Abstract
Below their ice shells, icy moons may offer a source of chemical energy that could support microbial life in the absence of light. In the Arctic, past and present glacial retreat leads to isostatic uplift of sediments through which cold and methane-saturated groundwater travels. This fluid reaches the surface and freezes as hill-shaped icings during winter, producing dark ice–water interfaces above water ponds containing chemical energy sources. In one such system characterized by elevated methane concentrations — the Lagoon Pingo in Adventdalen, Svalbard, Norway (~10 mg/L CH4, <0.3 mg/L O2, −0.25°C, pH 7.9), we studied amplicons of the bacterial and archaeal (microbial) 16S rRNA gene and transcripts in the water pond and overlaying ice. We found that active chemolithoautotrophic sulfur-oxidizing microorganisms (Sulfurimonas, Thiomicrorhabdus) dominate a niche at the bottom of the ice that is in contact with the anoxic water reservoir. There, the growing ice offers surfaces that interface with water and hosts favorable physico-chemical conditions for sulfide oxidation. The detection of anaerobic methanotrophs further suggests that throughout the winter, a steady-state dark and cold methane sink occurs under the ice in two steps: first, methane is oxidized to carbon dioxide and sulfates are concomitantly reduced to sulfides by the activity of anaerobic methanotrophs (ANME) ANME-1a and sulfate-reducing bacteria (SRB) SEEP-SRB1 consortia; and second, energy from sulfides is used by sulfur-oxidizing microorganisms to fix carbon dioxide into organic carbon. Our results underscore that ice-covered and dark ecosystems are hitherto overlooked oases of microbial life and emphasize the need to study microbial communities in icy habitats.
Keywords: chemoautotrophs; methane, sulfur; cold seep; Arctic; terrestrial ice
Introduction
Ice–water interfaces are targets in the search for life beneath the ice shells of icy moons because these interfaces may contain oxidants required for redox reactions, potentially available through ice irradiation [1]. On Earth, ecosystems that mirror the features of liquid oceans of icy moons such as Europa and Enceladus — characterized by the absence of light and presence of a chemical energy source in the form of methane and hydrogen sulfide [1] — are often encountered in the deep sea [2] or in terrestrial sulphidic environments such as cold springs [3, 4], cave ecosystems [5], or even stratified temperate lakes [6]. However, environments that also harbor ice–water interfaces are scarce [7]. These environments can exist under the ice of polar lakes characterized by a source of biogenic methane [8, 9]. This methane, together with carbon dioxide, is the final product of organic matter degradation by methanogenic archaea in sediments [10–12] and can also be recharged from the active layer of the permafrost [13]. The presence of methane supports the development of methanotrophs in sediments [14] and in the water column [15] as members of microbial consortia that often produce reduced sulfur-containing molecules as a by-product of anaerobic methane oxidation [14].
At Moskuslaguna on the Svalbard coast (Adventdalen, 78°N, Norway), geological structures specific to deglaciated landscapes harbor relevant features for studying life in the absence of light. There, groundwater seepage creates hydraulic pressure within the permafrost, leading to the formation of hill-shaped structures called open-system pingos [16]. In this environment, seasonal ice overlies an artesian source of reduced fluid originating below the permafrost. The reduced fluid is loaded with greenhouse gases from the anaerobic microbial degradation of permafrost organic matter or from the dissociation of gas hydrates [17]. At the Lagoon Pingo in Adventdalen, Svalbard, Norway (78°14′22.5 N; 015°45′16.0 E), an ice blister forms every winter above the reduced fluid seepage. This 1-m-thick ice layer encapsulates a pressurized and light-deprived water reservoir (<10-m-wide, 1-m-deep; Supplementary Fig. 1) that is saturated with methane (up to 0.9 mM) [16]. Unlike polar lakes for which the most common sources of methane and hydrogen sulfide are autochthonous production by methanogenesis and sulfate reduction (e.g. up to 1.5 μM under the ice [18]), at Lagoon Pingo the allochthonous supply of reduced fluid generates methane concentrations 1000 times higher. Sulfate concentrations in excess of those recorded in surface waters of the regions and occasional odors of H2S indicate a significant role of the microbial sulfur cycle [16], making this an ideal system to study chemolithoautotrophic psychrophilic organisms and the possibility of life in icy, light-deprived habitats.
The cryosphere is far from biologically inactive. Brine channels in sea ice harbor a microbial oasis where, despite low temperatures, photoautotrophic microorganisms bloom at the ice–water interface when the light returns to the Earth’s poles, triggering a solar energy-based food web [19, 20]. Microbial life is found where liquid water is available, such as in cryoconite holes, glacier ice surfaces, or subglacial environments where high carbon content, bedrock weathering, and oxygen from glacial meltwater provide energy for microbial metabolism, highlighting the importance of interfaces [21]. Subglacial environments are one example of a light-deprived icy environment; however, they are difficult to access. In other terrestrial icy environments devoid of light, such as ice-covered lakes, previous studies have highlighted the potential role of ice in hosting life based on methane oxidation, yet the microbiome of the ice associated with these systems remains to be investigated [18, 22, 23]. In this study, we explored whether the ice shell of the Lagoon Pingo could host microbial life supported by a chemical source of energy.
Materials and methods
The sampling design and methods used in this study are summarized in the main text and further described in the Supplementary Information.
Results and discussion
In March 2021, we collected three ice cores from the ice blister above the fluid seepage (Supplementary Fig. 1) and investigated microbial abundance and microbial community composition using quantitative polymerase chain reaction and 16S rRNA gene and transcript sequencing. The water in the reservoir was anoxic to microoxic, with an O2 concentration below 0.3 mg/L and a strong reductive potential (−371.4 mV). The high electrical conductivity (6.3 mS/cm) relative to natural surface waters of the region indicated that highly concentrated water was feeding the pond [16]. A pH of 7.9 indicated that the H2S, whose presence was assumed from the odor escaping the pond when drilling (Supplementary Fig. 2), was mostly in its ionic bisulfide form (HS−).
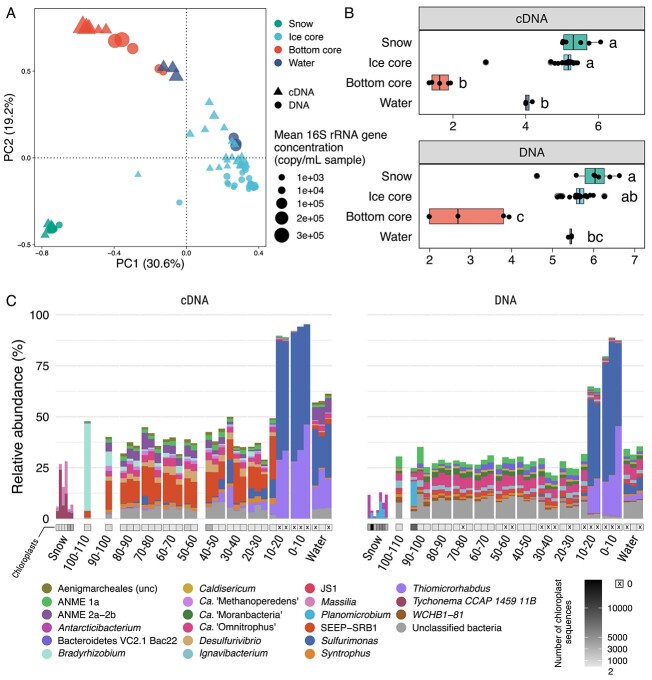
The three ice cores showed similar exponential increases in microbial 16S rRNA gene abundances with depth. The bottom 10 cm of the core exhibited a mean three-fold increase in 16S rRNA gene concentration (2.5 × 105 ± 9.9 × 104 copies/mL, n = 3) compared to the water reservoir (9.3 × 104 ± 2.9 × 104 copies/mL, n = 3), and a 100-fold increase compared to the top first 80 cm of the core (2.4 × 103 ± 2.4 × 103 copies/mL, n = 3) (Fig. 1A and Supplementary Fig. 3). Therefore, we concluded that a microbiologically active layer exists within 0 to 20 cm of ice from the interface with the water reservoir, although the actual activity of these bacteria within the ice remains uncertain [24]. Concurrently, Shannon’s diversity index dropped in the bottom core (defined in the Supplementary Information) compared to the core top (P value <.001) and underlying water reservoir (P value not significant) (Fig. 1B). This indicates the presence of a singular specialized ecological niche in the bottom ice.
Figure 1.
(A) Principal component analysis showing bacterial and archaeal beta diversity in the Lagoon Pingo ice-covered system. Colors show the sample’s environment (snow, water, ice), and the bottom core was defined based on the dominance of Sulfurimonas. The size of the points show the bacterial 16S rRNA gene concentration in copy number/mL of sample, as determined by quantitative polymerase chain reaction. (B) Shannon index for the four environments presented in (A). Letters indicate groups of significance (False Discovery Rate-corrected P value <.05) representing Dunn’s test output after Kruskal-Wallis significance tests. Observed ASV numbers are available in Supplementary Fig. 4. (C) Genus affiliation of the 20 most abundant ASVs in the cDNA (left) and DNA dataset (right). The x axis shows the height of the ice core (in cm) relative to the water–ice interface (0 cm). Shades of grey below the bars indicate the number of chloroplast reads identified in the sample; a cross on white background indicates the absence of chloroplast read. ASV, amplicon sequence variant.
The exponential increase of microbial abundance with depth in our samples resembles the increase in diatom abundances found in sea ice brine channels at the ocean–ice interface [19]. However, at the time of sampling, light had returned for only two weeks. The absence of chloroplast reads (Fig. 1C) and the presence of very low cyanobacteria relative abundances (< 0.025% DNA and cDNA in the bottom core) (Supplementary Fig. 5) attests to the lack of photosynthetic activity at the ice–reservoir interface. The bottom core community was, in fact, dominated by three amplicon sequence variants (ASVs) associated with the sulfur-oxidizing bacteria (SOB) genera Sulfurimonas (ASV_1, ASV_9) and Thiomicrorhabdus (ASV_2) (Fig. 1C), which accounted for 53% to 85% of the community (DNA) and 86% to 95% of the active community (cDNA). In the water, the ratio between the cDNA and DNA relative abundances of ASVs affiliated with SOBs was five times that in the bottom ice, indicating higher SOB activity in the reservoir (Supplementary Fig. 6). The reduced relative abundance in the DNA dataset suggests that SOBs might be limited in space in the water reservoir, whereas the bottom core likely provides surfaces in contact with water, suiting their niche.
Phylogenetic placement of ASVs affiliated with Sulfurimonas showed multiple disagreeing placements. Likelihood weight ratios were between 0.02 and 0.33 (ASV_1, n = 4 placements), and 0.05 and 0.60 (ASV_9, n = 4). The branch length indicated distant similarities with known species of the genera Sulfurimonas and Sulfuricurvum, suggesting that these two ASVs are related to SOBs but are likely from unknown species or genera (Supplementary Fig. 7 and Supplementary Table 1). ASV_2, representative of the Thiomicrorhabdus population inhabiting the bottom core, was closely related to Thiomicrorhabdus aquaedulcis [25] (Supplementary Fig. 7) (98.93% nucleotidic similarity, likelihood weight ratio = 0.99, n = 1). Sulfurimonas and Thiomicrorhabdus genera mostly consist of aerobic chemolithoautotrophs, which generally use O2 as the electron acceptor [26, 27]. Despite limited oxygen availability in the water, these species might benefit from punctual microoxic conditions through ice fissures, as efficient microaerophilic growth is reported for some Sulfurimonas spp. [28]. Alternative electron acceptors for Sulfurimonas spp. and related species include nitrate, nitrite [27, 29, 30], and manganese oxides [31]. One Thiomicrorhabdus sp. was reported to be capable of growing in anoxic conditions using nitrite as an electron acceptor [32]. In the Lagoon Pingo, nitrate concentrations are below detection limits in the reservoir [16]. This depletion could be explained by rapid use of nitrate by these SOBs and/or by denitrification involving Ca. “Methanoperedens” [33], which was consistently observed in the ice and water reservoir (Fig. 1C and Fig. 2).
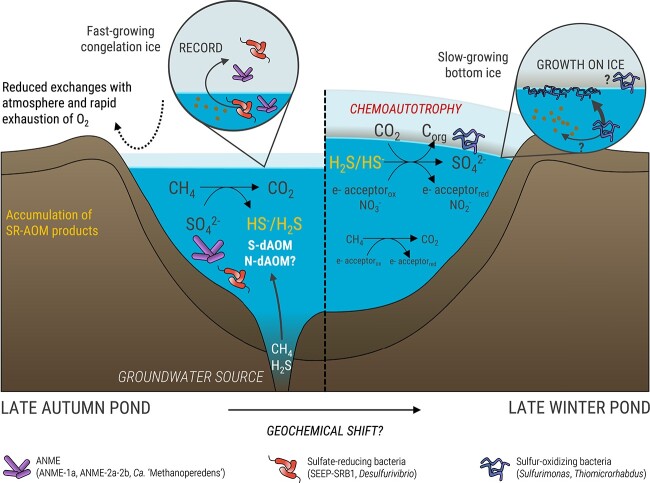
Figure 2.
Conceptual figure depicting the hypothetical functioning of the system during the ice-covered period. Left panel: Microbiological processes in the pingo water reservoir and ice cap just after the onset of the ice-cover formation, as hypothesized using the communities registered in the ice core. Right panel: Accumulated reduced sulfur species provide energy for SOB-mediated chemolithoautotrophy, which find a niche at the water–ice interface when ice has a slow growth at the end of the ice-covered period. SR, Sulfate reduction; AOM, anaerobic oxidation of methane; N-dAOM, nitrate-dependent AOM; S-dAOM, sulfate-dependent AOM.
Several factors could explain the repartition of sulfur oxidizers in the bottom core (Fig. 2): (1) electron acceptors for sulfur oxidation might be more available, potentially through cracks in the ice (O2) or background photosynthesis enabling efficient use of other electron acceptors under microoxic conditions as reported for many Sulfurimonas spp. [28]. (2) A shift in the reservoir geochemistry or redox balance — e.g. accumulation of reduced sulfur species, allowing SOBs to thrive at the end of the ice-covered period. However, the import or accumulation of electron acceptors and donors would benefit the reservoir’s communities as well and cannot explain the observed higher abundance of bacteria in the bottom ice versus water reservoir. (3) The ice structure might provide more surfaces for bacterial attachment. Young bottom ice forms much more slowly later in winter than at the beginning of the freezing process [34], leaving enough time for bacteria to settle on these surfaces [23]. This finding is consistent with the reported ability of Sulfurovum/Sulfurimonas-related and Thiomicrorhabdus spp. to attach to surfaces [35, 36] and the overall tendency of SOBs to form biofilms [37–39]. These results will need confirmation by in-depth studies confronting the geochemistry and microbiology of the ice and the reservoir throughout the winter period.
Reduced sulfur compounds (HS−) feeding sulfur oxidation could be sourced from the fluid or produced in the reservoir by consortia of ANMEs and SRBs. The concomitant presence of ANMEs and SRBs in the first 80 cm of the ice and in the water suggests that microbial anaerobic oxidation of methane (AOM) and sulfate reduction occurred throughout winter in the reservoir, with the water acting as a liquid incubator for ANMEs and SRBs (Fig. 2) that turn the system into a methane sink during the ice-covered period. Relative abundances of ANME-1a and SEEP-SRB1 were strongly correlated (Supplementary Fig. 8), suggesting that sulfate-dependent AOM (S-dAOM) was occurring [40]. The lower relative abundance of SRBs and higher abundance of ANME-2a-2b in the active community of the reservoir compared to the ice (Fig. 1C) suggest that S-dAOM likely occurred until a shift in the system opened a niche for ANMEs that use other electron acceptors. The discrepancy between DNA and cDNA relative abundances of SEEP-SRB1 (Fig. 1C,Supplementary Fig. 6) further suggests that the ice core recorded a seasonal shift in the reservoir, initially favoring sulfate reduction until SOBs dominated the community and contributed to the carbon sink through chemolithoautotrophy. More generally, the pool of reduced sulfur compounds might enhance carbon sequestration, as sulfurization of dissolved and particulate organic carbon generates refractory organic matter in oxygen-deprived environments [41].
This study demonstrated the significant role of the ice cap above a chemical energy-rich groundwater seepage in hosting active chemotrophic microorganisms that consume greenhouse gases. We found active anaerobic methanotrophs in the water reservoir, indicating a dynamic winter system where at least part of the greenhouse gases brought by the source is oxidized under the ice. An active community of SOBs thrives on the ice at the water interface. This finding strongly suggests that active microbial habitats at ice–water interfaces in dark environments have been overlooked and may provide valuable insights to the field of microbiology.
Supplementary Material
Acknowledgements
We acknowledge the Research Council of Norway grant no. 244906 and 326285, and the UNIS logistic team for their support. We also thank Alena Didriksen and Liabo Motleleng for laboratory support. The computations were performed thanks to the National Infrastructure for High Performance Computing and Data Storage in Norway, account nos. NN9639K and NS9593K.
Contributor Information
Lisa-Marie Delpech, LIENSs Littoral Environnement et Sociétés, UMRi 7266 CNRS–La Rochelle Université, La Rochelle, 17000, France; Department of Geosciences, UiT The Arctic University of Norway, Tromsø, 9010, Norway; Department of Biology, École Normale Supérieure de Lyon, Lyon, 69007, France.
Alexander T Tveit, Department of Arctic and Marine Biology, UiT The Arctic University of Norway, Tromsø, 9019, Norway.
Andrew J Hodson, Department of Arctic Geology, UNIS The University Center in Svalbard, Longyearbyen, 9170, Svalbard, Norway; Department of Civil Engineering and Environmental Science, Western Norway University of Applied Sciences, Sogndal, 6856, Norway.
Kevin P Hand, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA, 91109, United States.
Dimitri Kalenitchenko, LIENSs Littoral Environnement et Sociétés, UMRi 7266 CNRS–La Rochelle Université, La Rochelle, 17000, France; Department of Geosciences, UiT The Arctic University of Norway, Tromsø, 9010, Norway.
Author contributions
D.K. and A.T.T. designed field sampling and collected ice cores. L.M.D. extracted TNA and conducted quantitative polymerase chain reaction, analyzed data, and wrote the manuscript with D.K. All authors contributed to the manuscript and approved the final submitted version.
Conflicts of interest
None declared.
Funding
This study was funded by the Research Council of Norway through the projects Methanice grant number 326285, Climagas grant number 294764, and CAGE grant number 223259. K.P.H. acknowledges support from National Aeronautics and Space Administration PSTAR grant 80NSSC22K1312 and from the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (80NM0018D0004).
Data availability
The sequences reported in this paper have been deposited in the NCBI SRA database under the accession numbers SRR24977479–SRR24977560. Reproducible code for the figures and results presented in this study and in the Supplementary Information is available at https://github.com/lmdelpech/MethanIce-BriefReport-LP-ICE-2021.
References
- 1. Russell MJ, Murray AE, Hand KP. The possible emergence of life and differentiation of a shallow biosphere on irradiated icy worlds: the example of Europa. Astrobiology 2017;17:1265–73. 10.1089/ast.2016.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dubilier N, Bergin C, Lott C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nat Rev Microbiol 2008;6:725–40. 10.1038/nrmicro1992 [DOI] [PubMed] [Google Scholar]
- 3. Perreault NN, Greer CW, Andersen DTet al. Heterotrophic and autotrophic microbial populations in cold perennial springs of the high Arctic. Appl Environ Microbiol 2008;74:6898–907. 10.1128/AEM.00359-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Niederberger TD, Perreault NN, Lawrence JRet al. Novel sulfur-oxidizing streamers thriving in perennial cold saline springs of the Canadian high Arctic. Environ Microbiol 2009;11:616–29. 10.1111/j.1462-2920.2008.01833.x [DOI] [PubMed] [Google Scholar]
- 5. Macalady JL, Lyon EH, Koffman Bet al. Dominant microbial populations in limestone-corroding stream biofilms, Frasassi cave system, Italy. Appl Environ Microbiol 2006;72:5596–609. 10.1128/AEM.00715-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alfreider A, Baumer A, Bogensperger Tet al. CO2 assimilation strategies in stratified lakes: diversity and distribution patterns of chemolithoautotrophs. Environ Microbiol 2017;19:2754–68. 10.1111/1462-2920.13786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lorenz RD, Gleeson D, Prieto-Ballesteros Oet al. Analog environments for a Europa lander mission. Adv Space Res 2011;48:689–96. 10.1016/j.asr.2010.05.006 [DOI] [Google Scholar]
- 8. Vick-Majors TJ, Priscu JC, Amaral-Zettler LA. Modular community structure suggests metabolic plasticity during the transition to polar night in ice-covered Antarctic lakes. ISME J 2014;8:778–89. 10.1038/ismej.2013.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Savvichev AS, Kadnikov VV, Rusanov IIet al. Microbial processes and microbial communities in the water column of the polar meromictic Lake Bol’shie Khruslomeny at the White Sea coast. Front Microbiol 2020;11:1945. 10.3389/fmicb.2020.01945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bastviken D, Cole JJ, Pace MLet al. Fates of methane from different lake habitats: connecting whole-lake budgets and CH4 emissions. J Geophys Res Biogeosci 2008;113:G02024. 10.1029/2007JG000608 [DOI] [Google Scholar]
- 11. Cadieux SB, White JR, Sauer PEet al. Large fractionations of C and H isotopes related to methane oxidation in Arctic lakes. Geochim Cosmochim Acta 2016;187:141–55. 10.1016/j.gca.2016.05.004 [DOI] [Google Scholar]
- 12. Lofton DD, Whalen SC, Hershey AE. Vertical sediment distribution of methanogenic pathways in two shallow Arctic Alaskan lakes. Polar Biol 2015;38:815–27. 10.1007/s00300-014-1641-4 [DOI] [Google Scholar]
- 13. Paytan A, Lecher AL, Dimova Net al. Methane transport from the active layer to lakes in the Arctic using Toolik Lake, Alaska, as a case study. Proc Natl Acad Sci USA 2015;112:3636–40. 10.1073/pnas.1417392112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui M, Ma A, Qi Het al. Anaerobic oxidation of methane: an “active” microbial process. Microbiology 2015;4:1–11. 10.1002/mbo3.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabrol L, Thalasso F, Gandois Let al. Anaerobic oxidation of methane and associated microbiome in anoxic water of Northwestern Siberian lakes. Sci Total Environ 2020;736:139588. 10.1016/j.scitotenv.2020.139588 [DOI] [PubMed] [Google Scholar]
- 16. Hodson AJ, Nowak A, Redeker KRet al. Seasonal dynamics of methane and carbon dioxide evasion from an open system pingo: lagoon Pingo, Svalbard. Front Earth Sci 2019;7:30. 10.3389/feart.2019.00030 [DOI] [Google Scholar]
- 17. Hodson AJ, Nowak A, Hornum MTet al. Sub-permafrost methane seepage from open-system pingos in Svalbard. Cryosphere 2020;14:3829–42. 10.5194/tc-14-3829-2020 [DOI] [Google Scholar]
- 18. Ricão Canelhas M, Denfeld BA, Weyhenmeyer GAet al. Methane oxidation at the water-ice interface of an ice-covered lake. Limnol Oceanogr 2016;61:S78–90. 10.1002/lno.10288 [DOI] [Google Scholar]
- 19. Boetius A, Anesio AM, Deming JWet al. Microbial ecology of the cryosphere: sea ice and glacial habitats. Nat Rev Microbiol 2015;13:677–90. 10.1038/nrmicro3522 [DOI] [PubMed] [Google Scholar]
- 20. Arrigo KR, Perovich DK, Pickart RSet al. Phytoplankton blooms beneath the sea ice in the Chukchi Sea. Deep Sea Res II Top Stud Oceanogr 2014;105:1–16. 10.1016/j.dsr2.2014.03.018 [DOI] [Google Scholar]
- 21. Anesio AM, Lutz S, Chrismas NAMet al. The microbiome of glaciers and ice sheets. NPJ Biofilms Microbiomes 2017;3:10. 10.1038/s41522-017-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denfeld BA, Baulch HM, del Giorgio PAet al. A synthesis of carbon dioxide and methane dynamics during the ice-covered period of northern lakes. Limnol Oceanogr Lett 2018;3:117–31. 10.1002/lol2.10079 [DOI] [Google Scholar]
- 23. Spangenberg I, Overduin PP, Damm Eet al. Methane pathways in winter ice of a thermokarst lake–lagoon–coastal water transect in North Siberia. Cryosphere 2021;15:1607–25. 10.5194/tc-15-1607-2021 [DOI] [Google Scholar]
- 24. Paun VI, Icaza G, Lavin Pet al. Total and potentially active bacterial communities entrapped in a late glacial through Holocene ice core from Scarisoara ice cave. Romania Front Microbiol 2019;10:1193. 10.3389/fmicb.2019.01193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kojima H, Fukui M. 2019. Thiomicrorhabdus aquaedulcis sp. nov., a sulfur-oxidizing bacterium isolated from lake water. Int J Syst Evol Microbiol 2019;69:2849–53. 10.1099/ijsem.0.003567 [DOI] [PubMed] [Google Scholar]
- 26. Boden R, Scott KM, Williams Jet al. An evaluation of Thiomicrospira, Hydrogenovibrio and Thioalkalimicrobium: reclassification of four species of Thiomicrospira to each Thiomicrorhabdus gen. Nov. and Hydrogenovibrio, and reclassification of all four species of Thioalkalimicrobium to Thiomicrospira. Int J Syst Evol Microbiol 2017;67:1140–51. 10.1099/ijsem.0.001855 [DOI] [PubMed] [Google Scholar]
- 27. Wang S, Jiang L, Liu Xet al. Sulfurimonas xiamenensis sp. nov. and Sulfurimonas lithotrophica sp. nov., hydrogen- and sulfur-oxidizing chemolithoautotrophs within the Epsilonproteobacteria isolated from coastal sediments, and an emended description of the genus Sulfurimonas. Int J Syst Evol Microbiol 2020;70:2657–63. 10.1099/ijsem.0.004087 [DOI] [PubMed] [Google Scholar]
- 28. Han Y, Perner M. The globally widespread genus Sulfurimonas: versatile energy metabolisms and adaptations to redox clines. Front Microbiol 2015;6:989. 10.3389/fmicb.2015.00989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Wang S, Lai Qet al. Sulfurimonas marina sp. nov., an obligately chemolithoautotrophic, Sulphur-oxidizing bacterium isolated from a deep-sea sediment sample from the South China Sea. Int J Syst Evol Microbiol 2022;72:005582. 10.1099/ijsem.0.005582 [DOI] [PubMed] [Google Scholar]
- 30. Trivedi CB, Stamps BW, Lau GEet al. Microbial metabolic redundancy is a key mechanism in a sulfur-rich glacial ecosystem. mSystems 2020;5:e00504–20. 10.1128/mSystems.00504-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henkel JV, Dellwig O, Pollehne Fet al. A bacterial isolate from the Black Sea oxidizes sulfide with manganese(IV) oxide. Proc Natl Acad Sci USA 2019;116:12153–5. 10.1073/pnas.1906000116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Chen B, Lai Qet al. Thiomicrorhabdus sediminis sp. nov. and Thiomicrorhabdus xiamenensis sp. nov., novel sulfur-oxidizing bacteria isolated from coastal sediments and an emended description of the genus Thiomicrorhabdus. Int J Syst Evol Microbiol 2021;71:004660. 10.1099/ijsem.0.004660 [DOI] [PubMed] [Google Scholar]
- 33. Haroon MF, Hu S, Shi Yet al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 2013;500:567–70. 10.1038/nature12375 [DOI] [PubMed] [Google Scholar]
- 34. Leppäranta M., Leppäranta M.. Thermodynamics of seasonal lake ice. In: Leppäranta M (ed). Freezing of Lakes and the Evolution of Their Ice Cover. Berlin, Heidelberg: Springer, 2015, 91–135, 10.1007/978-3-642-29081-7_4. [DOI] [Google Scholar]
- 35. Meier DV, Pjevac P, Bach Wet al. Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents. ISME J 2017;11:1545–58. 10.1038/ismej.2017.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scott KM, Sievert SM, Abril FNet al. The genome of deep- sea vent chemolithoautotroph Thiomicrospira crunogena XCL-2. PLoS Biol 2006;4:e383. 10.1371/journal.pbio.0040383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Macalady JL, Dattagupta S, Schaperdoth Iet al. Niche differentiation among sulfur-oxidizing bacterial populations in cave waters. ISME J 2008;2:590–601. 10.1038/ismej.2008.25 [DOI] [PubMed] [Google Scholar]
- 38. Magnuson E, Mykytczuk NCS, Pellerin Aet al. Thiomicrorhabdus streamers and sulfur cycling in perennial hypersaline cold springs in the Canadian high Arctic. Environ Microbiol 2021;23:3384–400. 10.1111/1462-2920.14916 [DOI] [PubMed] [Google Scholar]
- 39. Okabe S, Ito T, Sugita Ket al. Succession of internal sulfur cycles and sulfur-oxidizing bacterial communities in microaerophilic wastewater biofilms. Appl Environ Microbiol 2005;71:2520–9. 10.1128/AEM.71.5.2520-2529.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knittel K, Boetius A. Anaerobic oxidation of methane: progress with an unknown process. Ann Rev Microbiol 2009;63:311–34. 10.1146/annurev.micro.61.080706.093130 [DOI] [PubMed] [Google Scholar]
- 41. Chen Q, Tang K, Chen Xet al. Microbial sulfurization stimulates carbon sequestration in marine oxygen minimum zones. Sci Bull 2022;67:895–8. 10.1016/j.scib.2022.01.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences reported in this paper have been deposited in the NCBI SRA database under the accession numbers SRR24977479–SRR24977560. Reproducible code for the figures and results presented in this study and in the Supplementary Information is available at https://github.com/lmdelpech/MethanIce-BriefReport-LP-ICE-2021.