Abstract
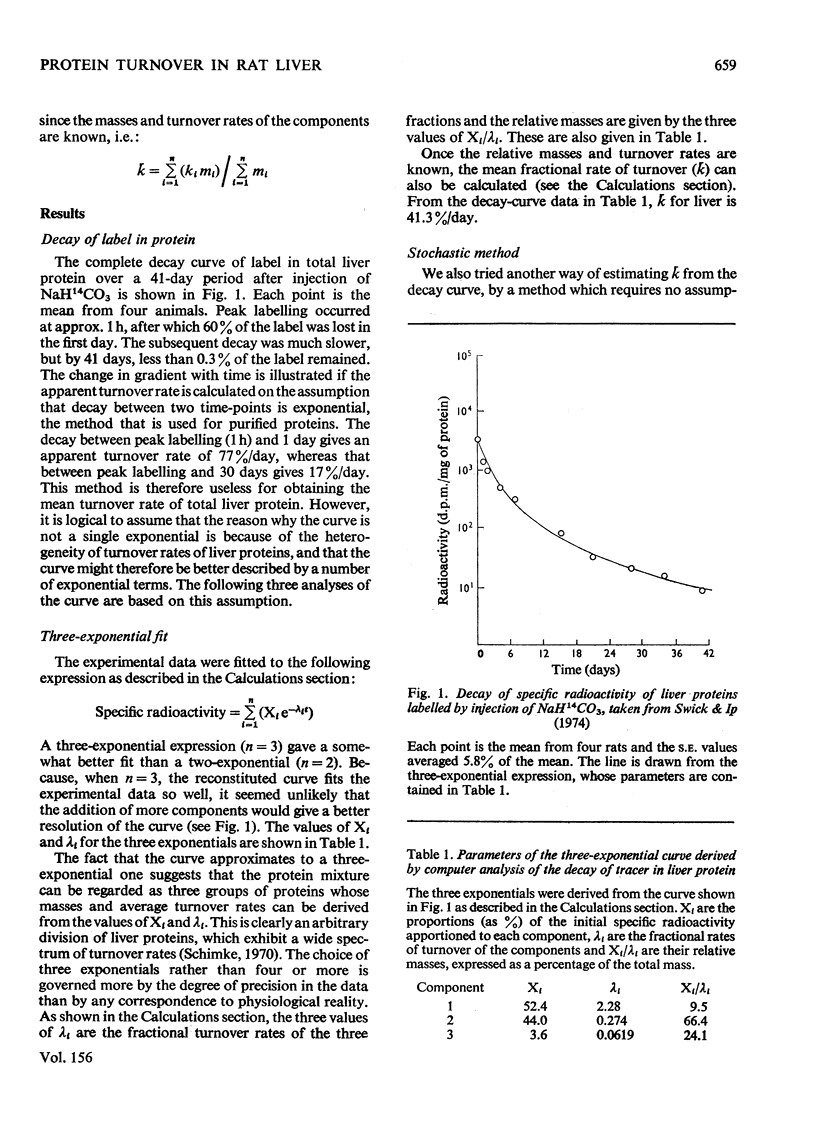
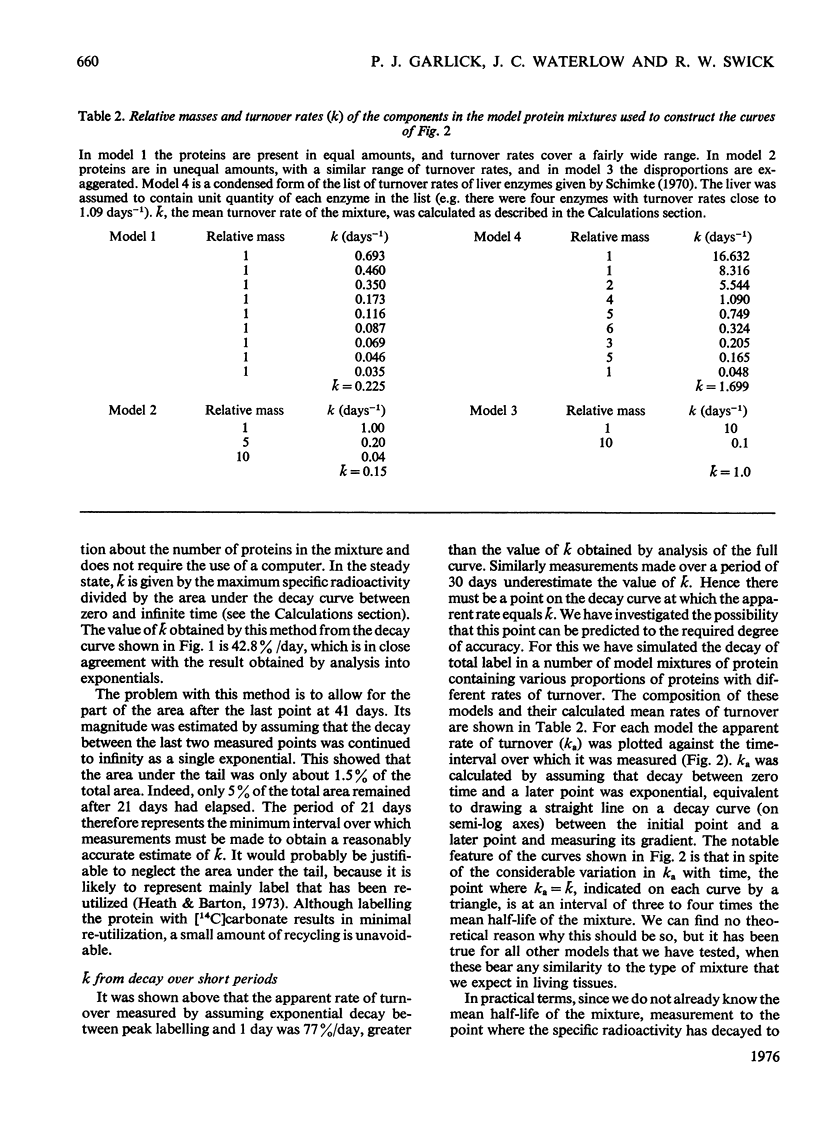
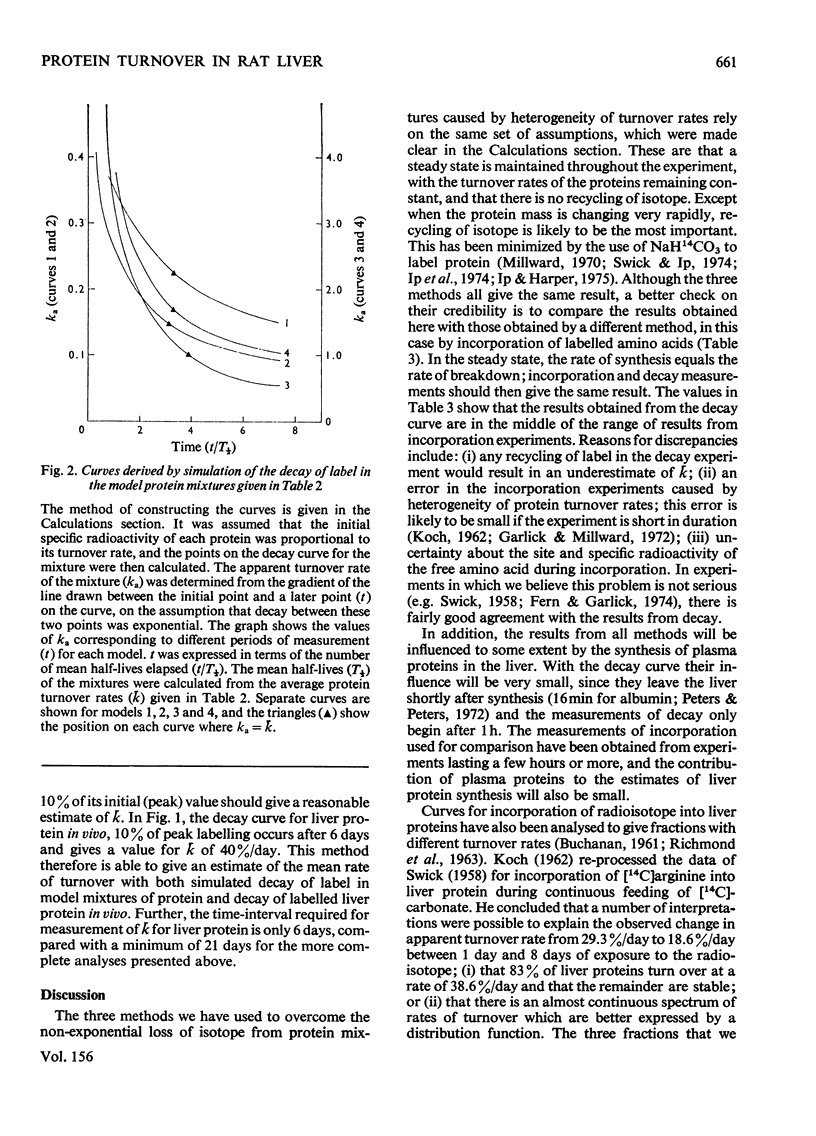
The curve for decay of 14C in rat liver protein labelled by injection of NaH14CO3 was analysed to obtain the average turnover rate of mixed liver protein. Three different methods of analysis were used. (1) Unlike decay curves from homogeneous proteins, the curve did not fit a single exponential, but a good fit was obtained with three exponentials. By assuming that the mixture contained three major components with different turnover rates, the calculated value for the average turnover rate (k) was close to 40% per day. (2) k was also calculated from the area under the decay curve, a method which makes no assumptions about the number of proteins in the mixture. This method also gave a value close to 40% per day. (3) It was shown empirically, both by simulation of decay of label in model mixtures of protein and with the decay curve measured in vivo, that k can be calculated from the time taken for the specific radioactivity to fall to 10% of its maximum value. This is an advantage, since the other two methods require the decay curve to be measured over a much longer period of time.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Doyle D., Schimke R. T. Studies on the synthesis and degradation of proteins of the endoplasmic reticulum of rat liver. J Biol Chem. 1969 Jun 25;244(12):3303–3315. [PubMed] [Google Scholar]
- Chee P. Y., Swick R. W. Effect of dietary protein and tryptophan and the turnover of rat liver ornithine aminotransferase. J Biol Chem. 1976 Feb 25;251(4):1029–1034. [PubMed] [Google Scholar]
- Fern E. B., Garlick P. J. The specific radioactivity of the tissue free amino acid pool as a basis for measuring the rate of protein synthesis in the rat in vivo. Biochem J. 1974 Aug;142(2):413–419. doi: 10.1042/bj1420413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J. An appraisal of techniques for the determination of protein turnover in vivo. Proc Nutr Soc. 1972 Dec;31(3):249–255. doi: 10.1079/pns19720048. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P., Waterlow J. C. The effect of protein deprivation and starvation on the rate of protein synthesis in tissues of the rat. Biochim Biophys Acta. 1975 Nov 18;414(1):71–84. doi: 10.1016/0005-2787(75)90126-4. [DOI] [PubMed] [Google Scholar]
- Haider M., Tarver H. Effect of diet on protein synthesis and nucleic acid levels in rat liver. J Nutr. 1969 Dec;99(4):433–445. doi: 10.1093/jn/99.4.433. [DOI] [PubMed] [Google Scholar]
- Heath D. F., Barton R. N. The design of experiments using isotopes for the determination of the rates of disposal of blood-borne substrates in vivo with special reference to glucose, ketone bodies, free fatty acids and proteins. Biochem J. 1973 Nov;136(3):503–518. doi: 10.1042/bj1360503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip C., Harper A. E. Protein synthesis in liver, muscle, and brain of rats fed a high tyrosine-low protein diet. J Nutr. 1975 Jul;105(7):885–893. doi: 10.1093/jn/105.7.885. [DOI] [PubMed] [Google Scholar]
- Ip M. M., Chee P. Y., Swick R. W. Turnover of hepatic mitochondrial ornithine aminotransferase and cytochrome oxidase using (14C)carbonate as tracer. Biochim Biophys Acta. 1974 Jun 20;354(1):29–38. doi: 10.1016/0304-4165(74)90049-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Millward D. J. Protein turnover in skeletal muscle. II. The effect of starvation and a protein-free diet on the synthesis and catabolism of skeletal muscle proteins in comparison to liver. Clin Sci. 1970 Nov;39(5):591–603. doi: 10.1042/cs0390591. [DOI] [PubMed] [Google Scholar]
- Peters T., Jr, Peters J. C. The biosynthesis of rat serum albumin. VI. Intracellular transport of albumin and rates of albumin and liver protein synthesis in vivo under various physiological conditions. J Biol Chem. 1972 Jun 25;247(12):3858–3863. [PubMed] [Google Scholar]
- Richmond J. E., Shoemaker W. C., Elwyn D. H. Rates of biosynthesis of plasma and liver proteins. Am J Physiol. 1963 Nov;205(5):848–856. doi: 10.1152/ajplegacy.1963.205.5.848. [DOI] [PubMed] [Google Scholar]
- SWICK R. W. Measurement of protein turnover in rat liver. J Biol Chem. 1958 Apr;231(2):751–764. [PubMed] [Google Scholar]
- Swick R. W., Ip M. M. Measurement of protein turnover in rat liver with (14C)carbonate. Protein turnover during liver regeneration. J Biol Chem. 1974 Nov 10;249(21):6836–6841. [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]


