study the Effect of Jasminum subtriplinerve Blume Oleaceae Leaves Extract on Anticancer Activity in Experimental Models' [grant number QG.22.69], for the preparation of data
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Zhang J, Wu Y, Li Y, Li S, Liu J, Yang X, et al. Natural products and derivatives for breast cancer treatment: from drug discovery to molecular mechanism. Phytomedicine. 2024:155600. doi: 10.1016/j.phymed.2024.155600. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Duan Y, Wang Y, Chen L, Abdelrahim ME, Yan J. The effect of Green green tea consumption on body mass index, lipoprotein, liver enzymes, and liver cancer: an updated systemic review incorporating a meta-analysis. Crit. Rev. Food Sci. Nutr. 2024;64:1043–1051. doi: 10.1080/10408398.2022.2113360. [DOI] [PubMed] [Google Scholar]
- 3.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever‐increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5.Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med. 2021;9:20503121211034366. doi: 10.1177/20503121211034366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khazir J, Mir BA, Pilcher L, Riley DL. Role of plants in anticancer drug discovery. Phytochem. Lett. 2014;7:173–181. doi: 10.1016/j.phytol.2013.11.010. [DOI] [Google Scholar]
- 7.Hue Ngan D, Hoai HTC, Mai Huong L, Hansen PE, Vang O. Bioactivities and chemical constituents of a Vietnamese medicinal plant Che Vang, Jasminum subtriplinerve Blume (Oleaceae) Nat. Prod. Res. 2008;22:942–949. doi: 10.1080/14786410701647119. [DOI] [PubMed] [Google Scholar]
- 8.Vang APDO. Bioactivities and chemical constituents of a Vietnamese medicinal plant Jasminum subtriplinerve Blume (Che Vang) Department of Chemistry and Life Science, Roskilde University; 2005. [DOI] [PubMed] [Google Scholar]
- 9.Dai DN, Thang TD, Ogunwande IA, Lawal OA. Study on essential oils from the leaves of two Vietnamese plants: Jasminum subtriplinerve CL Blume and Vitex quinata (Lour) FN Williams. Nat. Prod. Res. 2016;30:860–864. doi: 10.1080/14786419.2015.1071364. [DOI] [PubMed] [Google Scholar]
- 10.Huong NTH, Cu NKQ, Quy TV, Zidorn C, Ganzera M, Stuppner H. A new phenylpropanoid glycoside from Jasminum subtriplinerve blume. J. Asian Nat. Prod. Res. 2008;10:1035–1038. doi: 10.1080/10286020802320897. [DOI] [PubMed] [Google Scholar]
- 11.Thang Hoang D, Hien Truong TT, Viet Duc N, Anh Hoang LT, Do TT, Vinh LB, et al. Hepatoprotective effects of extract of Helicteres hirsuta Lour. on liver fibrosis induced by carbon tetrachloride in rats. Appl. Sci. 2021;11:8758. doi: 10.3390/app11188758. [DOI] [Google Scholar]
- 12.Vinh LB, Nguyet NTM, Yang SY, Kim JH, Thanh NV, Cuong NX, et al. Cytotoxic triterpene saponins from the mangrove Aegiceras corniculatum. Nat. Prod. Res. 2019;33:628–634. doi: 10.1080/14786419.2017.1402320. [DOI] [PubMed] [Google Scholar]
- 13.Vinh LB, Han YK, Park SY, Kim YJ, Phong NV, Kim E, et al. Identification of triterpenoid saponin inhibitors of interleukin (IL)-33 signaling from the roots of Astragalus membranaceus. J. Funct. Foods. 2023;101:105418. doi: 10.1016/j.jff.2023.105418. [DOI] [Google Scholar]
- 14.Han YK, Vinh LB, Nam M-h, Lee KY. Identification of compounds using HPLC-QTOF-MS online antioxidant activity mapping from aerial parts of Ligularia stenocephala. Appl. Biol. Chem. 2023;66:53. doi: 10.1186/s13765-023-00814-1. [DOI] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Rashidi M, Ziai SA, Moini ZT, Khalilnezhad A, Jamshidi H, Amani D. Umbelliprenin is potentially toxic against the HT29, CT26, MCF-7, 4T1, A172, and GL26 cell lines, potentially harmful against bone marrow-derived stem cells, and non-toxic against peripheral blood mononuclear cells. Iran. Red Crescent Med. J. 2016;18:e35167. doi: 10.5812/ircmj.35167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Augustine D, Rao RS, Anbu J, Chidambara MKN. In vitro antiproliferative effect of earthworm coelomic fluid of Eudrilus eugeniae, Eisenia foetida, and Perionyx excavatus on squamous cell carcinoma-9 cell line: a pilot study. Pharmacog. Res. 2017;9:S61–S66. doi: 10.4103/pr.pr_52_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimi B, Ashrafi M, Shomali T, Yektaseresht A. Therapeutic effect of simvastatin on DMBA‐induced breast cancer in mice. Fundam. Clin. Pharmacol. 2019;33:84–93. doi: 10.1111/fcp.12397. [DOI] [PubMed] [Google Scholar]
- 19.Duyen NT, Vinh LB, Phong NV, Khoi NM, Long PQ, Hien TT, et al. Steroid glycosides isolated from Paris polyphylla var. chinensis aerial parts and paris saponin II induces G1/S-phase MCF-7 cell cycle arrest. Carbohydr. Res. 2022;519:108613. doi: 10.1016/j.carres.2022.108613. [DOI] [PubMed] [Google Scholar]
- 20.Edmondson JM, Armstrong LS, Martinez AO. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J. Tissue Culture Methods. 1988;11:15–17. doi: 10.1007/BF01404408. [DOI] [Google Scholar]
- 21.Stiborová M, Černá V, Moserová M, Mrízová I, Arlt VM, Frei E. The anticancer drug ellipticine activated with cytochrome P450 mediates DNA damage determining its pharmacological efficiencies: studies with rats, hepatic cytochrome P450 reductase null (HRN™) mice and pure enzymes. Int. J. Mol. Sci. 2014;16:284–306. doi: 10.3390/ijms16010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 23.Paul W. Fundamental immunology 4th edition. 1999. [Google Scholar]
- 24.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc. Natl. Acad. Sci. USA. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massari F, Santoni M, Ciccarese C, Santini D. The immunocheckpoints in modern oncology: the next 15 years. Expert. Opin. Biol. Ther. 2015;15:917–921. doi: 10.1517/14712598.2015.1035251. [DOI] [PubMed] [Google Scholar]
- 26.Pels E. Comparison of saliva interleukin-2 concentration to the condition of gums in children with acute lymphoblastic leukaemia during anti-tumour treatment. Cancer Chemother. Pharmacol. 2015;76:205–210. doi: 10.1007/s00280-015-2750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finetti F, Travelli C, Ercoli J, Colombo G, Buoso E, Trabalzini L. Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biology (Basel) 2020;9:434. doi: 10.3390/biology9120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunhachan P, Banchonglikitkul C, Kajsongkram T, Khayungarnnawee A, Leelamanit W. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait."G. Duke of Tuscany". Evid. Based Complement. Alternative Med. 2012;2012:471312. doi: 10.1155/2012/471312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Budin SB, Siti Nor Ain SM, Omar B, Taib IS, Hidayatulfathi O. Acute and subacute oral toxicity of Litsea elliptica Blume essential oil in rats. J. Zhejiang Univ. Sci. B. 2012;13:783–790. doi: 10.1631/jzus.B1100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henn JG, Steffens L, de Moura Sperotto ND, de Souza Ponce B, Veríssimo RM, Boaretto FBM, et al. Toxicological evaluation of a standardized hydroethanolic extract from leaves of Plantago australis and its major compound, verbascoside. J. Ethnopharmacol. 2019;229:145–156. doi: 10.1016/j.jep.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat. Rev. Dis. Primers. 2019;5:1–31. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 32.Hermawan A, Putri H. Current report of natural product development against breast cancer stem cells. Int. J. Biochem. Cell Biol. 2018;104:114–132. doi: 10.1016/j.biocel.2018.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.