Graphical Abstract
Graphical Abstract.
An overview of elements comprising current digital twins in cardiovascular medicine (left panel), elements needed to build future digital twins in cardiovascular medicine (right panel), and key elements for implementation of the digital twin in healthcare (center). AI, artificial intelligence; CABG, coronary artery bypass graft; EHR, electronic health record.
Keywords: Digital twins, Generative artificial intelligence, Multi-modal models, Precision medicine
Abstract
Digital twins, which are in silico replications of an individual and its environment, have advanced clinical decision-making and prognostication in cardiovascular medicine. The technology enables personalized simulations of clinical scenarios, prediction of disease risk, and strategies for clinical trial augmentation. Current applications of cardiovascular digital twins have integrated multi-modal data into mechanistic and statistical models to build physiologically accurate cardiac replicas to enhance disease phenotyping, enrich diagnostic workflows, and optimize procedural planning. Digital twin technology is rapidly evolving in the setting of newly available data modalities and advances in generative artificial intelligence, enabling dynamic and comprehensive simulations unique to an individual. These twins fuse physiologic, environmental, and healthcare data into machine learning and generative models to build real-time patient predictions that can model interactions with the clinical environment to accelerate personalized patient care. This review summarizes digital twins in cardiovascular medicine and their potential future applications by incorporating new personalized data modalities. It examines the technical advances in deep learning and generative artificial intelligence that broaden the scope and predictive power of digital twins. Finally, it highlights the individual and societal challenges as well as ethical considerations that are essential to realizing the future vision of incorporating cardiology digital twins into personalized cardiovascular care.
Introduction
Digital twins in the era of generative artificial intelligence (AI) can enable true precision cardiovascular care.1,2 In Figure 1, we present a clinical scenario that envisions digital twin-enabled precision care. This contrasts with current practice, where most care decisions are driven by clinicians trialling different therapies without information on the expected therapeutic response for individuals and requiring frequent in-person evaluations with static instruments that are inconvenient for both the patient and clinician.3,4 Digital twins are dynamic virtual patient representations that can personalize care by integrating multi-modal data from the patient and simulating clinical scenarios and treatment strategies to forecast outcomes, health status changes, and solutions. These data include dynamic features tracked by environmental sensors, wearable fitness trackers, and at-home health monitors, along with immutable and historical health data such as genomics and medical records.5 The integration of these data into deep learning models developed in broad and diverse patient populations enables the comparison of similar care scenarios to define accurate and personalized predictions, which are updated in real-time with feedback. There is unrealized potential for digital twin technology in cardiovascular care, which has been limited to disease phenotyping and broad treatment strategy definitions, with a future role that provides a holistic and dynamic record and guide for a patient’s lifetime.
Figure 1.
A clinical vignette. A step-by-step clinical vignette modelling the future of healthcare with digital twins. HCTZ, hydrochlorothiazide; SGLT2, sodium–glucose cotransporter 2
What are digital twins?
Digital twins are in silico replicates of a subject, utilizing multi-modal real-time data to build models optimizing decision-making and forecasting an intervention’s influence on outcomes.6,7 These replicates build accurate simulations while simultaneously receiving feedback through a continuous connection with the subject, known as the cyber-physical space.6 The concept originated in the aerospace industry and has established utility in production workflows in industrial manufacturing and traffic management in smart cities, among other applications.2,6 The data representing the inputs for these digital twins vary in granularity, and the outputs are connected with the digital twin through wireless communication, enabling real-time feedback and adjustments.6 These digital twins are generally powered by mechanistic models that use established scientific principles in mechanical engineering, fluid dynamics, and electromagnetism more than data-driven factors.
Although the digital twin is the established state-of-the-art simulation architecture for engineering, it is still a developing field in healthcare. There are varying definitions of a healthcare digital twin, with some restricted to ‘passive’ data collection, wherein future health states are predicted based on models that leverage large scans of retrospective data. In contrast, ‘active’ feedback from the patient utilizes continuous, real-time data streams that reactively update predictions.8 The latter has been challenging because patient data are variably acquired, mechanistic models of disease pathophysiology are incomplete, and factors outside biological constraints, such as human behaviour that are not captured as inputs, influence the success of interventions. This contrasts domains such as aviation, which have set design and safety principles.9 For example, outcomes of cardiovascular interventions established through randomized clinical trials (RCTs) have not always had a clear biological explanation, such as the mortality benefit from sodium–glucose cotransporter 2 (SGLT2) inhibitors or the higher mortality with intensive blood glucose-lowering or with prescribing anti-arrhythmic medications after myocardial infarction.10–12 New advances in data acquisition, integration of real-time and static patient data in deep learning models, and evidence generation and translation through generative AI provide opportunities to broaden the scope of cardiovascular digital twins (Figure 2).
Figure 2.
Potential cardiovascular clinical applications of digital twins. An overview of data available and potential digital twin applications. ECG, electrocardiogram; EHR, electronic health record, ‘Omics: includes genomics, proteomics, and metabolomics data
Digital twins fill several important gaps. First, simulating several procedural approaches in one digital twin allows a safe and optimized procedure on the actual patient. It also allows the examination of interacting elements, such as an individual’s environment and genome, which might influence their outcome. In addition, it is infeasible to run RCTs necessary to test all permutations of an intervention, but digital twins can model these scenarios across broad populations with specific controls to address confounding. The eventual application is a pipeline that automates the analysis of multi-modal data and integrates them into clinical decision-making.13 Finally, the feedback from the success or failure of an intervention will improve the model performance compared with clinicians, whose diagnostic heuristics may affect decision-making.
In this review, we summarize the current role of digital twins in cardiovascular care and introduce advances in data acquisition and AI that further enable their capacity, discuss challenges in their implementation, and forecast future directions to achieve their immense potential (Graphical Abstract).
Digital twins in cardiology
Advancing precision medicine through digital twins spans deep phenotyping of complex cardiac disorders and individualizing treatment, such as coronary and structural interventions as well as electrophysiological procedures (Table 1).14–46 Below, we summarize these innovations, while also discussing the uses of digital twins combined with generative AI for generating synthetic data to improve the understanding of pathophysiology and conducting in silico clinical trials.
Table 1.
Summary of electrophysiological and electromechanical models
| Year | Study application | Data | Model type | Validation size | Simulation use | Authors |
|---|---|---|---|---|---|---|
| 2013 | HF | CMR/ECG | EM | Individual (n = 11) | Regional contractility LV | Marchesseau et al.14 |
| 2016 | CABG planning | CTA | CFD | Individual (n = 5) | Coronary HD | Ramachandra et al.15 |
| 2016 | Post-MI arrhythmia | CMR | EPM | Individual (n = 41) | SCD risk | Arevalo et al.16 |
| 2016 | Healthy | CMR | EPM | Individual (n = 3) | AP, MD, and TS of HB | Augustin et al.17 |
| 2017 | CABG planning | Ang./CT | CFD | Individual (n = 10) | Coronary HD | Ballarin et al.18 |
| 2017 | Healthy | Simulated data | EPM | Simulated data | AP, HD, MD, and TS of HB | Kosta et al.19 |
| 2018 | Post-MI arrhythmia | CT | EP | Individual (n = 7) | VT ablation planning | Cedilnik et al.20 |
| 2019 | PCI planning | Ang. | CFD | Individual (n = 54) | Post-PCI FFR | Gosling et al.21 |
| 2019 | PCI planning | CCTA/Ang. | CFD | Individual (n = 24) | Post-PCI FFR | Modi et al.22 |
| 2019 | AF | CMR | EPM | Individual (n = 10) | AF ablation planning | Boyle et al.23 |
| 2020 | CABG planning | CCTA | CFD | Cohort (n = 12) | Graft patency | Zhu et al.24 |
| 2020 | TAVR planning | CT | EM/FEM | Cohort (n = 9) | PVL | Dowling et al.25 |
| 2020 | TAVR planning | CT | EM | Cohort (n = 80) | PVL | El Faquir et al.26 |
| 2020 | Healthy | CMR | GP/FEM | Individual (n = 1) | AP of Atrium | Hu et al.27 |
| 2021 | Healthy | MRI/ECG | EP | Individual (n = 1) | 12-lead ECG | Gillette et al.28 |
| 2021 | Healthy/scar | CMR | EPM | Individual (n = 1) | Ablation scar modelling | Gerach et al.29 |
| 2022 | PCI | OCT | FEM | Individual (n = 2) | LA | Poletti et al.30 |
| 2022 | Healthy | CMR | EPM | Individual (n = 1) | PV, TS, strain | Stimm et al.31 |
| 2022 | Healthy | ECG/CMR | VAE | Cohort (n = 30) | Ventricular activation | Li et al.32 |
| 2022 | Aortic coarctation | CMR | EM | Individual (n = 7) | AP, MD, TS of HB | Jung et al.33 |
| 2023 | Healthy | N/A | FEM/EM | N/A | AP, MD, TS of HB | Fedele et al.34 |
| 2023 | Healthy | CMR | Hybrid GAN/U-net | Individual (n = 18) | Synthetic CMR | Xing et al.35 |
| 2023 | Healthy | CT | VAE/diffusion | Cohort (n = 512) | Volume | Kadry et al.36 |
| 2023 | Post-MI VT | CMR | EP | Individual (n = 21) | VT risk | Serra et al.37 |
| 2023 | HF with AF undergoing CRT | ECG-gated CT, IPM | EM | Individual (n = 1) | AP, HD, MD, TS of HB | Strocchi et al.38 |
| 2023 | Healthy | CMR/CT | SSM | Individual (n = 1) | Cardiac anatomy | Azzolin et al.39 |
| 2023 | Healthy | OFI | Hybrid CNN/PINN | Simulated/ex vivo | AP | Kashtanova et al.40 |
| 2024 | Healthy | Simulated data | PINN | Simulated data | AP | Jiang et al.41 |
| 2024 | MI scar from healthy | ECG/CMR | EP/VAE | Individual (n = 1) | Simulate MI scar | Li et al.42 |
| 2024 | Healthy | ECG/CMR | EP | Individual (n = 1) | Dofetilide dosing | Camps et al.43 |
| 2024 | LVAD/MS | ECG/US | PINN | Cohort (n = 200) | PV loop | Kuang et al.44 |
| 2024 | HCM | ECG/CMR | FEM/EP/CNN | Individual (n = 6) | Purkinje network | Camps et al.45 |
| 2024 | AF | CMR | EA | Individual (n = 26) | PV isolation ablation | Sakata et al.46 |
The first column describes the year of publication, the second column describes the study application, the third column describes the data used, the fourth column describes the type of model used, the fifth column describes the size and type of validation of the model, the sixth column describes the digital twin simulation use, and the final column is the publication reference.
AF, atrial fibrillation; Ang., angiography; AP, action potential; CCTA, coronary computed tomography angiography; CFD, computational fluid dynamics; CMR, cardiac magnetic resonance; CNN, convolutional neural networks; CT, computed tomography; EA, electroanatomical; ECG, electrocardiogram; EM, electromechanical; EP, electrophysiological; EPM, electrophysiological and electromechanical; FEM, finite element method; FFR, fractional flow reserve; GP, Gaussian process; HB, heartbeat; HD, haemodynamics; HF, heart failure; IPM, invasive pressure measurements; LA, lumen area; LV, left ventricular; LVAD, left ventricular assist device; MD, mechanical deformation; MI, myocardial infarction; MS, mitral stenosis; OCT, optical coherence tomography; OFI, optical fluorescence imaging; PINN, physics-informed neural network; PV, pressure volume; PVL, paravalvular leak; SCD, sudden cardiac death; TS, tissue structure; US, ultrasound; VAE, variational autoencoder; VT, ventricular tachycardia.
Digital twins for precision medicine in cardiology
Digital twins in cardiomyopathies
Digital twins enable granular phenotyping for heterogeneous disorders such as cardiomyopathies characterized by complex myocardial, mechanical, and electrical remodelling patterns.47 These distinct signatures have been detected on a myriad of data streams48,49 and increasingly have implications for the diagnosis, prognostication, and management of these disorders.49–52 They may also underlie some of the treatment effect heterogeneity described for various commonly used therapeutic approaches, such as those in the setting of cardiac resynchronization therapy (CRT),53 implantable cardioverter defibrillators,54 and disease-modifying therapeutics.55–57 The multimodal assessment of these patients, which generally encompasses 12-lead electrocardiography (ECG) along with echocardiography and cardiac magnetic resonance (CMR), further enables a granular comparative assessment of different patients and therapies.47,58
In recent years, the integration of geometrical and topological features from cardiac imaging and ECG has enabled personalized, in silico simulation of electromechanical coupling and its haemodynamic implications from abnormal conduction rhythms such as left bundle branch block (LBBB).59,60 Digital twin models have also been used to enhance disease-specific predictive models, such as stratifying the risk of ventricular arrhythmias in ischaemic cardiomyopathy by incorporating dynamic aspects of myocardial physiology and evolving anatomical substrates, triggers, and modulators.61 In addition, in a small-scale retrospective analysis of 45 patients with cardiac sarcoidosis, personalized fusion models of CMR and positron emission tomography improved the prediction of future arrhythmias.62
Digital twins may also guide more precise phenotyping of rare forms of cardiomyopathy. In an analysis of 313 patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), echocardiography-based digital twin models linked deformation abnormalities to the underlying myocardial disease substrate, enabling deeper insights into the mechanical underpinnings of progression and eventual prediction of ventricular arrhythmia events.63 In a separate study of patients with ARVC, a genotype-specific digital twin approach (geno-digital twin) combined genotype-derived cellular electrophysiological simulations with CMR to accurately predict the location and mechanism of ventricular tachycardia circuits.64 This paradigm has recently been extended to congenital heart diseases, where information from computed tomography (CT) scans and ECGs are integrated into a computational approach defining the electrophysiological activation and repolarization patterns in a patient with hypoplastic left heart syndrome.65
Coronary and structural heart simulations
Digital twins simulate procedural decision-making on patient physiology, ensuring a higher likelihood of success without invasive experimentation (Figure 3). For example, coronary CT angiography with fractional flow reserve (CT-FFR) is an imaging study that builds 3D reconstructions of atherosclerotic coronary vessels, replacing the need for invasive measurements when planning for percutaneous coronary interventions. Digital twins constructed from CT-FFR have combined reconstruction with haemodynamic modelling to accurately predict the degree of coronary artery stenosis and enable virtual coronary intervention simulations.21,22
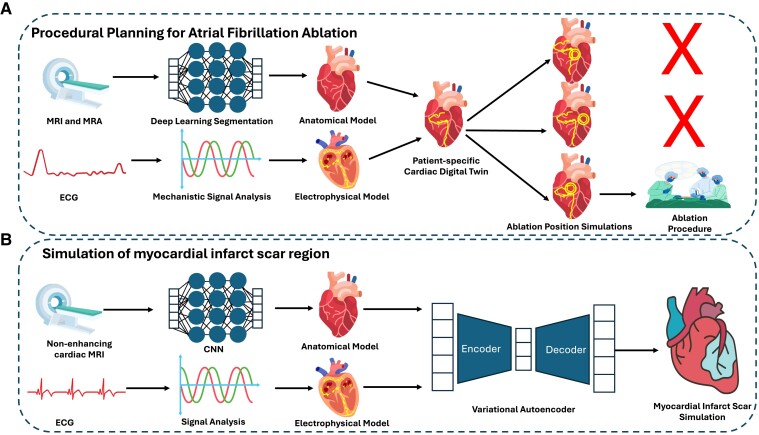
Figure 3.
Selected precision medicine applications of digital twins. (A) An example digital twins for procedural planning of atrial fibrillation ablation. 3D anatomical and electrophysiological models are derived from magnetic resonance imaging, magnetic resonance angiography imaging, and electrocardiograms to build a patient-specific digital twin. Different ablation locations are simulated, and the one most likely to stop atrial fibrillation is chosen. (B) An example simulation of a myocardial infarct scar. To substitute late-gadolinium enhancement magnetic resonance images, non-contrast magnetic resonance images and electrocardiograms can be combined to make an anatomical and electrophysiological cardiac digital twin. The digital twin can then be input into a variational autoencoder, which can generate simulations of 3D scarring, e.g. from myocardial infarction. ECG, electrocardiogram; CNN, convolutional neural network; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging
In addition, applying computational fluid dynamics and wall shear calculations to these CT-FFR digital twins can optimize the selection of bypass grafts with higher accuracy in predicting 1-year patency than selection without assistance from this technology.15,18,24 A pilot study of 114 patients, FASTTRACK CABG, is testing the use of CT-FFR digital twins for surgical planning for coronary artery bypass graft.66 In preventive cardiovascular care, the CREDENCE study of 303 patients combined CT-FFR with neural network models to predict atherosclerosis progression, with an accuracy of 84% for ≥50% stenosis prediction and a correlation of .73 with quantitative coronary angiography.67
Outside of coronary procedures, digital twin models for structural heart interventions, such as valve replacement, can align anatomical structures to prosthetic devices. A pair of proof-of-concept studies with 9 and 80 patients, respectively, undergoing transcatheter aortic valve replacement (TAVR) showed that CT-derived computer simulations could improve TAVR depth implantation estimates to reduce post-procedural paravalvular regurgitation.25,26
Electrophysiology and electromechanical models
Digital twins can also combine cardiac electrophysiology with cardiac electromechanics to advance procedural planning to treat cardiac arrhythmias. For example, simulating the physiological effects of CRT in the presence of LBBB60 can guide appropriate lead placement. In an analysis of 45 patients with heart failure referred for CRT, virtual pacing simulations using echocardiography-based digital twins predicted subsequent ventricular remodelling based on a reduction in the in silico septal-to-lateral work imbalance.68 Similar concepts are now increasingly used across all electrophysiological procedures, including the ablation of ventricular tachycardia and atrial fibrillation (AF)46,69,70 and identifying areas for radiofrequency ablation.29,34 Beyond predicting treatment response and prognosis, integrating ventricular anatomical information by CMR and electrophysiological function information based on a 12-lead ECG can simulate human physiology variation across a patient population,43 paving the path towards low-cost in silico clinical trials.
In one example, researchers integrated CMR and ECG recordings across two patients with hypertrophic cardiomyopathy and four controls to simulate individualized regional electromechanical coupling effects.45 In another application, a virtual assessment of anti-arrhythmic drug efficacy was simulated in a single-centre study of 232 individuals at high risk of recurrent AF following catheter ablation. By integrating CT and electroanatomical maps and knowledge of the pharmacological properties of distinct anti-arrhythmic drugs, the treatment that virtually terminated AF the fastest was associated with a lower risk of AF recurrence during follow-up relative to alternative agents.71
Data, models, and applications to advance digital twins in cardiology
As described above, current digital twin approaches in cardiovascular medicine generally use multi-modal data to define a phenotype with specified cardiac physiologic parameters to advance treatment strategy selection and prognostication of outcomes. These twins rarely have any dynamic connection to patients with the responsiveness to changing inputs and enable broader innovations in care. Digital twin models must adapt to external factors affecting the patient to predict treatment effects accurately. Specifically, with the new data and model advances summarized below, digital twins in cardiovascular medicine can advance the granularity of phenotypes, predict outcomes with dynamic adaptation, and build novel in silico clinical trials.
Contemporary digital health data to augment digital twins
An ideal medical digital twin incorporates information from the cellular to physiological level to build a genomic, biological, mechanical, and environmental representation of a patient. These data, along with a continuous connection to the patient, comprise the medical digital twin cyber-physical space.8 Population health data expand digital twins in cardiology from procedural planning to personalized prevention and intervention strategies in real-time. These include data in various historical formats, including tabular data from electronic health records (EHRs), imaging, ECGs, and genomics, and now dynamically available data from wearables and at-home health monitors used by the public. In addition, incorporating longitudinal data models changes a patient’s phenotype from interactions with external factors, such as the environment and medical interventions. This section discusses the data types and patient cohorts available to advance the cardiovascular digital twin.
Historical data that builds a comprehensive but static patient health profile can be found in the EHR, which provides a large, high-dimensional dataset of clinical information essential for building medical digital twins. These include demographics, physiological biomarkers such as vital signs and laboratory values, and administrative codes for medical conditions, procedures, and medications, and also unstructured components such as medical notes.72 The data are in chronological order and documented at varying time points. They also include digital data streams, such as ECGs; imaging, such as CT scans, magnetic resonance images (MRIs), and pathology images; and videos, such as point-of-care ultrasounds, echocardiograms, and cardiac catheterization.
In an attempt to make such multi-modal data part of characterizing individuals, large registries such as the UK Biobank and All of Us, have compiled some of these data by protocolized testing of a large number of individuals,73 therefore, enabling the compilation of EHR data with cellular omics data, whole-body CTs, and MRIs.74,75
None of these data sources are set up for a real-time connection between the patient and the digital twin. Continuous monitoring of vital signs, blood-based biomarkers, and cardiac rhythms is exclusively seen in critically ill patients in the intensive care unit and relies on special equipment and personnel gathering these data.76 Although this exemplifies the ideal data acquisition frequency, it limits the scalability and generalizability of digital twins of real-world patients. Wearable devices such as smartwatches, fitness trackers, continuous glucose monitors, and Bluetooth blood pressure monitors can complete the cyber-physical feedback loop with continuous metrics such as vital signs, arrhythmia screening, activity level, physical location, and environmental factors.77
Methodological basis for cardiovascular digital twins
Mechanistic modelling strategies to build digital twins
Digital twins employ computational models that vary from mechanistic to statistical, depending on data availability and the purpose of the model (Figure 4). Mechanistic models employ physiologic parameters and principles of fluid dynamics, electrophysiology, and electromagnetism to characterize cardiac physiology and to simulate interventions to correct cardiac pathophysiology.
Figure 4.
Methodological strategies for developing digital twins. An overview of mechanistic and statistical models used in cardiac digital twins
Statistical modelling strategies to build digital twins
Statistical models utilize probabilistic methods, including deep learning, to integrate high-dimensional multi-modal data and identify patterns across patient populations with the goal of predicting a wide range of cardiac pathology and processing longitudinal information.8,78 Beyond establishing mechanistic and statistical models for digital twins, hybrid twins utilize physiological parameters while integrating lightweight deep learning models to process multi-modal data.
A growing number of deep learning-based digital twins are emerging to reduce computational complexity for applications without well-defined pathophysiology and for gaps in evidence.35,41,42,79 The architecture of these deep learning models depends on the type of data (Figure 3). Current algorithms project multiple data sources to a shared embedding, a mathematical representation of the similarities and dissimilarities between different data types, such as ECGs, echocardiograms, and tabular data. This construct forms the basis of subsequent tasks and can be used to predict how changes in any of the input features may impact downstream events.80
Digital twins powered by generative AI models can advance the practice and understanding of cardiology through data generation. The most common deep learning method to generate digital twins of health data is a generative adversarial network (GAN), a deep learning model that comprises competing neural networks originally proposed for generating synthetic images.81–86 These models discover complex hidden correlations between features. Specialized recurrent neural network models called transformers are adept at preserving the temporal structure of data and have been applied to EHR-based digital twins.87
Cardiovascular digital twins are set to be further enhanced by large language models (LLMs) that can expand patient representations from unstructured data in medical notes through inferences found from conditions, social determinants of health, or patient-reported medical history. Exemplified by GPT-4 (the model behind the popular ChatGPT), LLMs are adept at processing vast amounts of textual data, including medical records and research papers, to extract valuable insights and aid in diagnosis.88
Once the digital twin has been generated, it enables simulations to guide care. Beyond treatment strategy, digital twins can enhance outcome prediction. The integration of continuous data sources can update predictions in real-time while also giving feedback to develop the model for future simulations. Advances in deep learning have demonstrated accurate screening of cardiac disease normally diagnosed on echocardiograms using electrocardiograms and one-lead ECGs on wearables and detecting coronary artery disease from facial features.89–92 This technology has also been incorporated into stethoscopes for screening at the point of care.93
Digital twins for data generation: synthetic cardiac data
Often, digital twins are created from a select few data types available to gain further insights into the missing data and the cardiac function and pathology. Electrocardiography models, based on computational simulations and patient-specific data, such as MRIs, simulate the behaviour of the heart to produce realistic ECG waveforms.28 By using anatomical information with known formulae based on the laws of electromagnetism, Gillette et al. have demonstrated the creation of synthetic ECG waveforms.28 The direct link between the design principles from the mechanistic modelling of cardiac anatomy and the ECG waveform has many potential implications, such as the ability to generate large datasets of synthetic ECGs, potentially out of distribution, representative of different populations for training models.94
This will be especially valuable for those cardiomyopathies such as hypertrophic cardiomyopathy that have a low prevalence, are underdiagnosed, and lack specific remarkable features on ECGs.95 Future studies will need to elucidate the degree to which such anatomical defects can be replicated in the model. In addition, generative models trained on one data modality can create realistic missing data. For example, CMRs have been generated from ECGs using an autoencoder trained with a small dataset of paired CMRs and ECGs (Figure 3).96 Moreover, when MRI and ECG data are available, information on genomic variants can also be inferred.96 The need for training data can also be reduced by enhancing datasets with generative models; however, whether the generated data has any useful new information is uncertain. Electrocardiography models pre-trained using a transformer model detected left ventricular dysfunction and hypertrophic cardiomyopathy with fewer examples, though their performance has been outperformed by other models trained directly with more examples.90,91,97–99
Digital twins for data generation: clinical trial efficiency and translation
Clinical trial efficiency
Beyond optimizing individual care, digital twins enhance the efficiency of the evidence-generation process itself (Figure 5). Randomized clinical trials represent the backbone of evidence-based practice but are challenged by the time and resources it takes to conduct them. Digital twins can enhance the efficiency of RCTs, such as the augmentation of the control arm based on the relevant patient population in the EHR. By enrolling fewer controls, digital twins could, therefore, reduce recruitment time and cost.
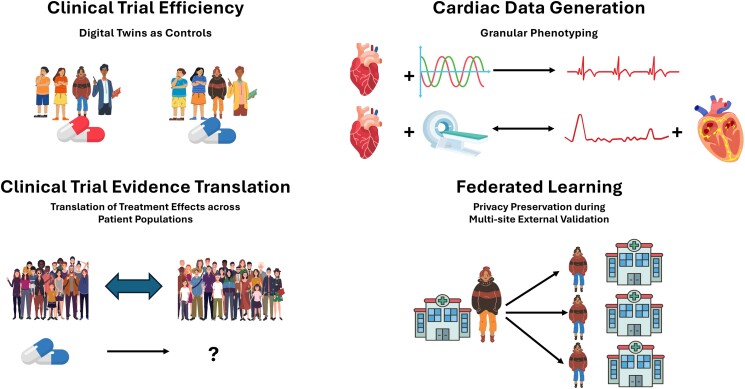
Figure 5.
Applications of digital twins for evidence generation. An overview of digital twin applications for evidence generation across clinical trial populations, for generating cardiac data, for estimating treatment effects of a clinical trial in a second patient population, and for safely testing machine learning models across multiple sites
Proof-of-concept studies of control arm augmentation with digital twins have demonstrated survival curves similar to the original participants, though some demographic and clinical value distributions differ between populations,100,101 suggesting the reliability of inference from these experiments. With observational cohort data, several studies have used GANs for feature and participant balance across the cohorts to improve treatment effect estimates.84,85,102,103 Finally, the entire trial enterprise will likely undergo significant upheaval with several applications of statistical machine learning and genomically enabled trial enrichment poised to transform how evidence is generated.104,105
Clinical trial translation
Digital twins of patient cohorts can also assess the generalizability of treatment effects in RCTs, particularly by projecting these effects in real-world populations (Figure 5). Randomized clinical trials estimate the average treatment effect of an intervention across all included individuals. However, there is often under-enrolment of key populations such as women, minorities, older adults, and patients with multiple comorbidities.106 Moreover, there is demonstrable heterogeneity of treatment effects in many trials.56,57,107 To translate evidence from one patient population to another, digital twins can simulate multiple patient population characteristics by constructing a synthetic cohort that accurately represents their covariate distributions. They utilize GANs, which simulate treatment effects in these synthetic patient populations, to reconcile discrepancies in treatment effects between different established patient populations.108 This has been demonstrated through studying a pair of RCTs with the same intervention and primary outcome, SPRINT and ACCORD, in which a SPRINT-conditioned ACCORD digital twin reproduced the SPRINT average treatment effect and vice versa.79
Challenges and ethical considerations
Challenges
Digital twins of medical data have special challenges and ethical considerations. These span the special privacy controls, fairness of algorithms, trust, reliability, and durability of the model predictions, and the infrastructure and cost required in developing these twins. The next section outlines approaches to these challenges to ensure responsible deployment in healthcare settings.109
Infrastructure and technological challenges
The digital twin infrastructure houses multi-modal data, stores and continually updates patient sensor data, and utilizes high-dimensional processing power to integrate the data and update prediction models. Storage and computing sources are expensive, but in healthcare settings, they also require compliance with patient security laws and provisions and adequate testing against attacks attempting to reverse-engineer source data.110
Acquiring the right data for the digital twins can also be challenging. Electronic health record data are sparse; often, these data are not missing at random, and their absence can be clinically informative. Prospective cohorts or registries such as the UK Biobank have a comprehensive diagnostic repository with data uniformly recorded in ‘well’ humans but are limited by a lack of global representation and low rates of pathology and outcomes.74 In addition, ‘omics’ data are not commonly acquired in hospitals or clinics, limiting their generalizability across digital twins. Fitness trackers rely on consumer uptake and constant use. Representative studies have shown, e.g., that patients who would benefit from wearable technology the most, such as those with cardiovascular disease, often do not use the technology.111,112
Multi-modal data vary across formats, frequency of capture, and storage, requiring a concerted effort to promote and build the infrastructure for standardized data organization and interoperability across healthcare devices and software.113 Strategies to address these challenges include broader compliance with interoperable data formats, such as DICOM,114 and standardized structure for EHR research across institutions through HL7 fast healthcare interoperability resources (FHIR) and the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM).115,116 Technical solutions that leverage processing disparate data with autoencoders or formatting images to resemble other imaging modalities are examples of successful integration of multi-modal data into AI models in cardiology.49,96
Ensuring data privacy
Digital twins in cardiovascular medicine use sensitive health information drawn from the EHR, genomic and other diagnostic testing, and continuously connected wearable devices, requiring special precautions for storage and access to prevent re-identification.117 The models must be evaluated frequently with differential privacy measures for any leakage of the training data.118 Digital twins that use decoder sampling or GAN models preserve covariate distributions and correlations between covariates of the real data without revealing original information.119–121 The model’s output can also be analysed to assess whether the generated data reveals specific information about individual training samples using techniques such as membership inference attacks.122 Employing anonymization methods such as data perturbation or tokenization also reduces the risk of the re-identification of individuals in the training data. Regular privacy impact assessments and audits throughout the model development lifecycle help identify and address potential privacy vulnerabilities.
The need for digital twin outcome data
Digital twins simulate many permutations of an intervention on a patient, but the efficacy of this intervention on patient health outcomes, along with interventions powered by AI, is only starting to be tested in healthcare settings. In particular, the single known RCT is evaluating Type 2 diabetes remission through digital twin monitoring.123 Depending on trial feasibility and clinical risk, testing for digital twin efficacy can vary for regulatory approvals for medical devices, including those with robust modelling results but no clinical testing.124 Without clinical validation, however, the accuracy and potential impacts on patient safety for predictions and treatment recommendations from digital twins are unknown.125 Therefore, RCTs are needed to establish the efficacy of clinical paradigms that leverage digital twins before their clinical implementation.
Ethical considerations
Fairness
In patient populations, under-representation and selection biases can impact the usability of synthetic datasets for downstream tasks. For example, EHR cohorts have high volume health data but are limited by biases in patient selection, data acquisition and documentation, and sparseness since the data is collected during healthcare encounters.126 Therefore, to evaluate whether generated data perpetuates biases present in the training set, particularly concerning sensitive attributes like race or gender, fairness metrics such as demographic parity and equalized odds can be implemented.127 Furthermore, diversity metrics can assess the variety and representativeness of the generated data across different demographic groups. Despite continued technical developments, bias correction for generative deep learning has been challenging to overcome. Recent developments align the consistency of the distribution of inputs and outputs128 with the intent to make the digital twins more reliable.
Disparities in healthcare and technology access can limit the information available for many populations, leading to suboptimal representation in digital twin model training. For instance, older adults, minorities, and individuals with low income use wearable devices as well as smart devices at a lower rate than their privileged counterparts.112,129 Moreover, in deployment, differential access to high bandwidth internet connectivity can further hamper equitable use. Digital twin development and deployment must, therefore, ensure the inclusion of safety-net and rural hospitals to avoid the widening of health disparities. This could be through many strategies, including incentivizing the reimbursement of models that are generalizable across health systems and demographic groups.130
Reliability and trust
Digital twins can integrate a vast amount of data and provide intervention recommendations, but the predictions must be reliable and trustworthy for clinicians to act upon them. Artificial intelligence algorithms historically are not explainable, making it difficult for physicians to understand or trust their output. Current methods such as Shapley Additive Explanations and Gradient-weighted Class Activation Mapping, which explain and visualize feature importance in deep learning models, enable a degree of explainability to black box AI models.131,132 However, more sophisticated explainability methods are essential to broaden trust. Moreover, patients must be fully informed of the privacy consequences of real-time health monitoring with potential interventions outside of a healthcare setting before consent is obtained to participate in care strategies that leverage digital twins.7 The AI Trust, Risk, and Security Management (TRiSM) framework has standardized how to address central ethical, practical, and implementable tenants of AI models.133
In addition, the accuracy of prediction models deployed in healthcare drifts over time, paradoxically, from constant retraining with new patients.134 The changes in the diagnostic and therapeutic landscape may contribute to this drift, along with false correlations between covariates and outcomes from past patients. This reiterates the need for more outcomes research with digital twin implementation.
Data ownership
Since the digital twins are created from patient data, ethics of data ownership, especially after a patient’s death, must be addressed.8 The European Union General Data Protection Regulation legislation and the US Health Insurance Portability and Accountability Act give citizens control of their data, but their application to digital twins is not established.135–137 An ethical framework must be developed to address data ownership, utilization, and patient autonomy.
Considerations for implementation at scale
Government regulation
In the USA, AI tools in healthcare, such as digital twins, are regulated by the Food and Drug Administration (FDA) as a Software as a Medical Device. They are classified based on the severity of the medical condition and the extent to which the software contributes to medical management.138 As digital twin models become embedded in healthcare, their regulation will establish new privacy standards for continuous and real-time syncing of patient wearables to data warehouses, developing patient autonomy over their digital twin, and promoting trust and safety in the models.139 The AI Act from the European Union has added measures to reduce the personal risks of AI and ensure constant re-evaluation and maintenance of the models.
Building international collaboration and federated learning
For regulatory clearance and subsequent use of digital wins, broad validation networks are needed. The charge for developers of models for any healthcare application, especially for digital twins, is that they reliably simulate scenarios across varying data availability, processing pipelines, healthcare systems, and patient populations. International collaborations utilize standardized data organization, such as the OMOP CDM, which is implemented by institutions and major data registries globally as a standard set of relational tables to map EHR data, allowing for multi-national characterizations of hundreds of millions of patients.140–142 Data-sharing consortiums such as the EUDAT Collaborative Data Infrastructure and the Information Commons established by the US 21st Century Cures Act are important steps in building the foundation for federated learning.135,143 UNESCO has also established an open data policy which emphasizes interoperability and accessibility through sharing of data resources.144 Federated learning bypasses data transfer and potential leakage across institutions by decentralizing model training and testing to the data site, and digital twins built from a distribution can prevent original data from being shared (Figure 5).110
Implementation in healthcare systems
Once clinically relevant and accurate models have been successfully developed and tested through RCTs, they will need to be implemented within healthcare systems. This will require the creation and maintenance of the infrastructure to store, organize, and process multi-modal data that are input to these models and to refine the models across diverse patient populations. In addition, clinicians will need to adjust their workflow and be trained on how to evaluate and act on recommendations from digital twin models.
Moreover, reimbursement models will need to be structured to align with the resources necessary to develop these models and the value they provide. Currently, there are no strategies for reimbursing digital twin-enabled care, so hospitals must invest in these emerging technologies. In the USA, the Center for Medicare and Medicaid Services (CMS) has reimbursed certain single-purpose FDA-cleared software, spanning AI diagnostics and assisted triage systems.130 European countries have varying reimbursement policies for digital health, but none for AI models.145 These predate the current movement towards comprehensive foundation and digital twin models.13 However, given the shift to value-based care in the USA and in nationalized health systems, the efficiency gained by physicians through increased patient volumes and better outcomes may factor into financial models that can support the broader use of digital twin technology.130
Future directions
The future of digital twins in cardiovascular care will move beyond the traditional static 3D models of patient physiology into expansive tools examining healthcare data in real-time and enabling the selection and deployment of personalized interventions to prevent adverse cardiovascular outcomes. The fusion of multi-modal medical data, advanced deep learning models, and generative AI will bring cardiology twins closer to effectively and bidirectionally transferring complex processes from the physical world to the in silico one. Through comprehensive registries, data from all health encounters in EHRs, and personalized health data available from wearable devices, digital twin models will have access to millions of examples to develop accurate predictions on individual patients. Despite these advances, digital twins will need to be pragmatic with the data available, clinicians will need to understand the uncertainties behind probabilistic recommendations, and models will need to be continuously re-evaluated. Prospective trials of the models with multicenter and multi-national validation will ensure the feasibility and generalizability of digital twin deployment, with the need for special emphasis on fairness. The tools for building a digital twin, complete with physiological, environmental, and systemic data to accelerate cardiovascular care, are available. The barriers to entry are surmountable with a co-ordinated, multi-national effort to prioritize data and model sharing to advance precision cardiovascular care through digital twins.
Contributor Information
Phyllis M Thangaraj, Section of Cardiology, Department of Internal Medicine, Yale School of Medicine, 789 Howard Ave., New Haven, CT, USA.
Sean H Benson, Department of Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands.
Evangelos K Oikonomou, Section of Cardiology, Department of Internal Medicine, Yale School of Medicine, 789 Howard Ave., New Haven, CT, USA.
Folkert W Asselbergs, Department of Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands; Institute of Health Informatics, University College London, London, UK; The National Institute for Health Research University College London Hospitals Biomedical Research Center, University College London, London, UK.
Rohan Khera, Section of Cardiology, Department of Internal Medicine, Yale School of Medicine, 789 Howard Ave., New Haven, CT, USA; Section of Health Informatics, Department of Biostatistics, Yale School of Public Health, 47 College St., New Haven, CT, USA; Department of Biomedical Informatics and Data Science, Yale School of Medicine, 100 College St. Fl 9, New Haven, CT, USA; Center for Outcomes Research and Evaluation, Yale-New Haven Hospital, 195 Church St. Fl 6, New Haven, CT 06510, USA.
Declarations
Disclosure of Interest
Dr Thangaraj, Dr Oikonomou, and Dr Khera are coinventors of a provisional patent related to the current work (63/606,203). Dr Khera is an Associate Editor of JAMA and receives research support, through Yale, from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL167858 and K23HL153775), the Doris Duke Charitable Foundation (2022060), the Blavatnik Foundation, Bristol-Myers Squibb, Novo Nordisk, and BridgeBio. He is a co-inventor of other pending patents, WO2023230345A1, US20220336048A1, 63/346,610, 63/484,426, 63/508,315, 63/580,137, 63/619,241, and 63/562,335, and a co-founder of Ensight-AI, Inc. and Evidence2Health, LLC. Dr Oikonomou is an academic co-founder of Evidence2Health LLC, and has been a consultant for Caristo Diagnostics, Ltd and Ensight-AI, Inc. He is a co-inventor in patent applications (US17/720,068, 63/619,241, 63/177,117, 63/580,137, 63/606,203, 63/562,335, WO2018078395A1, WO2020058713A1) and has received royalty fees from technology licensed through the University of Oxford. Dr Asselbergs is supported by Heart4Data, which received funding from the Dutch Heart Foundation and ZonMw (2021-B015), and UCL Hospitals NIHR Biomedical Research Centre.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
The work is supported by the National Heart, Lung, and Blood Institute (R01HL167858). Dr Thangaraj and Dr Oikonomou are also supported by grants from the National Heart, Lung, and Blood Institute (5T32HL155000-03 and 1F32HL170592-01, respectively). Prof. Asselbergs and Dr Benson are supported by the Dutch Research Council (MyDigiTwin 628.011.213). Dr Asselbergs received grant funding from the European Union Horizon scheme (AI4HF 101080430 and DataTools4Heart 101057849).
References
- 1. Winter PD, Chico TJA. Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) framework to identify barriers and facilitators for the implementation of digital twins in cardiovascular medicine. Sensors 2023;23:6333. 10.3390/s23146333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laubenbacher R, Niarakis A, Helikar T, An G, Shapiro B, Malik-Sheriff RS, et al. Building digital twins of the human immune system: toward a roadmap. NPJ Digit Med 2022;5:64. 10.1038/s41746-022-00610-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta LS, Elkind MSV, Achenbach S, Pinto FJ, Poppas A. Clinician well-being-addressing global needs for improvements in the health care field: a joint opinion from the American College of Cardiology, American Heart Association, European Society of Cardiology, World Heart Federation. Eur Heart J 2021;42:3122–6. 10.1093/eurheartj/ehab346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bank I, Vliegen HW, Bruschke AVG. The 200th anniversary of the stethoscope: can this low-tech device survive in the high-tech 21st century? Eur Heart J 2016;37:3536–43. 10.1093/eurheartj/ehw034 [DOI] [PubMed] [Google Scholar]
- 5. Khera R. Artificial intelligence-enhanced exposomics: novel insights into cardiovascular health. Eur Heart J 2024;45:1550–2. 10.1093/eurheartj/ehae159 [DOI] [PubMed] [Google Scholar]
- 6. Kamel Boulos MN, Zhang P. Digital twins: from personalised medicine to precision public health. J Pers Med 2021;11:745. 10.3390/jpm11080745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lal A, Dang J, Nabzdyk C, Gajic O, Herasevich V. Regulatory oversight and ethical concerns surrounding software as medical device (SaMD) and digital twin technology in healthcare. Ann Transl Med 2022;10:950. 10.21037/atm-22-4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coorey G, Figtree GA, Fletcher DF, Snelson VJ, Vernon ST, Winlaw D, et al. The health digital twin to tackle cardiovascular disease-a review of an emerging interdisciplinary field. NPJ Digit Med 2022;5:126. 10.1038/s41746-022-00640-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laubenbacher R, Mehrad B, Shmulevich I, Trayanova N. Digital twins in medicine. Nat Comput Sci 2024;4:184–91. 10.1038/s43588-024-00607-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 11. Echt Debra S, Liebson Philip R, Mitchell LB, Peters Robert W, Obias-Manno D, Barker Allan H, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. N Engl J Med 1991;324:781–8. 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- 12. Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khera R, Oikonomou EK, Nadkarni GN, Morley JR, Wiens J, Butte AJ, et al. Transforming cardiovascular care with artificial intelligence: from discovery to practice. J Am College Cardiol 2024;84:97–114. 10.1016/j.jacc.2024.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchesseau S, Delingette H, Sermesant M, Cabrera-Lozoya R, Tobon-Gomez C, Moireau P, et al. Personalization of a cardiac electromechanical model using reduced order unscented Kalman filtering from regional volumes. Med Image Anal 2013;17:816–29. 10.1016/j.media.2013.04.012 [DOI] [PubMed] [Google Scholar]
- 15. Ramachandra AB, Kahn AM, Marsden AL. Patient-specific simulations reveal significant differences in mechanical stimuli in venous and arterial coronary grafts. J Cardiovasc Transl Res 2016;9:279–90. 10.1007/s12265-016-9706-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arevalo HJ, Vadakkumpadan F, Guallar E, Jebb A, Malamas P, Wu KC, et al. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun 2016;7:11437. 10.1038/ncomms11437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Augustin CM, Neic A, Liebmann M, Prassl AJ, Niederer SA, Haase G, et al. Anatomically accurate high resolution modeling of human whole heart electromechanics: a strongly scalable algebraic multigrid solver method for nonlinear deformation. J Comput Phys 2016;305:622–46. 10.1016/j.jcp.2015.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballarin F, Faggiano E, Manzoni A, Quarteroni A, Rozza G, Ippolito S, et al. Numerical modeling of hemodynamics scenarios of patient-specific coronary artery bypass grafts. Biomech Model Mechanobiol 2017;16:1373–99. 10.1007/s10237-017-0893-7 [DOI] [PubMed] [Google Scholar]
- 19. Kosta S, Negroni J, Lascano E, Dauby PC. Multiscale model of the human cardiovascular system: description of heart failure and comparison of contractility indices. Math Biosci 2017;284:71–9. 10.1016/j.mbs.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 20. Cedilnik N, Duchateau J, Dubois R, Sacher F, Jaïs P, Cochet H, et al. Fast personalized electrophysiological models from computed tomography images for ventricular tachycardia ablation planning. Europace 2018;20:iii94–iii101. 10.1093/europace/euy228 [DOI] [PubMed] [Google Scholar]
- 21. Gosling RC, Morris PD, Silva Soto DA, Lawford PV, Hose DR, Gunn JP. Virtual coronary intervention: a treatment planning tool based upon the angiogram. JACC Cardiovasc Imaging 2019;12:865–72. 10.1016/j.jcmg.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Modi BN, Sankaran S, Kim HJ, Ellis H, Rogers C, Taylor CA, et al. Predicting the physiological effect of revascularization in serially diseased coronary arteries. Circ Cardiovasc Interv 2019;12:e007577. 10.1161/CIRCINTERVENTIONS.118.007577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH, et al. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–9. 10.1038/s41551-019-0437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu L, Pan Z, Li Z, Chang Y, Zhu Y, Yan F, et al. Can the wall shear stress values of left internal mammary artery grafts during the perioperative period reflect the one-year patency? Thorac Cardiovasc Surg 2020;68:723–9. 10.1055/s-0040-1714385 [DOI] [PubMed] [Google Scholar]
- 25. Dowling C, Firoozi S, Brecker SJ. First-in-human experience with patient-specific computer simulation of TAVR in bicuspid aortic valve morphology. JACC Cardiovasc Interv 2020;13:184–92. 10.1016/j.jcin.2019.07.032 [DOI] [PubMed] [Google Scholar]
- 26. El Faquir N, De Backer O, Bosmans J, Rudolph T, Buzzatti N, Bieliauskas G, et al. Patient-specific computer simulation in TAVR with the self-expanding evolut R valve. JACC Cardiovasc Interv 2020;13:1803–12. 10.1016/j.jcin.2020.04.018 [DOI] [PubMed] [Google Scholar]
- 27. Hu Z, Du D, Du Y. Gaussian process-based spatiotemporal modeling of electrical wave propagation in human atrium. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 20–24 July 2020. Montreal, QC, Canada: IEEE, 2020. [DOI] [PubMed]
- 28. Gillette K, Gsell MAF, Prassl AJ, Karabelas E, Reiter U, Reiter G, et al. A framework for the generation of digital twins of cardiac electrophysiology from clinical 12-leads ECGs. Med Image Anal 2021;71:102080. 10.1016/j.media.2021.102080 [DOI] [PubMed] [Google Scholar]
- 29. Gerach T, Schuler S, Fröhlich J, Lindner L, Kovacheva E, Moss R, et al. Electro-mechanical whole-heart digital twins: a fully coupled multi-physics approach. Mathematics 2021;9:1247. 10.3390/math9111247 [DOI] [Google Scholar]
- 30. Poletti G, Antonini L, Mandelli L, Tsompou P, Karanasiou GS, Papafaklis MI, et al. Towards a digital twin of coronary stenting: a suitable and validated image-based approach for mimicking patient-specific coronary arteries. Electronics (Basel) 2022;11:502. 10.3390/electronics11030502 [DOI] [Google Scholar]
- 31. Stimm J, Nordsletten DA, Jilberto J, Miller R, Berberoğlu E, Kozerke S, et al. Personalization of biomechanical simulations of the left ventricle by in-vivo cardiac DTI data: impact of fiber interpolation methods. Front Physiol 2022;13:1042537. 10.3389/fphys.2022.1042537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li L, Camps J, Banerjee A, Beetz M, Rodriguez B, Grau V. Deep computational model for the inference of ventricular activation properties. arXiv:2208.04028 [cs.CV], 10.48550/arXiv.2208.04028, 8 August 2022, preprint: not peer reviewed. [DOI]
- 33. Jung A, Gsell MAF, Augustin CM, Plank G. An integrated workflow for building digital twins of cardiac electromechanics-a multi-fidelity approach for personalising active mechanics. Mathematics (Basel) 2022;10:823. 10.3390/math10050823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fedele M, Piersanti R, Regazzoni F, Salvador M, Africa PC, Bucelli M, et al. A comprehensive and biophysically detailed computational model of the whole human heart electromechanics. Comput Methods Appl Mech Eng 2023;410:115983. 10.1016/j.cma.2023.115983 [DOI] [Google Scholar]
- 35. Xing X, Ser JD, Wu Y, Li Y, Xia J, Xu L, et al. HDL: hybrid deep learning for the synthesis of myocardial velocity maps in digital twins for cardiac analysis. IEEE J Biomed Health Inform 2023;27:5134–42. 10.1109/JBHI.2022.3158897 [DOI] [PubMed] [Google Scholar]
- 36. Kadry K, Gupta S, Nezami FR, Edelman ER. Probing the limits and capabilities of diffusion models for the anatomic editing of digital twins. arXiv:2401.00247 [cs.CV], 10.48550/arXiv.2401.00247, 30 December 2023, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 37. Serra D, Franco P, Romero P, Romitti G, Garcia-Fernandez I, Lozano M, et al. Assessment of risk for ventricular tachycardia based on extensive electrophysiology simulations. Conf Proc IEEE Eng Med Biol Soc 2023;2023:1–4. 10.1109/EMBC40787.2023.10340169 [DOI] [PubMed] [Google Scholar]
- 38. Strocchi M, Longobardi S, Augustin CM, Gsell MAF, Petras A, Rinaldi CA, et al. Cell to whole organ global sensitivity analysis on a four-chamber heart electromechanics model using Gaussian processes emulators. PLoS Comput Biol 2023;19:e1011257. 10.1371/journal.pcbi.1011257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azzolin L, Eichenlaub M, Nagel C, Nairn D, Sánchez J, Unger L, et al. Augmenta: patient-specific augmented atrial model generation tool. Comput Med Imaging Graph 2023;108:102265. 10.1016/j.compmedimag.2023.102265 [DOI] [PubMed] [Google Scholar]
- 40. Kashtanova V, Pop M, Ayed I, Gallinari P, Sermesant M. Simultaneous data assimilation and cardiac electrophysiology model correction using differentiable physics and deep learning. Interface Focus 2023;13:20230043. 10.1098/rsfs.2023.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang X, Vadhavkar S, Ye Y, Toloubidokhti M, Missel R, Wang L. HyPer-EP: Meta-learning hybrid personalized models for cardiac electrophysiology. arXiv:2403.15433 [eess.SP], 10.48550/arXiv.2403.15433, 15 March 2024, preprint: not peer reviewed. [DOI]
- 42. Li L, Camps J, Zhinuo W, Banerjee A, Beetz M, Rodriguez B, et al. Towards enabling cardiac digital twins of myocardial infarction using deep computational models for inverse inference. arXiv:2307.04421 [eess.SP] , 10.48550/arXiv.2307.04421, 14 February 2024, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 43. Camps J, Wang ZJ, Doste R, Holmes M, Lawson B, Tomek J, et al. Cardiac digital twin pipeline for virtual therapy evaluation . arXiv:2401.10029 [cs.CE], 10.48550/arXiv.2401.10029, 18 January 2024, preprint: not peer reviewed. [DOI]
- 44. Kuang K, Dean F, Jedlicki JB, Ouyang D, Philippakis A, Sontag D, et al. Non-invasive medical digital twins using physics -informed self-supervised learning. arXiv:2403.00177 [cs.LG], 10.48550/arXiv.2403.00177, 28 May 2024, preprint: not peer reviewed. [DOI]
- 45. Camps J, Berg LA, Wang ZJ, Sebastian R, Riebel LL, Doste R, et al. Digital twinning of the human ventricular activation sequence to clinical 12-lead ECGs and magnetic resonance imaging using realistic Purkinje networks for in silico clinical trials. Med Image Anal 2024;94:103108. 10.1016/j.media.2024.103108 [DOI] [PubMed] [Google Scholar]
- 46. Sakata K, Bradley RP, Prakosa A, Yamamoto CAP, Ali SY, Loeffler S, et al. Assessing the arrhythmogenic propensity of fibrotic substrate using digital twins to inform a mechanisms-based atrial fibrillation ablation strategy. Nat Cardiovasc Res 2024;3:857–68. 10.1038/s44161-023-00407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC guidelines for the management of cardiomyopathies. Eur Heart J 2023;44:3503–626. 10.1093/eurheartj/ehad194 [DOI] [PubMed] [Google Scholar]
- 48. Sangha V, Khunte A, Holste G, Mortazavi BJ, Wang Z, Oikonomou EK, et al. Biometric contrastive learning for data-efficient deep learning from electrocardiographic images. J Am Med Inform Assoc 2024;31:855–65. 10.1093/jamia/ocae002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oikonomou EK, Holste G, Yuan N, Coppi A, McNamara RL, Haynes NA, et al. A multimodal video-based AI biomarker for aortic stenosis development and progression. JAMA Cardiol 2024;9:534–44. 10.1001/jamacardio.2024.0595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holste G, Oikonomou EK, Mortazavi BJ, Coppi A, Faridi KF, Miller EJ, et al. Severe aortic stenosis detection by deep learning applied to echocardiography. Eur Heart J 2023;44:4592–604. 10.1093/eurheartj/ehad456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oikonomou EK, Sangha V, Dhingra LS, Aminorroaya A, Coppi A, Krumholz HM, et al. Artificial intelligence-enhanced risk stratification of cancer therapeutics-related cardiac dysfunction using electrocardiographic images. medRxiv:24304047, 10.1101/2024.03.12.24304047, 19 March 2024, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 52. Dhingra LS, Aminorroaya A, Sangha V, Camargos AP, Asselbergs FW, Brant LC, et al. Scalable risk stratification for heart failure using artificial intelligence applied to 12-lead electrocardiographic images: a multinational study. medRxiv:24305232, 10.1101/2024.04.02.24305232, 3 April 2024, preprint: not peer reviewed. [DOI]
- 53. Friedman DJ, Al-Khatib SM, Dalgaard F, Fudim M, Abraham WT, Cleland JGF, et al. Cardiac resynchronization therapy improves outcomes in patients with intraventricular conduction delay but not right bundle branch block: a patient-level meta-analysis of randomized controlled trials. Circulation 2023;147:812–23. 10.1161/CIRCULATIONAHA.122.062124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–30. 10.1056/NEJMoa1608029 [DOI] [PubMed] [Google Scholar]
- 55. Oikonomou EK, Aminorroaya A, Dhingra LS, Partridge C, Velazquez EJ, Desai NR, et al. Real-world evaluation of an algorithmic machine-learning-guided testing approach in stable chest pain: a multinational, multicohort study. Eur Heart J Digit Health 2014;5:303–13. 10.1093/ehjdh/ztae023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oikonomou EK, Van Dijk D, Parise H, Suchard MA, Lemos J, Antoniades C, et al. A phenomapping-derived tool to personalize the selection of anatomical vs. functional testing in evaluating chest pain (ASSIST). Eur Heart J 2021;42:2536–48. 10.1093/eurheartj/ehab223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oikonomou EK, Spatz ES, Suchard MA, Khera R. Individualising intensive systolic blood pressure reduction in hypertension using computational trial phenomaps and machine learning: a post-hoc analysis of randomised clinical trials. Lancet Digit Health 2022;4:e796–805. 10.1016/S2589-7500(22)00170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donal E, Delgado V, Bucciarelli-Ducci C, Galli E, Haugaa KH, Charron P, et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2019;20:1075–93. 10.1093/ehjci/jez178 [DOI] [PubMed] [Google Scholar]
- 59. Qian S, Ugurlu D, Fairweather E, Strocchi M, Toso LD, Deng Y, et al. Developing cardiac digital twins at scale: insights from personalised myocardial conduction velocity. medRxiv :23299435, 10.1101/2023.12.05.23299435, 5 January 2024, preprint: not peer reviewed. [DOI]
- 60. Viola F, Del Corso G, De Paulis R, Verzicco R. GPU accelerated digital twins of the human heart open new routes for cardiovascular research. Sci Rep 2023;13:8230. 10.1038/s41598-023-34098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lepper A, Buck CMA, Veer M, Huberts W, Vosse F, Dekker LRC. From evidence-based medicine to digital twin technology for predicting ventricular tachycardia in ischaemic cardiomyopathy. J R Soc Interface 2022;19:20220317. 10.1098/rsif.2022.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shade JK, Prakosa A, Popescu DM, Yu R, Okada DR, Chrispin J, et al. Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci Adv 2021;7:eabi8020. 10.1126/sciadv.abi8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kirkels FP, Osta N, Rootwelt-Norberg C, Chivulescu M, Loon T, Aabel EW, et al. Monitoring of myocardial involvement in early arrhythmogenic right ventricular cardiomyopathy across the age spectrum. J Am Coll Cardiol 2023;82:785–97. 10.1016/j.jacc.2023.05.065 [DOI] [PubMed] [Google Scholar]
- 64. Zhang Y, Zhang K, Prakosa A, James C, Zimmerman SL, Carrick R, et al. Predicting ventricular tachycardia circuits in patients with arrhythmogenic right ventricular cardiomyopathy using genotype-specific heart digital twins. Elife 2023;12:RP88865. 10.7554/eLife.88865.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Salvador M, Kong F, Peirlinck M, Parker DW, Chubb H, Dubin AM, et al. Digital twinning of cardiac electrophysiology for congenital heart disease. bioRxiv:568942, 10.1101/2023.11.27.568942, 28 November 2023, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 66. Kawashima H, Pompilio G, Andreini D, Bartorelli AL, Mushtaq S, Ferrari E, et al. Safety and feasibility evaluation of planning and execution of surgical revascularisation solely based on coronary CTA and FFRCT in patients with complex coronary artery disease: study protocol of the FASTTRACK CABG study. BMJ Open 2020;10:e038152. 10.1136/bmjopen-2020-038152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Griffin WF, Choi AD, Riess JS, Marques H, Chang H-J, Choi JH, et al. AI evaluation of stenosis on coronary CTA, comparison with quantitative coronary angiography and fractional flow reserve: a CREDENCE trial substudy. JACC Cardiovasc Imaging 2023;16:193–205. 10.1016/j.jcmg.2021.10.020 [DOI] [PubMed] [Google Scholar]
- 68. Koopsen T, Gerrits W, Osta NV, Loon TV, Wouters P, Prinzen FW, et al. Virtual pacing of a patient’s digital twin to predict left ventricular reverse remodelling after cardiac resynchronization therapy. Europace 2023;26:euae009. 10.1093/europace/euae009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. O’Hara RP, Binka E, Prakosa A, Zimmerman SL, Cartoski MJ, Abraham MR, et al. Personalized computational heart models with T1-mapped fibrotic remodeling predict sudden death risk in patients with hypertrophic cardiomyopathy. Elife 2022;11:e73325. 10.7554/eLife.73325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga H, et al. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng 2018;2:732–40. 10.1038/s41551-018-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hwang T, Kwon O, Lim B, Jin Z, Yang S, Kim D, et al. Clinical application of virtual antiarrhythmic drug test using digital twins in patients who recurred atrial fibrillation after catheter ablation. Europace 2023;25:euad122.076. 10.1093/europace/euad122.076 [DOI] [Google Scholar]
- 72. Dhingra LS, Shen M, Mangla A, Khera R. Cardiovascular care innovation through data-driven discoveries in the electronic health record. Am J Cardiol 2023;203:136–48. 10.1016/j.amjcard.2023.06.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aminorroaya A, Dhingra LS, Oikonomou EK, Saadatagah S, Thangaraj P, Vasisht Shankar S, et al. Development and multinational validation of an algorithmic strategy for high Lp(a) screening. Nat Cardiovasc Res 2024;3:558–66. 10.1038/s44161-024-00469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK biobank resource with deep phenotyping and genomic data. Nature 2018;562:203–9. 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lyles CR, Lunn MR, Obedin-Maliver J, Bibbins-Domingo K. The new era of precision population health: insights for the All of Us Research Program and beyond. J Transl Med 2018;16:211. 10.1186/s12967-018-1585-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Khera R. AI-enabled diagnosis from an electrocardiogram image: the next frontier of innovation in a century-old technology. Heart BMJ 2024;110:1065–6. 10.1136/heartjnl-2024-324299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oikonomou EK, Khera R. Artificial intelligence-enhanced patient evaluation: bridging art and science. Eur Heart J 2024;45:3204–18. 10.1093/eurheartj/ehae415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miotto R, Li L, Kidd BA, Dudley JT. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep 2016;6:1–10. 10.1038/srep26094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thangaraj PM, Shankar SV, Huang S, Nadkarni G, Mortazavi B, Oikonomou EK, et al. A novel digital twin strategy to examine the implications of randomized control trials for real-world populations. medRxiv :24304868, 10.1101/2024.03.25.24304868, 26 March 2024, preprint: not peer reviewed. [DOI]
- 80. Kline A, Wang H, Li Y, Dennis S, Hutch M, Xu Z, et al. Multimodal machine learning in precision health: a scoping review. NPJ Digit Med 2022;5:171. 10.1038/s41746-022-00712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Choi E, Biswal S, Malin B, Duke J, Stewart WF, Sun J. Generating multi-label discrete patient records using generative adversarial networks. arXiv:1703.06490 [cs.LG], 10.48550/arXiv.1703.06490, 11 January 2018, preprint: not peer reviewed. [DOI]
- 82. Esteban C, Hyland SL, Rätsch G. Real-valued (medical) time series generation with recurrent conditional GANs. arXiv :1706.02633 [stat.ML], 10.48550/arXiv.1706.02633, 4 December 2017, preprint: not peer reviewed. [DOI]
- 83. Li J, Cairns BJ, Li J, Zhu T. Generating synthetic mixed-type longitudinal electronic health records for artificial intelligent applications. NPJ Digit Med 2023;6:98. 10.1038/s41746-023-00834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yoon J, Jordon J, Schaar M. GANITE: Estimation of individualized treatment effects using generative adversarial nets. International Conference on Learning Representations. Vancover, BC, Canada, 2018.
- 85. Ghosh S, Boucher C, Bian J, Prosperi M. Propensity score synthetic augmentation matching using generative adversarial networks (PSSAM-GAN). Comput Methods Programs Biomed Update 2021;1:100020. 10.1016/j.cmpbup.2021.100020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bietsch D, Stahlbock R, Voß S. Synthetic data as a proxy for real-world electronic health records in the patient length of stay prediction. Sustain Sci Pract Policy 2023;15:13690. 10.3390/su151813690 [DOI] [Google Scholar]
- 87. Pang C, Jiang X, Kalluri KS, Spotnitz M, Chen R, Perotte A, et al. CEHR-BERT: Incorporating temporal information from structured EHR data to improve prediction tasks. arXiv:2111.08585 [cs.LG], 10.48550/arXiv.2111.08585, 10 November 2021, preprint: not peer reviewed. [DOI]
- 88. Boonstra MJ, Weissenbacher D, Moore JH, Gonzalez-Hernandez G, Asselbergs FW. Artificial intelligence: revolutionizing cardiology with large language models. Eur Heart J 2024;45:332–45. 10.1093/eurheartj/ehad838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Khunte A, Sangha V, Oikonomou EK, Dhingra LS, Aminorroaya A, Mortazavi BJ, et al. Detection of left ventricular systolic dysfunction from single-lead electrocardiography adapted for portable and wearable devices. NPJ Digit Med 2023;6:124. 10.1038/s41746-023-00869-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sangha V, Nargesi AA, Dhingra LS, Khunte A, Mortazavi BJ, Ribeiro AH, et al. Detection of left ventricular systolic dysfunction from electrocardiographic images. Circulation 2023;148:765–77. 10.1161/CIRCULATIONAHA.122.062646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–4. 10.1038/s41591-018-0240-2 [DOI] [PubMed] [Google Scholar]
- 92. Lin S, Li Z, Fu B, Chen S, Li X, Wang Y, et al. Feasibility of using deep learning to detect coronary artery disease based on facial photo. Eur Heart J 2020;41:4400–11. 10.1093/eurheartj/ehaa640 [DOI] [PubMed] [Google Scholar]
- 93. Attia ZI, Dugan J, Rideout A, Maidens JN, Venkatraman S, Guo L, et al. Automated detection of low ejection fraction from a one-lead electrocardiogram: application of an AI algorithm to an electrocardiogram-enabled Digital Stethoscope. Eur Heart J Digit Health 2022;3:373–9. 10.1093/ehjdh/ztac030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Minic A, Jovanovic L, Bacanin N, Stoean C, Zivkovic M, Spalevic P, et al. Applying recurrent neural networks for anomaly detection in electrocardiogram sensor data. Sensors 2023;23:9878. 10.3390/s23249878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Movahed MR, Irannejad K, Bates S. The majority of participants with suspected hypertrophic cardiomyopathy documented during screening echocardiography have a normal electrocardiogram. Crit Pathw Cardiol 2024;23:20–5. 10.1097/HPC.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 96. Radhakrishnan A, Friedman SF, Khurshid S, Ng K, Batra P, Lubitz SA, et al. Cross-modal autoencoder framework learns holistic representations of cardiovascular state. Nat Commun 2023;14:2436. 10.1038/s41467-023-38125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vaid A, Jiang J, Sawant A, Lerakis S, Argulian E, Ahuja Y, et al. A foundational vision transformer improves diagnostic performance for electrocardiograms. NPJ Digit Med 2023;6:108. 10.1038/s41746-023-00840-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sangha V, Dhingra LS, Oikonomou E, Aminorroaya A, Sikand NV, Sen S, et al. Identification of hypertrophic cardiomyopathy on electrocardiographic images with deep learning. medRxiv:23300490. 10.1101/2023.12.23.23300490, 28 December 2023, preprint: not peer reviewed [DOI]
- 99. Harmon DM, Carter RE, Cohen-Shelly M, Svatikova A, Adedinsewo DA, Noseworthy PA, et al. Real-world performance, long-term efficacy, and absence of bias in the artificial intelligence enhanced electrocardiogram to detect left ventricular systolic dysfunction. Eur Heart J Digit Health 2022;3:238–44. 10.1093/ehjdh/ztac028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fisher CK, Smith AM, Walsh JR. Coalition against major diseases. Sci Rep 2019;9:13622. 10.1038/s41598-019-49656-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eckardt J-N, Hahn W, Röllig C, Stasik S, Platzbecker U, Müller-Tidow C, et al. Mimicking clinical trials with synthetic acute myeloid leukemia patients using generative artificial intelligence. NPJ Digit Med 2024;7:76. 10.1038/s41746-024-01076-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Qian Z, Zhang Y, Bica I, Wood A, Schaar M. Synctwin: Treatment effect estimation with longitudinal outcomes. 35th Conference on Neural Information Processing Systems (NeurIPS 2021), Advances in Neural Information Processing Systems 34, 2021. Article No.: 243, p. 3178–90.
- 103. Averitt AJ, Vanitchanant N, Ranganath R, Perotte AJ. The counterfactual χ-GAN: finding comparable cohorts in observational health data. J Biomed Inform 2020;109:103515. 10.1016/j.jbi.2020.103515 [DOI] [PubMed] [Google Scholar]
- 104. Oikonomou EK, Thangaraj PM, Bhatt DL, Ross JS, Young LH, Krumholz HM, et al. An explainable machine learning-based phenomapping strategy for adaptive predictive enrichment in randomized clinical trials. NPJ Digit Med 2023;6:217. 10.1038/s41746-023-00963-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fahed AC, Philippakis AA, Khera AV. The potential of polygenic scores to improve cost and efficiency of clinical trials. Nat Commun 2022;13:2922. 10.1038/s41467-022-30675-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cho L, Vest AR, O’Donoghue ML, Ogunniyi MO, Sarma AA, Denby KJ, et al. Increasing participation of women in cardiovascular trials: JACC council perspectives. J Am Coll Cardiol 2021;78:737–51. 10.1016/j.jacc.2021.06.022 [DOI] [PubMed] [Google Scholar]
- 107. Oikonomou EK, Suchard MA, McGuire DK, Khera R. Phenomapping-derived tool to individualize the effect of canagliflozin on cardiovascular risk in type 2 diabetes. Diabetes Care 2022;45:965–74. 10.2337/dc21-1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Thangaraj PM, Shankar SV, Oikonomou EK, Khera R. RCT-twin-GAN generates digital twins of randomized control trials adapted to real-world patients to enhance their inference and application. medRxiv:23299464, 2023, 10.1101/2023.12.06.23299464, preprint: not peer reviewed. [DOI]
- 109. Marco-Ruiz L, Hernández MÁT, Ngo PD, Makhlysheva A, Svenning TO, Dyb K, et al. A multinational study on artificial intelligence adoption: clinical implementers’ perspectives. Int J Med Inform 2024;184:105377. 10.1016/j.ijmedinf.2024.105377 [DOI] [PubMed] [Google Scholar]
- 110. Rieke N, Hancox J, Li W, Milletarì F, Roth HR, Albarqouni S, et al. The future of digital health with federated learning. NPJ Digit Med 2020;3:119. 10.1038/s41746-020-00323-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dhingra LS, Aminorroaya A, Oikonomou EK, Nargesi AA, Wilson FP, Krumholz HM, et al. Use of wearable devices in individuals with or at risk for cardiovascular disease in the US, 2019 to 2020. JAMA Netw Open 2023;6:e2316634. 10.1001/jamanetworkopen.2023.16634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Aminorroaya A, Dhingra LS, Nargesi AA, Oikonomou EK, Krumholz HM, Khera R. Use of smart devices to track cardiovascular health goals in the United States. JACC Adv 2023;2:100544. 10.1016/j.jacadv.2023.100544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Armoundas AA, Ahmad FS, Bennett DA, Chung MK, Davis LL, Dunn J, et al. Data interoperability for ambulatory monitoring of cardiovascular disease: a scientific statement from the American Heart Association. Circ Genom Precis Med 2024;17:e000095. 10.1161/HCG.0000000000000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aiello M, Esposito G, Pagliari G, Borrelli P, Brancato V, Salvatore M. How does DICOM support big data management? Investigating its use in medical imaging community. Insights Imaging 2021;12:164. 10.1186/s13244-021-01081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hripcsak G, Shang N, Peissig PL, Rasmussen LV, Liu C, Benoit B, et al. Facilitating phenotype transfer using a common data model. J Biomed Inform 2019;96:103253. 10.1016/j.jbi.2019.103253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. HL7 International . FHIR v5.0.0. HL7 FHIR Release 7. https://www.hl7.org/fhir/ (2 July 2024).
- 117. Chikwetu L, Miao Y, Woldetensae MK, Bell D, Goldenholz DM, Dunn J. Does deidentification of data from wearable devices give us a false sense of security? A systematic review. Lancet Digit Health 2023;5:e239–47. 10.1016/S2589-7500(22)00234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sun C, Soest J, Dumontier M. Generating synthetic personal health data using conditional generative adversarial networks combining with differential privacy. J Biomed Inform 2023;143:104404. 10.1016/j.jbi.2023.104404 [DOI] [PubMed] [Google Scholar]
- 119. Gwon H, Ahn I, Kim Y, Kang HJ, Seo H, Choi H, et al. LDP-GAN: generative adversarial networks with local differential privacy for patient medical records synthesis. Comput Biol Med 2024;168:107738. 10.1016/j.compbiomed.2023.107738 [DOI] [PubMed] [Google Scholar]
- 120. Shen Z, Zhong T. Analysis of application examples of differential privacy in deep learning. Comput Intell Neurosci 2021;2021:4244040. 10.1155/2021/4244040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Beaulieu-Jones BK, Wu ZS, Williams C, Lee R, Bhavnani SP, Byrd JB, et al. Privacy-preserving generative deep neural networks support clinical data sharing. Circ Cardiovasc Qual Outcomes 2019;12:e005122. 10.1161/CIRCOUTCOMES.118.005122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shokri R, Stronati M, Song C, Shmatikov V. Membership inference attacks against machine learning models. arXiv:1610.05820 [cs.CR], 10.48550/arXiv.1610.05820, 31 March 2017, preprint: not peer reviewed. [DOI]
- 123. Shamanna P, Joshi S, Shah L, Dharmalingam M, Vadavi A, Damodaran S, et al. Remission of T2DM by digital twin technology with reduction of cardiovascular risk: interim results of randomised controlled clinical trial. Eur Heart J 2022;43:ehab849.177. 10.1093/eurheartj/ehab849.177 [DOI] [Google Scholar]
- 124. Faris O, Shuren J. An FDA viewpoint on unique considerations for medical-device clinical trials. N Engl J Med 2017;376:1350–7. 10.1056/NEJMra1512592 [DOI] [PubMed] [Google Scholar]
- 125. Thangaraj PM, Khera R. Accelerating chest pain evaluation with machine learning. Eur Heart J Acute Cardiovasc Care 2023;12:753–4. 10.1093/ehjacc/zuad117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Al-Sahab B, Leviton A, Loddenkemper T, Paneth N, Zhang B. Biases in electronic health records data for generating real-world evidence: an overview. J Healthc Inform Res 2024;8:121–39. 10.1007/s41666-023-00153-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bhanot K, Qi M, Erickson JS, Guyon I, Bennett KP. The problem of fairness in synthetic healthcare data. Entropy 2021;23:1165. 10.3390/e23091165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Grover A, Song J, Agarwal A, Tran K, Kapoor A, Horvitz E, et al. Bias correction of learned generative models using likelihood-free importance weighting. arXiv:1906.09531 [stat.ML], 10.48550/arXiv.1906.09531, 3 November 2019, preprint: not peer reviewed. [DOI]
- 129. Rodriguez JA, Shachar C, Bates DW. Digital inclusion as health care—supporting health care equity with digital-infrastructure initiatives. N Engl J Med 2022;386:1101–3. 10.1056/NEJMp2115646 [DOI] [PubMed] [Google Scholar]
- 130. Parikh RB, Helmchen LA. Paying for artificial intelligence in medicine. NPJ Digit Med 2022;5:63. 10.1038/s41746-022-00609-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lundberg S, Lee S-I. A unified approach to interpreting model predictions. arXiv:1705.07874 [cs.AI], 10.48550/arXiv.1705.07874, 25 November 2017, preprint: not peer reviewed. [DOI]
- 132. Selvaraju RR, Cogswell M, Das A, Vedantam R, Parikh D, Batra D. Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Computer Vision 2020;128:336–59. 10.1007/s11263-019-01228-7 [DOI] [Google Scholar]
- 133. Habbal A, Ali MK, Abuzaraida MA. Artificial Intelligence Trust, Risk and Security Management (AI TRiSM): frameworks, applications, challenges and future research directions. Expert Syst Appl 2024;240:122442. 10.1016/j.eswa.2023.122442 [DOI] [Google Scholar]
- 134. Vaid A, Sawant A, Suarez-Farinas M, Lee J, Kaul S, Kovatch P, et al. Implications of the use of artificial intelligence predictive models in health care settings: a simulation study. Ann Intern Med 2023;176:1358–69. 10.7326/M23-0949 [DOI] [PubMed] [Google Scholar]
- 135. Viceconti M, De Vos M, Mellone S, Geris L. Position paper From the digital twins in healthcare to the Virtual Human Twin: a moon-shot project for digital health research. IEEE J Biomed Health Inform 2024;28:491–501. 10.1109/JBHI.2023.3323688 [DOI] [PubMed] [Google Scholar]
- 136. EU General Data Protection Regulation (GDPR): Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), OJ 2016 L 119/1.
- 137. Office for Civil Rights (OCR) . Summary of the HIPAA Privacy Rule. Hhs.gov. US Department of Health and Human Services, 2008. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html (19 April 2024)
- 138. International Medical Device Regulators Forum Software as a Medical Device (SaMD) Working Group . Software as a Medical Device: Possible Framework for Risk Categorization and Corresponding Considerations. IMDRF/SaMD WG/N12:2014. 2014. https://www.imdrf.org/documents/software-medical-device-possible-framework-risk-categorization-and-corresponding-considerations
- 139. Bruynseels K, Santoni de Sio F, Hoven J van den. Digital twins in health care: ethical implications of an emerging engineering paradigm. Front Genet 2018;9:31. 10.3389/fgene.2018.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med 2010;153:600–6. 10.7326/0003-4819-153-9-201011020-00010 [DOI] [PubMed] [Google Scholar]
- 141. Hallinan CM, Ward R, Hart GK, Sullivan C, Pratt N, Ng AP, et al. Seamless EMR data access: integrated governance, digital health and the OMOP-CDM. BMJ Health Care Inform 2024;31:100953. 10.1136/bmjhci-2023-100953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Khera R, Dhingra LS, Aminorroaya A, Li K, Zhou JJ, Arshad F, et al. Multinational patterns of second line antihyperglycaemic drug initiation across cardiovascular risk groups: federated pharmacoepidemiological evaluation in LEGEND-T2DM. BMJ Med 2023;2:e000651. 10.1136/bmjmed-2023-000651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Majumder MA, Guerrini CJ, Bollinger JM, Cook-Deegan R, McGuire AL. Sharing data under the 21st Century Cures Act. Genet Med 2017;19:1289–94. 10.1038/gim.2017.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. UNESCO . Open Data. 2024. https://www.unesco.org/en/open-solutions/open-data (2 July 2024)
- 145. Kessel R, Srivastava D, Kyriopoulos I, Monti G, Novillo-Ortiz D, Milman R, et al. Digital health reimbursement strategies of 8 European countries and Israel: scoping review and policy mapping. JMIR MHealth UHealth 2023;11:e49003. 10.2196/49003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.