Abstract
With the recent stringent criteria for antibiotic susceptibility in probiotics, the presence of antibiotic resistance genes and plasmids associated with their transfer has become a limiting factor in the approval of probiotics. The need to remove genes related to antibiotic resistance and virulence through plasmid curing for the authorization of probiotics is increasing. In this study, we investigated the curing efficiency of ethidium bromide, acridine orange, and novobiocin at different concentrations and durations in five strains of plasmid-bearing lactic acid bacteria and examined the curing characteristics in each strain. Limosibacillus reuteri and Lacticaseibacillus paracasei exhibited curing efficiencies ranging from 5% to 44% following treatment with ethidium bromide (10–50 μg/ml) for 24–72 h, while Lactobacillus gasseri showed the highest efficiency at 14% following treatment with 10 μg/ml novobiocin for 24 h. Lactiplantibacillus plantarum, which harbors two or more plasmids, demonstrated curing efficiencies ranging from 1% to 8% after an additional 72-h treatment of partially cured strains with 10 μg/ml novobiocin. Plasmid curing in strains with larger plasmids exhibited lower efficiencies and required longer durations. In strains harboring two or more plasmids, a relatively low curing efficiency with a single treatment and a high frequency of false positives, wherein recovery occurred after curing, were observed. Although certain strains exhibited altered susceptibilities to specific antibiotics after curing, these outcomes could not be attributed to the loss of antibiotic resistance genes. Furthermore, the genomic data from the cured strains revealed minimal changes throughout the genome that did not lead to gene mutations.
Keywords: Lactic acid bacteria, plasmid curing, antibiotic resistance, curing agent, SNP
Introduction
Plasmids are independently replicating DNA molecules that exist independently of chromosomal DNA [1]. They are known to possess various functions, such as F plasmid (facilitates conjugation) [2] and R plasmid (which confers resistance to antibiotics or toxins) [3]. In widely used probiotic bacteria, various types of plasmids also exist. The fertility plasmid (F plasmid) is used to transfer DNA between bacteria, enabling gene transfer between cells, which promotes genetic exchange within bacterial populations. It regulates the necessary steps in the cell conjugation process and replicates the DNA to be transferred between cells [4]. When plasmids contain antibiotic resistance genes, neighboring bacteria can acquire these genes through such mechanisms, facilitating the widespread dissemination of antibiotic resistance within bacterial populations [5].
In recent decades, bacterial antibiotic resistance has rapidly escalated. Various organizations, including the World Health Organization [6], Food and Agriculture Organization [7], Food and Drug Administration [8], and the European Food Safety Authority (EFSA) [9] are promoting awareness of this matter, which is considered a globally significant medical and public health concern [10, 11]. The heightened potential for the transfer of antibiotic resistance genes within the gut, especially when probiotics are antibiotic resistant, has prompted the proposal of safety evaluation methods for addressing antibiotic resistance gene transmission [12]. Although probiotics with exceptional functionality show improved effectiveness, they cannot be used as functional probiotics if they fail safety assessments due to antibiotic resistance. Therefore, if the probiotic plasmid DNA contains antibiotic resistance genes, the expression and transmission of these genes must be inhibited through methods that eliminate antibiotic resistance, such as plasmid curing [13]. This process is essential for passing the safety assessment and enabling registration as a functional probiotic. As a prime example, Limosilactobacillus reuteri ATCC 55730, which harbors antibiotic resistance genes for penicillin, tetracycline, and lincomycin, was transformed into Lm. reuteri DSM 17938, which had its antibiotic susceptibility restored through plasmid curing[14]. This strain is currently used in various functional health products.
Plasmid curing methods have been extensively developed, employing curing compounds such as detergents, DNA-intercalating agents, biocides, antibiotics, and plant-derived compounds [15]. In addition, methods based on plasmid incompatibility principles [16, 17], anti-plasmid systems utilizing bacteriophages [18], and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein-based plasmid curing systems [19] have been used [15]. In particular, the use of DNA-intercalating agents such as acridine orange (AO) and ethidium bromide (EtBr) and DNA gyrase-inhibiting drugs such as novobiocin (Nv) and coumermycin A is a common example of a widely employed traditional curing method [1, 20].
CRISPR/Cas9 technology has emerged as a highly precise and efficient method for inducing targeted genetic modifications [21]. However, applying CRISPR technology to plasmid removal in lactic acid bacteria (LAB) presents several challenges. CRISPR only cuts specific fragments of the plasmid DNA, requiring multiple edits to achieve complete removal [22]. Additionally, this includes the need for species-specific guide RNA design, the potential for off-target effects, and the requirement for sophisticated laboratory infrastructure that may not be readily accessible in all research environments [23]. In contrast, traditional mutagenesis methods such as EtBr, AO, and Nv offer the advantage of being widely applicable to various bacteria without the need for species-specific guide RNA design. These methods also allow for the simultaneous removal of multiple plasmids. Although CRISPR is known as an effective method for knocking out specific genes, challenges exist in introducing genes in LAB, and it can only be utilized if the exact target gene is known [22]. In this study, we observed a decrease in antibiotic resistance following the removal of plasmids, even though the plasmids did not contain any known antibiotic resistance genes. This suggests that complete plasmid removal may be more effective in enhancing the safety of using LAB, compared to targeting and removing specific resistance genes with CRISPR. Therefore, this study aims to evaluate the effectiveness of traditional plasmid removal methods in LAB, providing a benchmark for future research using technologies like CRISPR.
For instance of using traditional curing agents, treatment of Escherichia coli K12 with 25 μg/ml AO for 72 h demonstrated a 99% curing probability [24], while treatment of E. coli 207940 with 100 μg/ml EtBr for 48 h resulted in approximately 21% curing efficiency [25]. Nv has been used as a curing agent not only for strains belonging to Enterobacteriaceae, such as E. coli and Shigella sonnei, but also for some LAB strains, including Lactiplantibacillus plantarum [26, 27], and excellent curing efficiencies ranging from 4.0% to 90% have been reported.
However, the concentration of the agents and treatment time for plasmid curing vary, and no protocol has been precisely defined. In preliminary studies wherein LAB strains carrying plasmids are treated with varying concentrations of curing agents, extremely low curing efficiencies or recovery of plasmids in the cured strains as false-positive results have been verified (Fig. S1). These problems require the reestablishment of curing methods applicable to LAB.
In this study, we investigated the curing efficiencies of widely used curing agents, namely EtBr, AO, and Nv, in 10 strains of LAB belonging to five different species, whose plasmid types, sizes, G+C contents, and coding sequences were elucidated through whole-genome analysis. We compared the curing efficiency in each strain and examined whether antibiotic susceptibility changed or genetic variations occurred after plasmid curing.
Materials and Methods
Strains and Culture Medium
The LAB strains were obtained from the Bio R&D Product program (https://biorp.kribb.re.kr/). The bacterial strains were cultivated in de Man, Rogosa, and Sharpe (MRS) media (BD, USA) under anaerobic conditions at 37°C for 24–48 h. The names, numbers of plasmids, plasmid sizes, and G+C content of the strains used in this study are listed in Table 1. Whole-genome sequences before and after plasmid curing have been deposited in the National Center for Biotechnology Information database (Table S1).
Table 1.
Scientific names, strain numbers, number of plasmids, plasmid size, and G+C contents of the LAB used in this study.
| Scientific name | Strain | Plasmids | Accession number for wild-type strain | Plasmid size (bp) | G+C content (%) |
|---|---|---|---|---|---|
| Lactiplantibacillus plantarum | DS1989 | Plasmid 1 Plasmid 2 Plasmid 3 |
CP146869 CP146870 CP146871 |
50,933 48,573 8,694 |
38.9 39.0 36.0 |
| DS0815 | Plasmid 1 Plasmid 2 Plasmid 3 |
CP146873 CP146874 CP146875 |
66,660 39,431 6,156 |
39.3 40.5 37.4 |
|
| DS1902 | Plasmid 1 Plasmid 2 |
CP146866 CP146867 |
7,845 2,410 |
36.9 38.2 |
|
| DS1073 | Plasmid 1 Plasmid 2 |
CP147893 CP147894 |
50,512 19,584 |
39.0 40.5 |
|
| Limosilactobacillus reuteri | DS0354 | Plasmid 1 | CP146877 | 19,051 | 36.9 |
| DS0384 | Plasmid 1 | CP090314 | 20,351 | 37.1 | |
| Lactobacillus gasseri | DS2831 | Plasmid 1 | CP146881 | 49,996 | 36.0 |
| Lacticaseibacillus paracasei | DS0725 | Plasmid 1 | CP151182 | 66,795 | 43.8 |
| DS2766 | Plasmid 1 | CP146879 | 6,196 | 38.9 | |
| Bifidobacterium longum | DS1566 | Plasmid 1 | CP146883 | 193,392 | 57.2 |
Plasmid Curing
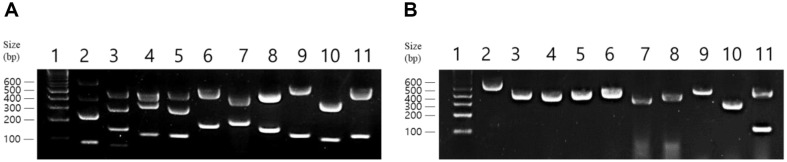
Plasmid curing was performed with EtBr, AO, and Nv as curing agents at concentrations of 10 μg/ml and 50 μg/ml. Treatment with the curing agent was performed for 24, 48, and 72 h until sufficient curing data were obtained. When complete curing was not achieved in the primary curing reaction, secondary curing was conducted using partially cured strains. For secondary curing, the reaction was conducted for up to 72 h using the most effective agent concentration determined from the primary reaction. The LAB strain (108 colony-forming unit CFU/ml) was inoculated (2% v/v) into the MRS broth containing the curing agent and incubated statically in an anaerobic chamber at 37°C for 24, 48, and 72 h. At each time point, the bacterial solution was spread onto MRS agar plates and incubated for 2–3 days. Colonies obtained from these plates were selected. To confirm the presence or absence of plasmids, colony PCR was performed by picking each colony using a toothpick and adding 10 μl of PCR Master Mix (Bioneer, Republic of Korea) containing 3.2 pmol of each primer, and distilled water was added to obtain a final volume of 20 μl. Specific primers for chromosomes and plasmids in each LAB strain were designed based on whole-genome sequencing data. The PCR conditions were set as follows: 35 cycles of denaturation at 94°C for 30 sec, annealing at 59°C for 30 sec, and extension at 72°C for 30 sec. The PCR products were analyzed via electrophoresis on 1.5% agarose gels. The amplified fragments were of different sizes, allowing simultaneous verification of the chromosome and plasmid products in a single electrophoresis run (Fig. 1, Table S2). Plasmid curing was successfully achieved if the PCR results showed amplification of the chromosomal DNA but not of the plasmid DNA. The plasmid-cured cells were then suspended in phosphate-buffered saline, spread onto MRS agar plates, and incubated. The resulting colonies were subjected to the same colony PCR procedure to verify plasmid recovery.
Fig. 1. Agarose gel electrophoresis of PCR-amplified DNA fragments from wild-type and cured strains.
(A) PCR-amplified products from the chromosomes and plasmids of wild-type LAB strains. (B) PCR-amplified products from the chromosomes and plasmids of plasmid-cured strains. Lane 1, 1 kb plus DNA ladder; lane 2, L. plantarum DS1989; lane 3, L. plantarum DS0815; lane 4, L. plantarum DS1902; lane 5, L. plantarum DS1073; lane 6, Lm. reuteri DS0354; lane 7, Lm. reuteri DS0384; lane 8, L. gasseri DS2831; lane 9, Lc. paracasei DS0725; lane 10, Lc. paracasei DS2766; lane 11, B. longum DS1566. Curing of B. longum DS1566 was not achieved.
Statistical Analysis
Statistical analyses were conducted to evaluate the significance of differences in plasmid removal rates among different agents and concentrations. An analysis of variance (ANOVA) was performed to determine if there were statistically significant differences between groups. Fisher’s exact test and chi-square test were used to calculate the probability values for categorical data. A p < 0.05 was considered statistically significant. All statistical analyses were conducted using SPSS (version 25.0) [28] and R (version 4.0.2) ( https://www.R-project.org/).
Principal Component Analysis (PCA)
Principal Component Analysis (PCA) was performed to reduce the dimensionality of the dataset and to identify patterns and relationships between different strains and agents. The data matrix consisted of plasmid removal rates for different strains treated with various agents at two concentrations (10 μg/ml and 50 μg/ml). Prior to PCA, the data were standardized to ensure each variable contributed equally to the analysis. PCA was conducted using the sklearn library in Python (version 0.24.2) [29], and the results were visualized in scatter plots. The first two principal components were used to create a two-dimensional plot where each point represents a strain-agent combination, colored by the removal rate.
DNA Extraction and Genomic Analysis
Bacterial genomic DNA was extracted using the phenol:chloroform:isoamyl alcohol method [30], and whole-genome sequencing was performed using a PacBio RS II platform at Macrogen Inc. (Republic of Korea). The sequencing data were assembled de novo using SPAdes (version 3.13.0). Genome annotation was performed using Prokka (version 1.14.6), and genome analysis was performed using ISfinder, NCBI Background Reference Gene DB, UnitProtKB DB, and HMM DB. Genomic G + C content was calculated by analyzing the draft genome [31]. For cured bacteria, whole-genome resequencing was performed on an Illumina platform (Macrogen). After mapping the reads, variants (insertions, deletions, and single nucleotide polymorphisms [SNPs]) were identified using SAMTools and compared to the genome of wild-type bacteria. In addition, the presence of genes related to antibiotic resistance or virulence in their chromosomes and plasmids in the LAB strains used in the study was determined using Resfinder (version 4.5.0) and Virulence Finder (version 2.0), provided by the Center for Genomic Epidemiology (http://www.genomicepidemiology.org/) [32, 33].
To confirm the copy number of the plasmid, RNA from the strains was extracted using the RNeasy extraction kit (Qiagen, Germany), and 1 μg of RNA was synthesized into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, USA). Quantitative real time polymerase chain reaction (qRT-PCR) was performed on a CFX-96 real-time PCR system (Bio-Rad) as previously described [34] using self-designed primers (Table S2). The 2-ΔΔCT method was employed to calculate the copy number of the plasmid per chromosome in each strain [35].
Analysis of Variation in Antibiotic Susceptibility for Plasmid-Cured LAB
To determine and compare the antibiotic susceptibility of each Lactobacillus strain before and after plasmid curing, minimum inhibitory concentration (MIC) test strips (Liofilchem s.r.l., Italy) for ampicillin, vancomycin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, and chloramphenicol were used according to the manufacturer's instructions. Bacteria cultured in MRS broth for 16–24 h were adjusted to 108 CFU/ml. A 100 μl aliquot of this suspension was spread onto MRS agar plates. Antibiotic strips were then placed on the plates, and they were incubated in an anaerobic chamber for 48 h. MIC values were determined by reading the intersection of the test strip and the lower part of the ellipse-shaped growth inhibition zone.
Result
Plasmid Curing
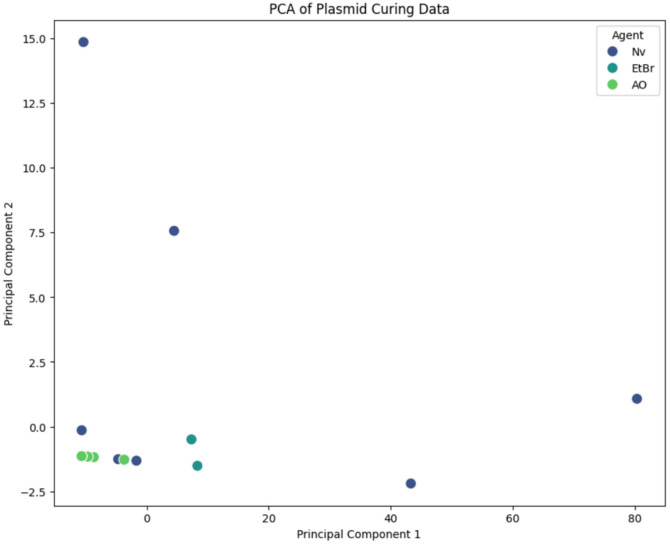
The curing efficiencies in Lm. reuteri strains DS0354 and DS0384 were 29% and 45%, respectively, after treatment with 10 μg/ml EtBr for 24 h and ranged from 3% to 10% after treatment with AO, whereas no growth was observed with 50 μg/ml EtBr or with Nv (Table 2). In the case of Lactobacillus gasseri DS2831, treatment with 10 μg/ml Nv for 24 h had a curing efficiency of 15%; however, DS2831 did not grow in a medium containing 50 μg/ml Nv, and curing was not achieved with AO or EtBr. Curing Lc. paracasei DS0725 had an efficiency of 5% after treatment with 50 μg/ml EtBr for 72 h but was not successful with AO treatment. For Lc. paracasei DS2766, a curing efficiency of 22% was achieved when cultured with 50 μg/ml EtBr or AO for 48 h. In both the DS0725 and DS2766 strains, bacterial growth was not observed following Nv treatment. Bifidobacterium longum DS1566 did not grow at any concentration of the tested agents, even when the concentration was reduced to 0.1 μg/ml, confirming that plasmid curing was not achievable. For Lp. plantarum strains carrying two or more plasmids, the plasmids could not be completely cured after 72 h of cultivation in a medium containing each agent (Table 3). However, a curing efficiency of 1-8% was observed upon retreatment of the partially cured strains with 10 μg/ml Nv for 72 h, which was found to be the most effective concentration for plasmid curing in Lp. plantarum strains (Table 2). Additionally, when comparing the curing efficiency based on plasmid size and copy number, it was observed in Lp. plantarum strains that even with plasmid sizes ranging from 2.4 kb to 66.8 kb, the curing efficiency sometimes increased when the copy number was lower, regardless of the larger plasmid size (Table 4). However, even when the copy number ranged from 1.9 to 142.2, there were cases where differences in curing efficiency were observed despite the small difference in copy number, and this was not consistent in all cases. In the case of Lc. paracasei, when comparing plasmids with the same number but with a size difference of about 10 times, the curing efficiency was lower for larger plasmid sizes (Table 4). Principal Component Analysis (PCA) was performed to identify patterns and relationships between various strains and agents concerning plasmid curing efficiency. The data matrix consisted of plasmid removal rates for strains treated with 10 μg/ml and 50 μg/ml concentrations of the agents. The PCA results are visualized in Fig. 2, where each point represents a strain-agent combination, color-coded according to plasmid removal efficiency. The first and second principal components account for the majority of the variance in the data, showing distinct clustering of strain-agent combinations based on their plasmid curing efficiency. According to the positioning of each cluster, AO and EtBr exhibited low curing efficiency, while Nv demonstrated high curing efficiency, allowing for a clear differentiation of curing efficiency among the agents.
Table 2.
Plasmid curing efficiency in the tested strains.
| Scientific name | Strain | Agent / (μg/ml) | Incubation time (h) | Curing rate (%) |
|---|---|---|---|---|
| Lactiplantibacillus plantarum | DS1989 | Nv / 10 | 72+72 | 1/96 (1%) |
| Lactiplantibacillus plantarum | DS0815 | Nv / 10 | 72+72 | 1/96 (1%) |
| Lactiplantibacillus plantarum | DS1902 | Nv / 10 | 72+72 | 2/96 (2%) |
| Lactiplantibacillus plantarum | DS1073 | Nv / 10 | 72+72 | 8/96 (8%) |
| Limosilactobacillus reuteri | DS0354 | EtBr / 10 | 24 | 28/96 (29%) |
| EtBr / 50 | - | |||
| AO / 10 | 24 | 3/96 (3%) | ||
| AO / 50 | 24 | 10/96 (10%) | ||
| Nv / 10 | - | |||
| Nv / 50 | - | |||
| Limosilactobacillus reuteri | DS0384 | EtBr / 10 | 24 | 43/96 (45%) |
| EtBr / 50 | - | |||
| AO / 10 | 24 | 4/96 (4%) | ||
| AO / 50 | - | |||
| Nv / 10 | - | |||
| Nv / 50 | - | |||
| Lactobacillus gasseri | DS2831 | EtBr / 10 | 24 | 0/96 |
| EtBr / 50 | 24 | 0/96 | ||
| AO / 10 | 24 | 0/96 | ||
| AO / 50 | 24 | 0/96 | ||
| Nv / 10 | 24 | 14/96 (15%) | ||
| Nv / 50 | - | |||
| Lacticaseibacillus paracasei | DS0725 | EtBr / 10 | 72 | 0/96 |
| EtBr / 50 | 72 | 5/96 (5%) | ||
| AO / 10 | 72 | 0/96 | ||
| AO / 50 | 72 | 0/96 | ||
| Nv / 10 | - | |||
| Nv / 50 | - | |||
| Lacticaseibacillus paracasei | DS2766 | EtBr / 10 | 24 | 4/96 (4%) |
| EtBr / 50 | 24 | 4/96 (4%) | ||
| EtBr / 10 | 48 | 7/96 (7%) | ||
| EtBr / 50 | 48 | 21/96 (22%) | ||
| AO / 10 | 48 | 17/96 (18%) | ||
| AO / 50 | 48 | 21/96 (22%) | ||
| Nv / 10 | - | |||
| Nv / 50 | - | |||
| Bifidobacterium longum | DS1566 | EtBr / 10 | - | - |
| EtBr / 50 | - | - | ||
| AO / 10 | - | - | ||
| AO / 50 | - | - | ||
| Nv / 10 | - | - | ||
| Nv / 50 | - | - |
–, the strain does not grow in medium containing a curing agent. EtBr, Ethidium bromide; AO, Acridine orange; Nv, Novobiocin.
Table 3.
Curing in LAB strains harboring more than two plasmids.
| Scientific name | Strain | Agent / (μg/ml) | Incubation time (h) | Plasmid 1 cured colonies | Plasmid 2 cured colonies | Plasmid 3 cured colonies | Plasmid 1&2 cured colonies | Plasmid 1&3 cured colonies | Plasmid 2&3 cured colonies | Plasmid 1&2&3 cured colonies |
|---|---|---|---|---|---|---|---|---|---|---|
| Lactiplantibacillus plantarum | DS1989 | Nv / 10 | 72 | 15/96 (16%) | 9/96 (9%) | 1/96 (1%) | 1/96 (1%) | 0/96 | 0/96 | 0/96 |
| Nv / 50 | 72 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| EtBr / 50 | 72 | 1/96 (1%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| EtBr/ 10 | 72 | 1/96 (1%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| AO / 10 | 72 | 2/96 (2%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| AO / 50 | 72 | 2/96 (2%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| Lactiplantibacillus plantarum | DS0815 | Nv / 10 | 72 | 91/96 (95%) | 4/96 (4%) | 4/96 (4%) | 4/96 (4%) | 4/96 (4%) | 0/96 | 0/96 |
| Nv / 50 | 72 | 6/6 (100%) | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | ||
| EtBr / 10 | 72 | 9/96 (9%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| EtBr/ 50 | 72 | 18/22 (82%) | 1/22 (5%) | 0/22 | 0/22 | 0/22 | 0/22 | 0/22 | ||
| AO / 10 | 72 | 9/96 (9%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| AO / 50 | 72 | 7/96 (7%) | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | 0/96 | ||
| Lactiplantibacillus plantarum | DS1073 | Nv / 10 | 72 | 54/96 (56%) | 0/96 | - | 0/96 | - | - | - |
| Nv / 50 | 72 | 9/96 (9%) | 0/96 | - | 0/96 | - | - | - | ||
| EtBr / 10 | 72 | 1/96 (1%) | 0/96 | - | 0/96 | - | - | - | ||
| EtBr / 50 | 72 | 19/96 (20%) | 0/96 | - | 0/96 | - | - | - | ||
| AO / 10 | 72 | 1/96 (1%) | 0/96 | - | 0/96 | - | - | - | ||
| AO / 50 | 72 | 0/96 | 0/96 | - | 0/96 | - | - | - | ||
| Lactiplantibacillus plantarum | DS1902 | Nv / 10 | 72 | 0/96 | 16/96 (17%) | - | 0/96 | - | - | - |
| Nv / 50 | 72 | 0/96 | 1/96 (1%) | - | 0/96 | - | - | - | ||
| EtBr / 10 | 72 | 0/96 | 0/96 | - | 0/96 | - | - | - | ||
| EtBr / 50 | 72 | 0/96 | 0/96 | - | 0/96 | - | - | - | ||
| AO / 10 | 72 | 0/96 | 0/96 | - | 0/96 | - | - | - | ||
| AO / 50 | 72 | 0/96 | 0/96 | - | 0/96 | - | - | - |
–, the strain does not possess plasmid 3. EtBr, Ethidium bromide; AO, Acridine orange; Nv, Novobiocin.
Table 4.
Plasmid curing efficiency based on plasmid size.
| Strain | Number of plasmids | Plasmid size (bp) | Copy number | G+C content (%) | Agent / (μg/ml) | Incubation time (h) | Cured colonies | Curing (%) |
|---|---|---|---|---|---|---|---|---|
| DS1989 | 3 | 50,933 | 38.9 | 38.9 | EtBr/ 10 | 72 | 1/96 | 1 |
| EtBr / 50 | 72 | 1/96 | 1 | |||||
| AO / 10 | 72 | 2/96 | 2 | |||||
| AO / 50 | 72 | 2/96 | 2 | |||||
| Nv / 10 | 72 | 15/96 | 15 | |||||
| 48,573 | 121.5 | 39.0 | Nv / 10 | 72 | 9/96 | 9 | ||
| 8,694 | 142.2 | 36.0 | Nv / 10 | 72 | 1/96 | 1 | ||
| DS0815 | 3 | 66,660 | 4.3 | 39.3 | EtBr/ 10 | 72 | 9/96 | 9 |
| EtBr / 50 | 72 | 18/22 | 82 | |||||
| AO / 10 | 72 | 9/96 | 9 | |||||
| AO / 50 | 72 | 7/96 | 7 | |||||
| Nv / 10 | 72 | 91/96 | 95 | |||||
| Nv / 50 | 72 | 6/6 | 100 | |||||
| 39,431 | 3.6 | 40.5 | EtBr / 50 | 72 | 1/22 | 5 | ||
| Nv / 10 | 72 | 4/96 | 4 | |||||
| 6,156 | 8.6 | 37.4 | Nv / 10 | 72 | 4/96 | 4 | ||
| DS1073 | 2 | 50,512 | 1.9 | 39.0 | EtBr / 10 | 72 | 1/96 | 1 |
| EtBr / 50 | 72 | 19/96 | 20 | |||||
| AO / 10 | 72 | 1/96 | 1 | |||||
| Nv / 10 | 72 | 54/96 | 56 | |||||
| Nv / 50 | 72 | 9/96 | 9 | |||||
| 19,584 | 4.2 | 40.5 | ALL | 72 | 0/96 | 0 | ||
| DS1902 | 2 | 7,845 | 3.4 | 36.9 | ALL | 72 | 0/96 | 0 |
| 2,410 | 9.8 | 38.2 | Nv / 10 | 72 | 16/96 | 17 | ||
| Nv / 50 | 72 | 1/96 | 1 | |||||
| DS0725 | 1 | 66,795 | 43.8 | EtBr / 50 | 72 | 5/96 | 5 | |
| DS2766 | 1 | 6,196 | 38.9 | EtBr / 10 | 24 | 4/96 | 4 | |
| EtBr / 50 | 24 | 4/96 | 4 | |||||
| EtBr / 10 | 48 | 7/96 | 7 | |||||
| EtBr / 50 | 48 | 21/96 | 22 | |||||
| AO / 10 | 48 | 17/96 | 18 | |||||
| AO / 50 | 48 | 21/96 | 22 |
EtBr, Ethidium bromide; AO, Acridine orange; Nv, Novobiocin.
Fig. 2. Principal Component Analysis (PCA) of plasmid curing data.
The scatter plot shows the first and second principal components (PC1 and PC2) based on the plasmid curing rates of various strains treated with agents at 10 μg/ml and 50 μg/ml concentrations. Each point represents a strain-agent combination, color-coded according to the curing rate (%), visually illustrating patterns and relationships.
Variation in Antibiotic Susceptibility for Plasmid-Cured LAB
After plasmid curing, three Lp. plantarum strains (DS1989, DS1902, and DS1073) showed a decrease in the MIC values for gentamicin by more than half (Table 5). Susceptibility of Lp. plantarum (DS1902 and DS1073) to tetracycline decreased up to 1/10-fold after curing, whereas that of Lm. reuteri DS0384 increased. Lm. reuteri DS0384 and DS0354 and L. gasseri DS2831 had decreased MIC values for streptomycin. In terms of susceptibility to chloramphenicol, four strains (Lp. plantarum DS1902, L. gasseri DS2831, and Lc. paracasei DS2766 and DS0725) had MIC values that decreased by half, while Lm. reuteri DS0384 had an increased MIC value, as listed in Table 5.
Table 5.
Changes in the antibiotic susceptibility of each strain after plasmid curing.
| Strain Antibiotic | DS1989 Lp plantarum | DS0815 Lp plantarum | DS1073 Lp plantarum | DS1902 Lp plantarum | DS0354 Lm. reuteri | DS0384 Lm. reuteri | DS2831 L. gasseri | DS0725 Lc. paracasei | DS2766 Lc. paracasei | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | C | WT | C | WT | C | WT | C | WT | C | WT | C | WT | C | WT | C | WT | C | |
| Ampicillin | 0.125 | 0.125 | 0.125 | 0.064 | 0.94 | 0.94 | 0.19 | 0.19 | 3 | 4 | 4 | 8 | 0.38 | 0.25 | 0.75 | 0.75 | 1 | 1 |
| Vancomycin | - | - | - | - | - | - | - | - | - | - | - | - | 1.5 | 1 | - | - | - | - |
| Gentamycin | 64 | 24 | 48 | 64 | 100 | 24 | 48 | 24 | 12 | 6 | 16 | 12 | 32 | 32 | 24 | 24 | 64 | 64 |
| Kanamycin | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 256 | 128 | 256 | 256 | 96 | 256 | 256 |
| Streptomycin | - | - | - | - | - | - | - | - | 96 | 48 | 128 | 96 | 16 | 12 | 32 | 48 | 256 | 256 |
| Erythromycin | 1 | 1 | 1 | 0.75 | 1.5 | 1 | 1 | 1 | 0.75 | 0.5 | 0.5 | 1 | 0.5 | 0.38 | 0.38 | 0.38 | 0.5 | 0.5 |
| Clindamycin | 1 | 1 | 0.75 | 0.75 | 0.75 | 0.75 | 1 | 1 | 0.125 | 0.047 | 0.094 | 0.125 | 8 | 12 | 0.047 | 0.94 | 0.38 | 0.38 |
| Tetracycline | 16 | 16 | 4 | 4 | 12 | 2 | 64 | 6 | 16 | 16 | 12 | 96 | 1.5 | 1.5 | 0.38 | 0.5 | 0.75 | 0.5 |
| Chloramphenicol | 8 | 8 | 4 | 4 | 6 | 6 | 12 | 6 | 3 | 4 | 2 | 4 | 6 | 3 | 8 | 4 | 12 | 6 |
WT, wild-type strain; C, plasmid-cured strain. Instances where antibiotic resistance has significantly decreased or increased are highlighted in bold.
Genomic Variation of Plasmid-Cured LAB
Plasmid-cured strains, including Lp. plantarum DS1902 and DS1073, Lm. reuteri DS0384, Lc. paracasei DS0725, and L. gasseri DS2831, were sequenced again to investigate the presence of mutations in the genome induced by treatment with a curing agent. Lm. reuteri DS0384 showed the greatest number of variations, with 40 different variants due to new base insertions (Table 6). Other strains exhibited 2 to 14 variants, with single nucleotide polymorphisms (SNPs) and new base insertions occurring frequently, while deletions were relatively rare.
Table 6.
Analysis of genomic variation induced by treatment with the curing agents.
| Strain | Scientific name | Number of SNPs | Number of insertions | Number of deletions | Variants |
|---|---|---|---|---|---|
| DS1073 | Lactiplantibacillus plantarum | 6 | 7 | 1 | 14 |
| DS1902 | Lactiplantibacillus plantarum | 2 | 0 | 0 | 2 |
| DS0384 | Limosilactobacillus reuteri | 0 | 40 | 0 | 40 |
| DS0725 | Lacticaseibacillus paracasei | 3 | 0 | 0 | 3 |
| DS2831 | Lactobacillus gasseri | 4 | 5 | 1 | 10 |
Discussion
Research on curing LAB has been reported less frequently than that on pathogenic strains or species showing multi-drug resistance. Cases of plasmid curing in LAB using 100 μg/ml of AO [36], 2–10 μg/ml of acriflavine [37, 38], 8–10 μg/ml of EtBr [26], and 0.1–40 μg/ml of Nv [26, 27, 39] are representative examples. In this study, three curing agents (EtBr, AO, and Nv) were applied to 10 LAB strains from five species that are widely used as probiotics, whose plasmid numbers, sizes, and gene contents were determined through whole-genome analysis. This study aimed to reassess the differences in susceptibility to each curing agent among species and strains and to evaluate the efficiency of curing. Additionally, this study aimed to explore useful approaches for achieving complete curing of strains harboring multiple plasmids.
The DNA-intercalating agents EtBr and AO, and the DNA gyrase inhibitor Nv were used as curing agents at concentrations ranging from 10–50 μg/ml. No microbial growth was observed in the medium containing Nv for Lm. reuteri (DS0354 and DS0384) and Lc. paracasei (DS0725 and DS2766). Interestingly, B. longum DS1566 did not exhibit microbial growth in the presence of curing agents at any concentration (Table 2), even when diluted to 1/100. However, other strains of B. longum (AM54 and DS4107) grown in the laboratory were able to grow in MRS broth containing all curing agents (data not shown), suggesting that the phenomenon observed in the DS1566 strain is specific to it. Analysis of whole-genome sequencing data revealed that the DS1566 strain harbors the serine/threonine-protein kinase toxin HipA gene on its plasmid, which is distinct from the other LAB strains used in this study. HipA, derived from E. coli, acts as a toxic gene and is expressed in response to external factors such as antibiotics or stressors [40]. Expression of this gene inhibits the growth of the strain, and this effect is sometimes counteracted by an effective antidote, HipB [41, 42]. Ultimately, the sensitivity of LAB strains to each curing agent may vary, including in specific cases such as B. longum DS1566. Further accumulation of cases is necessary to determine the plasmid-curing characteristics of each species.
Following plasmid curing, 10 μg/ml EtBr exhibited the highest curing efficiency at 29–40% in Lm. reuteri. For L. gasseri, a curing efficiency of 15% was determined with 10 μg/ml Nv. For Lc. paracasei, relatively high concentrations of EtBr and AO exhibited superior curing efficiency after 48–72 h of treatment (Table 2). During the plasmid curing of Lp. plantarum, complete elimination of all plasmids was not achieved even after treatment with the three agents for up to 72 h. Additionally, despite the absence of amplification products for all plasmids via PCR analysis, false-positive instances were observed upon cultivation and repeated PCR (Fig. S1). Based on plasmid 1, which is the most easily cured plasmid among two or more plasmids, the curing efficiency of 10 μg/ml Nv in L. plantarum DS1989, 0815, and 1073 was 16%, 95%, and 56%, respectively. However, plasmid 1 was not cured even after 72 h of treatment in L. plantarum DS1902 (Table 3). These curing efficiencies are inconsistent with previously reported cases wherein 94–100% curing was achieved via treatment with 0.125–0.25 μg/ml Nv [26]. In studies that report high curing efficiency, successful curing was confirmed even if only one of the plasmids, among those ranging from 5–16 plasmids and varying in size from 2–68 kb, underwent curing [26]. Therefore, it is challenging to consider cases of partial curing success as typical examples of plasmid curing in L. plantarum. In this study, we examined the curing efficiency of 0.2–1 μg/ml Nv treatment in Lp. plantarum strains, and Nv at a concentration of 10 μg/ml was deemed more efficient as it led to curing in all Lp. plantarum strains. Furthermore, an additional treatment of 10 μg/ml Nv for 72 h was conducted on strains harboring two to three plasmids to achieve complete curing of all plasmids. Through this process, complete curing was achieved in only 1–8% of the cases, indicating a very low success rate (Table 2).
During plasmid curing in Lp. plantarum strains harboring two or more plasmids, strains with plasmid sizes of 48–66 kb showed efficiencies ranging from 9–95%, indicating that they were generally more easily cured than those with plasmids smaller than 20 kb (Table 4). However, the maximum curing efficiency for the 6.2 kb plasmid of Lc. paracasei DS2766 and the 66.7 kb plasmid of Lc. paracasei DS0725 was 22% after 48 hours and 5% after 72 h in contrast to Lp. plantarum. This suggests that other factors may influence plasmid curing efficiency. For example, previous studies have shown that the cell growth phase can affect plasmid removal [43]. Plasmids are more stably maintained when cells are in the early growth phase. However, as cells progress to the mid-growth phase and cell division becomes more active, plasmid removal can be enhanced. The plasmid copy numbers in Lp. plantarum strains varied widely, ranging from approximately 1.9 to 142.2 copies, while the G+C content ranged from 36.0% to 40.5%, indicating a relatively similar range. However, these two factors did not significantly affect plasmid curing (Table 4).
Additionally, the genomes of 10 LAB strains from 5 species used in this study were analyzed using Resfinder and Virulence Finder. The analysis revealed no genes related to antibiotic resistance or virulence in the chromosomes or plasmids. However, some strains exhibited resistance that exceeded the antibiotic susceptibility guidelines proposed by the EFSA (Table S3). A comparison of antibiotic susceptibility before and after plasmid curing revealed a decrease in resistance to gentamicin by 1/2- and 1/4-fold in Lp. plantarum (Table 5). In the case of Lm. reuteri, both strains showed a tendency toward decreased resistance to streptomycin, while Lc. paracasei showed a tendency toward decreased resistance to chloramphenicol. However, for the cured strain of Lm. reuteri DS0384, tetracycline resistance increased more than six fold. This is in contrast to the results for Lp. plantarum DS1703 and DS1902 strains, wherein the resistance decreased by 1/6-fold (Table 5). These results indicate the involvement of factors other than the currently known antibiotic resistance-related genes and their mechanisms, which remain to be elucidated. In such cases, the plasmid removal process can cause stress to the cells, potentially leading to genetic recombination or mutations, which may increase antibiotic resistance [44, 45].
The curing agents AO and EtBr, which are DNA-intercalating agents, are well-known mutagens that induce genetic mutations upon prolonged exposure[46]. Sequencing and variant calling of the five cured strains revealed that Lm. reuteri DS0384, which underwent curing with 10 μg/mL EtBr for 24 h, had the highest number of variants among the strains, having 40 SNP mutations (Table 6). However, for Lc. paracasei DS0725, which underwent curing with a higher concentration of EtBr at 50 μg/ml, only three mutations were observed despite treatment for 72 h. In addition, in strains cured using Nv, which induces changes in plasmid topology by acting as a DNA gyrase inhibitor, 2–14 variants were detected. However, variations of 2–40 nucleotides are considered to occur in LAB, which typically has an average genome size of approximately 2.6 GB. Therefore, it is presumed that mutations induced by treatment with curing agents do not significantly affect the functional characteristics of the strains, unless they alter the expression of important genes.
In conclusion, when considering the curing probability with the three agents across different LAB species, it is more efficient to use Nv for curing in Lp. plantarum and L. gasseri. For Lm. reuteri, EtBr is more effective, while Lc. paracasei can benefit from curing with EtBr and AO to increase the success rate of plasmid removal. Additionally, when the plasmid size is large, higher agent concentrations and longer treatment times are required compared to smaller plasmids. Moreover, if there are multiple plasmids, achieving complete curing may require undergoing the curing process twice. Our study demonstrates that traditional mutagenic agents such as EtBr, AO, and Nv can effectively remove plasmids from various LAB strains, achieving up to 45% curing efficiency under optimal conditions. These results are important as they provide a benchmark for evaluating technologies like CRISPR. While CRISPR offers precise and targeted genetic modifications, traditional methods are extremely useful in initial screenings and broad applications due to their wide applicability and relative simplicity. Additionally, genome analysis after plasmid removal showed that off-target effects were minimized, highlighting the specificity of traditional mutagenic agents when used under controlled conditions. This offers a practical alternative to using CRISPR technology for ensuring the safety of probiotic strains.
Supplemental Materials
Supplementary data for this paper are available on-line only at http://jmb.or.kr.
Acknowledgments
This work was carried out with the support of a Korea Innovation Foundation (INNPOLIS) grant (2021-DD-UP-0380-03-203), a grant from the National Research Foundation of Korea (2022M3H9A1084279) funded by the Ministry of Science and ICT, and Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (KGM5232423).
Footnotes
Authors Contributions
C-HP performed the experiments and wrote the manuscript; HY and SHK assisted with the experiments and data interpretation; C-SY and B-CJ participated in the analysis of the experimental data; and Y-JH and D-SP contributed to the conception of the study and revised the manuscript.
Ethics Approval
This study was approved by the Public Institutional Bioethics Committee designated by the MOHW (P01-201703-31-007).
Abbreviations
LAB Lactic acid bacteria
Lm. reuteri Limosilactobacillus reuteri
Lp plantarum Lactiplantibacillus plantarum
L. gasseri Lactobacillus gasseri
Lc. paracasei Laticaseibacillus paracasei
B. longum Bifidobacterium longum
MRS de Man, Rogosa, and Sharpe
AO Acridine Orange
EtBr Ethidium bromide
Nv Novobiocin
MIC Minimum Inhibitory Concentration
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Trevors J. Plasmid curing in bacteria. FEMS Microbiol. Rev. 1986;1:149–157. doi: 10.1016/0378-1097(86)90286-7. [DOI] [Google Scholar]
- 2.Wong JJ, Lu J, Glover JM. Relaxosome function and conjugation regulation in F‐like plasmids-a structural biology perspective. Mol. Mcrobiol. 2012;85:602–617. doi: 10.1111/j.1365-2958.2012.08131.x. [DOI] [PubMed] [Google Scholar]
- 3.Foster T. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol. Rev. 1983;47:361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 1986;192:1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- 5.Lerminiaux NA, Cameron AD. Horizontal transfer of antibiotic resistance genes in clinical environments. Canadian J. Microbiol. 2019;65:34–44. doi: 10.1139/cjm-2018-0275. [DOI] [PubMed] [Google Scholar]
- 6.Sabtu N, Enoch D, Brown N. Antibiotic resistance: what, why, where, when and how? Br. Med. Bull. 2015;116:105–113. doi: 10.1093/bmb/ldv041. [DOI] [PubMed] [Google Scholar]
- 7.Caniça M, Manageiro V, Abriouel H, Moran-Gilad J, Franz CM. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019;84:41–44. doi: 10.1016/j.tifs.2018.08.001. [DOI] [Google Scholar]
- 8.Briceno VK. Superbug me: the FDA's role in the fight against antibiotic resistance. NYUJ Legis. Pub. Pol'y. 2005;9:521. [Google Scholar]
- 9.ECDC EPoBH, Use ECfMPfV, author. ECDC, EFSA and EMA Joint Scientific Opinion on a list of outcome indicators as regards surveillance of antimicrobial resistance and antimicrobial consumption in humans and food‐producing animals. EFSA J. 2017;15:e05017. doi: 10.2903/j.efsa.2017.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson DI. Persistence of antibiotic resistant bacteria. Curr. Opin. Microbiol. 2003;6:452–456. doi: 10.1016/j.mib.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Böttger EC, Springer B, Pletschette M, Sander P. Fitness of antibiotic-resistant microorganisms and compensatory mutations. Nat. Med. 1998;4:1343–1344. doi: 10.1038/3906. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Yoon Y, Oh S. Suggestion of a safety evaluation procedure to improve probiotic safety. J. Dairy Sci. Biotechnol. 2020;38:99–111. doi: 10.22424/jdsb.2020.38.2.99. [DOI] [Google Scholar]
- 13.Hassanshahian M, Saadatfar A, Masoumipour F. Formulation and characterization of nanoemulsion from Alhagi maurorum essential oil and study of its antimicrobial, antibiofilm, and plasmid curing activity against antibiotic-resistant pathogenic bacteria. J. Environ. Health Sci. Eng. 2020;18:1015–1027. doi: 10.1007/s40201-020-00523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl. Environ. Microbiol. 2008;74:6032–6040. doi: 10.1128/AEM.00991-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner MM, Ciusa ML, Piddock LJ. Strategies to combat antimicrobial resistance: anti-plasmid and plasmid curing. FEMS Microbiol. Rev. 2018;42:781–804. doi: 10.1093/femsre/fuy031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringel F, Frey L, Hubert JC. Characterization, cloning, curing, and distribution in lactic acid bacteria of pLP1, a plasmid from Lactobacillus plantarum CCM 1904 and its use in shuttle vector construction. Plasmid. 1989;22:193–202. doi: 10.1016/0147-619X(89)90002-4. [DOI] [PubMed] [Google Scholar]
- 17.Posno M, Leer R, Van Luijk N, Van Giezen M, Heuvelmans P, Lokman B, et al. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 1991;57:1822–1828. doi: 10.1128/aem.57.6.1822-1828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalasvuori M, Friman VP, Nieminen A, Bamford JK, Buckling A. Bacteriophage selection against a plasmid-encoded sex apparatus leads to the loss of antibiotic-resistance plasmids. Biol. Lett. 2011;7:902–905. doi: 10.1098/rsbl.2011.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternberg SH, Doudna JA. Expanding the biologist's toolkit with CRISPR-Cas9. Mol. Cell. 2015;58:568–574. doi: 10.1016/j.molcel.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 20.Chatterji M, Unniraman S, Mahadevan S, Nagaraja V. Effect of different classes of inhibitors on DNA gyrase from Mycobacterium smegmatis. J. Antimicrob. Chemother. 2001;48:479–485. doi: 10.1093/jac/48.4.479. [DOI] [PubMed] [Google Scholar]
- 21.Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 22.Song X, Zhang Xy, Xiong Zq, Liu Xx, Xia Yj, Wang Sj, et al. CRISPR-Cas-mediated gene editing in lactic acid bacteria. Mol. Biol. Rep. 2020;47:8133–8144. doi: 10.1007/s11033-020-05820-w. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, He D, Li B, Guo Y, Wang W, Luo X, et al. Eliminating mcr-1-harbouring plasmids in clinical isolates using the CRISPR/Cas9 system. J. Antimicrob. Chemother. 2019;74:2559–2565. doi: 10.1093/jac/dkz246. [DOI] [PubMed] [Google Scholar]
- 24.Salisbury V, Hedges R, Datta N. Two modes of 'curing'transmissible bacterial plasmids. Microbiology. 1972;70:443–452. doi: 10.1099/00221287-70-3-443. [DOI] [PubMed] [Google Scholar]
- 25.Zaman M, Pasha M, Akhter M. Plasmid curing of Escherichia coli cells with ethidium bromide, sodium dodecyl sulfate and acridine orange. Bangladesh J. Microbiol. 2010;27:28–31. doi: 10.3329/bjm.v27i1.9165. [DOI] [Google Scholar]
- 26.Ruiz‐Barba JL, Piard JC, Jiménez‐Díaz R. Plasmid profiles and curing of plasmids in Lactobacillus plantarum strains isolated from green olive fermentations. J. Appl. Microbiol. 1991;71:417–421. doi: 10.1111/j.1365-2672.1991.tb03810.x. [DOI] [PubMed] [Google Scholar]
- 27.Chin CS, Norhani A, Wen ST, Yin WH. Plasmid profiling and curing of Lactobacillus strains isolated from the gastrointestinal tract of chicken. J. Microbiol. 2005;43:251–256. [PubMed] [Google Scholar]
- 28.IBMCorp Ibm S. statistics for windows, version 25.0. Armonk, NY: IBM Corp., USA; 2017. [Google Scholar]
- 29.Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in python. J. Machine Learning Res. 2011;12:2825–2830. [Google Scholar]
- 30.Wilson KH, Blitchington RB, Greene RC. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J. Clin. Microbiol. 1990;28:1942–1946. doi: 10.1128/jcm.28.9.1942-1946.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 32.Nwaiwu O, Aduba CC. An in silico analysis of acquired antimicrobial resistance genes in Aeromonas plasmids. AIMS Microbiol. 2020;6:75. doi: 10.3934/microbiol.2020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jang YJ, Gwon HM, Jeong WS, Yeo SH, Kim SY. Safety evaluation of Weissella cibaria JW15 by phenotypic and genotypic property analysis. Microorganisms. 2021;9:2450. doi: 10.3390/microorganisms9122450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng YT, Li HB, Lu MX, Du YZ. Evaluation and validation of reference genes for qRT-PCR normalization in Frankliniella occidentalis (Thysanoptera: Thripidae) PLoS One. 2014;9:e111369. doi: 10.1371/journal.pone.0111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Adeyemo S, Onilude A. Plasmid curing and its effect on the growth and physiological characteristics of Lactobacillus plantarum isolated from fermented cereals. J. Microbiol. Res. 2015;5:11–22. [Google Scholar]
- 37.Chassy BM, Gibson EM, Guiffrida A. Evidence for plasmid-associated lactose metabolism in Lactobacillus casei subsp. casei. Curr. Microbiol. 1978;1:141–144. doi: 10.1007/BF02601666. [DOI] [PubMed] [Google Scholar]
- 38.Axelsson LT, Ahrné SE, Andersson MC, Ståhl SR. Identification and cloning of a plasmid-encoded erythromycin resistance determinant from Lactobacillus reuteri. Plasmid. 1988;20:171–174. doi: 10.1016/0147-619X(88)90023-6. [DOI] [PubMed] [Google Scholar]
- 39.McHugh GL, Swartz MN. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob. Agents Chemother. 1977;12:423–426. doi: 10.1128/AAC.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korch SB, Hill TM. Ectopic overexpression of wild-type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J. Bacteriol. 2006;188:3826–3836. doi: 10.1128/JB.01740-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Semanjski M, Germain E, Bratl K, Kiessling A, Gerdes K, Macek B. The kinases HipA and HipA7 phosphorylate different substrate pools in Escherichia coli to promote multidrug tolerance. Sci. Signal. 2018;11:eaat5750. doi: 10.1126/scisignal.aat5750. [DOI] [PubMed] [Google Scholar]
- 42.Gerdes K, Bærentsen R, Brodersen DE. Phylogeny reveals novel HipA-homologous kinase families and toxin-antitoxin gene organizations. mBio. 2021;12:e0105821. doi: 10.1128/mBio.01058-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litake GM. Plant-assisted plasmid curing strategies for reversal of antibiotic resistance. 2022. pp. 559–575. Antimicrob. Res. [Google Scholar]
- 44.Lauritsen I, Porse A, Sommer MO, Nørholm MH. A versatile one-step CRISPR-Cas9 based approach to plasmid-curing. Microb. Cell Fact. 2017;16:135. doi: 10.1186/s12934-017-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letchumanan V, Chan KG, Lee LH. An insight of traditional plasmid curing in Vibrio species. Front. Microbiol. 2015;6:735. doi: 10.3389/fmicb.2015.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waring M. Complex formation between ethidium bromide and nucleic acids. J. Mol. Biol. 1965;13:269–282. doi: 10.1016/S0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.