Abstract
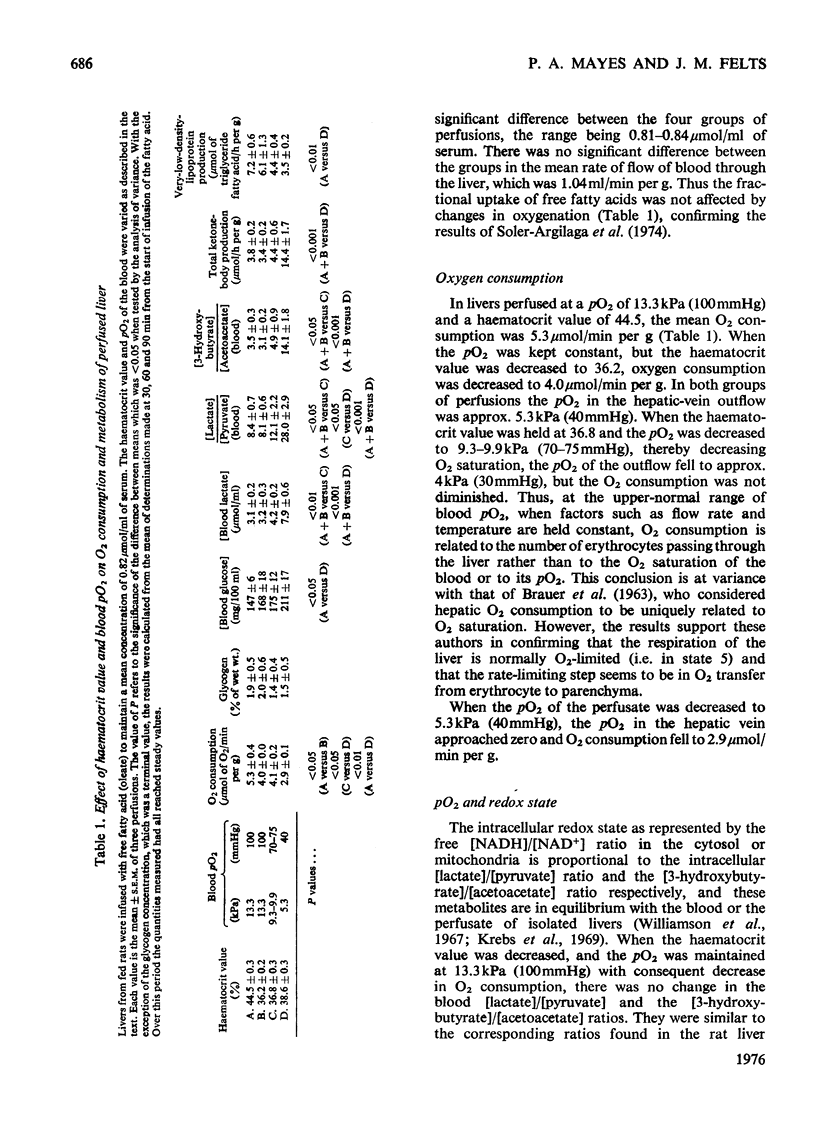
1. The haematocrit value and pO2 of blood perfusing the isolated liver were varied. Provided O2 content of the blood was not rate-limiting, O2 consumption was related to haemotocrit value rather than O2 saturation or pO2. 2. Hypoxia caused the blood-glucose concentration and ketogenesis to increase and the output of very-low-density (d less than 1.006) lipoproteins to decrease. 3. A decrease in pO2 caused an increase in both the (lactate)/(pyruvate) and (3-hydroxybutyrate)/(acetoacetate) and a decrease in (ATP)/(ADP) ratios, independently of O2 consumption. 4. The more reduced redox state was associated with a shift in the balance between the oxidation and esterification of free fatty acids in favour of oxidation. 5. Acetoacetate may be an important hydrogen acceptor during hypoxia of the liver.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton S. D., Ishida T. Effect of insulin on potassium and glucose movement in perfused rat liver. Am J Physiol. 1965 Dec;209(6):1145–1151. doi: 10.1152/ajplegacy.1965.209.6.1145. [DOI] [PubMed] [Google Scholar]
- Felts J. M., Whayne T. F., Jr A small oxygenator for use with an organ perfusion system. IEEE Trans Biomed Eng. 1973 Sep;20(5):382–384. doi: 10.1109/TBME.1973.324236. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Oxidative metabolism at low PO 2 . Fed Proc. 1972 Sep-Oct;31(5):1404–1413. [PubMed] [Google Scholar]
- Krebs H. A., Wallace P. G., Hems R., Freedland R. A. Rates of ketone-body formation in the perfused rat liver. Biochem J. 1969 May;112(5):595–600. doi: 10.1042/bj1120595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Infante R., Polonovski J. Lipid metabolism of rat liver isolated and perfused in hypoxia. Biomedicine. 1974 Mar;20(2):154–159. [PubMed] [Google Scholar]
- Solymar M., Rucklidge M. A., Prys-Roberts C. A modified approach to the polarographic measurement of blood O2 content. J Appl Physiol. 1971 Feb;30(2):272–275. doi: 10.1152/jappl.1971.30.2.272. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem J. 1972 Jan;126(2):295–311. doi: 10.1042/bj1260295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


