Abstract
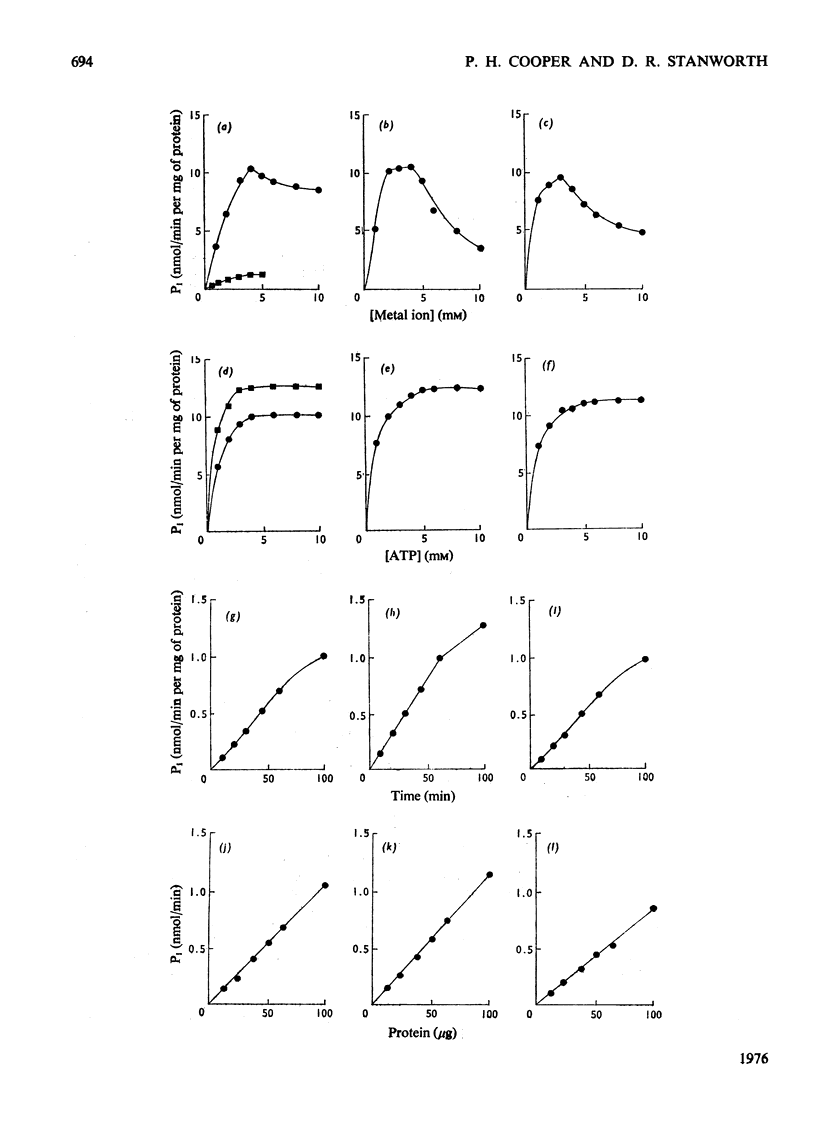
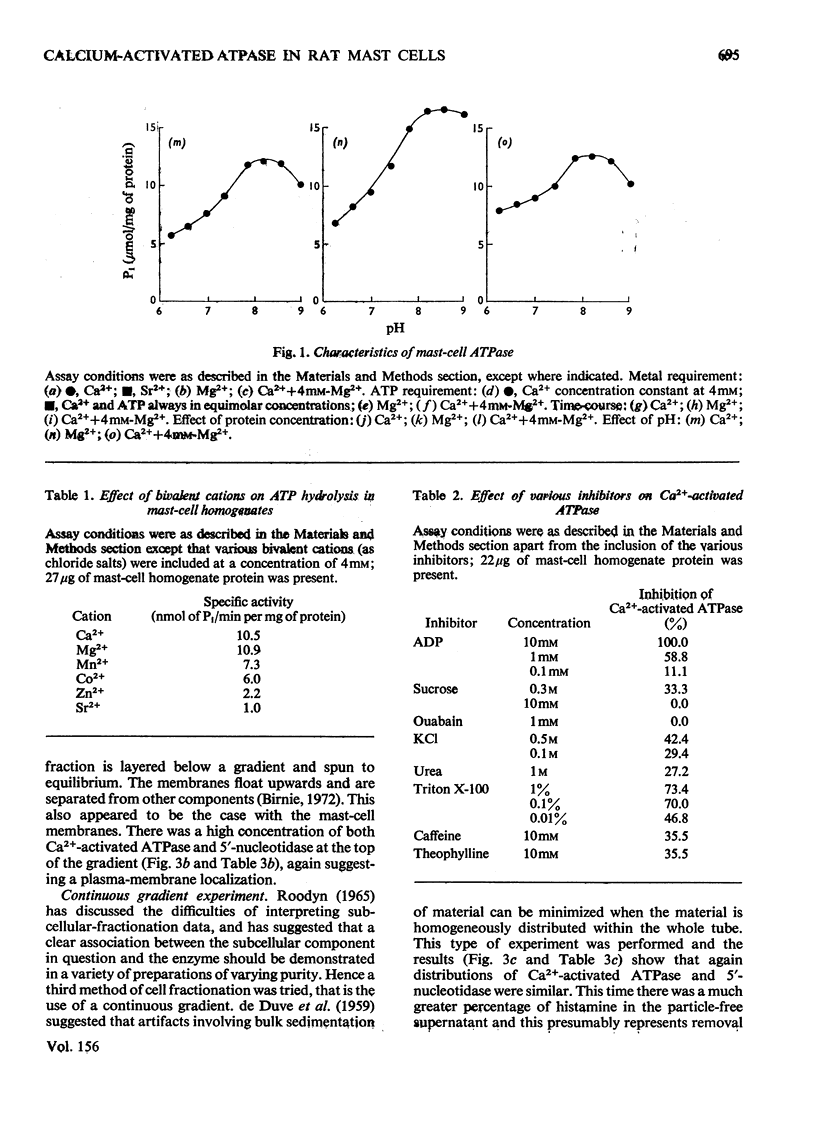
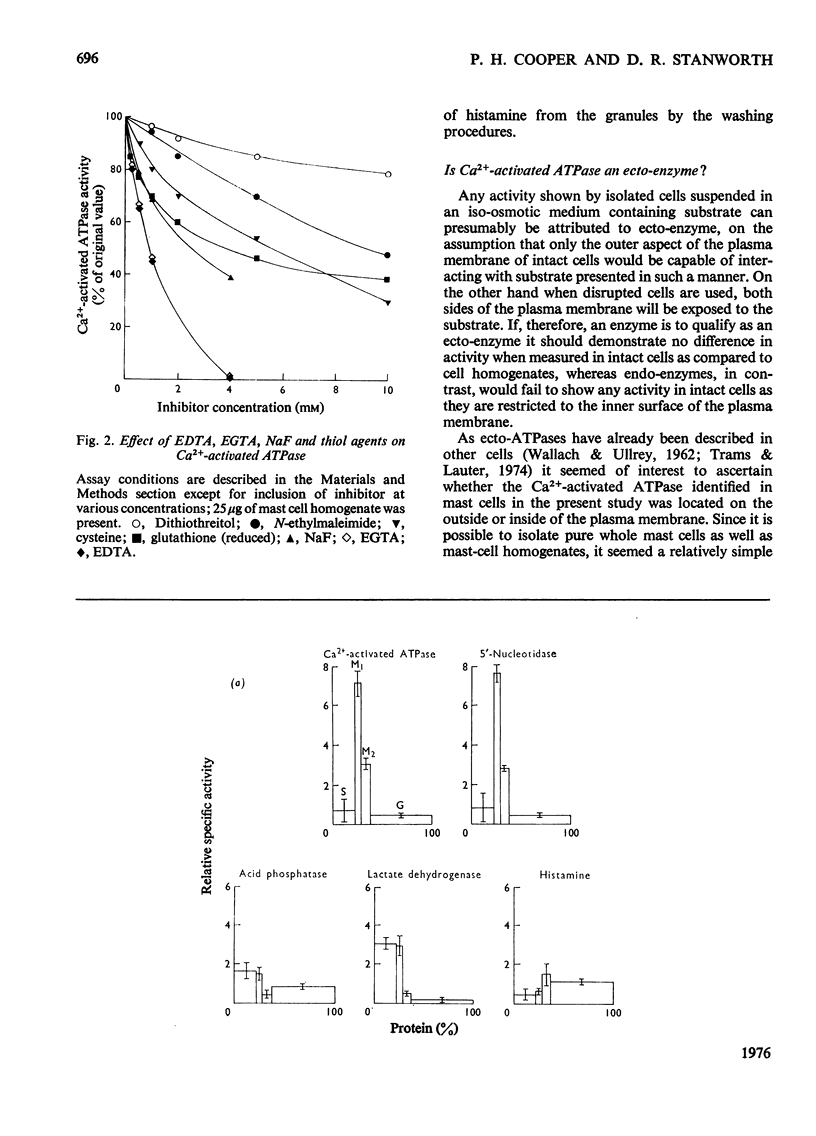
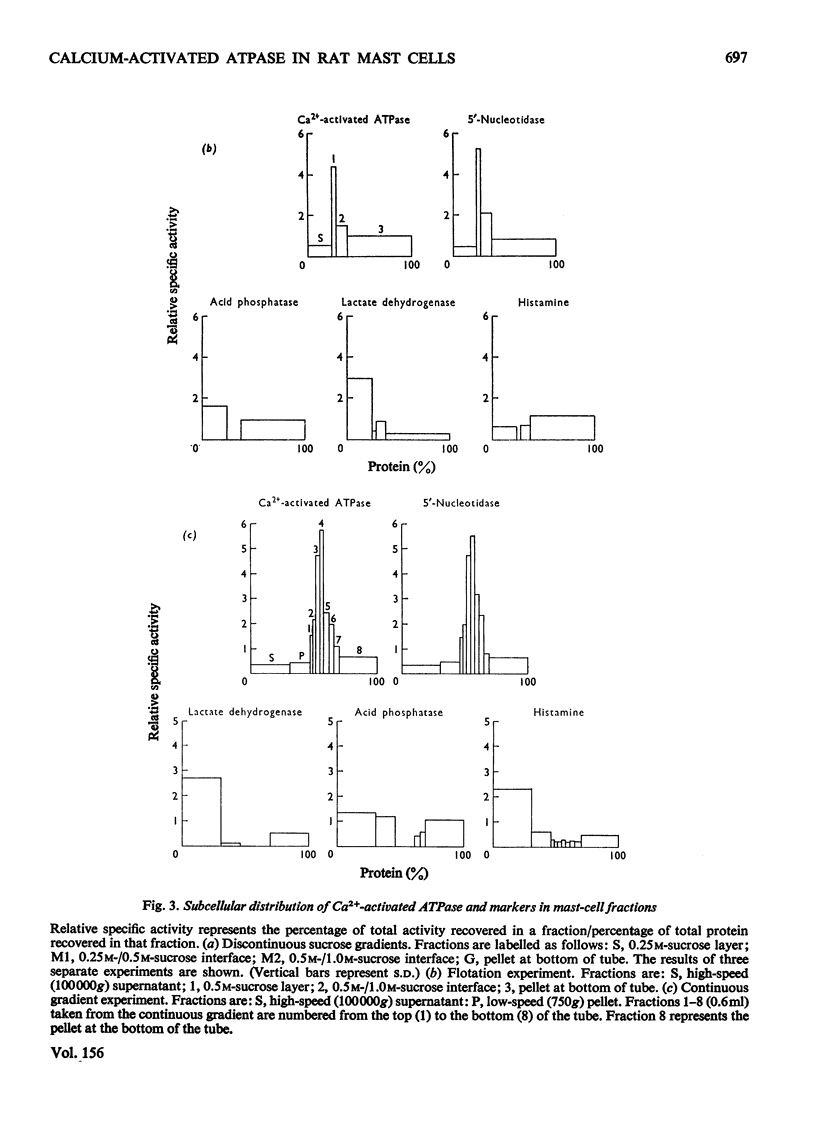
The properties of a Ca2+ activated adenosine triphosphatase shown to be present in homogenates of purified rat peritoneal mast cells were investigated. The enzyme was activated by Ca2+, Mg2+, and to a lesser extent by Mn2+ and Co2+. Ca2+ alone was necessary for full activity and the further addition of Mg2+ did not have any effect. The chelating agents EGTA (ethanedioxybis(ethylamine)tetra-acetate) and EDTA completely inhibited the reaction. The pH optimum was 7.8. Reduced glutathione, cysteine, dithiothreitol, N-ethylmaleimide, urea, ADP, NaF, increasing ionic strength and Triton X-100 all inhibited the reaction. On subcellular fractionation of mast-cell homogenates by density-gradient centrifugation, the distribution of Ca2+ activated adenosine triphosphatase resembled that of 5'-nucleotidase, but differed from that of the other markers used, suggesting localization in the plasma membrane. Further experiments indicated that the enzyme is present on the external surface of the plasma membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agren G., Pontén J., Ronquist G., Westermark B. Formation of extracellular adenosine triphosphate by normal and neoplastic human cells in culture. J Cell Physiol. 1971 Jun;77(3):331–336. doi: 10.1002/jcp.1040770307. [DOI] [PubMed] [Google Scholar]
- Bach M. K. A molecular theory to explain the mechanisms of allergic histamine release. J Theor Biol. 1974 May;45(1):131–151. doi: 10.1016/0022-5193(74)90047-2. [DOI] [PubMed] [Google Scholar]
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- Coleman R. Membrane-bound enzymes and membrane ultrastructure. Biochim Biophys Acta. 1973 Apr 3;300(1):1–30. doi: 10.1016/0304-4157(73)90010-5. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Hawthorne J. N. Phosphomonoesterase hydrolysis of polyphosphoinositides in rat kidney: Properties and subcellular localization of the enzyme system. Biochem J. 1975 Sep;150(3):537–551. doi: 10.1042/bj1500537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. H., Stanworth D. R. A simple and reproducible method of isolating rat peritoneal mast cells in high yield and purity. Prep Biochem. 1974;4(2):105–114. doi: 10.1080/00327487408068766. [DOI] [PubMed] [Google Scholar]
- Diamant B., Krüger P. G. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5'-triphosphate. Acta Physiol Scand. 1967 Dec;71(4):291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- EDMAN K. A., MONGAR J. L., SCHILD H. O. THE ROLE OF SH AND S-S GROUPS AND OXYGEN IN THE ANAPHYLACTIC REACTION OF CHOPPED GUINEA-PIG LUNG. J Physiol. 1964 Jan;170:124–137. doi: 10.1113/jphysiol.1964.sp007318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford B. C. Independent routes for Na transport across dog red cell membranes. Nature. 1975 Aug 14;256(5518):580–582. doi: 10.1038/256580a0. [DOI] [PubMed] [Google Scholar]
- Evans D. P., Lewis J. A., Thomson D. S. An automated fluorimetric assay for the rapid determination of histamine in biological fluids. Life Sci II. 1973 Apr 8;12(7):327–336. doi: 10.1016/0024-3205(73)90366-4. [DOI] [PubMed] [Google Scholar]
- Fenselau A., Long C. On the extracellular synthesis of adenosine triphosphate by mammalian cells. FEBS Lett. 1974 Sep 15;46(1):251–254. doi: 10.1016/0014-5793(74)80380-7. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFISCHER S., ESSNER E., NOVIKOFF A. B. THE LOCALIZATION OF PHOSPHATASE ACTIVITIES AT THE LEVEL OF ULTRASTRUCTURE. J Histochem Cytochem. 1964 Feb;12:72–95. doi: 10.1177/12.2.72. [DOI] [PubMed] [Google Scholar]
- Goth A. Effect of drugs on mast cells. Adv Pharmacol. 1967;5:47–78. doi: 10.1016/s1054-3589(08)60654-7. [DOI] [PubMed] [Google Scholar]
- Green J. P. Uptake, storage and release of histamine. Uptake and binding of histamine. Fed Proc. 1967 Jan-Feb;26(1):211–218. [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Harris G. L., Cove D. H., Crawford N. Effect of divalent cations and chelating agents on the ATPase activity of platelet contractile protein, "thrombosthenin". Biochem Med. 1974 Sep;11(1):10–25. doi: 10.1016/0006-2944(74)90090-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagunoff D. Membrane fusion during mast cell secretion. J Cell Biol. 1973 Apr;57(1):252–259. doi: 10.1083/jcb.57.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Perry D. W., Mustard J. F. Interactions of nucleoside di- and triphosphates with rabbit platelets. Am J Physiol. 1974 Nov;227(5):1143–1148. doi: 10.1152/ajplegacy.1974.227.5.1143. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion. Biochim Biophys Acta. 1973 Dec 28;300(4):421–465. doi: 10.1016/0304-4157(73)90015-4. [DOI] [PubMed] [Google Scholar]
- Randolph M. L., Ryan R. R. A Slicer for Sampling Liquids. Science. 1950 Nov 3;112(2914):528–528. doi: 10.1126/science.112.2914.528. [DOI] [PubMed] [Google Scholar]
- Roodyn D. B. The classification and partial tabulation of enzyme studies on subcellular fractions isolated by differential centrifuging. Int Rev Cytol. 1965;18:99–190. doi: 10.1016/s0074-7696(08)60553-7. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhlich P. Membrane-associated actin filaments in the cortical cytoplasm of the rat mast cell. Exp Cell Res. 1975 Jul;93(2):293–298. doi: 10.1016/0014-4827(75)90453-x. [DOI] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., ULLREY D. The hydrolysis of ATP and related nucleotides by Ehrlich ascites carcinoma cells. Cancer Res. 1962 Feb;22:228–234. [PubMed] [Google Scholar]


