Abstract
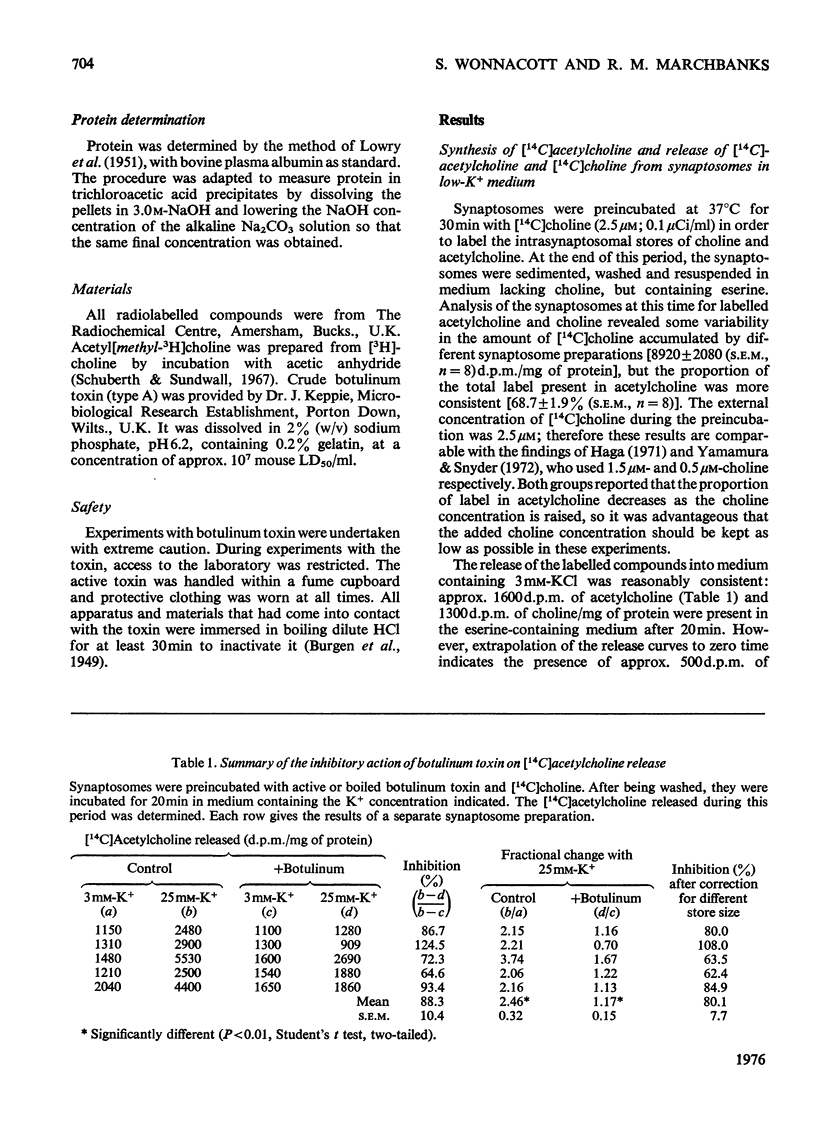
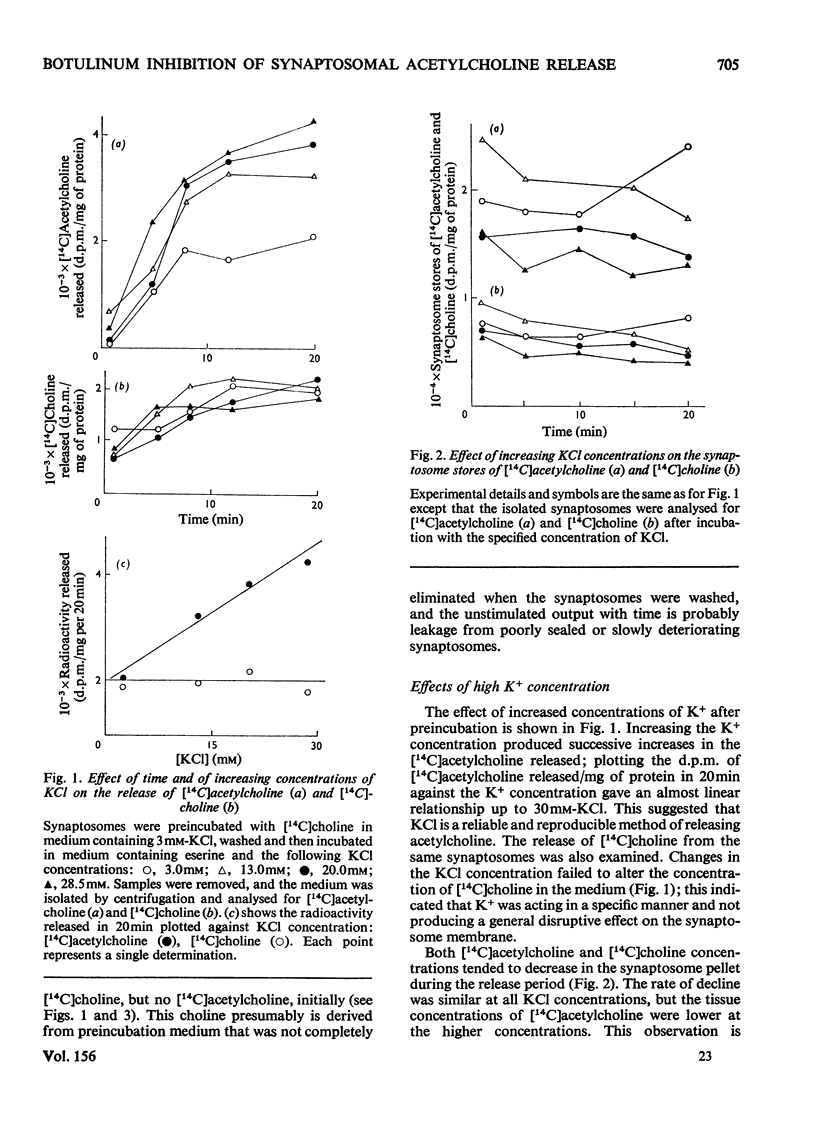
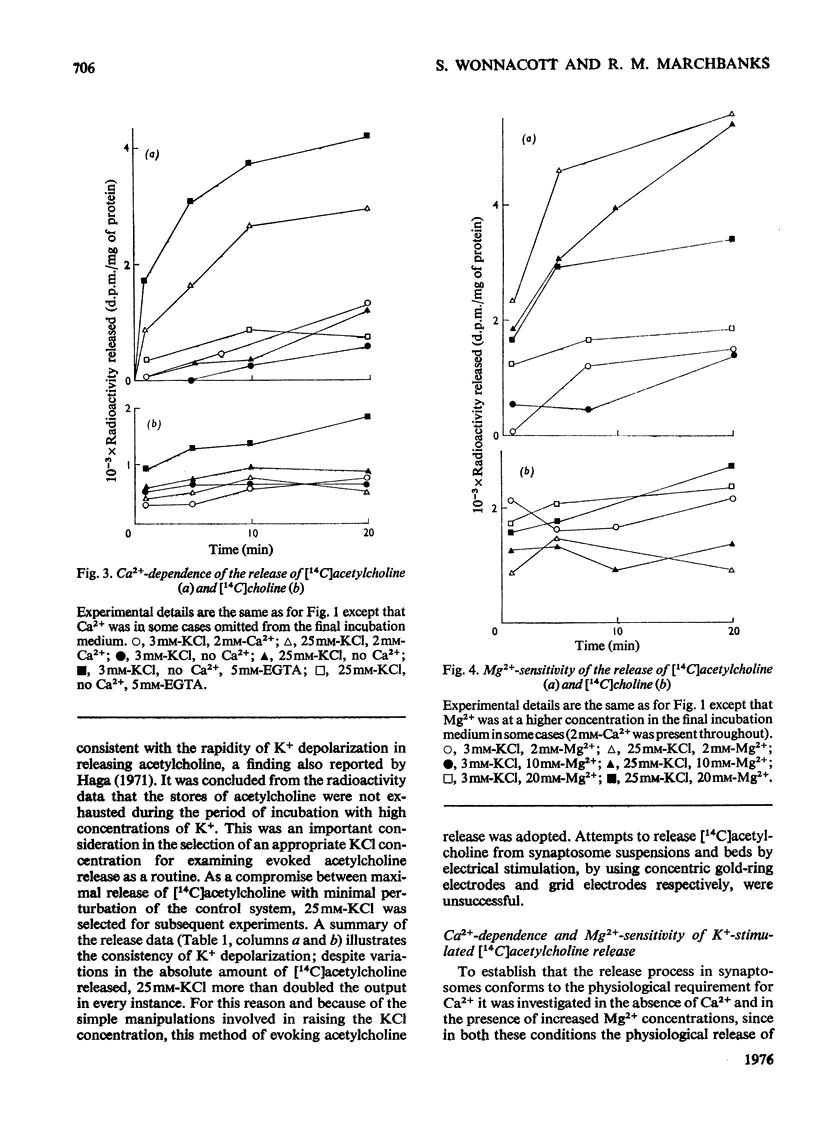
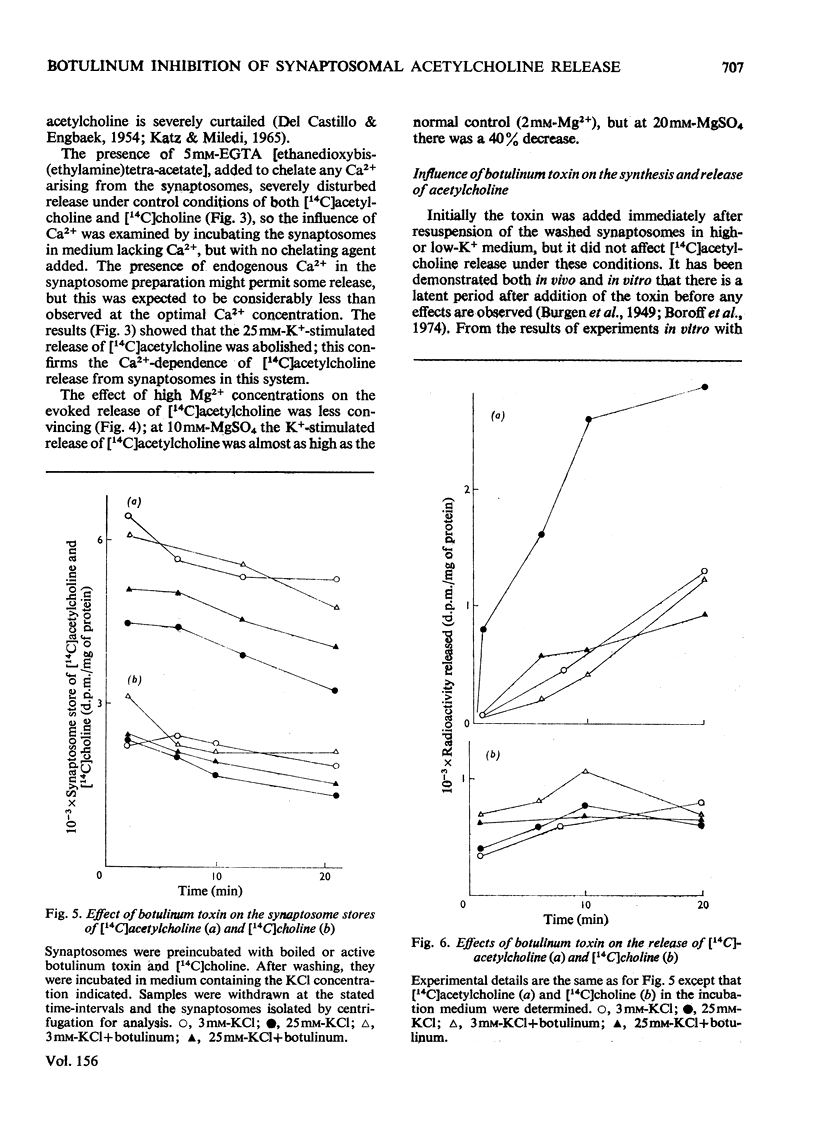
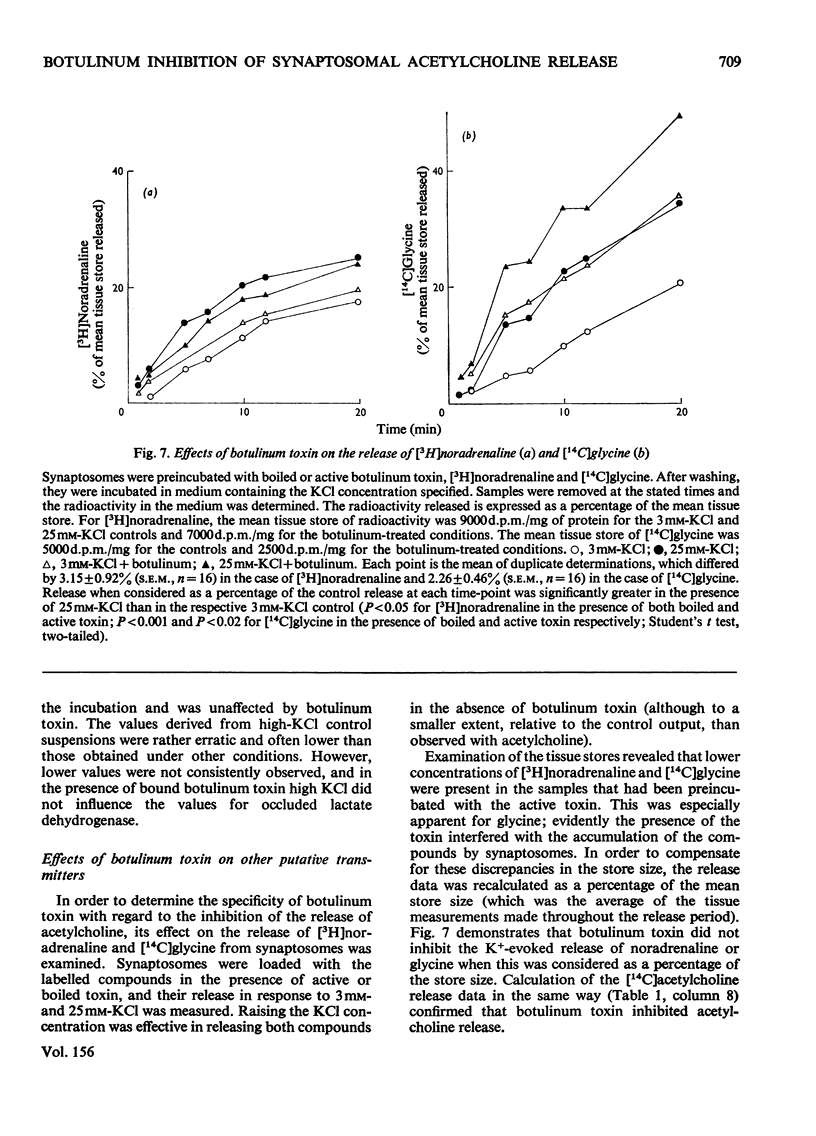
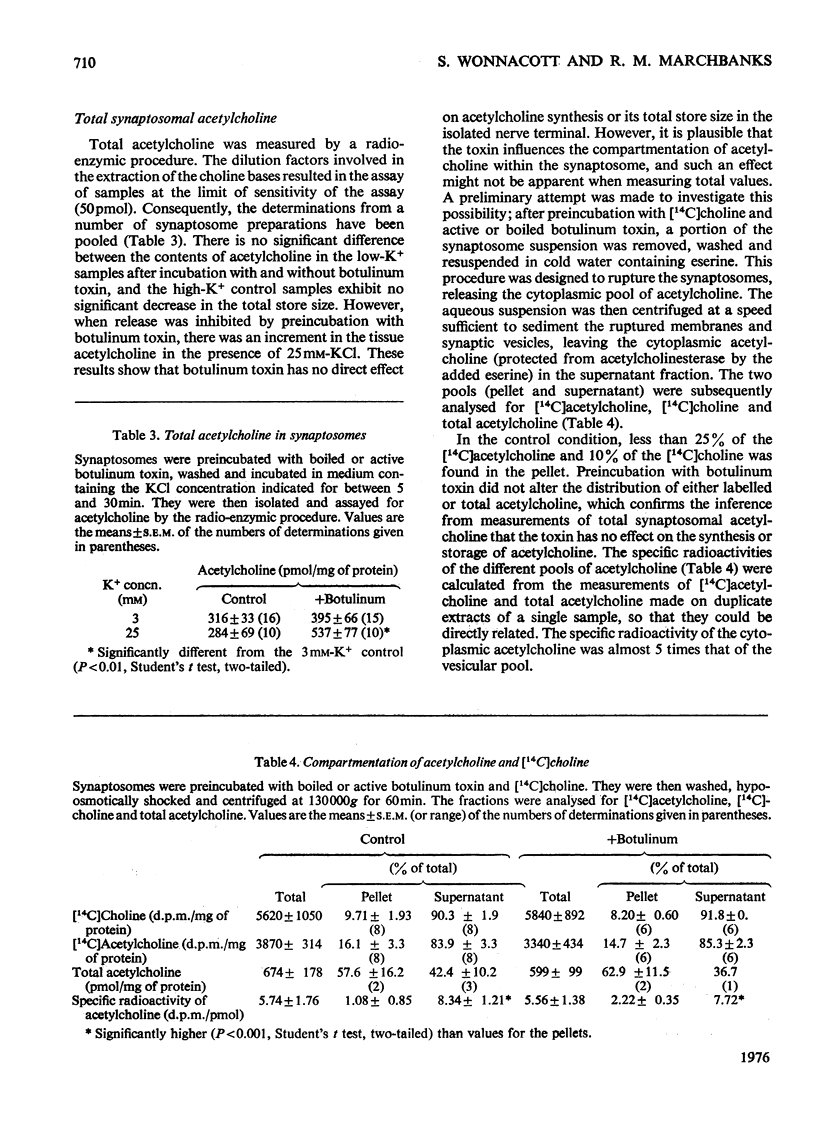
1. Cerebral-cortex synaptosomes were shown to synthesize (14C)acetylcholine after incubation with (14C)choline, and 25mM-KCl released (14C)acetylcholine (but not (14C)choline) into the medium by a Ca2+-dependent and Mg2+-sensitive process. 2. The K+-stimulated release of (14C)acetylcholine was inhibited by more than 80% after preincubation of the synaptosomes with 10(5) mouse lethal doses of botulinum toxin/ml. (14C)choline uptake, (14C)acetylcholine synthesis, intrasynaptosomal K+ and occluded lactate dehydrogenase were unaffected by the toxin. It also failed to prevent the K+-stimulated release of (3H)noradrenaline and (14C)glycine from synaptosomes. 3. Fractionation of hypo-osmotically shocked synaptosomes revealed that more than 75% of the radioactive acetylcholine was in the cytoplasmic compartment, although the vesicle pellet contained more total acetylcholine than the cytoplasmic pool. Consequently the specific radioactivity of acetylcholine in the cytoplasmic pool was almost 5 times that of the vesicles. This distribution was unaffected by preincubation with botulinum toxin. It is concluded that the toxin acts directly on the release of acetylcholine, rather than influencing its storage. 4. After K+-stimulation, toxin-inhibited synaptosomes contained increased amounts of total acetylcholine, which suggests that its rate of synthesis is controlled by depolarization rather than release.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. A further survey of the action of Clostridium botulinum toxin upon different types of autonomic nerve fibre. J Physiol. 1951 Mar;113(1):1–17. doi: 10.1113/jphysiol.1951.sp004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Effects of potassium, veratridine, and scorpion venom on calcium accumulation and transmitter release by nerve terminals in vitro. J Physiol. 1975 Jun;247(3):617–655. doi: 10.1113/jphysiol.1975.sp010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Johnson E. M., Jr, Needleman P. Calcium-dependent norepinephrine release from presynaptic nerve endings in vitro. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2237–2240. doi: 10.1073/pnas.69.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P. Preganglionic stimulation increases calcium uptake by sympathetic ganglia. Science. 1971 Apr 23;172(3981):391–393. doi: 10.1126/science.172.3981.391. [DOI] [PubMed] [Google Scholar]
- Boroff D. A., del Castillo J., Evoy W. H., Steinhardt R. A. Observations on the action of type A botulinum toxin on frog neuromuscular junctions. J Physiol. 1974 Jul;240(2):227–253. doi: 10.1113/jphysiol.1974.sp010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Belleroche J. S., Bradford H. F. The stimulus-induced release of acetylcholine from synaptosome beds and its calcium dependence. J Neurochem. 1972 Jul;19(7):1817–1819. doi: 10.1111/j.1471-4159.1972.tb06229.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Isolation of choline esters from aqueous solutions by extraction with sodium tetraphenylboron in organic solvents. Biochem J. 1969 Jun;113(2):291–298. doi: 10.1042/bj1130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Haga T. Synthesis and release of ( 14 C)acetylcholine in synaptosomes. J Neurochem. 1971 Jun;18(6):781–798. doi: 10.1111/j.1471-4159.1971.tb12008.x. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. K. The intracellular distribution of glycolytic and other enzymes in rat-brain homogenates and mitochondrial preparations. Biochem J. 1960 Dec;77:610–618. doi: 10.1042/bj0770610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON M. K., WHITTAKER V. P. LACTATE DEHYDROGENASE AS A CYTOPLASMIC MARKER IN BRAIN. Biochem J. 1963 Sep;88:404–409. doi: 10.1042/bj0880404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchbanks R. M., Israël M. Aspects of acetylcholine metabolism in the electric organ of Torpedo marmorata. J Neurochem. 1971 Mar;18(3):439–448. doi: 10.1111/j.1471-4159.1971.tb11971.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The conversion of 14C-choline to 14C-acetylcholine in synaptosomes in vitro. Biochem Pharmacol. 1969 Jul;18(7):1763–1766. doi: 10.1016/0006-2952(69)90165-8. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M. The uptake of [14C] choline into synaptosomes in vitro. Biochem J. 1968 Dec;110(3):533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Stimulation by atropine of acetylcholine release and synthesis in cortical slices from rat brain. Br J Pharmacol. 1970 Nov;40(3):406–417. doi: 10.1111/j.1476-5381.1970.tb10622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson I. W., Szerb J. C. The release of labelled acetylcholine and choline from cerebral cortical slices stimulated electrically. Br J Pharmacol. 1974 Dec;52(4):499–507. doi: 10.1111/j.1476-5381.1974.tb09717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. A., Marchbanks R. M. Isolation of ( 3 H) acetylcholine pools by subcellular fractionation of cerebral cortex slices incubated with ( 3 H) choline. J Neurochem. 1971 May;18(5):705–712. doi: 10.1111/j.1471-4159.1971.tb12000.x. [DOI] [PubMed] [Google Scholar]
- Richter J. A., Marchbanks R. M. Synthesis of radioactive acetylcholine from ( 3 H)choline and its release from cerebral cortex slices in vitro. J Neurochem. 1971 May;18(5):691–703. doi: 10.1111/j.1471-4159.1971.tb11999.x. [DOI] [PubMed] [Google Scholar]
- Simpson L. L. Ionic requirements for the neuromuscular blocking action of botulinum toxin: implications with regard to synaptic transmission. Neuropharmacology. 1971 Nov;10(6):673–684. doi: 10.1016/0028-3908(71)90082-7. [DOI] [PubMed] [Google Scholar]
- Vincenzi F. F. Effect of botulinum toxin on autonomic nerves in a dually innervated tissue. Nature. 1967 Jan 28;213(5074):394–395. doi: 10.1038/213394a0. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S., Marchbanks R. M. A radioenzymic assay for acetylcholine and the problems encountered during its development and application. Biochem Soc Trans. 1975;3(1):102–106. doi: 10.1042/bst0030102. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Choline: high-affinity uptake by rat brain synaptosomes. Science. 1972 Nov 10;178(4061):626–628. doi: 10.1126/science.178.4061.626. [DOI] [PubMed] [Google Scholar]


