Abstract
Background:
The TAS1R2 receptor, known for its role in taste perception, has also emerged as a key regulator of muscle physiology. Previous studies have shown that genetic ablation of TAS1R2 in mice enhances muscle fitness mimicking responses to endurance exercise training. However, the translational relevance of these findings to humans remains uncertain.
Methods:
We explored responses to endurance exercise training in mice and humans with genetic deficiency of TAS1R2. First, we assessed the effects of muscle-specific deletion of TAS1R2 in mice (mKO) or wild type controls (mWT) following 4 weeks of voluntary wheel running (VWR). Next, we investigated the effects of the TAS1R2−Ile191val (rs35874116) partial loss-of-function variant on responses to a 6-month diet-induced weight loss with exercise training (WLEX), weight loss alone (WL), or education control (CON) interventions in older individuals with obesity. Participants were retrospectively genotyped for the TAS1R2−Ile191Val polymorphism and classified as conventional function (Ile/Ile) or partial loss-of-function (Val carriers: Ile/Val and Val/Val). Body composition, cardiorespiratory fitness, and skeletal muscle mitochondrial function were assessed before and after the intervention.
Results:
In response to VWR, mKO mice demonstrated enhanced running endurance and mitochondrial protein content. Similarly, TAS1R2 Val carriers exhibited distinctive improvements in body composition, including increased muscle mass, along with enhanced cardiorespiratory fitness and mitochondrial function in skeletal muscle following the WLEX intervention compared to Ile/Ile counterparts. Notably, every Val carrier demonstrated substantial responses to exercise training and weight loss, surpassing all Ile/Ile participants in overall performance metrics.
Conclusions:
Our findings suggest that TAS1R2 partial loss-of-function confers beneficial effects on muscle function and metabolism in humans in response to exercise training, akin to observations in TAS1R2 muscle-deficient mice. Targeting TAS1R2 may help enhancing exercise training adaptations in individuals with compromised exercise tolerance or metabolic disorders, presenting a potential avenue for personalized exercise interventions.
Keywords: TAS1R2, Sweet taste receptor, Polymorphism, rs35874116, Obesity, Aging, Muscle metabolism, Mitochondria, Exercise, Running, Weight loss, Genetics, NAD, Muscle mass, HbA1c, PDE
1. Introduction
Aging and obesity are associated with detrimental changes in muscle mass and function. Aging is characterized by a progressive decline in muscle mass and strength, known as sarcopenia, coupled with impaired muscle function, that contributes to diminished physical performance and increased risk of disability and mortality [1]. Similarly, obesity often exacerbates muscle dysfunction through various mechanisms including chronic low-grade inflammation, insulin resistance, and altered lipid metabolism [2]. These overlapping physiological alterations underscore the urgent need for effective interventions to mitigate muscle decline in older adults with obesity.
Exercise training and weight loss represent cornerstone interventions to combat the deleterious effects of aging and obesity on muscle health. Regular physical activity, encompassing endurance, resistance, and combination training modalities, has been consistently shown to preserve muscle mass, enhance cardiorespiratory fitness, and improve mitochondrial function in skeletal muscle of both older adults and individuals with obesity [3]. Moreover, concomitant weight loss achieved through exercise and dietary modifications further augments these beneficial adaptations, leading to improved metabolic health and physical function [4]. However, the extent of individual response to exercise interventions varies widely [5]. The specific factors and their interactions that account for this phenomenon remain unclear. This knowledge gap has sparked considerable interest in uncovering the underlying contributors to such variability [6].
Genetic predisposition is increasingly recognized as a significant determinant of individual response to exercise interventions. Genome-wide association studies (GWAS) have identified numerous genetic variants associated with differential responses to various aspects of exercise training, including cardiorespiratory fitness, muscle hypertrophy, and metabolic adaptations [7]. These genetic variants may influence diverse physiological processes such as mitochondrial biogenesis, muscle fiber composition, inflammatory responses, and insulin sensitivity, thereby shaping an individual’s adaptive capacity to exercise stimuli [8]. For instance, the identification of specific genetic variants, particularly within genes encoding for components of signaling pathways involved in exercise-induced adaptations, has provided insight into the inter-individual variability in response to exercise interventions [9]. Despite the growing body of evidence implicating specific genetic variants in exercise training responses, further elucidation of the underlying determinants of inter-individual variability is crucial for tailoring personalized exercise prescriptions, optimizing training outcomes, and promoting health and wellness across diverse populations.
While traditionally known for its role in taste perception on the tongue [10], the TAS1R2 G-protein coupled receptor (GPCR) is expressed in various extra-oral tissues, including intestinal L-cells [11,12], and pancreatic β-cells [13,14], suggesting broader physiological functions beyond gustation. Recently, the taste receptor TAS1R2 has also been implicated in the regulation of skeletal muscle fitness [15]. Muscle-specific genetic deficiency of TAS1R2 suppressed PARP1 activity leading to increased NAD levels and subsequent beneficial adaptations in mitochondrial density and capacity. Consequently, mice with TAS1R2 loss-of-function demonstrated increased running endurance, suggesting that genetic inhibition of TAS1R2 may also modulate exercise training adaptations. Although the extra-oral function of TAS1R2 in humans is less clear, genetic studies have revealed associations between the TAS1R2−Ile191Val (rs35874116) variant and altered nutrient sensing, plasma insulin, and metabolic responses to dietary intake in obesity [16–18]. Notably, these associations cannot be attributed to differences in taste perception [19], implicating TAS1R2 as a potential modulator of peripheral nutrient metabolism and energy homeostasis. Through a combination of biochemical analyses, we showed that the human TAS1R2−Ile191Val variant induces a partial loss-of-function phenotype in the TAS1R2 receptor [20]. Subsequent observational studies confirmed the biochemical findings by demonstrating that carriers of this partial loss-of-function variant (Val) in humans exhibit a similar phenotype to that observed in mice [20]. Nevertheless, aside from a few observational studies, the clinical relevance of this loss-of-function variant of the TAS1R2 glucose receptor has not been evaluated following relevant interventions, such as diet or exercise.
Considering that TAS1R2 deficiency in mouse skeletal muscle mimics adaptations to endurance exercise, investigating the role of TAS1R2−Ile191Val variant in shaping the responses to exercise training holds promise for personalizing interventions and optimizing outcomes in diverse populations [21]. The aim of this translational study is to evaluate the role of TAS1R2−Ile191Val variant in enhancing physiological adaptations to chronic exercise. Using mouse and human genetics, we show that the TAS1R2 partial loss-of-function in older adults who are obese substantially improved responses to an exercise-training program, recapitulating the effects seen in TAS1R2 deficient mice.
2. Materials and methods
2.1. Animal and cell studies
2.1.1. Animals
All animal procedures adhered to The Ohio State University institutional animal care and use committee (IACUC). Conditional Tas1r2 knockouts were previously generated from Myogenin-Cre and Tas1r2 floxed mice [15]. Whole body Tas1r2 knockout mice (bKO) were a gift from Dr. Zuker. Leptin deficient Lepob mice (ob) (Jax #000632) were obtained from the Jackson Lab. bKO and ob mice were crossed to generate obese mice deficient in Tas1r2. A cohort of bWT and bKO mice was aged 27 months. All mice were housed on a 12h light/dark cycle with free access to water and standard diet (Tecklad 7912) and were used after reaching sexual maturity at 12 weeks.
2.1.2. Mouse genotyping
PCR genotyping was performed on ear samples digested with NaOH. Cre presence was determined by PCR with ChoiceTaq (Sigma) using CCGGTGAACGTGCAAAACAGGCTCTA and CTTCCAGGGCGCGAGTTGATAGC as forward and reverse primers. The leptin allele was genotyped with TaqReady Mix (Sigma) using TGAGTTTGTCCAAGATGGACC and GCCATCCAGGCTCTCTGG as forward and reverse primers, combined with AATGACCTGGAGAATCTCC for the detection of the wild-type allele or GCAGATGGAGGAGGTCTCA for the detection of the ob mutation.
2.1.3. Body composition
Body composition was measured using an EchoMRI instrument and percent composition was calculated after subtraction of free water.
2.1.4. Running endurance
Running endurance was performed using an Exer 6 treadmill (Columbus Instruments) after 2 sessions of acclimatization. Endurance was evaluated before and after exercise training with a ramp protocol, starting 5 min-0°-10 m/min, followed by 5 min-0°-20 m/min, 5 min-10°-20 m/min and then increasing 1 m/min every 5 min until exhaustion. Obese mice were tested at 0°-10 m/min until exhaustion. Old mice were tested with a mild ramp protocol to accommodate mice with gait problems, starting 5 min-0°-5 m/min, followed by 5 min-10°-5 m/min, 5 min-10°-10 m/min and then increasing 1 m/min every 5 min until exhaustion. Exhaustion was defined as the inability to run >3 s for 3 consecutive trials.
2.1.5. Exercise training
Mice were housed individually for 30 days in standard cages containing a running wheel (STARR Life Sciences) to provide voluntary access to physical exercise. Wheel revolutions were recorded with VitalView data acquisition software (STARR Life Sciences) to calculate training volume.
2.1.6. Echocardiography
Cardiac function was determined by 2-dimensional echocardiography using a Visual Sonics Vevo 3100 Ultrasound (Visual Sonics) under isoflurane anesthesia. M-mode tracings provided left ventricular dimensions used to calculate ejection fraction.
2.1.7. Harvest and tissue processing
Tissues were harvested in vivo under 165 mg/kg pentobarbital anesthesia, snap-frozen in liquid nitrogen and lyophilized overnight.
2.1.8. Human myotubes
Primary human skeletal myoblasts were provided by Translational Research Institute Advent Health (Florida, USA). Myoblasts were grown to a confluence of approximately 80 % to 90 % and differentiated into myotubes as previously described [22].
2.1.9. Glycogen assay
Muscle glycogen was measured with a commercial kit (abcam ab65620) and normalized by mg of dry tissue after subtraction of free glucose.
2.1.10. NAD cyclic assay
NAD was determined by a cycling assay based on alcohol dehydrogenase after the acidic destruction of NADH as previously described [15]. Results were normalized by dry tissue weight.
2.1.11. Protein extraction
Protein was extracted from lyophilized tissue as previously described [15]. Extracts were spun and protein was measured with a BCA kit (Thermo) before storage at −20 °C with reducing buffer.
2.1.12. Immunoblotting
Samples were heated (65 °C for mitochondrial proteins, 85 °C otherwise) and electrophoresis and immunoblotting were performed as previously described [15]. Loading from Ponceau staining (Sigma) was used as normalizing factor.
2.2. Human studies
2.2.1. Study design and participant characteristics
The study was a randomized controlled trial with a parallel group design conducted between 2012 and 2017 at the University of Pittsburgh and the AdventHealth Translational Research Institute (AH TRI). The protocol was approved by the institutional boards and registered on ClinicalTrials.gov (NCT02230839). Eighty-four physically inactive obese men and women aged between 60 and 80 were recruited. Participants were randomly assigned to three 6-month interventions: health education control (CON), calorie restriction-induced weight loss (WL), and weight loss with exercise (WLEX), with equal allocation. Participants provided written informed consent, and randomization was done electronically using a permuted-blocks approach. The study coordinator managed enrollment and group assignment, and outcome assessors were blinded. Eligibility and exclusion criteria are reported in [23].
2.2.2. Interventions
CON participants received bi-weekly health education seminars on medication and type 2 diabetes management without specific exercise or dietary education. WL aimed for a 10 % reduction in body weight through a 500–1000 kcal daily deficit coupled with a low-fat diet. Details and compliance are reported in [23]. Participant weights remained stable during the final two weeks of the intervention. WLEX participants exercised 4–5 days weekly for 45 min each session. Activities mainly comprised walking (outdoors and on a treadmill) with options for stationary cycling, elliptical, and rowing machines. From week 8, the group engaged in two non-consecutive 30-minute resistance training sessions weekly as reported in [23]. Dietary guidance was provided by the Registered Dietitian, as in the WL group.
2.2.3. Blood analyses
Glucose and HbA1C were measured from fasting blood and analyzed in the clinical chemistry laboratory at AH TRI using standard assays. Buffy coat was collected and frozen for DNA isolation and genotyping.
2.2.4. Cardiorespiratory fitness, strength, and functional performance
A cardiopulmonary graded exercise test was conducted by an exercise physiologist on the cycle ergometer using open circuit indirect calorimetry to measure maximal oxygen uptake (VO2peak). Participants exercised at a moderate intensity and resistance increased gradually until volitional fatigue [24].
2.2.5. Body composition
Weight, height, BMI and waist circumference were measured before and after the intervention. Fat mass (FM) and fat-free mass (FFM) were determined using dual-energy X-ray absorptiometry (DXA) with a GE Lunar machine (GE Healthcare, UK). Skeletal muscle index was calculated as appendicular lean mass normalized by height squared. Abdominal and thigh adipose tissue (AT) and muscle volume were measured via MRI using a 3 Tesla magnet (Philips Achieva) at AH TRI. Analyze 11.0 software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) was used to analyze images.
2.2.6. Muscle biopsy
Muscle biopsies were taken from the vastus lateralis after an overnight fast and 48-hour exercise avoidance. Specimens were blotted dry and trimmed of visible adipose tissue using a standard dissecting microscope (Leica EZ4; Leica Microsystems, Heerbrugg, Switzerland). Samples were preserved in BIOPS buffer for mitochondrial analysis.
2.2.7. Mitochondrial respiration assay
Biopsies were permeabilized with saponin (50 μg/mL) and treated with blebbistatin (25 μM) before placing them in the 37 °C Oxygraph 2 K chamber. Leak respiration was measured with glutamate (5 mM), malate (2 mM), and succinate (10 mM). Maximal coupled respiration (OXPHOS) was assessed with ADP (4 mM), and Electron Transport System (ETS) respiration was determined with p-trifluoromethoxy carbonyl cyanide phenyl hydrazone (2 μM). Cytochrome C (10 μM) was used to check mitochondrial membrane integrity. OXPHOS and ETS values were obtained by subtracting leak respiration and analyzed with DatLab software, normalized to fiber bundle wet weight.
2.2.8. Genotyping
The Ile191Val TAS1R2 gene polymorphism (rs35874116) was determined by a TaqMan allelic discrimination assay (#4351379, ThermoFisher Scientific) following specifications, including non-template controls and reference samples for each genotype. The assay was performed independently twice, leading to concordant results with no ambiguous sample. Out of the 61 allocated participants, 41 had available blood samples and complete pre- and post- outcome data to conduct a retrospective genotyping (CON, n = 15; WL, n = 12; WLEX, n = 14).
2.3. Statistics
Statistics were performed at a significance level of 0.05. Preclinical data was analyzed with GraphPad Prism 9. Delta values after the exercise intervention were tested for a change with one-sample t-tests and genotype effects were compared by two-tailed t-tests. 2-factor designs were analyzed by two-way ANOVAs. Linear regressions and F-tests were used to test association between variables (slopes), or to compare two groups (intercepts).
Clinical data was analyzed with jamovi 2.2.5. Genotyping results were tested for Hardy-Weinberg equilibrium and allele distribution against a standard population using Chi square tests. Retrospective genotype effects were evaluated through factorial design, correcting for sex, age and diabetic status. Delta values were used to analyze the intervention effect as recommended for retrospective studies [25]. Data was checked for covariate balance, allocation differences, and baseline genotype equivalence. Intervention and genotype effects were described using GLM and coded contrasts. Genotype responses to the intervention were compared against the pooled control group to overcome an imbalance on the genotype subpopulations (HED: Ile n = 10; Val n = 5). The primary analysis assessed differential responses to exercise intervention between genotype groups using composite indexes. Individual responses were also examined. Additional linear regressions tested outcome associations, assessing significant slopes and group differences. Correlations were evaluated using Pearson’s r on adjusted delta values.
3. Results
3.1. Ablation of Tas1r2 in skeletal muscle enhances responses to endurance exercise training in mice
Our studies revealed that the deletion of the TAS1R2 receptor in the muscles of mice induces adaptations similar to those produced by chronic endurance exercise training, such as increased NAD levels, enhanced mitochondrial function, and improved exercise endurance [15]. Consequently, we investigated whether an exercise training intervention could further enhance the muscle phenotype of mKO mice. Both genotypes exercised similarly during the intervention (Fig. S1A). After four weeks of voluntary wheel running (VWR), fat mass decreased in both genotypes, but lean mass increased only in mKO mice (Fig. 1A,B,C; Fig. S1B,C,D). VWR induced several expected adaptations in both mKO and mWT mice, including improved running endurance (Fig. 1D,E; Fig. S1E), increased muscle content of mitochondrial proteins (Fig. 1F), markers of mitochondrial dynamics (Fig. S1F), and CAMKII, a downstream target of calcium and GPCR signaling (Fig. S1F). Additionally, VWR increased muscle NAMPT protein (Fig. S1F) and NAD levels (Fig. 1H), but not SIRT1 protein, which also influences NAD homeostasis (Fig. S1F). These findings align with the exercise-induced NAD synthesis through the salvage pathway [26]. However, TAS1R2 deficiency further potentiated several of the adaptive responses to VWR. Specifically, mKO mice exhibited enhanced running endurance (Fig. 1D) independent of training volume (Fig. 1E). This effect was accompanied by greater mitochondrial protein (Fig. 1F) and glycogen content (Fig. 1G) in mKO muscles. Notably, muscle NAD levels were also enhanced (Fig. 1H) in mKO muscles, suggesting synergistic beneficial contributions of TAS1R2 deficiency and chronic exercise training on NAD homeostasis and muscle fitness.
Fig. 1.
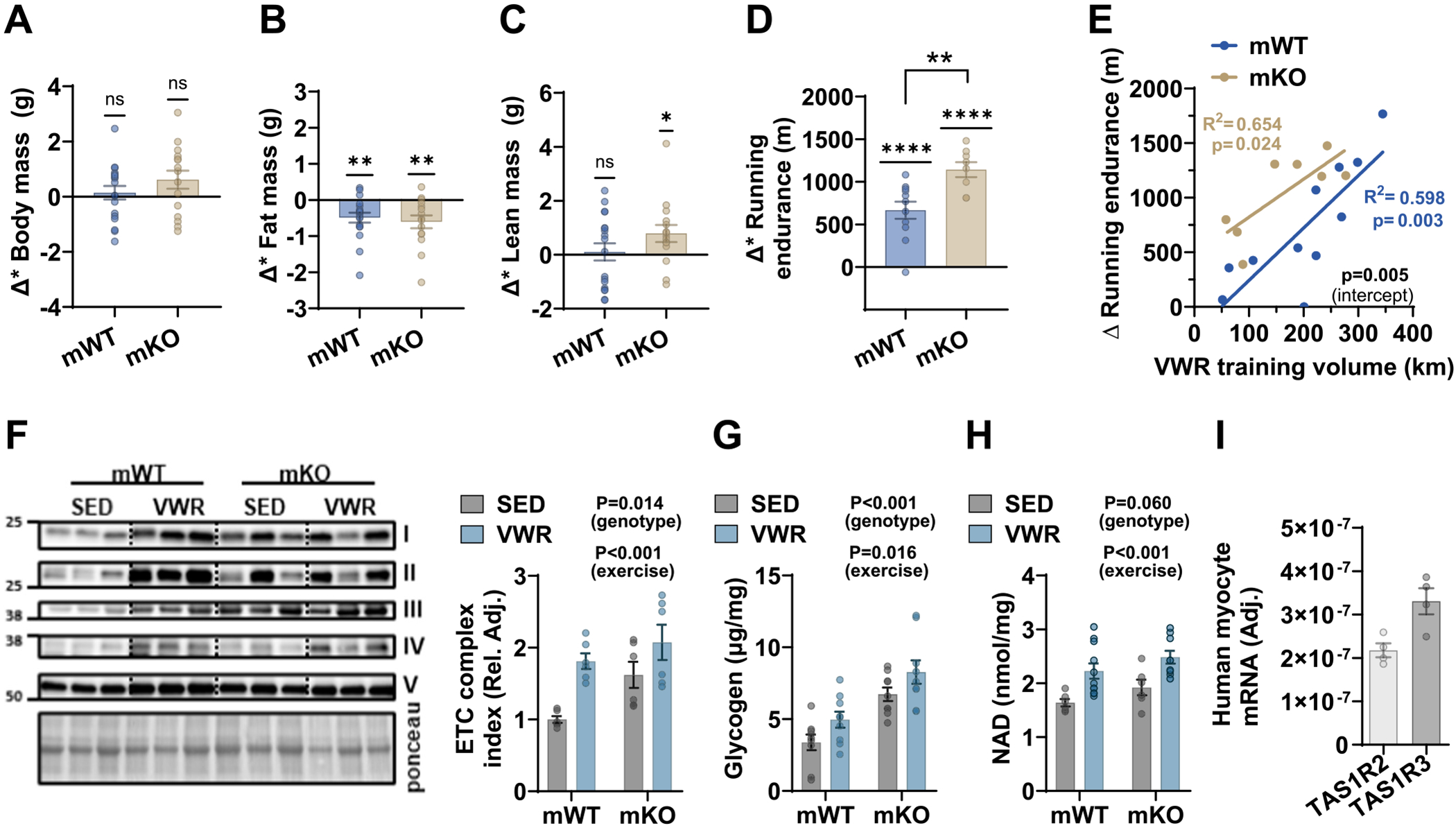
Loss-of-function of TAS1R2 in muscle enhances responses to endurance exercise training in mice.
Effects of 4-weeks of voluntary wheel training (VWR) in mWT and mKO mice or sedentary (SED) controls. Absolute changes (Δ; post-pre) in (A) body mass, (B) fat mass, (C) lean mass and (D) running endurance following VWR. Variables are adjusted by VWR training volume. One-sample t-test for a significant change. P value over brackets indicates genotype effect in two-sided t-test. (E) Simple linear regression between changes in running endurance and total training volume in mWT and mKO mice. Slope P value for each genotype (in color). Elevation contrast P value between mWT and mKO mice (intercept). Immunoblotting and quantitation of (F) mitochondrial electron transport chain (ETC) proteins. Two-way ANOVA. (G) Glycogen concentrations in muscles of mWT and mKO mice. Two-way ANOVA. (H) NAD concentrations in acidic extracts from muscles of mWT and mKO mice. Two-way ANOVA. (I) Expression of TAS1R2 and TAS1R3 in differentiated human myocyte cultures.
3.2. TAS1R2 genotype-dependent effect on baseline variables in humans
Genetic loss of TAS1R2 in mouse skeletal muscle improved muscle fitness [15] and enhanced the response to exercise training (Fig. 1). This observation suggested that targeting TAS1R2 may be beneficial for individuals with low exercise tolerance, capacity, or response, such as those who are obese, older, or have diabetes [6]. Similar to mice, TAS1R2/TAS1R3 is expressed in human cultured myocytes (Fig. 1I), pointing to an equivalent role in human biology. However, it remains uncertain whether a corresponding phenotype can be observed in humans. Through biochemical and clinical approaches, we have previously shown that the TAS1R2-Ile191Val (rs35874116) polymorphism leads to a partial loss-of-function of the TAS1R2 receptor [20], recapitulating metabolic effects previously seen in mice with whole-body deletion of TAS1R2 (bKO) [12]. Therefore, we performed a retrospective analysis to evaluate the impact of this TAS1R2 variant in older adults with obesity who completed a 6-month randomized trial of diet-induced weight loss with exercise training (WLEX), diet-induced weight loss alone (WL), or an education control (CON) [23,27]. This interventional study was originally designed to examine outcomes, such as body composition, aerobic endurance, and mitochondrial capacity, which mimic key parameters of the exercise training intervention used to evaluate TAS1R2 muscle-deficient mice. Participants within each group (e.g., CON, WL, and WLEX) were retrospectively genotyped and classified as TAS1R2 conventional function (Ile/Ile) or TAS1R2 partial loss-of-function (Val/_; Val carriers: Ile/Val and Val/Val) (Fig. S2). First, we assessed the allele frequency and distribution of TAS1R2−Ile191Val in the studied population and found it exhibits the predicted patterns compared to reference population (Table S1). Next, we evaluated the combined effects of TAS1R2−Ile191Val variant on the assessed measures independent of the assigned groups before the intervention (baseline) (Table S2). No significant differences in the assessed variables were observed between the combined Ile/Ile and Val/_ participants at baseline. Also, no major genotype effects were noted across or within groups prior to the interventions (Table S2).
3.3. Main effects of the WL and WLEX interventions independent of TAS1R2 genotype
Before assessing the specific contributions of TAS1R2−Ile191Val variant we evaluated the primary intervention effect in the study population without accounting for the genotype within each group. Compared to CON group, the dietary intervention (WL) caused an anticipated reduction in absolute body and fat mass, but no other effects on the measured outcomes were noted (Table S3). In contrast, addition of exercise training (WLEX) significantly reduced body mass and caused further improvements in body composition (Table S3 and Fig. 2A–C). The WLEX treatment also led to specific decreases in the subcutaneous adipose tissue (SAT) of the thigh and the abdomen (Table S3). WLEX also resulted in increases in cardiorespiratory fitness and corresponding improvements in mitochondrial and oxidative capacity (Table S3 and Fig. 2D–F). These findings suggest that the combination of diet-induced weight loss and exercise training (WLEX) caused significant beneficial effects on body composition and induced the anticipated increases in cardiorespiratory performance [27].
Fig. 2.
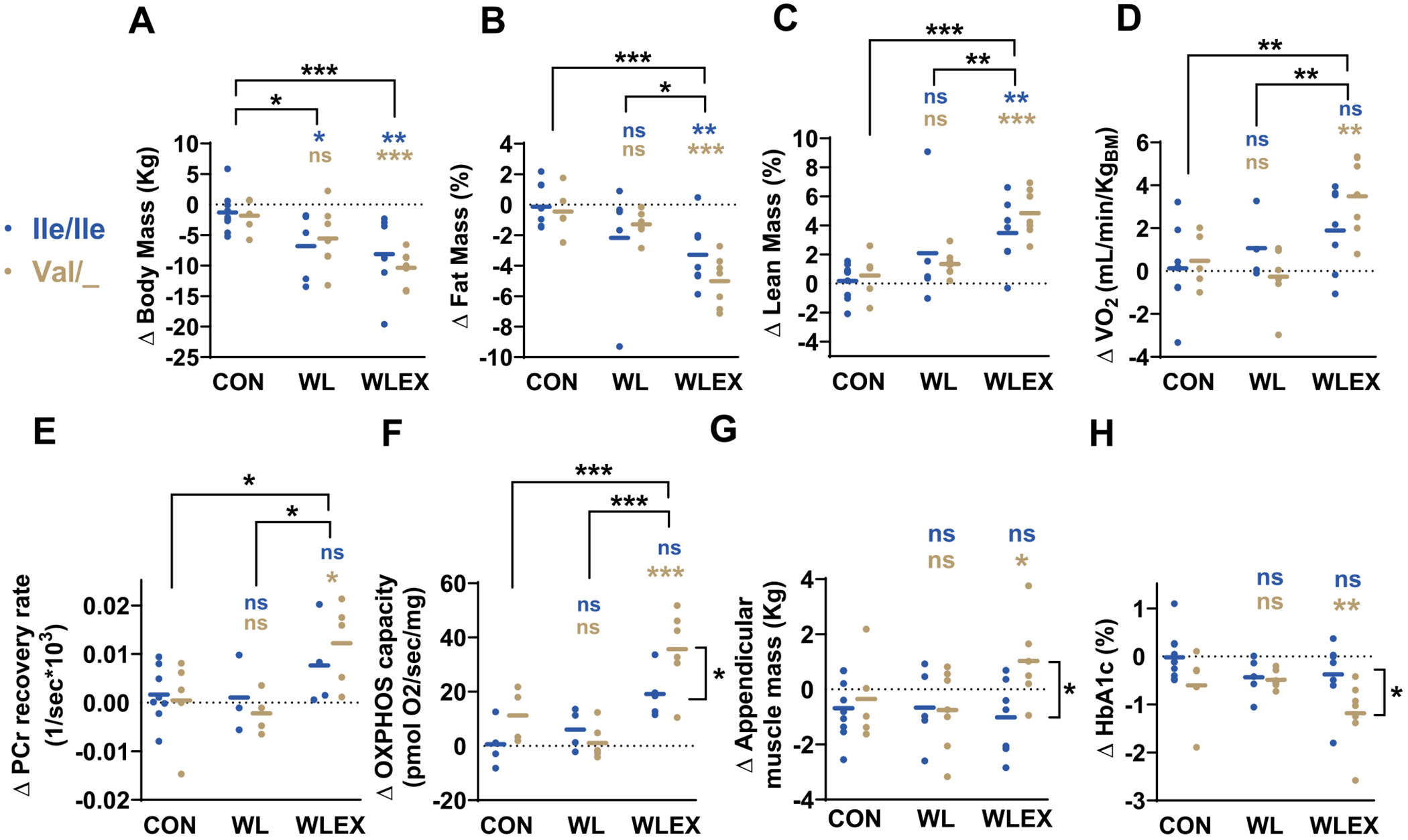
Partial loss-of-function of TAS1R2 enhances responses to endurance exercise training in humans.
Effects of TAS1R2−Ile191Val variant in older obese individuals subjected to 6-months of diet-induced weight loss with exercise training (WLEX), diet-induced weight loss alone (WL), or education control (CON). Blue symbols show Ile/Ile (TAS1R2 normal function) and brown symbols show Val/_ (Val carriers; TAS1R2 partial loss-of-function). Data are shown as absolute changes (Δ; post-pre) following the interventions. (A) Body mass, (B) fat mass, and (C) lean mass. (D) Peak oxygen consumption (VO2) during cycle ergometer testing. (E) In vivo muscle phosphocreatine (PCr) recovery rate using 31P-MRS. (F) Respiratory capacity of isolated myofibers in the ADP-activated state of oxidative phosphorylation (OXPHOS;P). (G) Appendicular muscle mass, and (H) glycated hemoglobin (HbA1c). Statistics: Values are adjusted for sex, age and diabetic status with a two-way ANCOVA. Blue font (Ile/Ile) or brown font (Val/_) P-values (* < 0.05; ** < 0.01; *** < 0.001) show contrasts of the corresponding genotype between a treatment group and the CON group. ns, non-significant. Horizontal or vertical brackets indicate effects between treatment groups or between genotypes within WLEX group, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Effects of TAS1R2−Ile191Val variant on responses to WL and WLEX intervention
Next, we used the TAS1R2−Ile191Val genotype (rs35874116) to evaluate its impact following the interventions. We assessed whether TAS1R2 partial loss-of-function differentially affected responses to WL and WLEX compared to CON group. Although Ile/Ile and Val carriers (Val/_) demonstrated similar improvements in measures of body composition following the WLEX intervention (Table 1 and Fig. 2A–C), the TAS1R2 partial loss-of-function in Val/_ participants yielded distinctive adaptive effects, which were absent among Ile/Ile counterparts (Table 1). For instance, Val/_ carriers exhibited increased appendicular muscle mass (Fig. 2G) and reduced thigh SAT mass (Table 1), despite a similar decrease in body mass. Moreover, unlike Ile/Ile participants, Val/_ improved maximal oxygen consumption (Fig. 2D) and other indicators of cardiorespiratory fitness (Table 1). In addition, the TAS1R2 partial loss-of-function in Val/_ participants was associated with improved mitochondrial oxidative capacity in muscle in vivo (Fig. 2E) and in isolated myofibers (Fig. 2F). Strikingly, Val carriers also demonstrated significant reductions in fasting glucose (Table 1) and HbA1c [28] (Fig. 2H). For all assessed variables, the genotype by intervention interaction explained between 4 and 41 % of the variability (Table S4). These findings demonstrate prominent TAS1R2 genotype-dependent adaptations to exercise training which, like in mice, show that TAS1R2 partial loss-of-function in humans confers beneficial effects associated with muscle function and metabolism.
Table 1.
Effects of TAS1R2 genotype on the responses to treatment.
| CON |
CON |
WL |
WLEX |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ile/Ile | Val/_ | P (geno) | Ile/Ile + Val/_ | Ile/Ile | P (CON) | Val/_ | P (CON) | Ile/Ile | P (CON) | Val/_ | P (CON) | P (geno) | |
| Body Composition | |||||||||||||
| Δ BMI (Kg/m2) | −0.36 ± 0.62 | −0.50 ± 0.86 | 0.884 | −0.43 ± 0.55 | −2.42 ± 0.82 | 0.040 | −1.56 ± 0.75 | 0.190 | −2.69 ± 0.68 | 0.014 | −3.82 ± 0.73 | <0.001 | 0.262 |
| Δ Body mass (Kg) | −1.30 ± 1.64 | −1.83 ± 2.28 | 0.844 | −1.56 ± 1.47 | −6.81 ± 2.17 | 0.041 | −5.57 ± 2.00 | 0.083 | −8.12 ± 1.80 | 0.008 | −10.37 ± 1.94 | <0.001 | 0.396 |
| Δ Fat mass (Kg) | −0.34 ± 1.31 | −0.48 ± 1.82 | 0.947 | −0.41 ± 1.17 | −4.33 ± 1.73 | 0.054 | −2.86 ± 1.59 | 0.179 | −6.35 ± 1.43 | 0.003 | −9.20 ± 1.54 | <0.001 | 0.182 |
| Δ Fat mass (%) | −0.13 ± 0.75 | −0.45 ± 1.05 | 0.793 | −0.29 ± 0.67 | −2.18 ± 0.99 | 0.103 | −1.29 ± 0.92 | 0.335 | −3.28 ± 0.82 | 0.008 | −5.02 ± 0.89 | <0.001 | 0.159 |
| Δ Lean mass (Kg) | −0.62 ± 0.73 | −0.10 ± 1.02 | 0.667 | −0.36 ± 0.65 | −0.90 ± 0.96 | 0.630 | −1.00 ± 0.89 | 0.529 | −0.22 ± 0.80 | 0.892 | 0.10 ± 0.86 | 0.652 | 0.783 |
| Δ Lean mass (%) | 0.19 ± 0.75 | 0.56 ± 1.04 | 0.762 | 0.37 ± 0.67 | 2.09 ± 0.99 | 0.136 | 1.34 ± 0.91 | 0.349 | 3.48 ± 0.82 | 0.006 | 4.84 ± 0.88 | <0.001 | 0.265 |
| Δ Appendicular lean mass (Kg) | −0.69 ± 0.50 | −0.35 ± 0.70 | 0.687 | −0.52 ± 0.45 | −0.66 ± 0.67 | 0.852 | −0.75 ± 0.62 | 0.737 | −1.02 ± 0.55 | 0.487 | 1.03 ± 0.60 | 0.036 | 0.016 |
| Δ Thigh SAT (cm3) | −38 ± 100 | −14 ± 147 | 0.885 | −26 ± 95 | 47 ± 184 | 0.727 | −170 ± 169 | 0.455 | −361± 122 | 0.051 | −502 ± 115 | 0.004 | 0.430 |
| Δ Abdomen SAT (cm3) | −162 ± 378 | −916 ± 552 | 0.236 | −539 ± 359 | 274 ± 695 | 0.309 | −97 ± 639 | 0.551 | −2162 ± 458 | 0.015 | −2246 ± 435 | 0.005 | 0.901 |
| Δ Abdomen VAT (cm3) | −126 ± 545 | −433 ± 797 | 0.734 | −280 ± 517 | −987 ± 1002 | 0.536 | −778 ± 921 | 0.632 | −1865 ± 661 | 0.086 | −1679 ± 627 | 0.089 | 0.848 |
| Δ Abdomen AT (cm3) | −288 ± 826 | −1350 ± 1208 | 0.441 | −819 ± 784 | −713 ± 1519 | 0.951 | −1749 ± 1397 | 0.556 | −4027 ± 1002 | 0.026 | −3926 ± 951 | 0.017 | 0.945 |
| Glucose Control | |||||||||||||
| Δ Glucose fasting (mg/dL) | 8.37 ± 6.63 | −1.51 ± 9.55 | 0.375 | 3.43 ± 6.12 | −4.63 ± 9.05 | 0.437 | −2.39 ± 8.33 | 0.533 | −0.17 ± 7.51 | 0.711 | −25.26 ± 8.07 | 0.005 | 0.029 |
| Δ HbA1c (%) | −0.02 ± 0.20 | −0.60 ± 0.29 | 0.091 | −0.31 ± 0.19 | −0.43 ± 0.28 | 0.688 | −0.49 ± 0.25 | 0.533 | −0.37 ± 0.23 | 0.823 | −1.18 ± 0.25 | 0.005 | 0.021 |
| Aerobic Capacity | |||||||||||||
| Δ VO2 peak (mL/min/KgFFM) | 0.12 ± 1.09 | 0.06 ± 1.45 | 0.970 | 0.09 ± 0.94 | 0.24 ± 1.54 | 0.930 | −1.89 ± 1.26 | 0.172 | 0.96 ± 1.14 | 0.555 | 3.35 ± 1.22 | 0.033 | 0.160 |
| Δ VO2 peak (mL/min/KgBM) | 0.13 ± 0.67 | 0.48 ± 0.89 | 0.745 | 0.31 ± 0.58 | 1.07 ± 0.94 | 0.469 | −0.26 ± 0.77 | 0.517 | 1.90 ± 0.70 | 0.086 | 3.49 ± 0.75 | 0.001 | 0.129 |
| Δ RER peak | −0.02 ± 0.02 | −0.05 ± 0.03 | 0.533 | −0.03 ± 0.02 | 0.02 ± 0.03 | 0.109 | −0.01 ± 0.03 | 0.341 | 0.01 ± 0.02 | 0.162 | 0.07 ± 0.03 | 0.001 | 0.078 |
| Δ Ergometer work peak (W) | 7.53 ± 7.48 | 4.33 ± 9.89 | 0.789 | 5.93 ± 6.46 | −0.12 ± 10.51 | 0.604 | −13.34 ± 8.65 | 0.056 | 24.21 ± 7.78 | 0.078 | 35.01 ± 8.37 | 0.007 | 0.349 |
| Mitochondrial Oxidative Capacity in vivo (31P-MRS) | |||||||||||||
| Δ k; PCr recovery rate constant (1/sec*103) | 1.65 ± 3.07 | 0.45 ± 4.27 | 0.806 | 1.05 ± 2.83 | 1.05 ± 4.68 | 1.000 | −2.19 ± 4.75 | 0.498 | 7.65 ± 4.14 | 0.231 | 12.22 ± 3.86 | 0.027 | 0.437 |
| Δ Qmax; ATP rate (mM/sec) | 0.07 ± 0.07 | −0.01 ± 0.10 | 0.452 | 0.03 ± 0.07 | 0.02 ± 0.11 | 0.929 | −0.09 ± 0.11 | 0.303 | 0.15 ± 0.10 | 0.341 | 0.22 ± 0.09 | 0.113 | 0.663 |
| Δ PDE1/2 (mM) | 0.91 ± 0.45 | 0.34 ± 0.63 | 0.427 | 0.62 ± 0.42 | 0.29 ± 0.69 | 0.678 | 0.00 ± 0.70 | 0.380 | −0.62 ± 0.61 | 0.128 | −1.97 ± 0.57 | 0.001 | 0.127 |
| ex vivo (Oxygraph) | |||||||||||||
| Δ P; OXPHOS capacity net (pmol O2/sec/mg) | 0.65 ± 5.59 | 11.23 ± 6.11 | 0.211 | 5.94 ± 4.18 | 6.01 ± 5.65 | 0.991 | 1.10 ± 4.97 | 0.416 | 19.19 ± 4.88 | 0.053 | 35.69 ± 4.90 | <0.001 | 0.026 |
| Δ E; ETS capacity net (pmol O2/sec/mg) | 6.58 ± 6.77 | 13.24 ± 7.40 | 0.510 | 9.91 ± 5.07 | 2.01 ± 6.85 | 0.334 | 1.31 ± 6.02 | 0.238 | 17.92 ± 5.92 | 0.316 | 36.31 ± 5.94 | 0.002 | 0.039 |
| Δ P/E; OXPHOS control ratio net | −0.07 ± 0.05 | 0.04 ± 0.06 | 0.193 | −0.02 ± 0.04 | 0.10 ± 0.05 | 0.073 | −0.09 ± 0.06 | 0.267 | −0.01 ± 0.05 | 0.897 | 0.16 ± 0.05 | 0.005 | 0.032 |
All values are mean ± SEM of post-pre treatment change (Δ), obtained after adjustments for sex, age,and diabetes status using a general linear model. All contrasts were computed within the model. (Pgeno) values under CON and WLEX represent the within- group genotype effect, while P(CON) values under WL and WLEX represent differences against the pooled control group. p values <0.05 are shown in bold. CON, health education only control; WL, diet-induced weight loss; WLEX, WL with exercise training; BMI, body mass index; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; AT, total adipose tissue; HbA1c, glycated hemoglobin A1c; VO2, O2 consumption during peak exercise; RER, respiratory exchange ratio during peak exercise; 31P-MRS, phosphorus magnetic resonance spectroscopy; PCr, phosphocreatine; PDE, phosphodiesters; OXPHOS, oxidative phosphorylation; ETS, electron transport system.
3.5. TAS1R2 genotype-dependent effects on variable associations
First, we assessed the dependency among relevant measured variables. We found a strong positive correlation between changes in total fat and abdominal fat mass (Fig. S3A), suggesting that the different methods for the assessment of fat mass (DXA) and abdominal fat mass (MRI) can be interpreted together. Next, we assessed the relationships between cardiorespiratory fitness and measures of muscle oxidative capacity. The outcomes for the assessment of muscle mitochondrial oxidative capacity in vivo (31P-MRS) and ex vivo (Oxygraph) were positively correlated (Fig. S3B), supporting the high relevance and reliability of the two complementary methods. Consequently, the changes in aerobic performance were also significantly correlated with the changes in muscle mitochondrial function (Fig. S3C,D) consistent with the documented exercise-dependent effect on improving muscle mitochondrial function and, subsequently, cardiorespiratory fitness. These associations were not affected by the genotype.
Next, we specifically assessed possible contributions of the TAS1R2−Ile191Val genotype, so we performed simple linear regression analysis using the genotype as a factor. Compared to Ile/Ile participants, Val/_ carriers demonstrated proportionally higher changes in muscle mass relative to the changes in total lean mass (Fig. 3A) followed by a genotype-dependent dissociation between the relative gains and losses in muscle and fat mass, respectively (Fig. 3B). These findings suggest TAS1R2−Ile191Val genotype-specific contributions in the regulation of muscle mass adaptations.
Fig. 3.
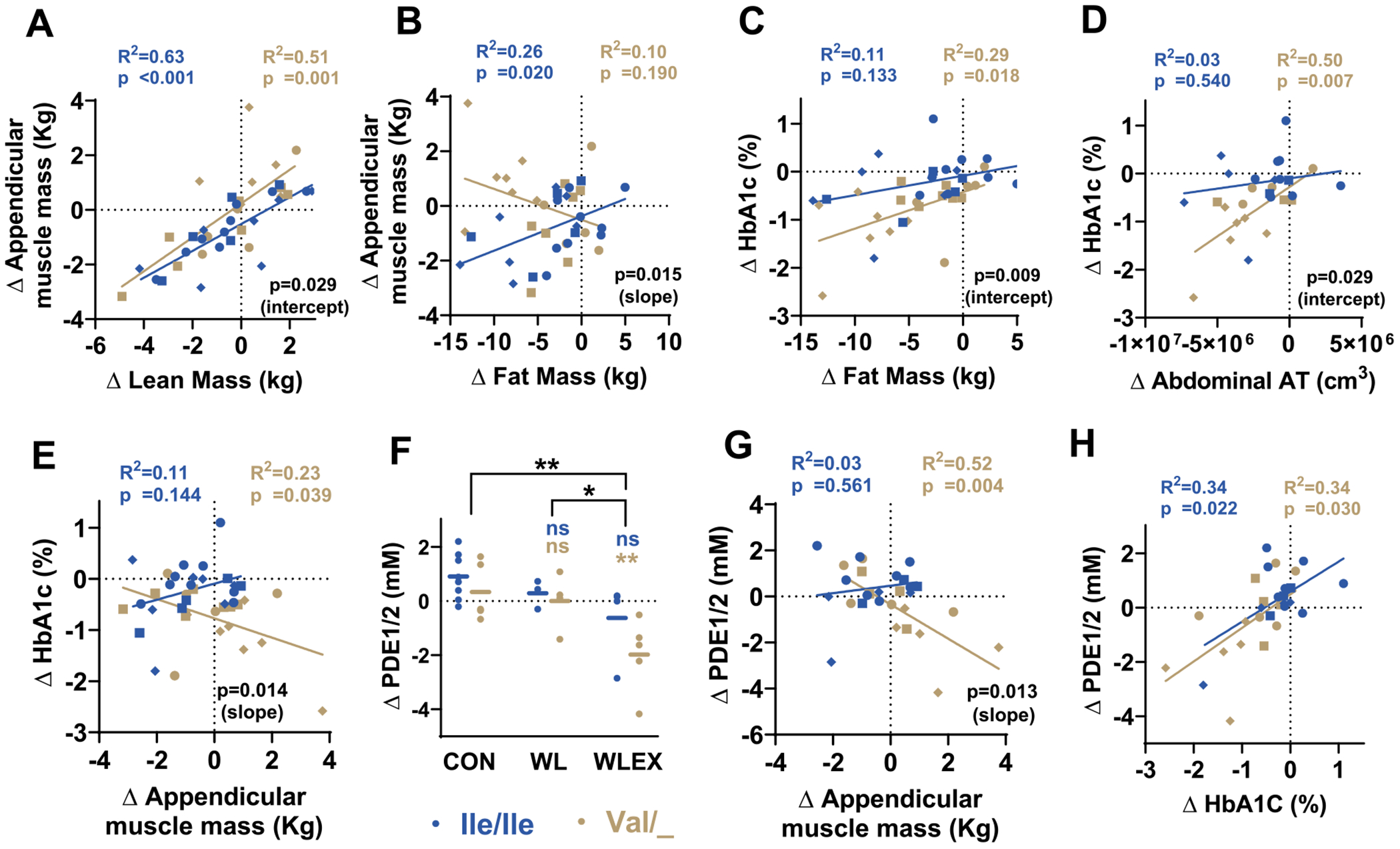
Partial loss-of-function of TAS1R2 affects the associations between measured outcomes.
TAS1R2−Ile191Val genotype effect on simple linear regressions between select variables of older obese individuals subjected to 6-months of diet-induced weight loss with exercise training (WLEX), diet-induced weight loss alone (WL), or education control (CON). Blue symbols show Ile/Ile (TAS1R2 normal function), and brown symbols show Val/_ (Val carriers; TAS1R2 partial loss-of-function). Squares = CON; Circles = WL; Rhombus = WLEX. Data are shown as absolute changes (Δ; post-pre) following the interventions. Effects of changes in (A) lean mass or (B) fat mass on the changes in appendicular muscle mass. Effects of changes in (C) fat mass, (D) abdominal adipose tissue (AT) volume, or (E) appendicular muscle mass on the changes in glycated hemoglobin (HbA1c). Effects of changes in (G) appendicular muscle mass or (H) HbA1c on the changes in muscle phosphodiester 1/2 (PDE1/2) content. (F) Changes in muscle PDE1/2 content of Ile/Ile or Val/_ participants subjected to 6-months of CON, WL, WLEX interventions. Statistics: Values are adjusted for sex, age and diabetic status with a two-way ANCOVA. For panel (F), blue font (Ile/Ile) or brown font (Val/_) P-values (* < 0.05; ** < 0.01) show contrasts of the corresponding genotype between a treatment group and the CON group. ns, non-significant. Horizontal brackets indicate effects between treatment groups. For panels (A,B,C,D,E,G, and H), Slope P value and coefficient of determination (R2) for each genotype (in corresponding color). Contrast of the slope values for each genotype (in black). If slopes were not statistically different (panels A, C, D), contrast of the intercepts for each genotype using a pooled slope (in black). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Because of the prominent reductions in HbA1c in Val/_ carriers, we evaluated whether the changes in other accessed variables can account for these effects. Stinkingly, we observed a clear Val/_ genotype effect on the association between the reduction in HbA1c and the improvements in total and abdominal fat mass (Fig. 3C,D), and in muscle mass (Fig. 3E). These associations, which were absent in Ile/Ile participants, suggest that changes in fat or muscle mass alone can only partially explain the robust improvements in HbA1c in Val/_ carriers.
Notably, Val/_ carriers demonstrated a significant reduction in muscle phosphodiester1/2 (PDE1/2) content following the WLEX intervention (Fig. 3F). Elevated PDE content has been proposed as an in vivo readout for cellular membrane damage [29], and aging is positively associated with increases in skeletal muscle PDE content [30]. In agreement, we found a strong association between muscle mass gains and the decrease in muscle PDE1/2 content which was only present in Val/_ carriers (Fig. 3G), but the reduction in HbA1c positively associated with the reduction in PDE1/2 content in both genotypes (Fig. 3H), further confirming previous observations [31].
3.6. Effects of TAS1R2−Ile191Val variant on cumulative performance to the WLEX intervention
The magnitude of responses varies between participants and individual variables (Table S5). Therefore, we considered cumulative effects of all major measured outcomes to rank the performance of participants from both genotypes. For this, we ranked the overall individual performance of all participants post-interventions by combining normalized responses (z-scores of Δ) across multiple outcomes related to body composition, glucose control, aerobic performance, and mitochondrial capacity (Fig. 4A). Strikingly, every Val/_ participant in the WLEX group responded substantially better to the exercise intervention than any of the Ile/Ile participant (Fig. 4B). Contingency analysis based on the median WLEX response revealed that 7/7 Val/_ carriers were classified as high responders (SD > 1.33), compared to 1/7 Ile/Ile counterparts (Fig. 4C). Our findings demonstrate that TAS1R2 partial loss-of-function in humans enhances exercise training adaptations, mirroring the traits observed in the mKO mice.
Fig. 4.
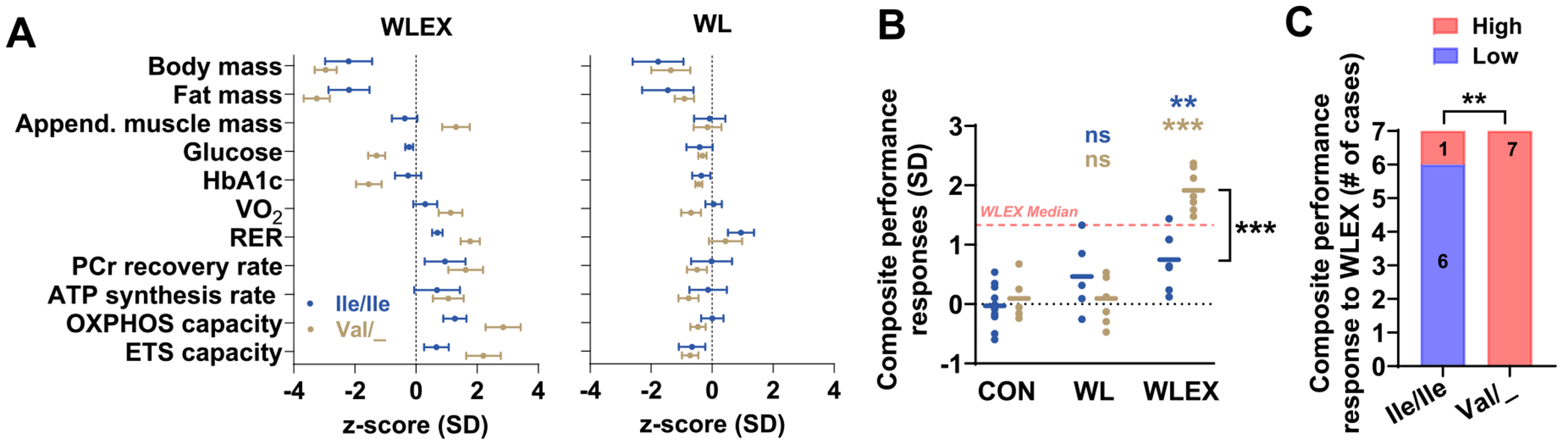
Partial loss-of-function of TAS1R2 enhances the cumulative performance of the WLEX intervention.
(A) CON-adjusted z-scores of changes (Δ; post-pre) in assessed variables of Ile/Ile (TAS1R2 normal function), or Val/_ (Val carriers; TAS1R2 partial loss-of-function) individuals subjected to 6-months of diet-induced weight loss with exercise training (WLEX) or diet-induced weight loss alone (WL). Glycated hemoglobin (HbA1c); peak oxygen consumption (VO2) and respiratory exchange ratio (RER) during cycle ergometer testing; phosphocreatine (PCr) recovery rate and ATP synthesis rate in muscles in vivo using 31P-MRS; respiratory capacity (OXPHOS) of isolated myofibers in the ADP-activated state of oxidative phosphorylation; respiratory electron-transfer-pathway capacity (ETS capacity) of isolated myofibers measured as oxygen consumption in the noncoupled state. (B) Composite performance responses of Ile/Ile or Val/_ participants subjected to 6-months of WLEX, WL, or CON calculated as the average of absolute z-scores of variables shown in (A). Red dotted line shows median (SD) responses of all participants in the WLEX group. (C) Number (shown in colored bars) of Ile/Ile or Val/_ participants with high (above the median) or low (below the median) composite performance responses to the WLEX intervention. Statistics: For panel (B), blue font (Ile/Ile) or brown font (Val/_) P-values (** < 0.01; *** < 0.001) show contrasts between the corresponding genotype within a treatment group and the CON group. ns, non-significant. Bracket indicates effect between genotypes within WLEX group. For panel C, P-value (** < 0.01) shows TAS1R2’−Ile191Val genotype differences between high and low responders using Fisher’s exact test. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. Tas1r2 deficiency in mice mitigates the decline in exercise performance associated with obesity or aging
The TAS1R2 partial loss-of-function variant in humans could potentially affect the receptor’s function in tissues other than skeletal muscle. To address this possibility, we studied mice with whole-body deletion of Tas1r2 (bKO) and WT controls (bWT). Young lean bKO mice improved running endurance compared to bWT counterparts (Fig. S4A), indicating that TAS1R2 deficiency in tissues other than skeletal muscle does not mask or alter the muscle-specific benefits. These benefits were not due to changes in cardiac function (ejection fraction bWT = 59.6 ± 4.4, bKO = 60.6 ± 3.0; n = 5/8; t-test P = 0.84). Additionally, advanced age or obesity in the studied population might have influenced the genotype-dependent effects, so we aged bKO mice to 27 months or crossed them with ob mice, a genetic model of obesity. The increased endurance observed with TAS1R2 deficiency was also preserved in old or obese mice (Fig. S4B,C), further validating the clinical significance of genotype-dependent responses in the human cohort of older obese participants.
4. Discussion
Habitual exercise leads to a number of well-documented health benefits [32] through adaptive responses involving multiple organs and molecular pathways [33]. Critical for these adaptations is the involvement of several nutrient sensing mechanisms that respond and integrate the substantial energy demands of exercise [34]. For instance, exercise triggers the activation of sirtuins [35], a class of NAD-dependent protein deacetylases, which regulate cellular metabolism, stress resistance, and longevity. Recently, our research identified TAS1R2, initially known as a mediator of sweet taste perception [10], as a glucose sensor in skeletal muscle that regulates muscle fitness through NAD-dependent adaptations [15]. TAS1R2 deficiency in mouse skeletal muscle produces similar effects to endurance exercise training, including increases in NAD levels, enhanced mitochondrial function, and improved endurance. A 4-week VWR exercise intervention yielded anticipated improvements in muscle fitness in mWT mice, but these effects were significantly potentiated in mKO mice, suggesting that TAS1R2 inhibition could enhance the effects of a conventional endurance exercise program. To test these findings in humans, we studied the TAS1R2−Ile191Val polymorphism (rs35874116), which partially impairs TAS1R2 function [12,20]. This approach allowed us to directly compare human and rodent loss-of-function phenotypes in relevant clinical interventions. Specifically, we assessed the impact of TAS1R2−Ile191Val in enhancing the responses to chronic exercise training in older obese adults [23,27].
The weight loss (WL) intervention alone reduced body and fat mass significantly, aligning with existing research [36,37]. However, it did not significantly improve other outcomes, suggesting that diet alone may not be sufficient for broader improvements in body composition or cardiorespiratory performance. Conversely, combining exercise with diet (WLEX) led to greater reductions in body and fat mass, especially in subcutaneous adipose tissue (SAT) of the thigh and abdomen, and improved cardiorespiratory fitness, mitochondrial function, and oxidative capacity. These findings are consistent with previous research demonstrating the synergistic effects of diet and exercise in promoting weight loss and reducing adiposity [38], underscoring the beneficial effects of exercise training on cardiorespiratory fitness and metabolic health.
However, when we considered the TAS1R2−Ile191Val variant as a factor, we observed significant differences in adaptive responses to the exercise. Val carriers, despite similar reductions in body mass, exhibited increased muscle mass compared to CON group and Ile/Ile counterparts. While some studies support the idea that endurance exercise can attenuate muscle loss during weight loss interventions [39,40], others have reported that aerobic exercise alone may not be sufficient to preserve skeletal muscle mass [41,42]. In addition to the beneficial effects on preserving muscle mass, Val carrier demonstrated lower muscle phosphodiesters (i.e. PDE1/2) content, a proposed readout of cell membrane damage [30]. PDE has been negatively associated with lower extremity function [43,44] and muscle volume in older obese individuals [44]. We also found a negative association between muscle mass and PDE content in Val carriers, but not in their Ile/Ile participants. This data indicates that TAS1R2 genotype may also influence the rate and magnitude of muscle mass decline and damage associated with advanced aging. The clinical impact of this possibility should be explored in cohorts with appropriate evaluations of muscle-associated outcomes.
Similarly, compared to CON group, the Val carriers of the WLEX group demonstrated significant improvements in aerobic and muscle mitochondrial oxidative capacity, unlike their Ile/Ile counterparts who did not significantly improve. The variability on mitochondrial adaptations has been previously documented in similar interventions and populations with some studies showing improvements [45,46], while other indicate no measurable changes [47,48], despite positive effects on cardiorespiratory fitness or glucose homeostasis. These effects have been attributed to heterogeneity of responses to exercise training among participants [23,49]. Our data suggests that the group improvement in cardiorespiratory and muscle mitochondrial oxidative capacity of the WLEX intervention was primarily driven by the TAS1R2 partial loss-of-function individuals of the group. Notably, combining the measured outcomes for the assessment of cumulative individual responses to WLEX revealed Ile/Ile and Val carriers being low and high responders to exercise, respectively. This variability in responses [5,6] can be ascribed to a combination of environmental, behavioral, genetic, and epigenetic factors [6,49], emphasizing the importance of identifying robust predictors of individual responses to tailor effective personalized exercise interventions.
In this regard, several candidate genes have been associated with favorable adaptations following aerobic exercise [50], suggesting a strong genetic basis for the observed range in aerobic responses [51]. In our study, the effect size (Cohen’s d = 0.80) of cardiorespiratory fitness (VO2peak) following WLEX was comparable to that seen with other polymorphisms (e.g., ACE, CS, COX4I1) affecting exercise adaptations (average d = 0.82) [50]. Although the predictive value of our partial loss-of-function TAS1R2 variant should be validated in larger cohorts, the magnitude and consistency of effects underscore the potential significance of the TAS1R2−Ile191Val variant as a candidate gene for enhancing responses to exercise training. The clinical significance of this effect should not be underestimated, especially considering that as little as a 1 % improvement in VO2peak is associated with a 2 % decline in cardiovascular mortality [52]. More importantly, older and obese populations have lower VO2peak and typically experience a gradual decline to the point where cardiovascular fitness no longer supports ambulation, resulting in mobility disability. In this context, an increase in VO2peak due to a genotype effect may delay these detrimental effects in these populations. Consistent with the human data, the differences in running performance between mWT and mKO mice following 4 weeks of VWR (63 %) were similar or superior to the differences reported after nicotinamide riboside supplementation (NAD precursor) [53] or treatments with PARP1 inhibitors [54]. Thus, TAS1R2 inhibition in skeletal muscle could exert effects comparable to those seen with other interventions aiming to raise NAD levels. Therefore, it is possible that the TAS1R2 receptor is a candidate gene for promoting favorable adaptations following endurance training, potentially accounting for the observed variation in exercise responses among individuals.
One unexpected observation that emerged from our analysis is the robust reduction of HbA1c levels in the Val carriers of the WLEX group. While some lifestyle interventions demonstrate positive effects on glycemic control in obese [55], others do not report improvements in HbA1c levels despite improvements in other outcomes [56]. In our study participants were older, sedentary, obese, and slightly hyperglycemic. These baseline characteristics may have enhanced the effect of genotype on plasma glucose and HbA1c, which underscores the clinical significance of our findings. Notably, the effects on HbA1c following the WLEX intervention are consistent with independent observations showing that TAS1R2-Ile191Val variant is associated with reduced HbA1c in a cohort with variable levels of glucose homeostasis and BMI [28] and reduced glucose excursions in a healthy lean cohort following an OGTT [20]. These outcomes were not seen in the Ile/Ile counterparts, even though both genotype groups had similar baseline glycemic profiles and lost the same amount of body and fat mass in response to the intervention. Strikingly, the improvements in HbA1c strongly correlated with the improvements in muscle PDE content, supporting a role of glycemia in regulating skeletal muscle membrane damage [31]. The unexpected but consistent association of TAS1R2 partial loss of function with favorable effects on HbA1c may have significant clinical implications for individuals at risk of developing diabetes or for diabetic populations who are less responsive to traditional pharmacological treatments [57,58]. Similar to other tissues [14,59], we confirmed the expression of TAS1R2 and TAS1R3 specifically in human cultured myotubes isolated from biopsies and cross-referenced public expression databases [60]. Nevertheless, it is not apparent which tissues expressing TAS1R2 contributed to the effects on glucose control, but it is possible that, beyond skeletal muscle, inhibition of TAS1R2 may have independent effects on glucose homeostasis. So, a potential limitation of our study is that factors other than TAS1R2 function in skeletal muscle may have influenced our results in humans. In addition, part of the effects observed could be attributed to the weight loss in the exercise intervention group. However, weight loss intervention alone (WL) had no effect on the measured outcomes, suggesting the exercise intervention as the main driver of these adaptations.
Our clinical data suggests that TAS1R2 function in humans is a crucial determinant of muscle adaptations following endurance exercise training, but it is not yet clear whether partial loss-of-function of TAS1R2 is linked to any negative clinical outcomes. Nevertheless, given that GPCRs are excellent targets for pharmacological interventions, a TAS1R2 antagonist or an inhibitor of the TAS1R2 signaling cascade could be used to enhance the effects of exercise training in skeletal muscle. Such effects would be particularly beneficial for individuals with low exercise tolerance and response [6] or those experiencing muscle dysfunction associated with aging or other conditions.
Supplementary Material
Funding
This work was supported by the National Institutes of Health (R01DK127444 to GAK; R01AG021961 to BHG), the National Institute of Food and Agriculture (NIFA-2018-67001-28246 to GAK), The American Heart Association (AHA904048 to JS), and Advent Health (to GAK and REP).
Abbreviations:
- ACE
angiotensin-converting enzyme
- ADH
alcohol dehydrogenase
- ANOVAs
analysis of variance
- AT
adipose tissue
- bKO
body knockout
- BMI
body mass index
- BSA
bovine serum albumin
- CAMKII
calmodulin-dependent protein kinase II
- CON
education control
- COX4I1
cytochrome c oxidase subunit 4 isoform 1
- CS
citrate synthase
- DXA
dual-energy X-ray absorptiometry
- FMN
flavin mononucleotide
- GLM
general linear model
- GPCR
G protein-coupled receptor
- GWAS
genome-wide association studies
- HbA1c
hemoglobin A1c
- IACUC
Institutional Animal Care and Use Committee
- IMAT
intermuscular adipose tissue
- mKO
muscle knockout
- MRI
magnetic resonance imaging
- mWT
muscle wild type
- NAD
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyl-transferase
- ob
obese mice due to a leptin mutation
- PARP1
poly(ADP-ribose) polymerase 1
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PDE
phosphodiester
- SAT
subcutaneous adipose tissue
- SIRT1
sirtuin 1
- SBP
systolic blood pressure
- TaqMan
allelic discrimination assay
- TAS1R2
taste receptor type 1 member 2
- VO2peak
maximal (peak) oxygen consumption
- VWR
voluntary wheel running
- WL
weight loss
- WLEX
weight loss with exercise training
Footnotes
CRediT authorship contribution statement
Joan Serrano: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Saki Kondo: Investigation. Grace M. Link: Investigation. Ian S. Brown: Investigation. Richard E. Pratley: Resources, Methodology. Kedryn K. Baskin: Resources, Methodology. Bret H. Goodpaster: Resources, Methodology, Funding acquisition, Formal analysis, Conceptualization. Paul M. Coen: Writing – review & editing, Supervision, Resources, Methodology, Formal analysis, Data curation, Conceptualization. George A. Kyriazis: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare no competing interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2024.156045.
References
- [1].Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol (1985) 2007;102:919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bouchard C, Antunes-Correa LM, Ashley EA, Franklin N, Hwang PM, Mattsson CM, et al. Personalized preventive medicine: genetics and the response to regular exercise in preventive interventions. Prog Cardiovasc Dis 2015;57:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sparks LM. Exercise training response heterogeneity: physiological and molecular insights. Diabetologia 2017;60:2329–36. [DOI] [PubMed] [Google Scholar]
- [7].Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc 2001;33. S446–51; discussion S52-3. [DOI] [PubMed] [Google Scholar]
- [8].Bouchard C. Genomic predictors of trainability. Exp Physiol 2012;97:347–52. [DOI] [PubMed] [Google Scholar]
- [9].Rankinen T, Bouchard C. Gene-exercise interactions. Prog Mol Biol Transl Sci 2012;108:447–60. [DOI] [PubMed] [Google Scholar]
- [10].Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 2001;106:381–90. [DOI] [PubMed] [Google Scholar]
- [11].Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 2007;104:15069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smith K, Karimian Azari E, LaMoia TE, Hussain T, Vargova V, Karolyi K, et al. T1R2 receptor-mediated glucose sensing in the upper intestine potentiates glucose absorption through activation of local regulatory pathways. Mol Metab 2018;17: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kyriazis GA, Smith KR, Tyrberg B, Hussain T, Pratley RE. Sweet taste receptors regulate basal insulin secretion and contribute to compensatory insulin hypersecretion during the development of diabetes in male mice. Endocrinology 2014:155:2112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 2012;109. E524–E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Serrano J, Boyd J, Brown IS, Mason C, Smith KR, Karolyi K, et al. The TAS1R2 G-protein-coupled receptor is an ambient glucose sensor in skeletal muscle that regulates NAD homeostasis and mitochondrial capacity. Nat Commun 2024;15:4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eny KM, Wolever TM, Corey PN, El-Sohemy A. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am J Clin Nutr 2010;92:1501–10. [DOI] [PubMed] [Google Scholar]
- [17].Melo SV, Agnes G, Vitolo MR, Mattevi VS, Campagnolo PDB, Almeida S. Evaluation of the association between the TAS1R2 and TAS1R3 variants and food intake and nutritional status in children. Genet Mol Biol 2017;40:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ramos-Lopez O, Panduro A, Martinez-Lopez E, Roman S. Sweet taste receptor TAS1R2 polymorphism (Val191Val) is associated with a higher carbohydrate intake and hypertriglyceridemia among the population of West Mexico. Nutrients 2016;8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dias AG, Eny KM, Cockburn M, Chiu W, Nielsen DE, Duizer L, et al. Variation in the TAS1R2 gene, sweet taste perception and intake of sugars. J Nutrigenet Nutrigenomics 2015;8:81–90. [DOI] [PubMed] [Google Scholar]
- [20].Serrano J, Seflova J, Park J, Pribadi M, Sanematsu K, Shigemura N, et al. The Ile191Val is a partial loss-of-function variant of the TAS1R2 sweet-taste receptor and is associated with reduced glucose excursions in humans. Mol Metab 2021;54:101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Noone J, Mucinski JM, DeLany JP, Sparks LM, Goodpaster BH. Understanding the variation in exercise responses to guide personalized physical activity prescriptions. Cell Metab 2024;36:702–24. [DOI] [PubMed] [Google Scholar]
- [22].Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, et al. Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One 2011;6:e21068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brennan AM, Standley RA, Yi F, Carnero EA, Sparks LM, Goodpaster BH. Individual response variation in the effects of weight loss and exercise on insulin sensitivity and cardiometabolic risk in older adults. Front Endocrinol (Lausanne) 2020;11:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].American College of Sports Medicine, Liguori G, Feito Y, Fountaine C, Roy B. ACSM’s guidelines for exercise testing and prescription. Eleventh edition. ed. Philadelphia: Wolters Kluwer; 2021. p. 1 [online resource.]. [Google Scholar]
- [25].Van Breukelen GJ. ANCOVA versus change from baseline: more power in randomized studies, more bias in nonrandomized studies [corrected]. J Clin Epidemiol 2006;59:920–5. [DOI] [PubMed] [Google Scholar]
- [26].Ji LL, Yeo D. Maintenance of NAD+ homeostasis in skeletal muscle during aging and exercise. Cells 2022:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brennan AM, Standley RA, Anthony SJ, Grench KE, Helbling NL, DeLany JP, et al. Weight loss and exercise differentially affect insulin sensitivity, body composition, cardiorespiratory fitness, and muscle strength in older adults with obesity: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2022;77:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Serrano J, Yi F, Smith J, Pratley RE, Kyriazis GA. The Ile191Val variant of the TAS1R2 subunit of sweet taste receptors is associated with reduced HbA1c in a human cohort with variable levels of glucose homeostasis. Front Nutr 2022;9:896205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Burt CT, Ribolow HJ. A hypothesis: noncyclic phosphodiesters may play a role in membrane control. Biochem Med 1984;31:21–30. [DOI] [PubMed] [Google Scholar]
- [30].Satrustegui J, Berkowitz H, Boden B, Donlon E, McLaughlin A, Maris J, et al. An in vivo phosphorus nuclear magnetic resonance study of the variations with age in the phosphodiester content of human muscle. Mech Ageing Dev 1988;42:105–14. [DOI] [PubMed] [Google Scholar]
- [31].Ripley EM, Clarke GD, Hamidi V, Martinez RA, Settles FD, Solis C, et al. Reduced skeletal muscle phosphocreatine concentration in type 2 diabetic patients: a quantitative image-based phosphorus-31 MR spectroscopy study. Am J Physiol Endocrinol Metab 2018;315. E229–E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].MoTr PACSG, Lead A, MoTr PACSG. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 2024;629:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab 2017;25:1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 2010;11:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005;353:2111–20. [DOI] [PubMed] [Google Scholar]
- [37].Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755–67. [DOI] [PubMed] [Google Scholar]
- [38].Donnelly JE, Smith B, Jacobsen DJ, Kirk E, Dubose K, Hyder M, et al. The role of exercise for weight loss and maintenance. Best Pract Res Clin Gastroenterol 2004;18:1009–29. [DOI] [PubMed] [Google Scholar]
- [39].Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, Piner LW, et al. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab 2011;301:E1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Willis LH, Slentz CA, Bateman LA, Shields AT, Piner LW, Bales CW, et al. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J Appl Physiol (1985) 2012;113:1831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004;12:789–98. [DOI] [PubMed] [Google Scholar]
- [42].Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27(1212–21):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- [44].Hinkley JM, Cornnell HH, Standley RA, Chen EY, Narain NR, Greenwood BP, et al. Older adults with sarcopenia have distinct skeletal muscle phosphodiester, phosphocreatine, and phospholipid profiles. Aging Cell 2020;19:e13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS. Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age. J Clin Endocrinol Metab 2015;100:1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Larsen S, Danielsen JH, Sondergard SD, Sogaard D, Vigelsoe A, Dybboe R, et al. The effect of high-intensity training on mitochondrial fat oxidation in skeletal muscle and subcutaneous adipose tissue. Scand J Med Sci Sports 2015;25:e59–69. [DOI] [PubMed] [Google Scholar]
- [47].Schrauwen-Hinderling VB, Schrauwen P, Hesselink MK, van Engelshoven JM, Nicolay K, Saris WH, et al. The increase in intramyocellular lipid content is a very early response to training. J Clin Endocrinol Metab 2003;88:1610–6. [DOI] [PubMed] [Google Scholar]
- [48].Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol 2007;292:R1271–8. [DOI] [PubMed] [Google Scholar]
- [49].Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, et al. Precision exercise medicine: understanding exercise response variability. Br J Sports Med 2019;53:1141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chung HC, Keiller DR, Roberts JD, Gordon DA. Do exercise-associated genes explain phenotypic variance in the three components of fitness? A systematic review & meta-analysis. PLoS One 2021;16:e0249501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, et al. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE family study. J Appl Physiol (1985) 1999;87:1003–8. [DOI] [PubMed] [Google Scholar]
- [52].Vanhees L, Fagard R, Thijs L, Amery A. Prognostic value of training-induced change in peak exercise capacity in patients with myocardial infarcts and patients with coronary bypass surgery. Am J Cardiol 1995;76:1014–9. [DOI] [PubMed] [Google Scholar]
- [53].Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 2012;15:838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Pirinen E, Canto C, Jo YS, Morato L, Zhang H, Menzies KJ, et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab 2014;19:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004;27:2067–73. [DOI] [PubMed] [Google Scholar]
- [56].Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, et al. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med 2012;366:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gan S, Dawed AY, Donnelly LA, Nair ATN, Palmer CNA, Mohan V, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and Asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 2020;43:1948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sheng CS, Tian J, Miao Y, Cheng Y, Yang Y, Reaven PD, et al. Prognostic significance of long-term HbA(1c) variability for all-cause mortality in the ACCORD trial. Diabetes Care 2020;43:1185–90. [DOI] [PubMed] [Google Scholar]
- [59].Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, et al. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 2009;58:337–46. [DOI] [PubMed] [Google Scholar]
- [60].Pillon NJ, Gabriel BM, Dollet L, Smith JAB, Sardon Puig L, Botella J, et al. Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity. Nat Commun 2020;11:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


