Abstract
A cottontail rabbit papillomavirus (CRPV) E6 DNA vaccine that induces significant protection against CRPV challenge was used in a superior vaccination regimen in which the cutaneous sites of vaccination were primed with an expression vector encoding granulocyte-macrophage colony-stimulating factor (GM-CSF), a cytokine that induces differentiation and local recruitment of professional antigen-presenting cells. This treatment induced a massive influx of major histocompatibility complex class II-positive cells. In a vaccination-challenge experiment, rabbit groups were treated by E6 DNA vaccination, GM-CSF DNA inoculation, or a combination of both treatments. After two immunizations, rabbits were challenged with CRPV at low, moderate, and high stringencies and monitored for papilloma formation. As expected, all clinical outcomes were monotonically related to the stringency of the viral challenge. The results demonstrate that GM-CSF priming greatly augmented the effects of CRPV E6 vaccination. First, challenge sites in control rabbits (at the moderate challenge stringency) had a 0% probability of remaining disease free, versus a 50% probability in E6-vaccinated rabbits, and whereas GM-CSF alone had no effect, the interaction between GM-CSF priming and E6 vaccination increased disease-free survival to 67%. Second, the incubation period before papilloma onset was lengthened by E6 DNA vaccination alone or to some extent by GM-CSF DNA inoculation alone, and the combination of treatments induced additive effects. Third, the rate of papilloma growth was reduced by E6 vaccination and, to a lesser extent, by GM-CSF treatment. In addition, the interaction between the E6 and GM-CSF treatments was synergistic and yielded more than a 99% reduction in papilloma volume. Finally, regression occurred among the papillomas that formed in rabbits treated with the E6 vaccine and/or with GM-CSF, with the highest regression frequency occurring in rabbits that received the combination treatment.
Human papillomaviruses (HPVs) cause common and plantar warts in 10% of the population at large (20). A subset of HPVs also cause cervical cancer and appear to be etiologically involved in over 50% of other anogenital cancers, as well as some cancers of the skin and oronnasal cavity (73). Premalignant HPV-associated lesions are extremely difficult to treat, and no current medical or surgical therapy cures all lesions in all patients. The development of an effective vaccine against HPV to prevent and treat papillomavirus-induced disease would be a significant contribution to human health.
The use of plasmid DNAs as subunit vaccines is a simple yet powerful new approach to vaccine science (39). DNA vaccines have potential for use as HPV vaccines because they generally induce strong, specific, and persistent cell-mediated immunity and humoral immunity conferring prophylactic and therapeutic effects (12, 17, 44, 49, 52). In addition, DNA vaccines can be combined to generate multivalent vaccines against several gene products and/or viral types. More than 15 types of HPV are associated with cervical cancer, so a prophylactic HPV vaccine will need to be multivalent. On the other hand, an individual usually is infected with a single HPV type, so a therapeutic vaccine will probably be most effective if it targets the same viral type. Given the large number of HPV types, a corresponding large number of therapeutic vaccines may be required.
Cottontail rabbit papillomavirus (CRPV) infection of domestic rabbits is a powerful model for examining potential vaccine candidates (3). The papillomas induced by CRPV are similar to the papillomas induced by HPVs. The genomes of CRPV and HPV are conserved, and their genes encode proteins with homologous functions (4, 13, 23, 24, 46). Immunosuppression inhibits spontaneous regression in rabbits, as in humans, suggesting that the control of CRPV and HPV infections involves similar immunologic defense mechanisms (45, 55). Host genetics appear to influence the outcome of a CRPV infection (6, 25, 26, 29), as they do an HPV infection (2, 32; P. K. Magnusson, P. Sparen, and U. B. Gyllensten, Letter, Nature 400:29–30, 1999). The domestic rabbit-CRPV model is easy to manipulate, allows repeated clinical monitoring of disease development, including malignant progression, and produces highly quantifiable data for multiple outcome measurements. Sundaram et al. (60) and Donnelly et al. (16) used the CRPV-rabbit model to demonstrate that DNA vaccination with a DNA vaccine encoding the CRPV L1 major capsid protein induced virtually complete protection against CRPV challenge. Protection was accompanied by high-titer, L1-specific neutralizing antibodies.
Papillomavirus proteins such as E6 and E7, in contrast, are likely to be superior targets for a therapeutic vaccine because they are expressed in all papillomavirus-associated lesions, including genital condylomas (33, 57), intraepithelial neoplasias (31), and carcinomas (40, 57) in humans and papillomas and carcinomas in rabbits (67). In benign lesions, the E6 and E7 genes are generally transcribed at low levels in the basal cell layer and at high levels in the differentiated epithelium (31, 72). An inverse pattern in domestic rabbit papillomas has been reported (72). How the epithelial distribution of E6 and E7 transcripts affects the ability of an immune response to recognize or respond to an infection is unknown. The E6 and E7 genes are each essential to initiate papilloma formation during primary infection (5, 46, 70). Therefore, an effective vaccine against E6 or E7 could have both prophylactic and therapeutic applications.
The possibility of E6 vaccination in the CRPV-rabbit model has been investigated. Over 10 years ago, Lathe et al. reported that a vaccinia virus-based CRPV E6 vaccine, delivered before CRPV challenge, did not prevent the formation or subsequent growth of papillomas (38). Research from our laboratory has shown that an E6 DNA vaccine delivered by a gene gun into the skin provided significant, although partial, protection (61). Similar findings were reported by Han et al., who also showed that an intramuscular route of CRPV E6 DNA vaccination was not effective (27, 28). These studies demonstrate that an E6 DNA vaccine can induce prophylactic immunity but that its efficacy must be improved to be useful.
Immune responses generated by DNA vaccination are initiated by professional antigen-presenting cells (APCs) (9, 15, 21, 36, 39). APC presentation of antigen in the context of major histocompatibility complex (MHC) class I and MHC class II molecules leads to cell-mediated and humoral immunity. The primary APCs in skin are Langerhans cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that induces maturation, activation, and local recruitment of Langerhans cells and other APCs both in vivo and in vitro (8, 30, 34, 51, 53, 54, 58, 59, 62, 65, 68) and is one of many cytokines that show promise as genetic adjuvants for DNA vaccination (for reviews, see references 41 and 50). GM-CSF has been applied in a variety of clinical and research settings. Recombinant human GM-CSF is used as a bone marrow stimulant in immunosuppressed patients, particularly after chemotherapy (19). Expression vectors encoding GM-CSF have been used in several ways to augment immune responses; e.g., they have been transfected into weakly antigenic tumors to increase their antigenicity prior to use of the cells as tumor vaccines (1, 14, 18, 33, 42). Effective experimental vaccines have used GM-CSF as part of an antigen fusion product (64), as a separate DNA molecule for coinoculation with a vaccine (22, 35, 48, 56, 58, 62, 63, 71), and as a priming agent for vaccination (10). When inoculated directly into skin, a vector encoding GM-CSF leads to localized GM-CSF protein expression and subsequent inflammatory responses (37). Conry et al. demonstrated that GM-CSF had immune enhancing effects, especially for generating cell-mediated immunity, when used as a priming agent with a DNA vaccine against carcinoembryonic antigen (10). Maximal efficacy occurred with a 3-day interval between priming and vaccination. Other investigators found that inoculation of GM-CSF DNA prior to vaccination with DNA encoding rabies virus glycoprotein enhanced both B- and T-helper-cell activities (71).
We tested the hypothesis that intracutaneous delivery of a GM-CSF expression vector to rabbits would induce a local population of differentiated APCs and that this procedure could be used to prime sites of E6 DNA vaccination and enhance the induction of prophylactic immunity to CRPV challenge. The data presented here confirm our hypothesis and additionally show that these treatments also increased the frequency of subsequent regression.
MATERIALS AND METHODS
DNA expression vectors.
Four plasmids were used in this study: pdCMV-E6, which encodes the full-length CRPV E6 protein (61); pCMV-β, which encodes β-galactosidase (Clontech, Palo Alto, Calif.); pPJV3226, which encodes mouse GM-CSF (a gift from PowderJect Vaccines, Madison, Wis.); and empty vector pcDNA3.0 (Invitrogen, Carlsbad, Calif.).
Preparation and in vivo delivery of DNAs.
DNA-coated gold beads were prepared for in vivo inoculation as described previously (59, 60), except that the gold beads in this study were 1.9 μm in diameter. DNAs were delivered intracutaneously at a concentration of 1 μg of DNA per site by use of a helium-driven gene gun (PowderJect XR-1 device; PowerJect Vaccines) at 350 lb/in.2.
Rabbit skin biopsies.
Two-millimeter punch biopsies of GM-CSF DNA-inoculated, vector pcDNA3.0-inoculated, and uninoculated rabbit skin were obtained under local lidocaine anesthesia. In one experiment, rabbits were inoculated with DNAs at separate sites 1, 2, 3, 5, 7, 9, 11, 13, 15, and 17 days prior to euthanasia. In another experiment, rabbits were inoculated on day 0 and biopsies were collected on days 1, 2, 3, 5, 7, and 9. Tissues were frozen in cryopreservation medium or fixed in formalin and embedded in paraffin.
Generation of riboprobes.
Recombinant plasmid pcDNA1mGMCSF (kindly provided by Drew Pardoll, Johns Hopkins University) was linearized with HindIII or XbaI and transcribed in vitro with Sp6 or T7 RNA polymerase in the presence of digoxigenin-UTP (Boehringer) to generate sense or antisense riboprobes. In reactions with digoxigenin, 1% of the transcription products of 1 μg of the plasmid DNA were compared by dot blot hybridization to known quantities of a digoxigenin-labeled RNA provided by Boehringer. In each case, the signal associated with the plasmid RNA was comparable to or exceeded that of the 0.2 ng of control RNA supplied by Boehringer.
In situ hybridization.
Formalin-fixed tissue sections were hybridized in situ as described previously (69). An antisense mouse GM-CSF probe was prepared by in vitro transcription and labeled with digoxigenin. Briefly, 5-μm tissue sections were floated in a bath of distilled water onto acid-cleaned, 3-aminopropyltriethoxysilane-coated slides and heated in a 65°C incubator. They were dewaxed in xylene, rehydrated in serial graded ethanol washes (100, 95, and 70%), and digested with proteinase K (20 μg/ml) for 30 min at 37°C. The sections were treated with triethanolamine-acetic anhydride and dehydrated again in serial graded ethanol washes (70, 95, and 100%). The digoxigenin-labeled riboprobes were applied in formamide solution [50% formamide, 0.1 M piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 7.8), 0.01 M EDTA] in a volume, usually 20 μl, sufficient to cover the section. An acid-washed, siliconized coverslip was placed over the section and sealed with rubber cement. The slides were hybridized for 6 h at 50°C. After hybridization, the slides were submerged in 4× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and the coverslips were removed. The tissue sections were washed at room temperature with frequent changes of 1× SSC for 30 min and 0.1× SSC for 15 min. The slides were incubated in 10 μg of RNase A per ml in 2× SSC at 37°C for 15 min and then dehydrated through a graded ethanol series. Air-dried slides hybridized with the digoxigenin-labeled riboprobes were incubated in buffer 1 (100 mM Tris-HCl, 150 mM NaCl [pH 7.5]) for 1 min at room temperature, washed in buffer 1 containing 2% blocking agent (from the Boehringer digoxigenin detection kit) for 1 h, incubated with antidigoxigenin antibody-conjugate (1:500 with buffer 1) containing 1% normal sheep serum and 0.3% Triton X-100 for 1 h at room temperature, washed twice in buffer 1 for 15 min each time, and equilibrated for 10 min with buffer 2 (100 mM Tris-HCl, 100 mM NaCl, 50 mM MgCl2 [pH 9.5]). Color solution was prepared with 45 μl of nitroblue tetrazolium salt solution and 35 μl of X-phosphate solution (both from the Boehringer digoxigenin detection kit) and added to 10 ml of buffer 2. The reaction was stopped after 1 h, and the slides were washed for 5 min with buffer 3 (10 mM Tris HCl [pH 8.0], 1 mM EDTA). The slides were counterstained with nuclear red.
Immunohistochemical analysis.
Immunohistochemical analysis was performed on periodate-lysine-paraformaldehyde-fixed frozen sections with alkaline phosphatase-conjugated mouse anti-rabbit MHC class II monoclonal antibody clone 45-3 (Accurate Chemical and Scientific Corp., Waterbury, N.Y.) and HistoMark Red (Kirkegaard and Perry Laboratories, Gaithersburg, Md.). Negative control sections were treated in the same manner, except without primary antibody. Sections were counterstained with contrast blue.
DNA-based priming and vaccination.
Two-kilogram Pasteurella-free New Zealand White rabbits (Oryctolagus cuniculus) were used. Each experimental and control treatment consisted of a DNA priming agent and a DNA vaccine agent (Table 1). Priming agents were delivered on days 1 and 22, and vaccine agents were delivered on days 4 and 25. All DNAs were delivered intracutaneously with a PowderJect XR-1 gene gun on days 1 and 4 (primary vaccination) and days 22 and 25 (booster vaccination). Ten sites per rabbit on the left back were inoculated with 1 μg of DNA for each treatment (60). Prior to inoculation of a priming agent, rabbits were anesthetized with ketamine (30 mg/kg of body weight) and xylazine (3 mg/kg), hair was clipped from the left back, and residual hair and superficial keratin were treated with a depilatory (Nair, Division of Carter-Wallace, Inc., New York, N.Y.). Prior to inoculation of a vaccine agent, the primed sites were prepared by clipping only in order to minimize nonspecific depilatory-induced inflammation. DNA vaccine agents were administered directly to the previously primed sites.
TABLE 1.
Experimental design
| Rabbit group (no. of rabbits) | DNA treatmenta
|
|||
|---|---|---|---|---|
| Priming agent
|
Vaccine agent
|
|||
| GM-CSF | Plasmid | E6 | Plasmid | |
| Control (3) | − | pcDNA3 | − | pCMV-β |
| E6 only (4) | − | pcDNA3 | + | pdCMV-E6 |
| GM-CSF only (6) | + | pPJV3226 | − | pCMV-β or pcDNA3 |
| GM-CSF + E6 (6) | + | pPJV3226 | + | pdCMV-E6 |
Vector pcDNA3 contains no insert; pPJV3226 encodes mouse GM-CSF; pCMV-β encodes β-galactosidase; pdCMV-E6 encodes the full-length CRPV E6 protein (55).
Challenge with CRPV.
Two weeks after the booster immunization, all rabbits were challenged by CRPV infection on the right side of the back (contralateral to the vaccinated side). Clipped dorsal skin of anesthetized rabbits was infected with a stock of live CRPV diluted in phosphate-buffered saline–glycerol (1:1). Each of three sites per rabbit was challenged at a high, moderate, or low stringency (a total of nine sites per rabbit) using, per site, 30 μl of a 1:50, 1:150, or 1:450 dilution, respectively, as described previously (60, 61). In previous experiments, this CRPV stock induced papillomas at 100% of challenge sites in control rabbits infected with dilutions ranging from 1:10 to 1:160 (60) or from 1:30 to 1:270 (61).
Monitoring papilloma formation.
Rabbits were monitored for papilloma formation beginning 18 days after CRPV challenge and continuing until 116 days after challenge. At each inspection, the location and number of papillomas and the dimensions of each one (length, width, and height) were measured with a Digimatic caliper (Mitutoyo Corp., Aurora, Ill.). The volume of each papilloma was calculated using the mathematical formula for a geode [(4/3)II(length/2)(width/2)(height/2)]. The data from the 19 rabbits were analyzed for resistance to papilloma formation, as measured by three outcomes. (i) Frequency of complete protection, i.e., the percentage of challenge sites that remained papilloma free at a given time period, was determined for each of the nine time periods. (ii) Length of incubation period, i.e., the number of days between CRPV challenge and the time period in which at least one clinically visible papilloma was noted, was determined. When no growth was noted, an extremely conservative approach was used whereby it was assumed that a papilloma did, in fact, appear at 116 days. (iii) Rate of papilloma growth was determined by comparing the average papilloma volumes from onset through 116 days.
The data also were analyzed for susceptibility to papilloma regression using three additional outcomes: (i) frequency of regressing rabbits in a group, i.e., the fraction in which one or more papillomas regressed; (ii) frequency of regressing papillomas in a group, i.e., the fraction of papilloma-forming sites that subsequently regressed completely; and (iii) papilloma duration prior to regression, i.e., the number of days between the time period in which a papilloma was first visible and the time period in which all signs of papilloma (or other lesion) had disappeared. None of the regressed papillomas in this study reformed.
Statistical analysis.
Indicator variables were created for GM-CSF and E6. Each CRPV dilution was analyzed separately. The interaction of GM-CSF DNA inoculation and E6 DNA vaccination was included in all models. The incubation period was analyzed using proportional hazards regression. The volumes of the papillomas were modeled as linear in time. The numbers of papillomas were analyzed using logistic regression. Initially, we examined the nine sites within each rabbit using generalized estimating equations with exchangeable covariance matrices. Inference was almost identical when we treated the nine sites as independent within the same animal. These independence analyses are reported here. All of the statistical analyses performed for this study were done with multivariate models. The significance level of each parameter is the effect of that parameter in the presence of all other parameters, i.e., a type 3 analysis in SAS. This type of analysis provides more accurate significance levels than marginal models for parameters in the final developed models. In all cases, rabbit groups treated with either E6 vaccination or GM-CSF inoculation were compared to the control group. Additionally, the effects of E6 vaccination and GM-CSF inoculation were compared in a purely additive model to determine if E6 vaccination and GM-CSF inoculation acted additively (i.e., without interaction) as opposed to synergistically.
RESULTS
Effects of GM-CSF DNA inoculation.
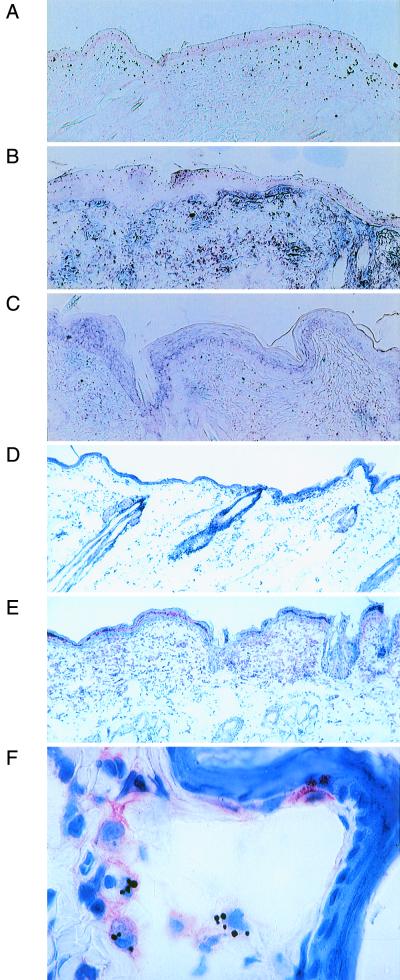
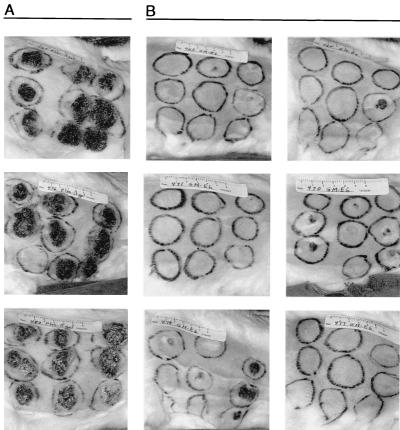
To evaluate the local effects of GM-CSF DNA inoculation, in situ hybridization was performed on sections of GM-CSF-treated skin harvested 1, 2, 3, and 5 days after treatment. Specific expression of GM-CSF mRNA occurred throughout the dermis and epidermis as soon as 1 day after GM-CSF DNA inoculation (Fig. 1A), with the most abundant signals occurring on day 2 (Fig. 1B). GM-CSF mRNA signals decreased on day 3 (data not shown) and were nearly absent on day 5 (Fig. 1C). Clinically, all DNA-inoculated sites developed redness, warmth, and induration (data not shown). Clinical reactions were more extreme at GM-CSF-inoculated sites than at vector-inoculated sites. The clinical reactions peaked approximately 3 days after DNA inoculation. Microscopically, inflammation was observed at all DNA-inoculated sites and almost never at uninoculated sites. Substantial inflammatory responses were elicited following GM-CSF DNA inoculation, beginning 24 h after inoculation. Lesser responses were elicited by the empty vector. Immunohistochemical analysis demonstrated the presence of large numbers of MHC class II-positive cells, including cells with the morphology of macrophages, dermal dendritic cells, and Langerhans cells in GM-CSF-treated skin (Fig. 1E and F). This effect was present 24 h after inoculation, remained strong throughout the first week, and gradually subsided in the second week. Rare MHC class II-positive cells were still detectable 17 days after inoculation. Distinctly less dramatic responses were elicited by the empty vector. These results implied that priming of intracutaneous vaccination sites with the GM-CSF vector prior to administration of an antigen-specific vaccine could enhance the immune response to the vaccine.
FIG. 1.
GM-CSF DNA inoculation induces GM-CSF mRNA expression and MHC class II protein expression. (A to C) The detection of GM-CSF mRNA expression (black grains) by in situ hybridization is shown for rabbit skin 1 (A), 2 (B), and 5 (C) days after GM-CSF DNA inoculation. Normal control skin showed no signal (data not shown). (D to F) The detection of cell surface expression of rabbit MHC class II protein (pink stain) by immunohistochemical analysis is shown for normal control skin (D) and rabbit skin 3 days following GM-CSF DNA inoculation (E and F). The tissue sections in panels E and F were photographed at magnifications of ×8.4 and ×84, respectively.
Resistance to papilloma formation.
Rabbits were primed and vaccinated as shown in Table 1. Two weeks after the booster treatment, each rabbit was challenged with CRPV at low, moderate, and high stringencies as described in Materials and Methods. As expected, all clinical outcomes were monotonically related to the stringency of the viral challenge (see Fig. 4 to 6). As the stringency of the challenge decreased, the probability of disease-free survival increased, the time to papilloma onset increased, and the rate of papilloma growth decreased.
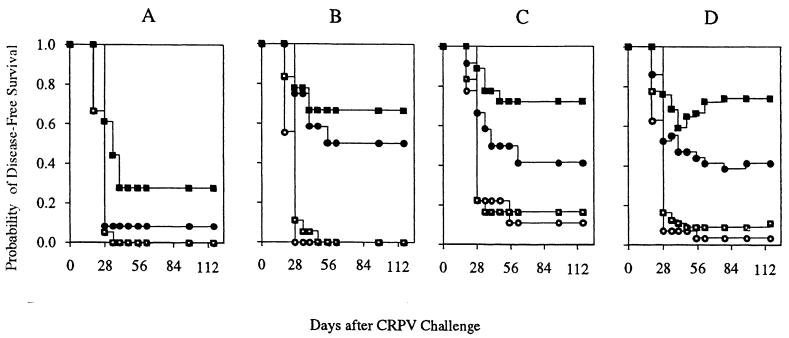
FIG. 4.
Persistence of disease-free status following CRPV challenge. Rabbit groups shown in the Kaplan-Meier curves are control (open circles), GM-CSF DNA only (open squares), E6 DNA vaccine only (filled circles), or GM-CSF DNA plus E6 DNA vaccine (filled squares). (A, B, and C) Probability of a papilloma never forming after challenge at high, moderate, and low stringencies, respectively. (D) Modified Kaplan-Meier curve showing the probability of being disease free at each time point and taking regression into account.
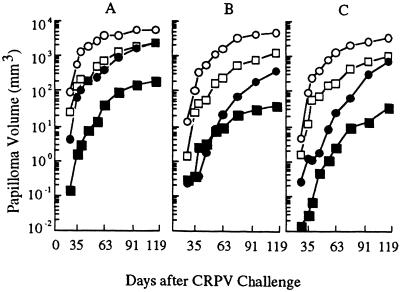
FIG. 6.
Papilloma growth following CRPV challenge. Rabbit groups are control (open circles), GM-CSF DNA only (open squares), E6 DNA vaccine only (filled circles), or GM-CSF DNA plus E6 DNA vaccine (filled squares). (A, B, and C) Outcomes after CRPV challenge at high, moderate, and low stringencies, respectively.
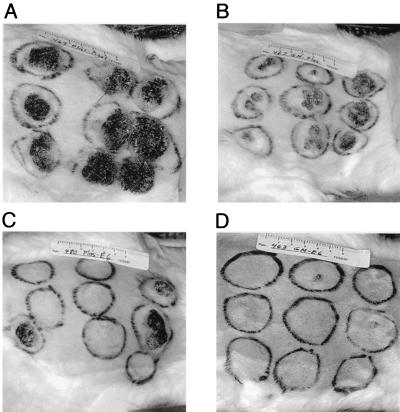
Clinical outcomes in each of the four rabbit groups are shown in Fig. 2. Skin sites that were challenged at low, moderate, and high stringencies (with 1:450, 1:150, and 1:50 dilutions of virus, respectively) are shown on the left, middle, and right of each photograph, respectively. Clinical outcomes in the four groups varied greatly according to treatment. Outcomes among individual rabbits within a group, however, were relatively consistent (Fig. 3). For example, large, rapidly growing papillomas developed at 26 of the 27 challenge sites in the three rabbits treated with control DNAs only (Fig. 3A). In contrast, all six rabbits that were primed with GM-CSF and vaccinated with the E6 gene had fewer and smaller papillomas, whose numbers and sizes also reflected the stringency of the CRPV challenge (Fig. 3B).
FIG. 2.
Clinical outcomes in a representative rabbit from each group 116 days after CRPV challenge. Skin sites that were challenged at low, moderate, and high stringencies are shown on the left, middle, and right of each rabbit's back, respectively. Rabbit groups are control DNA only (A), GM-CSF DNA only (B), E6 vaccine only (C), and GM-CSF DNA plus E6 vaccine (D).
FIG. 3.
Clinical outcomes in all rabbits from two groups 116 days after CRPV challenge. (A) All rabbits treated with control DNA only. (B) All rabbits primed with GM-CSF DNA and immunized with the CRPV E6 DNA vaccine. For each rabbit, the sites challenged at low, moderate, and high stringencies are shown on the left, middle, and right of each rabbit's back, respectively.
Papilloma formation was analyzed by treatment group with respect to three outcomes: (i) the probability of challenge sites remaining disease free; (ii) the length of the incubation period, i.e., the time between CRPV challenge and the first clinical signs of a papilloma; and (iii) the rate of papilloma growth. Kaplan-Meier curves were generated to determine the probability of challenge sites remaining clinically free of disease throughout the experiment (Fig. 4A to C). Among the sites challenged at a moderate stringency, for example, the chance of never forming a papilloma was 50% in rabbits vaccinated with E6 alone versus 0% in control rabbits. GM-CSF alone had no effect, but the combination of GM-CSF plus E6 by far yielded the highest probability of disease-free survival, with 72.2% of sites never forming a papilloma.
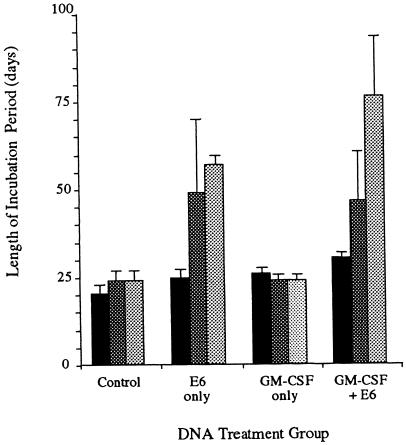
Although neither the E6 vaccine nor the GM-CSF treatment alone or in combination completely protected against papilloma formation, the papillomas that did form had longer incubation periods and slower rates of growth. The effects of treatment on the incubation period are depicted graphically in Fig. 5, and the magnitude and significance level of these effects are provided in Table 2. In the control group, the average incubation period was 20.3 to 24.0 days, depending on the challenge stringency. With E6 vaccination alone, the average incubation period increased by 4.5, 25.2, and 32.8 days at the high, moderate, and low challenge stringencies, respectively. GM-CSF DNA inoculation alone increased the incubation period only at the high challenge stringency, by 5.5 days. The combination of E6 vaccination plus GM-CSF priming increased the length of incubation by 10.0, 25.2, and 32.8 days at the high, moderate, and low challenge stringencies, respectively. These effects were additive.
FIG. 5.
Incubation period following DNA treatment. Bars represent low (pale gray)-, moderate (dark gray)-, and high (black)-stringency CRPV challenges, respectively. Data are the means and standard errors of the means.
TABLE 2.
Magnitude and significance of DNA effects on incubation perioda
| DNA treatment | Effect of treatment at the following CRPV challenge stringency:
|
|||||
|---|---|---|---|---|---|---|
| High
|
Moderate
|
Low
|
||||
| Dayb | P | Dayb | P | Dayb | P | |
| Control | 20.3 | NA | 24.0 | NA | 24.0 | NA |
| E6 only | 24.8 | 0.0700 | 49.2 | 0.0503 | 56.8 | 0.0148 |
| GM-CSF only | 25.8 | 0.0334 | 24.0 | NS | 24.0 | 0.0490 |
| GM-CSF + E6 | 30.3 | No interaction | 46.7 | No interaction | 76.3 | No interaction |
The data are based on maximum-likelihood estimates. NS, not significant at a P value of <0.10. NA, not applicable. No interaction, an additive model fits the data.
Mean number of days after CRPV challenge preceding papilloma onset.
E6 DNA vaccination, GM-CSF DNA inoculation, or a combination of both treatments also had strong effects on papilloma growth, as shown in Fig. 6 and Table 3. These effects were greater in rabbits treated by E6 DNA vaccination than by GM-CSF DNA inoculation and, by far, the greatest effects occurred in rabbits receiving the combined GM-CSF–E6 treatment. One month after challenge under the least stringent condition, for example, papillomas in the E6-vaccinated group were only 1.3 mm3, whereas papillomas in the control group had reached a volume of 89 mm3. GM-CSF treatment also reduced the rate of papilloma growth, but the magnitude of its effect, resulting in an average papilloma volume of 12 mm3, was smaller than that of E6 treatment. The strongest effects were observed in rabbits treated with the GM-CSF–E6 combination; their papillomas, on average, were only 0.03 mm3 (>99.9% reduction from the control group). Results at subsequent times also showed more than a 99% reduction in volume in this group, and similar results were obtained under the moderate- and high-stringency conditions.
TABLE 3.
Magnitude and significance of DNA effects on papilloma growtha
| Dayb | DNA treatment | Effect of treatment at the following CRPV challenge stringency:
|
|||||
|---|---|---|---|---|---|---|---|
| High
|
Moderate
|
Low
|
|||||
| Vol | P | Vol | P | Vol | P | ||
| 35 | Control | 566 | NA | 104 | NA | 89 | NA |
| E6 only | 54 | 0.0002 | 0.4 | 0.004 | 1.3 | 0.0070 | |
| GM-CSF only | 141 | 0.0006 | 25 | 0.0136 | 12 | 0.0096 | |
| GM-CSF + E6 | 1.5 | 0.0134 | 0.07 | 0.0575 | 0.03 | 0.0478 | |
| 62 | Control | 3,919 | NA | 1,744 | NA | 1,323 | NA |
| E6 only | 372 | 0.0001 | 22 | 0.0001 | 25 | 0.0002 | |
| GM-CSF only | 733 | 0.0001 | 256 | 0.0001 | 175 | 0.0002 | |
| GM-CSF + E6 | 42 | 0.0023 | 3.1 | 0.0001 | 2.5 | 0.0035 | |
| 96 | Control | 5,784 | NA | 4,153 | NA | 2,689 | NA |
| E6 only | 1,701 | 0.0026 | 214 | 0.0001 | 306 | 0.0013 | |
| GM-CSF only | 1,917 | 0.0022 | 806 | 0.0001 | 711 | 0.003 | |
| GM-CSF + E6 | 147 | 0.1248 | 11 | 0.0019 | 14 | 0.0422 | |
Vol, mean papilloma volume per site in cubic millimeters. A significant P value for the GM-CSF-E6 category means that a synergistic model fits the data. NA, not applicable.
Number of days after CRPV challenge.
Susceptibility to papilloma regression.
During the course of the experiment, a total of 16 sites, distributed across all CRPV challenge sites in the experimental rabbits, formed papillomas that subsequently underwent complete regression (Table 4). No papilloma in the control rabbits regressed. A modified Kaplan-Meier curve combining all challenge sites and taking regression into account is depicted in Fig. 5D. Papilloma regression occurred in 75, 33, and 83% of rabbits per group following treatment with the E6 vaccine only, GM-CSF only, and the GM-CSF–E6 combination, respectively. Within the regressor rabbits of each group, the rates at which papillomas completely regressed were 13, 6, and 71%, respectively. In the same groups, the mean durations of papillomas that ultimately regressed were 43.7, 27.3, and 18.9 days per group, respectively. These data indicate that regression was primarily attributable to E6 vaccination and secondarily to GM-CSF treatment and that by far the strongest effects occurred with GM-CSF priming plus E6 vaccination.
TABLE 4.
Papilloma regression
| Rabbit group | Regression
|
||||
|---|---|---|---|---|---|
| By rabbits
|
By papillomas
|
Papilloma durationa
|
|||
| No.b | % | No.c | % | Mean ± SEM | |
| Control | 0/3 | 0/26 | NA | ||
| E6 only | 3/4 | 75 | 3/21 | 13 | 27.3 ± 20.3 |
| GM-CSF only | 2/6 | 33 | 3/48 | 6.3 | 43.7 ± 19.5 |
| GM-CSF + E6 | 5/6 | 83 | 10/14 | 71 | 18.9 ± 1.3 |
Mean number of days between papilloma onset and complete regression. NA, not applicable.
Number of rabbits with regression/number of rabbits per group.
Number of papillomas that regressed/number of papillomas that formed.
DISCUSSION
High-risk HPV infection is a prerequisite for the development of cervical cancer. Host immune status is a critical determinant of the outcome of papillomavirus infections (45, 47; Magnusson et al., Letter). Vaccination to induce immunity to HPV could prevent primary infection and/or treat established disease (66). While an HPV L1-based vaccine should prevent primary HPV infection and help contain the spread of an established infection, it is not likely to have major therapeutic effects on the lesions themselves. This is because L1 expression is generally restricted to terminally differentiated keratinocytes, which produce virus particles, and because L1 expression decreases with premalignant changes, being virtually absent in papillomavirus-associated cancers.
Previous studies with the CRPV-rabbit model of high-risk HPV infection have demonstrated that CRPV E6 vaccination can induce significant protection against subsequent CRPV challenge when DNA vaccines are used (28, 61). This result indicates that the previously reported lack of protection with a vaccinia virus-based CRPV E6 vaccine must have been due to factors other than the targeted protein. Contributing factors might include the level of expression or intracellular processing of E6 antigens and might be associated with the dose, schedule, and/or route of vaccine administration. The genetic backgrounds of the rabbits and/or the particular strain of CRPV also might have been factors.
The current study was undertaken to determine whether the prophylactic efficacy of our CRPV E6 DNA vaccine (61) could be augmented by priming the sites of vaccination with a GM-CSF expression vector. GM-CSF is a potent cytokine that induces the maturation and migration of professional APCs to a site of GM-CSF expression. We chose to test GM-CSF treatment prior to E6 DNA vaccination as an augmentation strategy because DNA vaccine-induced immune responses depend on APCs and because GM-CSF treatments have been used successfully in other vaccine studies (7, 42, 43, 48, 56).
APCs express MHC class II and costimulatory proteins on their surfaces (11, 21, 39). First, we demonstrated that GM-CSF mRNA expression and local recruitment of activated MHC class II-positive dendritic cells were induced by DNA inoculation of rabbit skin with a GM-CSF expression vector. Activated cells were abundant by 3 days after inoculation and persisted for at least 9 days, gradually decreasing in number. Local loss of MHC class II-positive cells was preceded by a loss of GM-CSF mRNA; other studies have shown that such local decreases in dendritic APC numbers at the inoculation site are paralleled by their increased migration into draining lymph nodes (9).
Next, we performed a prophylactic vaccination experiment that involved treating rabbits with control DNA only, GM-CSF DNA only, the CRPV E6 DNA vaccine only, or a combination of GM-CSF DNA plus the E6 DNA vaccine (Table 1). CRPV challenge of each rabbit was performed at three stringencies with serial dilutions of virus. As would be predicted for causal relationships, the effects of the experimental treatments on papilloma formation were directly proportional to the dose of CRPV. E6 DNA vaccination prolonged the incubation period, increased the probability of disease-free survival, and inhibited papilloma growth. GM-CSF DNA inoculation had lesser effects on the incubation period and on the rate of growth and had no effect on disease-free survival. This GM-CSF effect, in the absence of E6 vaccination, was probably a consequence of increased numbers and activity of APCs circulating systemically at the time of CRPV challenge, 17 days after the GM-CSF booster. APCs could migrate to the (contralateral) sites of CRPV infection and rapidly respond to early viral antigens expressed in infected cells. This interpretation is consistent with the fact that the major effect of GM-CSF was on papilloma growth. While the effects of E6 vaccination alone were clearly stronger than the more modest effects of GM-CSF inoculation alone, these effects were dramatically enhanced by priming the sites of E6 DNA vaccination with GM-CSF. The combination treatment resulted in more than a 99% reduction in papilloma volume relative to the results for the control group. In conclusion, GM-CSF priming is an effective strategy for augmenting the efficacy of CRPV E6 DNA vaccination in the CRPV-rabbit model. This and other complementary strategies may ultimately be combined to produce the most effective HPV vaccines. One such strategy is to combine a CRPV E6 DNA vaccine with additional DNA vaccines for the CRPV E1, E2, and E7 proteins (28).
In the battle between papillomaviruses and their hosts, immunity to the papillomavirus E6 protein (and other early viral proteins) can act only at the level of infected cells. Immune responses to early antigens cannot affect viral attachment or penetration because the early proteins are not part of the virion. They cannot affect virion uncoating or trafficking to the cell nucleus because the early proteins can be synthesized only after these processes are complete. On the other hand, foreign proteins within a cell, including papillomavirus early proteins, will be degraded into peptides which subsequently form complexes with MHC proteins; these complexes, as subsequently expressed on the cell surface, can be recognized by T lymphocytes, triggering a cell-mediated immune response. A vigorous immune response to early viral antigens in a prophylactic setting would attack newly infected cells to preclude the formation of a papilloma or delay its onset and slow its growth. Indeed, E6 vaccination alone increased the probability of disease-free survival, prolonged the incubation period, and reduced the rate of papilloma growth. Moreover, the use of GM-CSF priming together with E6 vaccination greatly enhanced these effects. Depending on the stringency of CRPV challenge, the GM-CSF–E6 treatment increased disease-free survival by up to 66.7%, prolonged the incubation period by up to 54 days, and decreased the rate of papilloma growth by up to 99.9%, compared to the results for the control group. In a therapeutic setting, this kind of immunity might well suppress or eliminate viral lesions and the infection itself.
In this study, papillomas regressed in 75% of the E6 DNA-vaccinated rabbits (Table 4). A similar result was reported by Lathe et al., who observed regression in 80% of rabbits vaccinated with a vaccinia virus-based CRPV E6 vaccine (38). However, regression in that study also occurred in 30% of rabbits treated with the vaccinia virus vector alone, suggesting that part of the effect was nonspecific. In the current study, regression occurred only in rabbits with prophylactic immunity to papilloma formation, induced as a result of E6 vaccination and/or GM-CSF inoculation. The more important factor for inducing regression was the E6 vaccine, and the strongest effects occurred in the GM-CSF–E6 treatment group.
Regression was a delayed effect that probably required an augmentation of immune responses to E6 antigens, the induction of new immune responses to other viral antigens, or both. Some papillomavirus-infected cells in the less strongly protected rabbits were probably continually being killed by immunologic mechanisms. This activity would provide a cytokine-rich environment and a source of viral antigens to support the further development of CRPV-specific immunity, which ultimately would be able to cause papilloma regression. It is interesting to note that the papillomas destined to regress persisted initially for 2 to 6 weeks, an appropriate time frame for new immune responses to develop. This associated regression shows that the E6 vaccine induced therapeutic as well as prophylactic effects, suggesting that the vaccine might also be efficacious against preexisting lesions.
In summary, vaccination against the CRPV E6 protein prevented or suppressed papilloma formation in rabbits subsequently challenged with CRPV. Moreover, these effects were dramatically enhanced when GM-CSF priming was combined with E6 vaccination. Future in vitro studies will seek to identify immune responses to specific CRPV antigens that develop following CRPV E6 vaccination and following CRPV challenge. Given our current understanding of the immune system and of the role that the E6 protein plays in papillomavirus infections, the primary mechanism by which the E6 vaccine induced its effects was most likely cytotoxicity to papillomavirus-infected cells. This type of immunity is thought to be required for immunotherapy of papillomavirus infections and suggests that an E6 vaccine has the potential for therapeutic applications.
REFERENCES
- 1.Aruga E, Aruga A, Arca M J, Lee W M, Yang N S, Smith J W N, Chang A E. Immune responsiveness to a murine mammary carcinoma modified to express B7-1, interleukin-12, or GM-CSF. Cancer Gene Ther. 1997;4:157–166. [PubMed] [Google Scholar]
- 2.Bontkes H J, van Duin M, de Gruijl T D, Duggan-Keen M F, Walboomers J M, Stukart M J, Verheijen R H, Helmerhorst T J, Meijer C J, Scheper R J, Stevens F R, Dyer P A, Sinnott P, Stern P L. HPV 16 infection and progression of cervical intra-epithelial neoplasia: analysis of HLA polymorphism and HPV 16 E6 sequence variants. Int J Cancer. 1998;78:166–171. doi: 10.1002/(sici)1097-0215(19981005)78:2<166::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Brandsma J L. Animal models for human papillomavirus vaccine development. In: Lacey C, editor. Papillomavirus reviews: current research on papillomaviruses. Leeds, England: Leeds University Press; 1996. pp. 69–78. [Google Scholar]
- 4.Brandsma J L. Animal models of human-papillomavirus-associated oncogenesis. Intervirology. 1994;37:189–200. doi: 10.1159/000150377. [DOI] [PubMed] [Google Scholar]
- 5.Brandsma J L, Yang Z H, Barthold S W, Johnson E A. Use of a rapid, efficient inoculation method to induce papillomas by cottontail rabbit papillomavirus DNA shows that the E7 gene is required. Proc Natl Acad Sci USA. 1991;88:4816–4820. doi: 10.1073/pnas.88.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breitburd F, Salmon J, Orth G. The rabbit viral skin papillomas and carcinomas: a model for the immunogenetics of HPV-associated carcinogenesis. Clin Dermatol. 1997;15:237–247. doi: 10.1016/s0738-081x(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 7.Bueler H, Mulligan R C. Induction of antigen-specific tumor immunity by genetic and cellular vaccines against MAGE: enhanced tumor protection by coexpression of granulocyte-macrophage colony-stimulating factor and B7-1. Mol Med. 1996;2:545–555. [PMC free article] [PubMed] [Google Scholar]
- 8.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 9.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 10.Conry R M, Widera G, LoBuglio A F, Fuller J T, Moore S E, Barlow D L, Turner J, Yang N S, Curiel D T. Selected strategies to augment polynucleotide immunization. Gene Ther. 1996;3:67–74. [PubMed] [Google Scholar]
- 11.Corr M, Lee D J, Carson D A, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis H L. Plasmid DNA expression systems for the purpose of immunization. Curr Opin Biotechnol. 1997;8:635–646. doi: 10.1016/s0958-1669(97)80041-9. [DOI] [PubMed] [Google Scholar]
- 13.Defeo-Jones D, Vuocolo G A, Haskell K M, Hanobik M G, Kiefer D M, McAvoy E M, Ivey-Hoyle M, Brandsma J L, Oliff A, Jones R E. Papillomavirus E7 protein binding to the retinoblastoma protein is not required for viral induction of warts. J Virol. 1993;67:716–725. doi: 10.1128/jvi.67.2.716-725.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilloo D, Bacon K, Holden W, Zhong W, Burdach S, Zlotnik A, Brenner M. Combined chemokine and cytokine gene transfer enhances antitumor immunity. Nat Med. 1996;2:1090–1095. doi: 10.1038/nm1096-1090. [DOI] [PubMed] [Google Scholar]
- 15.Doe B, Selby M, Barnett S, Baenziger J, Walker C M. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly J J, Martinez D, Jansen K U, Ellis R W, Montgomery D L, Liu M A. Protection against papillomavirus with a polynucleotide vaccine. J Infect Dis. 1996;173:314–320. doi: 10.1093/infdis/173.2.314. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 18.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edmonson J H, Hartmann L C, Long H J, Colon-Otero G, Fitch T R, Jefferies J A, Braich T A, Maples W J. Granulocyte-macrophage colony-stimulating factor. Preliminary observations on the influences of dose, schedule, and route of administration in patients receiving cyclophosphamide and carboplatin. Cancer. 1992;70:2529–2539. doi: 10.1002/1097-0142(19921115)70:10<2529::aid-cncr2820701023>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. Dermatology and general medicine. 4th ed. Vol. 2. New York, N.Y: McGraw-Hill Book Co.; 1993. [Google Scholar]
- 21.Fu T M, Ulmer J B, Caulfield M J, Deck R R, Friedman A, Wang S, Liu X, Donnelly J J, Liu M A. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3:362–371. [PMC free article] [PubMed] [Google Scholar]
- 22.Geissler M, Gesien A, Tokushige K, Wands J R. Enhancement of cellular and humoral immune responses to hepatitis C virus core protein using DNA-based vaccines augmented with cytokine-expressing plasmids. J Immunol. 1997;158:1231–1237. [PubMed] [Google Scholar]
- 23.Giri I, Yaniv M. Structural and mutational analysis of E2 trans-activating proteins of papillomaviruses reveals three distinct functional domains. EMBO J. 1988;7:2823–2829. doi: 10.1002/j.1460-2075.1988.tb03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giri I, Yaniv M. Study of the E2 gene product of the cottontail rabbit papillomavirus reveals a common mechanism of transactivation among papillomaviruses. J Virol. 1988;62:1573–1581. doi: 10.1128/jvi.62.5.1573-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han R, Breitburd F, Marche P N, Orth G. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature. 1992;356:66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- 26.Han R, Cladel N M, Reed C A, Christensen N D. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology. 1998;251:253–263. doi: 10.1006/viro.1998.9416. [DOI] [PubMed] [Google Scholar]
- 27.Han R, Reed C A, Cladel N M, Christensen N D. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces T cell-mediated but not humoral immune responses in rabbits. Vaccine. 1999;17:1558–1566. doi: 10.1016/s0264-410x(98)00356-9. [DOI] [PubMed] [Google Scholar]
- 28.Han R, Cladel N M, Reed C A, Peng X, Christensen N D. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J Virol. 1999;73:7039–7043. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helland A, Borresen A L, Kristensen G, Ronningen K S. DQA1 and DQB1 genes in patients with squamous cell carcinoma of the cervix: relationship to human papillomavirus infection and prognosis. Cancer Epidemiol Biomarkers Prev. 1994;3:479–486. [PubMed] [Google Scholar]
- 30.Heufler C, Koch F, Schuler G. Granulocyte/macrophage colony-stimulating factor and interleukin 1 mediate the maturation of murine epidermal Langerhans cells into potent immunostimulatory dendritic cells. J Exp Med. 1988;167:700–705. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins G D, Uzelin D M, Phillips G E, McEvoy P, Marin R, Burrell C J. Transcription patterns of human papillomavirus type 16 in genital intraepithelial neoplasia: evidence for promoter usage within the E7 open reading frame during epithelial differentiation. J Gen Virol. 1992;73:2047–2057. doi: 10.1099/0022-1317-73-8-2047. [DOI] [PubMed] [Google Scholar]
- 32.Hildesheim A, Schiffman M, Scott D R, Marti D, Kissner T, Sherman M E, Glass A G, Manos M M, Lorincz A T, Kurman R J, Buckland J, Rush B B, Carrington M. Human leukocyte antigen class I/II alleles and development of human papillomavirus-related cervical neoplasia: results from a case-control study conducted in the United States. Cancer Epidemiol Biomarkers Prev. 1998;7:1035–1041. [PubMed] [Google Scholar]
- 33.Iftner, T., M. Oft, S. Bohm, S. P. Wilczynski, and H. Pfister. Transcription of the E6 and E7 genes of human papillomavirus type 6 in anogenital condylomata is restricted to undifferentiated cell layers of the epithelium. J. Virol. 66:4639–4646. [DOI] [PMC free article] [PubMed]
- 34.Irvine K R, Rao J B, Rosenberg S A, Restifo N P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996;156:238–245. [PMC free article] [PubMed] [Google Scholar]
- 35.Iwasaki A, Stiernholm B J, Chan A K, Berinstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 36.Iwasaki A, Torres C A, Ohashi P S, Robinson H L, Barber B H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 37.Keller E T, Burkholder J K, Shi F, Pugh T D, McCabe D, Malter J S, MacEwen E G, Yang N S, Ershler W B. In vivo particle-mediated cytokine gene transfer into canine oral mucosa and epidermis. Cancer Gene Ther. 1996;3:186–191. [PubMed] [Google Scholar]
- 38.Lathe R, Kieny M P, Dott K, Gautier C, Clertant P, Cuzin F, Breitburd F, Orth G, Meneguzzi G. Vaccination against polyoma- and papillomavirus-induced tumors using vaccinia recombinants expressing non-structural proteins. In: Mehens A, Spier R E, editors. Vaccines for sexually transmitted diseases. London, England: Butterworths; 1989. pp. 166–176. [Google Scholar]
- 39.Leachman S A, Brandsma J L. DNA vaccines for papillomaviruses. In: Tindle R W, editor. Human papillomavirus vaccines. Vol. 14. Austin, Tex: R. G. Landes Company; 1999. pp. 105–148. [Google Scholar]
- 40.Lowy D R, Kirnbauer R, Schiller J T. Genital human papillomavirus infection. Proc Natl Acad Sci USA. 1994;91:2436–2440. doi: 10.1073/pnas.91.7.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maecker H T, Umetsu D T, DeKruyff R H, Levy S. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine. 1997;15:1687–1696. doi: 10.1016/s0264-410x(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 42.Mahvi D M, Burkholder J K, Turner J, Culp J, Malter J S, Sondel P M, Yang N S. Particle-mediated gene transfer of granulocyte-macrophage colony-stimulating factor cDNA to tumor cells: implications for a clinically relevant tumor vaccine. Hum Gene Ther. 1996;7:1535–1543. doi: 10.1089/hum.1996.7.13-1535. [DOI] [PubMed] [Google Scholar]
- 43.Mahvi D M, Sondel P M, Yang N S, Albertini M R, Schiller J H, Hank J, Heiner J, Gan J, Swain W, Logrono R. Phase I/IB study of immunization with autologous tumor cells transfected with the GM-CSF gene by particle-mediated transfer in patients with melanoma or sarcoma. Hum Gene Ther. 1997;8:875–891. doi: 10.1089/hum.1997.8.7-875. [DOI] [PubMed] [Google Scholar]
- 44.Manickan E, Karem K L, Rouse B T. DNA vaccines—a modern gimmick or a boon to vaccinology? Crit Rev Immunol. 1997;17:139–154. doi: 10.1615/critrevimmunol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 45.McMichael H. Inhibition by methylprednisolone of regression of the Shope rabbit papilloma. J Natl Cancer Inst. 1967;39:55–63. [PubMed] [Google Scholar]
- 46.Meyers C, Harry J, Lin Y L, Wettstein F O. Identification of three transforming proteins encoded by cottontail rabbit papillomavirus. J Virol. 1992;66:1655–1664. doi: 10.1128/jvi.66.3.1655-1664.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz N, Bosch F X. Cervical cancer and human papillomavirus: epidemiological evidence and perspectives for prevention. Salud Publica Mex. 1997;39:274–282. doi: 10.1590/s0036-36341997000400005. [DOI] [PubMed] [Google Scholar]
- 48.Okada E, Sasaki S, Ishii N, Aoki I, Yasuda T, Nishioka K, Fukushima J, Miyazaki J, Wahren B, Okuda K. Intranasal immunization of a DNA vaccine with IL-12- and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–3647. [PubMed] [Google Scholar]
- 49.Pardoll D M, Beckerleg A M. Exposing the immunology of naked DNA vaccines. Immunity. 1995;3:165–169. doi: 10.1016/1074-7613(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 50.Pasquini S, Xiang Z, Wang Y, He Z, Deng H, Blaszczyk-Thurin M, Ertl H C. Cytokines and costimulatory molecules as genetic adjuvants. Immunol Cell Biol. 1997;75:397–401. doi: 10.1038/icb.1997.62. [DOI] [PubMed] [Google Scholar]
- 51.Reid C D, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992;149:2681–2688. [PubMed] [Google Scholar]
- 52.Robinson H L, Torres C A. DNA vaccines. Semin Immunol. 1997;9:271–283. doi: 10.1006/smim.1997.0083. [DOI] [PubMed] [Google Scholar]
- 53.Santiago-Schwarz F, Borrero M, Tucci J, Palaia T, Carsons S E. In vitro expansion of CD13+CD33+ dendritic cell precursors from multipotent progenitors is regulated by a discrete fas-mediated apoptotic schedule. J Leukoc Biol. 1997;62:493–502. doi: 10.1002/jlb.62.4.493. [DOI] [PubMed] [Google Scholar]
- 54.Siena S, Di Nicola M, Bregni M, Mortarini R, Anichini A, Lombardi L, Ravagnani F, Parmiani G, Gianni A M. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–1471. [PubMed] [Google Scholar]
- 55.Sillman F H, Sedlis A. Anogenital papillomavirus infection and neoplasia in immunodeficient women: an update. Dermatol Clin. 1991;9:353–369. [PubMed] [Google Scholar]
- 56.Sin J I, Sung J H, Suh Y S, Lee A H, Chung J H, Sung Y C. Protective immunity against heterologous challenge with encephalomyocarditis virus by VP1 DNA vaccination: effect of coinjection with a granulocyte-macrophage colony stimulating factor gene. Vaccine. 1997;15:1827–1833. doi: 10.1016/s0264-410x(97)88856-1. [DOI] [PubMed] [Google Scholar]
- 57.Stoler M H, Broker T R. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol. 1986;17:1250–1258. doi: 10.1016/s0046-8177(86)80569-x. [DOI] [PubMed] [Google Scholar]
- 58.Strobl H, Riedl E, Bello-Fernandez C, Knapp W. Epidermal Langerhans cell development and differentiation. Immunobiology. 1998;198:588–605. doi: 10.1016/S0171-2985(98)80080-6. [DOI] [PubMed] [Google Scholar]
- 59.Strunk D, Rappersberger K, Egger C, Strobl H, Kromer E, Elbe A, Maurer D, Stingl G. Generation of human dendritic cells/Langerhans cells from circulating CD34+ hematopoietic progenitor cells. Blood. 1996;87:1292–1302. [PubMed] [Google Scholar]
- 60.Sundaram P, Tigelaar R E, Brandsma J L. Intracutaneous vaccination of rabbits with the cottontail rabbit papillomavirus (CRPV) L1 gene protects against virus challenge. Vaccine. 1997;15:664–671. doi: 10.1016/s0264-410x(96)00237-x. [DOI] [PubMed] [Google Scholar]
- 61.Sundaram P, Tigelaar R E, Xiao W, Brandsma J L. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine. 1998;16:613–623. doi: 10.1016/s0264-410x(97)84510-0. [DOI] [PubMed] [Google Scholar]
- 62.Svanholm C, Lowenadler B, Wigzell H. Amplification of T-cell and antibody responses in DNA-based immunization with HIV-1 Nef by co-injection with a GM-CSF expression vector. Scand J Immunol. 1997;46:298–303. doi: 10.1046/j.1365-3083.1997.d01-130.x. [DOI] [PubMed] [Google Scholar]
- 63.Syrengelas A D, Chen T T, Levy R. DNA immunization induces protective immunity against B-cell lymphoma. Nat Med. 1996;2:1038–1041. doi: 10.1038/nm0996-1038. [DOI] [PubMed] [Google Scholar]
- 64.Tao M H, Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993;362:755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- 65.Tazi A, Bouchonnet F, Grandsaigne M, Boumsell L, Hance A J, Soler P. Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. J Clin Investig. 1993;91:566–576. doi: 10.1172/JCI116236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tindle R W. Immunomanipulative strategies for the control of human papillomavirus associated cervical disease. Immunol Res. 1997;16:387–400. doi: 10.1007/BF02786401. [DOI] [PubMed] [Google Scholar]
- 67.Wettstein F O. Papillomaviruses and malignant progression. I. Cottontail rabbit (Shope) papillomavirus. In: Salzman N P, Howley P M, editors. The Papovaviridae. 2. The papillomaviruses. New York, N.Y: Plenum Press; 1987. pp. 167–186. [Google Scholar]
- 68.Witmer-Pack M D, Olivier W, Valinsky J, Schuler G, Steinman R M. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987;166:1484–1498. doi: 10.1084/jem.166.5.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu T C, Mann R B, Epstein J I, MacMahon E, Lee W A, Charache P, Hayward S D, Kurman R J, Hayward G S, Ambinder R F. Abundant expression of EBER1 small nuclear RNA in nasopharyngeal carcinoma. A morphologically distinctive target for detection of Epstein-Barr virus in formalin-fixed paraffin-embedded carcinoma specimens. Am J Pathol. 1991;138:1461–1469. [PMC free article] [PubMed] [Google Scholar]
- 70.Wu X, Xiao W, Brandsma J L. Papilloma formation by cottontail rabbit papillomavirus requires E1 and E2 regulatory genes in addition to E6 and E7 transforming genes. J Virol. 1994;68:6097–6102. doi: 10.1128/jvi.68.9.6097-6102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xiang Z, Ertl H C. Manipulation of the immune response to a plasmid-encoded viral antigen by coinoculation with plasmids expressing cytokines. Immunity. 1995;2:129–135. doi: 10.1016/s1074-7613(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 72.Zeltner R, Borenstein L A, Wettstein F O, Iftner T. Changes in RNA expression pattern during the malignant progression of cottontail rabbit papillomavirus-induced tumors in rabbits. J Virol. 1994;68:3620–3630. doi: 10.1128/jvi.68.6.3620-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]