Abstract
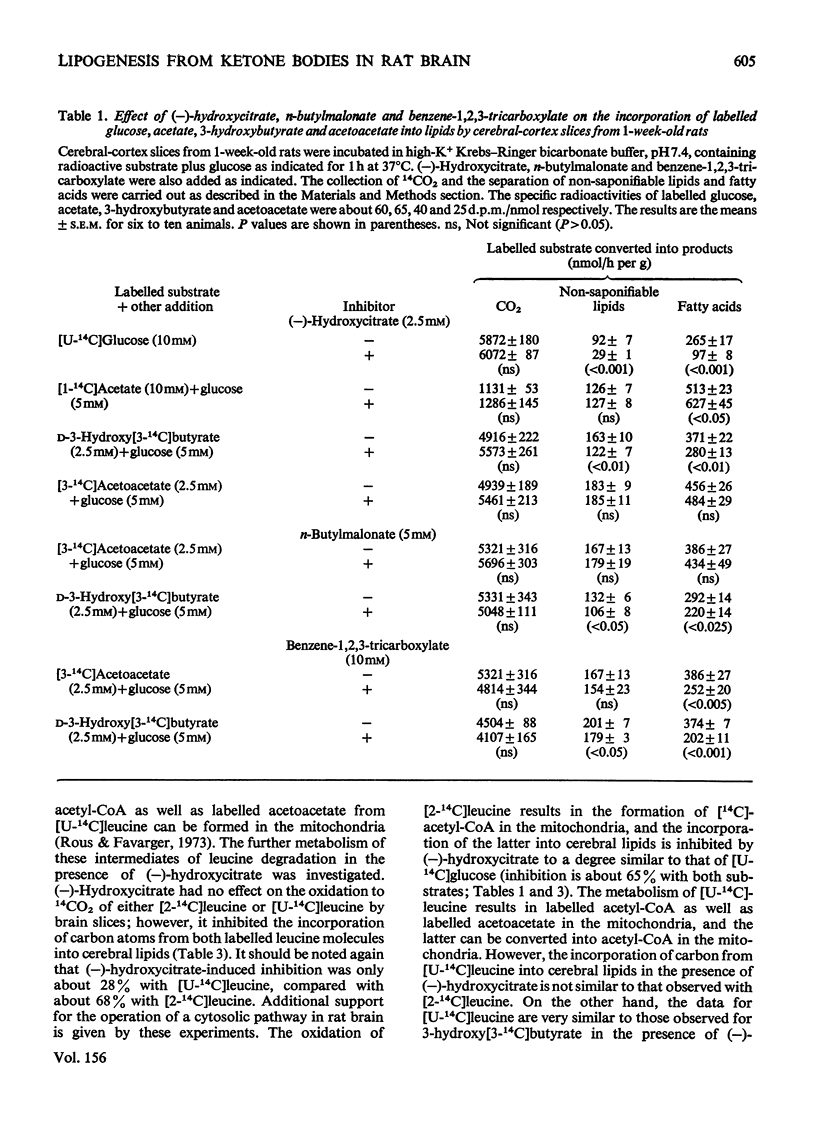
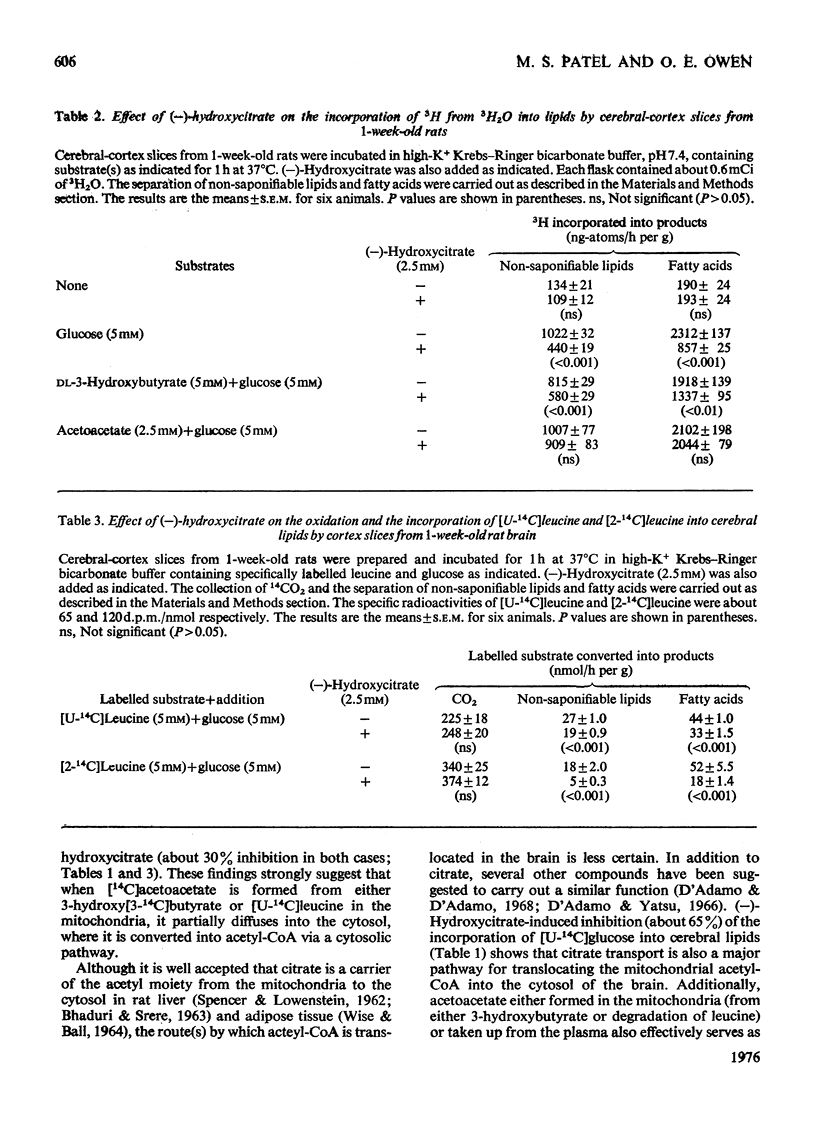
The metabolism of acetoacetate via a proposed cytosolic pathway in brain of 1-week-old rats was investigated. (-)-Hydroxycitrate, an inhibitor of ATP citrate lyase, markedly inhibited the incorporation of carbon from labelled glucose and 3-hydroxybutyrate into cerebral lipids, but had no effect on the incorporation of labelled acetate and acetoacetate into brain lipids. Similarly, n-butylmalonate and benzene-1,2,3-tricarboxylate inhibited the incorporation of labelled 3-hydroxybutyrate but not of acetoacetate into cerebral lipids. These inhibitors had no effect on the oxidation to 14CO2 of the labelled substrates used. (-)-Hydroxycitrate decreased the incorporation of 3H from 3H2O into cerebral lipids by slices metabolizing either glucose or 3-hydroxybutyrate, but not in the presence of acetoacetate. (-)-Hydroxycitrate also differentially inhibited the incorporation of [2-14C]-leucine and [U-14C]leucine into cerebral lipids. The data show that, although the acetyl moiety of acetyl-CoA generated in brain mitochondria is largely translocated as citrate from these organelles to the cytosol, a cytosolic pathway exists by which acetoacetate is converted directly into acetyl-COA in this cellular compartment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHADURI A., SRERE P. A. The incorporation of citrate carbon into fatty acids. Biochim Biophys Acta. 1963 Jun 18;70:221–230. doi: 10.1016/0006-3002(63)90747-9. [DOI] [PubMed] [Google Scholar]
- Berl S., Nicklas W. J., Clarke D. D. Compartmentation of glutamic acid metabolism in brain slices. J Neurochem. 1968 Feb;15(2):131–140. doi: 10.1111/j.1471-4159.1968.tb06184.x. [DOI] [PubMed] [Google Scholar]
- Buckley B. M., Williamson D. H. Acetoacetate and brain lipogenesis: developmental pattern of acetoacetyl-coenzyme A synthetase in the soluble fraction of rat brain. Biochem J. 1973 Mar;132(3):653–656. doi: 10.1042/bj1320653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo A. F., Jr, D'Adamo A. P. Acetyl transport mechanisms in the nervous system. The oxoglutarate shunt and fatty acid synthesis in the developing rat brain. J Neurochem. 1968 Apr;15(4):315–323. doi: 10.1111/j.1471-4159.1968.tb11616.x. [DOI] [PubMed] [Google Scholar]
- D'Adamo A. F., Jr, Yatsu F. M. Acetate metabolism in the nervous system. N-acetyl-L-aspartic acid and the biosynthesis of brain lipids. J Neurochem. 1966 Oct;13(10):961–965. doi: 10.1111/j.1471-4159.1966.tb10292.x. [DOI] [PubMed] [Google Scholar]
- Edmond J. Ketone bodies as precursors of sterols and fatty acids in the developing rat. J Biol Chem. 1974 Jan 10;249(1):72–80. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Foster D. W., Katz J. The distribution of tritium in fatty acids synthesized from tritiated glucose and tritiated water by rat adipose tissue. Biochim Biophys Acta. 1966 Dec 7;125(3):422–427. doi: 10.1016/0005-2760(66)90030-0. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., GOLDMAN D. S., MII S., BEINERT H. The acetoacetate activation and cleavage enzyme system. J Biol Chem. 1953 May;202(1):137–150. [PubMed] [Google Scholar]
- Hackenschmidt J., Barth C., Decker K. Stimulation of acetyl-CoA carboxylase by (-)-Hydroxycitrate. FEBS Lett. 1972 Oct 15;27(1):131–133. doi: 10.1016/0014-5793(72)80425-3. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H., Krebs H. A. Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J. 1971 Mar;122(1):13–18. doi: 10.1042/bj1220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Quastel J. H. Acetoacetate metabolism in infant and adult rat brain in vitro. Biochem J. 1970 Feb;116(4):641–655. doi: 10.1042/bj1160641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., BUNKER W. E. Preparation of tissue slices for metabolic studies: a hand-microtome especially suitable for brain. J Neurochem. 1957;2(1):11–14. doi: 10.1111/j.1471-4159.1957.tb12347.x. [DOI] [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr, Markus H., Boden G., Mozzoli M. A., Shuman C. R. Rapid intravenous sodium acetoacetate infusion in man. Metabolic and kinetic responses. J Clin Invest. 1973 Oct;52(10):2606–2616. doi: 10.1172/JCI107453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. A., Krebs H. A., Williamson D. H. Activities of enzymes of ketone-body utilization in brain and other tissues of suckling rats. Biochem J. 1971 Jan;121(1):49–53. doi: 10.1042/bj1210049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S. Inhibition by the branched-chain 2-oxo acids of the 2-oxoglutarate dehydrogenase complex in developing rat and human brain. Biochem J. 1974 Oct;144(1):91–97. doi: 10.1042/bj1440091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Johnson C. A., Rajan R., Owen O. E. The metabolism of ketone bodies in developing human brain: development of ketone-body-utilizing enzymes and ketone bodies as precursors for lipid synthesis. J Neurochem. 1975 Dec;25(6):905–908. doi: 10.1111/j.1471-4159.1975.tb04428.x. [DOI] [PubMed] [Google Scholar]
- Patel M. S., Owen O. E. Effect of hyperphenylalaninaemia on lipid synthesis from ketone bodies by rat brain. Biochem J. 1976 Feb 15;154(2):319–325. doi: 10.1042/bj1540319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Williams G. R., Halperin M. L., Leznoff C. C. The sensitivity of the exchange reactions of tricarboxylate, 2-oxoglutarate and dicarboxylate transporting systems of rat liver mitochondria to inhibition by 2-pentylmalonate, p-iodobenzylmalonate, and benzene 1,2,3-tricarboxylate. Eur J Biochem. 1971 May 11;20(1):65–71. doi: 10.1111/j.1432-1033.1971.tb01363.x. [DOI] [PubMed] [Google Scholar]
- Rous S., Favarger P. The role of acetoacetate in the transfer of acetyl units outside the mitochondria in liver and adipose tissue of rats or mice. FEBS Lett. 1973 Dec 1;37(2):231–234. doi: 10.1016/0014-5793(73)80466-1. [DOI] [PubMed] [Google Scholar]
- SPENCER A. F., LOWENSTEIN J. M. The supply of precursors for the synthesis of fatty acids. J Biol Chem. 1962 Dec;237:3640–3648. [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. A., Fang M., Lowenstein J. M. Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch Biochem Biophys. 1969 Dec;135(1):209–217. doi: 10.1016/0003-9861(69)90532-3. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]


