Abstract
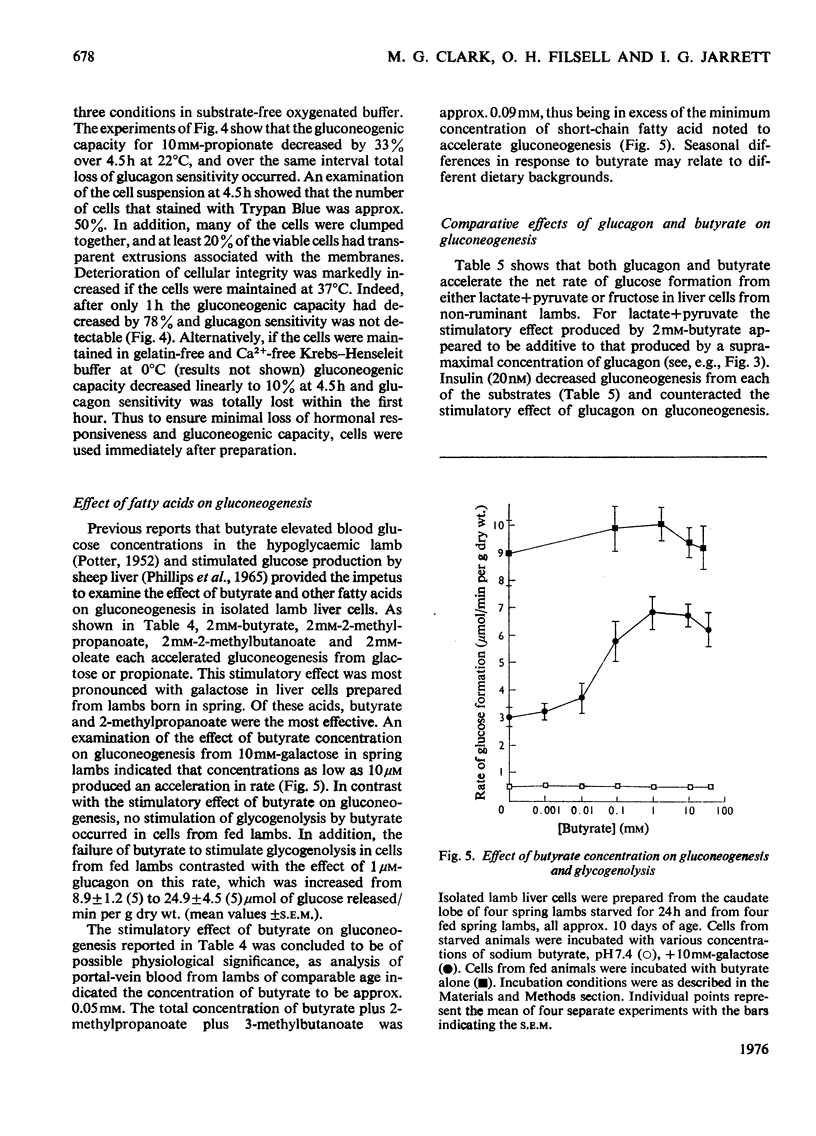
1. Isolated lamb liver cells were prepared from 24-h-starved animals by venous perfusion of the excised caudate lobe with buffer containing collagenase. On the basis of Trypan-Blue exclusion, rate of O2 uptake, adenine nucleotide content and retention of constitutive enzymes, these cells were judged to be intact. 2. Isolated caudate-lobe liver cells showed rates of gluconeogenesis from 10 mM-propionate and 10 mM-lactate that compared favourably with rates determined in isolated median-lobe cells and with rates determined with the isolated perfused lamb liver. 3. The gluconeogenic potential of substrates tested depended on the lamb's age. Cells prepared from suckling lambs (up to 20 days of age and essentially non-ruminant) showed highest rates from galactose, serine and alanine; those prepared from post-weaned lambs (older than 30 days of age and ruminant) showed highest rates from propionate, lactate and fructose. 4. Gluconeogenic rates from endogeneous precursors, 10 mM-propionate and 10mM-galactose, were linear for 1 h and were both stimulated by 1 muM-glucagon. Provided the endogenous rate of gluconeogenesis remained unchanged after substrate addition, glucagon caused a net stimulation of gluconeogenesis from each of these substrates. 5. Gluconeogenic capacity and glucagon sensitivity were examined in cells maintained in substrate-free oxygenated buffer at 37 degrees, 22 degrees and * degrees C. Even under the best of the three conditions of storage that were tested (i.e. at 22 degrees C in gelatin-containing buffer) deterioration of the lamb cells proceeded rapidly, and loss of glucagon responsiveness preceeded the loss of ability to convert precursor into glucose. 6. n-Butyric acid, 2-methylpropanoic acid and 3-methylbutanoic acid at concentrations comparable with those found in lamb portal-vein blood each stimulated gluconeogenesis from 10mM-galactose or 10mM-propionate; gluconeogenesis from galactose was stimulated to the greater extent. 7. The regulatory effects of glucagon and sodium butyrate on lamb liver-cell gluconeogenesis and glycogenolysis were compared. Glucagon (1 muM) and 2mM-butyrate accelerated the rate of glucose formation of liver cells of 24h-starved animals from lactate+pyruvate or fructose. Insulin (20nM) decreased both gluconeogenesis and the efficacy of 1 muM-glucagon. For lactate+pyruvate as substrate, the stimulatory effect of butyrate was additive to that of 1muM-glucagon and for both lactate+pyruvate and fructose the stimulatory effect of butyrate was not influenced by 20nM-insulin. In contrast with glucagon, which stimulated the rate of glycogenolysis in cells prepared from fed lambs, butyrate (0.1-20mM) had no effect. 8. It is concluded that glucagon and butyrate stimulate lamb liver-cell gluconeogenesis by different mechanisms.
Full text
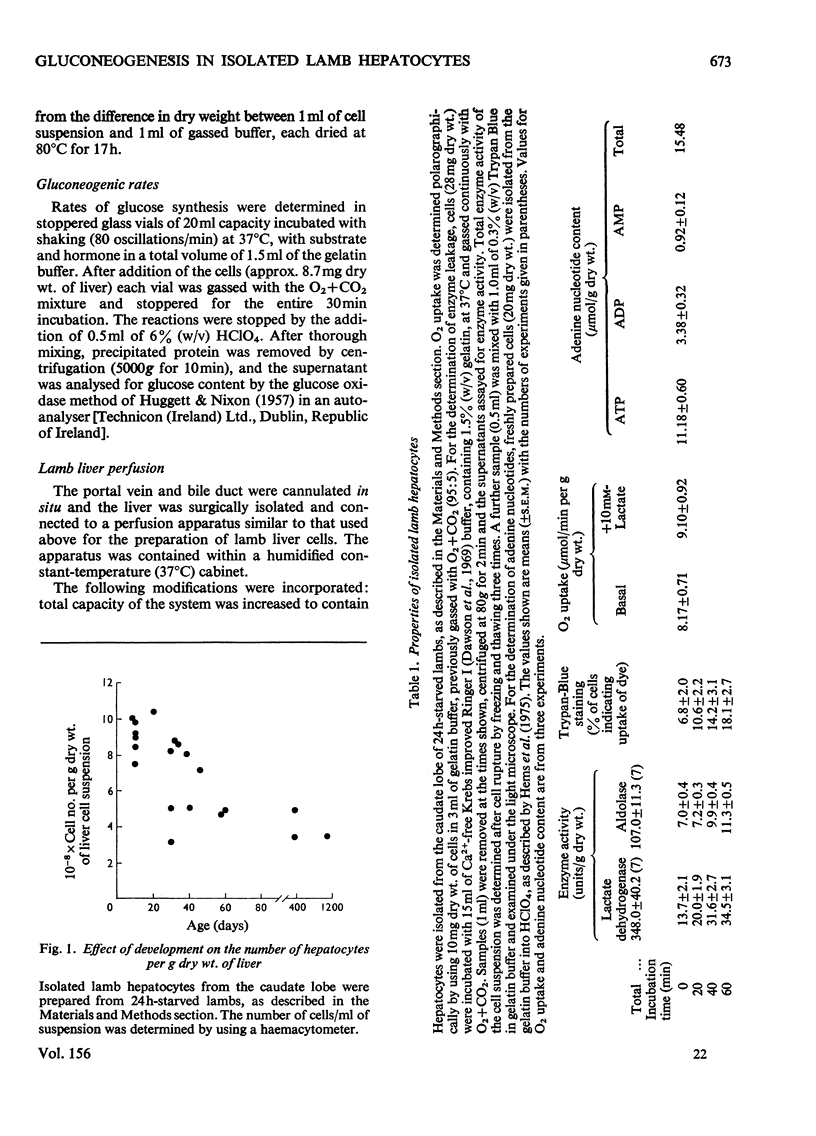
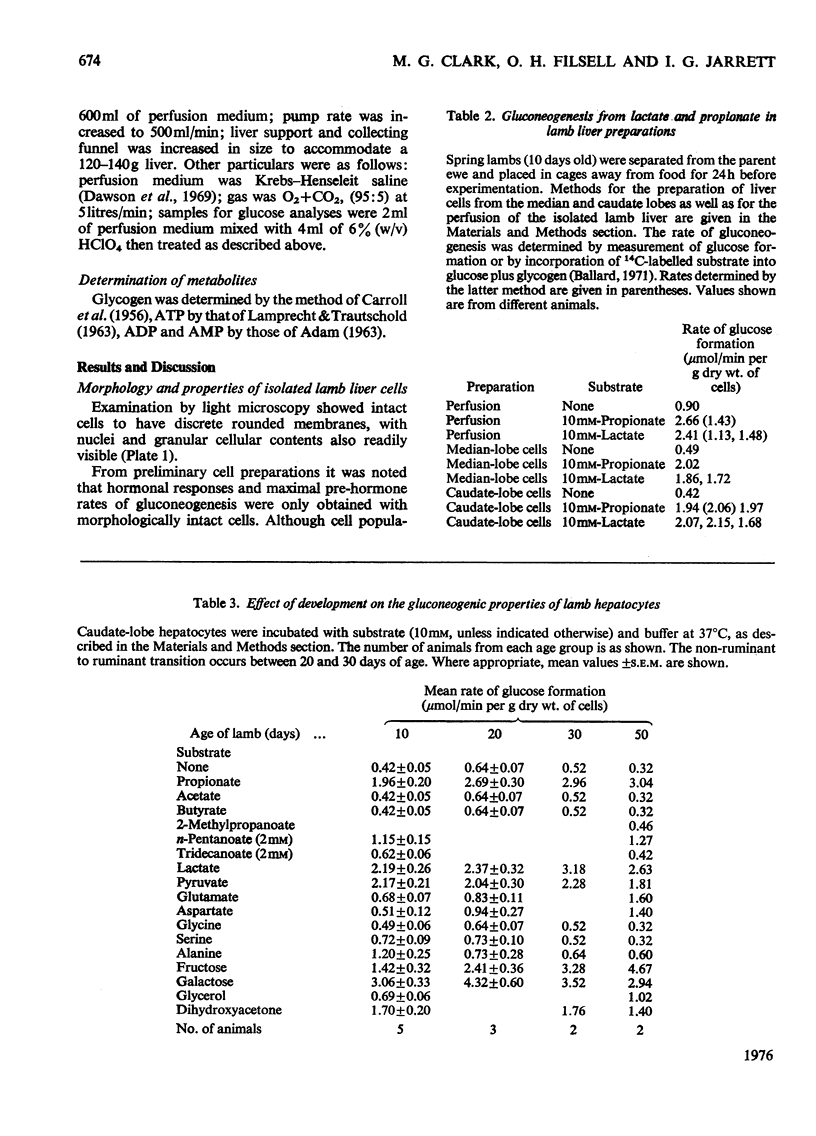
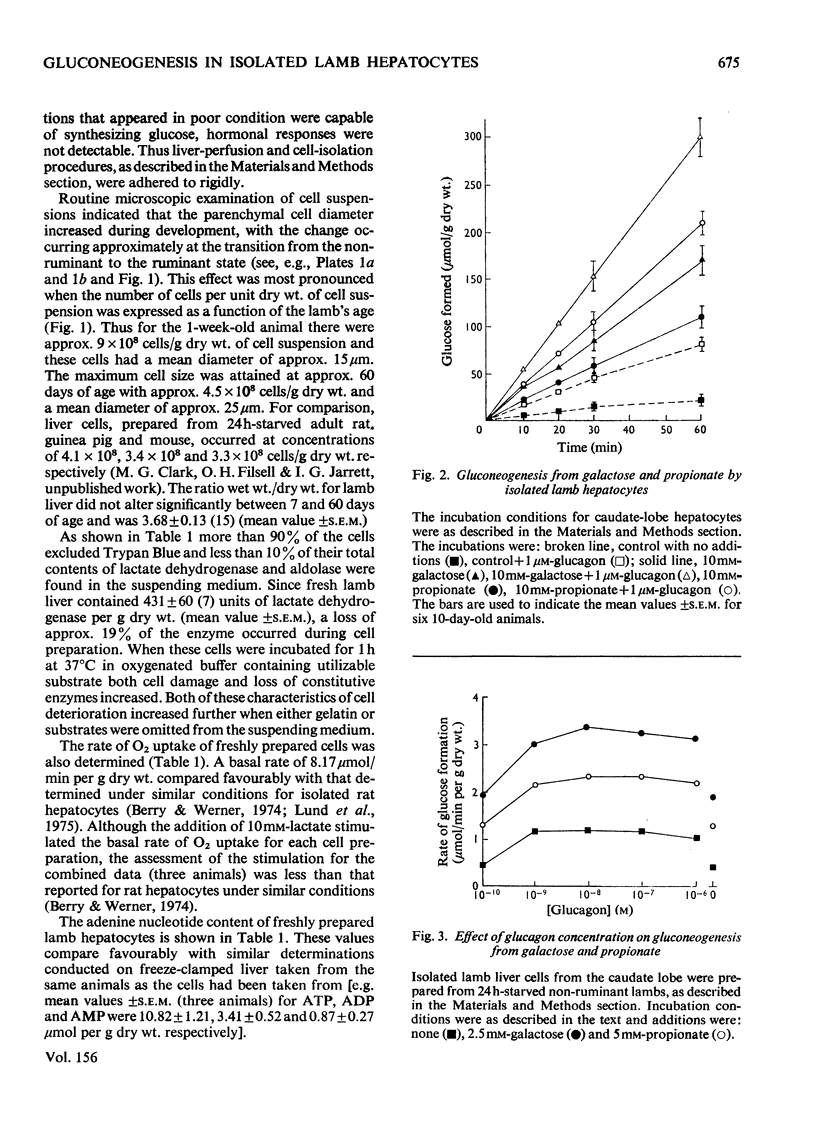
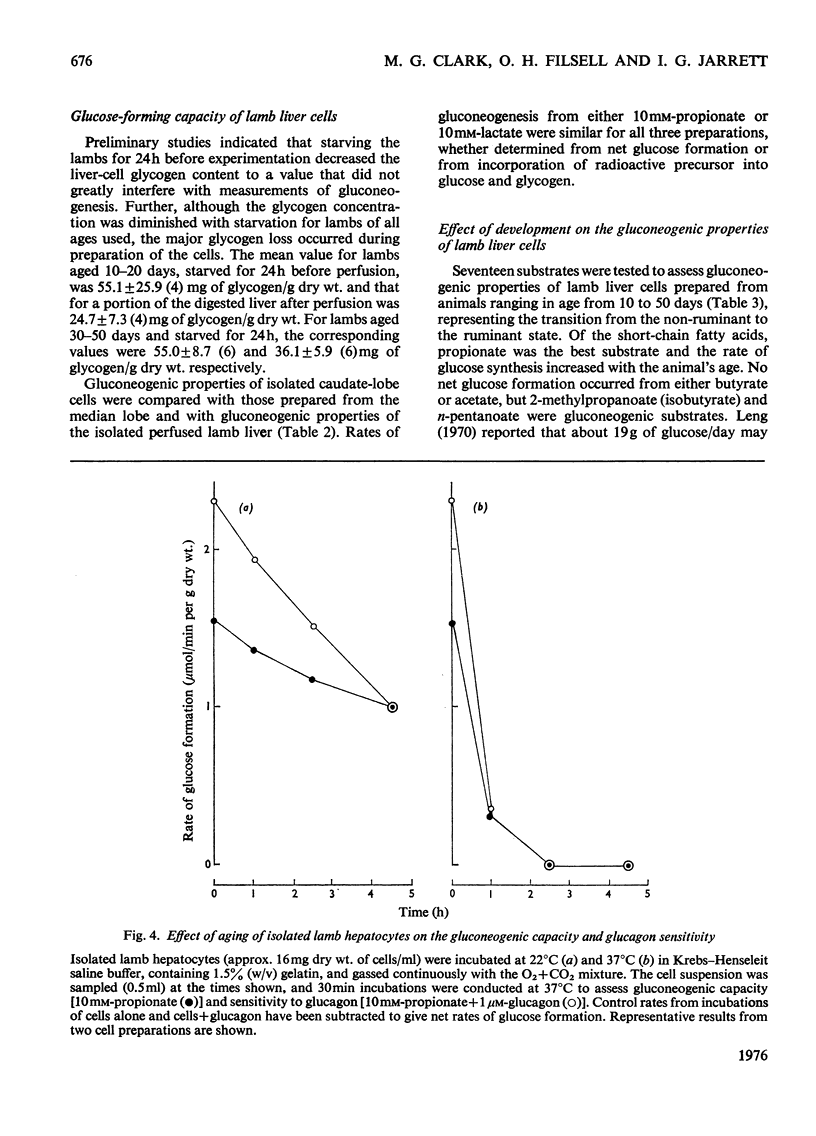
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J. Regulation of gluconeogenesis during exposure of young rats to hypoxic conditions. Biochem J. 1971 Jan;121(2):169–178. doi: 10.1042/bj1210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL N. V., LONGLEY R. W., ROE J. H. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956 Jun;220(2):583–593. [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hems R., Lund P., Krebs H. A. Rapid separation of isolated hepatocytes or similar tissue fragments for analysis of cell constituents. Biochem J. 1975 Jul;150(1):47–50. doi: 10.1042/bj1500047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett I. G., Jones G. B., Potter B. J. Changes in glucose utilization during development of the lamb. Biochem J. 1964 Jan;90(1):189–194. doi: 10.1042/bj0900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng R. A. Glucose synthesis in ruminants. Adv Vet Sci Comp Med. 1970;14:209–260. [PubMed] [Google Scholar]
- Lund P., Cornell N. W., Krebs H. A. Effect of adenosine on the adenine nucleotide content and metabolism of hepatocytes. Biochem J. 1975 Dec;152(3):593–599. doi: 10.1042/bj1520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS R. W., BLACK A. L., MOLLER F. BUTYRATE INDUCED GLYCOGENOLYSIS IN HYPOGLYCEMIC LAMBS. Life Sci. 1965 Mar;4:521–525. doi: 10.1016/0024-3205(65)90260-2. [DOI] [PubMed] [Google Scholar]
- POTTER B. J. Relief of hypoglycaemic convulsions with butyric acid. Nature. 1952 Sep 27;170(4326):541–541. doi: 10.1038/170541a0. [DOI] [PubMed] [Google Scholar]
- Wolff J. E., Bergman E. N. Gluconeogenesis from plasma amino acids in fed sheep. Am J Physiol. 1972 Aug;223(2):455–460. doi: 10.1152/ajplegacy.1972.223.2.455. [DOI] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W. The isolation of hormone-sensitive rat hepatocytes by a modified enzymatic technique. Arch Biochem Biophys. 1974 Aug;163(2):600–608. doi: 10.1016/0003-9861(74)90519-0. [DOI] [PubMed] [Google Scholar]



