Abstract
Objective
There are numerous reports of people substituting medical cannabis (MC) for medications. Our obejctive was to investigate the degree to which this substitution occurs among people with rheumatic conditions.
Methods
In a secondary analysis from a cross‐sectional survey conducted with patient advocacy groups in the US and Canada, we investigated MC use and medication substitution among people with rheumatic conditions. We subgrouped by whether participants substituted MC for medications and investigated differences in perceived symptom changes and use patterns, including methods of ingestion, cannabinoid content (cannabidiol vs delta‐9‐tetrahydrocannabinol [THC]), and use frequency.
Results
Among 763 participants, 62.5% reported substituting MC products for medications, including nonsteroidal anti‐inflammatory drugs (54.7%), opioids (48.6%), sleep aids (29.6%), and muscle relaxants (25.2%). Following substitution, most participants reported decreases or cessation in medication use. The primary reasons for substitution were fewer adverse effects, better symptom management, and concerns about withdrawal symptoms. Substitution was associated with THC use and significantly higher symptom improvements (including pain, sleep, anxiety, and joint stiffness) than nonsubstitution, and a higher proportion of substitutors used inhalation routes than those who did not.
Conclusion
Although the determination of causality is limited by our cross‐sectional design, these findings suggest that an appreciable number of people with rheumatic diseases substitute medications with MC for symptom management. Inhalation of MC products containing some THC was most commonly identified among those substituting, and disease characteristics did not differ by substitution status. Further study is needed to better understand the role of MC for symptom management in rheumatic conditions.
INTRODUCTION
The burden of rheumatic diseases is growing in the United States and Canada, with nearly 50% of adults age ≥65 years reporting at least one rheumatic condition. 1 , 2 These conditions often present with considerable pain and associated symptoms of sleep disturbance, mood changes, and disability. Unfortunately, many existing pain medications (eg, opioids and nonsteroidal anti‐inflammatory drugs [NSAIDs]) provide insufficient benefit and are accompanied by undesirable side effects. 3 As a result, some people with rheumatic conditions have reportedly turned to medical cannabis (MC) products. Indeed, the use of MC has grown substantially in recent years, from approximately 680,000 patients in 2016 to nearly 3 million in 2020 in the United States, with chronic pain being the most common qualifying condition for MC licensure. 4
MC products contain cannabinoids, such as cannabidiol (CBD) and delta‐9‐tetrahydrocannabinol (THC). In preclinical studies, both CBD and THC display analgesic and anti‐inflammatory effects, 5 , 6 , 7 and, in observational clinical studies, the use of MC products offers benefits for chronic pain management, sleep, and mood symptoms. 8 , 9 , 10 , 11 , 12 , 13 , 14 Further, there are increasing reports that MC use allows people to reduce their use of pain medications, including opioids, with better symptom management and fewer adverse effects. 8 , 9 , 10 , 11 , 12 However, MC products are not without risk. THC‐containing products in particular have been associated with unwanted side effects (eg, dizziness, disorientation, and sedation) and addiction potential. 15 , 16 , 17 Clinical research into the merits of therapeutic cannabis has been slow, mainly because of cannabis criminalization through its status as a Schedule I drug under the federal Controlled Substances Act in the United States. 18 Despite potential benefits, such scarcity of clinical trial literature on cannabinoids among people with rheumatic conditions has resulted in the existing evidence being insufficient to support standardized use. 19
With growing societal use of MC, understanding the current trends in why and how people use MC in the context of rheumatic conditions is critical to complement the limited clinical trial literature. Only a handful of observational studies have investigated MC use among people with rheumatic conditions, a group that may have unique challenges owing to age, substantial use of concomitant medications, and high symptom burden. Thus, we investigated patterns of MC product substitution for symptom management among people reporting current use of MC for rheumatic conditions in the United States and Canada.
This is a secondary data analysis of an existing survey sample of people with rheumatic conditions collected in partnership with the Arthritis Foundation and Arthritis Society Canada. 20 Based on our previous surveys of people using MC for chronic pain, 10 , 11 , 12 we hypothesized that the majority of participants would report substituting MC products for at least one medication class, largely for reasons associated with harm reduction (fewer negative side effects). We also hypothesized that the majority of those who substituted would report decreases or cessation in their use of other pharmacologic products. Lastly, we explored differences in the MC use patterns and clinical characteristics of those who substituted with MC compared with those who did not.
PATIENTS AND METHODS
As previously described, adult residents of the United States and Canada were invited to participate in an online, anonymous, confidential survey in Qualtrics via social media advertisement and email contacts list for the Arthritis Foundation and Arthritis Society Canada. 20 This study received institutional review board approval, and participation in the study was acknowledged as consent. Responses were anonymous, and participants were not compensated. We used the “prevent ballot stuffing” feature in Qualtrics to prevent participants from taking the survey more than one time. The survey included current and past MC use (eg, preferences and decision‐making), sociodemographic information, medication taken and substituted, substance use (eg, alcohol and cigarette), and patient‐reported outcomes on symptoms. Of the 1,727 who completed the survey, 655 had never used cannabis, 268 had used cannabis but discontinued, and 763 currently used cannabis. Only those reporting current use were included in this analysis.
Independent subgrouping
Medication substitution subgroups
We asked participants whether they substituted MC for other medication(s). This question was used to split the sample into subgroups of participants who substituted medication for MC and those that did not.
Measures
Sociodemographic and clinical characteristics and concomitant medication intake
We queried participant age, sex at birth, gender, race and ethnicity, marital status, annual income, education level, and diagnosed rheumatic conditions. We also assessed substance and concomitant medication use. Cigarette use responses included current smoker, former smoker, or never smoker. Alcohol intake responses included “none,” “monthly or less,” “2 to 4 times a month,” “2 to 3 times a week,” and “4 or more times a week.” Participants selected whether they took any of the following pain medication classes: NSAIDs, opioid analgesics, serotonin norepinephrine uptake inhibitors, selective serotonin reuptake inhibitors, gabapentinoids, benzodiazepines, muscle relaxants, sleeping pills, and any other medications, which could include disease‐modifying antirheumatic drugs (DMARDs); we reported other medications taken as a text response.
Substitution and change in concomitant medication
We asked participants whether they had substituted MC for another medication, including for NSAIDs, opioid analgesics, serotonin norepinephrine uptake inhibitors, selective serotonin reuptake inhibitors, gabapentinoids, benzodiazepines, muscle relaxants, sleeping pills, and DMARDs. Those who reported substitution then answered about changes in medication use since starting MC, with the response options “stopped using,” “decreased a lot,” “decreased a little,” “no change,” “increased a little,” and “increased a lot.” They also reported reasons for substituting in a “select all that apply” format, with reasons of “fewer adverse side effects,” “fewer withdrawal effects,” “ability to obtain MC versus medication,” “better symptom management,” and “other.”
Cannabis‐related characteristics
Participants reported their current duration of MC use and anticipated duration of use. Participants also reported the cannabinoid content (CBD, THC, combination, or other). We assessed daily and weekly frequency of use. We also assessed the routes of administration used via a “select all that apply”, with options including smoking, vaporizing, edible, topical applications, tincture/oil, and other route(s); when “other” was selected, a text response was asked for and relevant responses were included in the results. We calculated the number of administration routes and included this value as a continuous outcome. We also asked participants to report their most commonly used administration route.
Change in symptoms since the use of MC
Participants reported their change in symptoms since initiating MC as “very much worse,” “much worse,” “slightly worse,” “no change,” “slightly improved,” “much improved,” and “very much improved.” We analyzed responses continuously on a −3 to 3 scale. The symptoms assessed included pain, sleep, anxiety, fatigue, depression, memory, joint stiffness, spasm, and inflammation.
Clinical measures
2011 fibromyalgia survey criteria
The American College of Rheumatology 2011 fibromyalgia survey criteria consists of two subscale scores: the symptom severity score, a 12‐point measure of symptom burden, and the Widespread Pain Index, a 19‐point score representing painful body areas during the past week. Together, the sum of the symptom severity score and Widespread Pain Index ranges from 0 to 31, with higher scores indicative of worse symptoms. 21
Physical and mental health
We assessed mental and physical health using the PROMIS Global v1.2, a 10‐item measure that assesses an adult's global health. From this measure, we calculated two subscores: the Global Mental Health score and the Global Physical Health score. We then converted raw scores into t‐scores, with a mean of 50 and SD of 10. Better mental and physical functioning are indicated by higher scores. 22
Neuropathic pain
The PainDETECT is a validated 12‐item screening tool for the presence of neuropathic pain. Higher scores indicate a higher likelihood of neuropathic pain. 23
Statistical analysis
Independent subgrouping included medication substitution groups (ie, those reporting substitution of medication for MC compared with those without any substitution). We first used descriptive statistics to characterize the data and reported categorical and continuous data as frequency (n) and percentage (%) and mean ± SD, respectively. We used independent samples t‐tests to assess for significant differences in continuous variables between subgroups with effect sizes are reported as Cohen's d. We used Levine's test to assess the equality of variances. We used Pearson's chi‐square test to assess categorical variable differences. For binary outcomes, we used binary logistic regression to obtain odds ratios (ORs), which are reported with 95% confidence intervals (CIs). All tests were two‐sided tests with significance set at α = 0.05. Adjustment for multiple comparisons was not performed. Analysis was completed using IBM SPSS 28 (2021). 24
RESULTS
Sample characteristics
The study sample (N = 763) was mostly female (n = 642, 84.9%), White (n = 654, 90.8%) participants, with mean ± SD age of 59.0 ± 14.9 years. Most participants were college educated, married, and retired or working full‐time (Table 1). Overall, 151 (19.8%) reported a single rheumatic condition, 116 (15.2%) reported two, 125 (16.4%) reported three, and 371 (48.6%) reported more than three rheumatic conditions, with inflammatory rheumatic disease reported in 501 (68%) and fibromyalgia in 291 (38%). Most participants (75.2%) reported previous recreational cannabis use.
Table 1.
Sample demographic makeup and clinical characteristics*
| Total | No substitution | Substitution | P | |
|---|---|---|---|---|
| Frequency, n (%) | 763 (100.0) | 286 (37.5) | 477 (62.5) | |
| Age, mean (SD), y | 59.0 (14.5) | 61.7 (14.9) | 59.6 (13.8) | 0.098 |
| Sex, n (%) | ||||
| Male | 118 (15.5) | 45 (15.7) | 73 (15.3) | 0.877 |
| Female | 642 (84.1) | 240 (83.9) | 402 (84.3) | |
| Missing | 3 (0.4) | 1 (0.3) | 2 (0.4) | |
| Race and ethnicity, n (%) | ||||
| White/Caucasian | 654 (85.7) | 246 (86.0) | 408 (85.5) | 0.676 |
| Black/African American | 23 (3.0) | 7 (2.4) | 16 (3.4) | |
| Hispanic or Latino | 20 (2.6) | 9 (3.1) | 11 (2.3) | |
| Other | 22 (2.9) | 10 (3.5) | 12 (2.5) | |
| Missing | 44 (5.7) | 14 (4.9) | 30 (6.3) | |
| Relationship, n (%) | ||||
| Single | 83 (10.9) | 20 (7.0) | 63 (13.2) | 0.070 |
| Married | 431 (56.5) | 163 (57.0) | 268 (56.2) | |
| Living with partner | 83 (10.9) | 30 (10.5) | 53 (11.1) | |
| Divorced | 104 (13.6) | 44 (15.4) | 60 (12.6) | |
| Widowed | 44 (5.8) | 20 (7.0) | 24 (5.0) | |
| Missing | 18 (2.4) | 9 (3.1) | 9 (1.9) | |
| Annual income, n (%) | ||||
| $0–$49,999 | 229 (30.0) | 80 (28.0) | 149 (31.2) | 0.933 |
| $50,000–$99,999 | 220 (28.8) | 82 (28.7) | 138 (28.9) | |
| $100,000–$149,999 | 94 (12.3) | 36 (12.6) | 58 (12.2) | |
| $150,000+ | 57 (7.5) | 21 (7.3) | 36 (7.5) | |
| Missing | 163 (21.4) | 67 (23.4) | 96 (20.1) | |
| Education, n (%) | ||||
| High school or less | 84 (11.0) | 37 (12.9) | 47 (9.9) | 0.499 |
| Some college or associate degree | 207 (27.1) | 75 (26.2) | 132 (27.7) | |
| Bachelor's or university degree | 308 (40.4) | 115 (40.2) | 193 (40.5) | |
| Masters, professional, or doctoral degree | 155 (20.3) | 53 (18.5) | 102 (21.4) | |
| Missing | 9 (1.2) | 6 (2.1) | 3 (0.6) | |
| Employment, n (%) | ||||
| Unemployed | 32 (4.2) | 7 (2.4) | 25 (5.2) | <0.001 a |
| Student | 12 (1.6) | 6 (2.1) | 6 (1.3) | |
| Employed | 259 (33.9) | 92 (32.2) | 167 (35.0) | |
| Retired | 319 (41.8) | 145 (50.7) | 174 (36.5) | |
| Unable to work | 128 (16.8) | 33 (11.5) | 95 (19.9) | |
| Missing | 13 (1.7) | 3 (1.0) | 10 (2.1) | |
| Cigarettes, n (%) | ||||
| Never used | 383 (50.2) | 155 (54.2) | 228 (47.8) | 0.242 |
| Used in past | 316 (41.4) | 109 (38.1) | 207 (43.4) | |
| Currently use | 63 (8.3) | 22 (7.7) | 41 (8.6) | |
| Missing | 1 (0.1) | 0 (0.0) | 1 (0.2) | |
| Alcohol, n (%) | ||||
| Nondrinker | 227 (29.8) | 83 (29.0) | 144 (30.2) | 0.719 |
| Drinker | 535 (70.1) | 203 (71.0) | 332 (69.6) | |
| Missing | 1 (0.1) | 0 (0.0) | 1 (0.2) | |
| Country of residence, n (%) | ||||
| United States | 437 (57.3) | 146 (51.0) | 291 (61.0) | 0.254 |
| Canada | 326 (42.7) | 140 (49.0) | 186 (39.0) | |
| Condition(s), n (%) | ||||
| Ankylosing spondylitis | 72 (9.4) | 26 (9.1) | 46 (9.6) | 0.800 |
| Chronic low back pain | 329 (43.1) | 113 (39.5) | 216 (45.3) | 0.119 |
| Chronic upper back pain | 132 (17.3) | 37 (12.9) | 95 (19.9) | 0.014 a |
| Chronic fatigue syndrome | 112 (14.7) | 35 (12.2) | 77 (16.1) | 0.140 |
| Chronic neck pain | 217 (28.4) | 65 (22.7) | 152 (31.9) | 0.007 a |
| Crohn disease–associated arthritis | 16 (2.1) | 5 (1.8) | 11 (2.3) | 0.603 |
| Degenerative disc disorder | 223 (29.2) | 80 (28.0) | 143 (30.0) | 0.555 |
| Dermatomyositis | 3 (0.4) | 1 (0.4) | 2 (0.4) | 0.882 |
| Ehler Danlos syndrome | 17 (2.2) | 2 (0.7) | 15 (3.1) | 0.027 a |
| Fibromyalgia | 291 (38.1) | 94 (32.9) | 197 (41.3) | 0.020 a |
| Gout | 26 (3.4) | 6 (2.1) | 20 (4.2) | 0.123 |
| Juvenile arthritis | 38 (5.0) | 15 (5.2) | 23 (4.8) | 0.795 |
| Lupus | 22 (2.9) | 5 (1.8) | 17 (3.6) | 0.147 |
| Osteoarthritis (hand) | 163 (21.4) | 66 (23.1) | 97 (20.3) | 0.371 |
| Osteoarthritis (hip) | 201 (26.3) | 73 (25.5) | 128 (26.8) | 0.691 |
| Osteoarthritis (knee) | 253 (33.2) | 91 (31.8) | 162 (34.0) | 0.543 |
| Osteoarthritis (joint) | 278 (36.4) | 111 (38.8) | 167 (35.0) | 0.291 |
| Osteoporosis | 99 (13.0) | 36 (12.6) | 63 (13.2) | 0.805 |
| Psoriatic arthritis | 64 (8.4) | 18 (6.3) | 46 (9.6) | 0.106 |
| Raynaud syndrome | 76 (10.0) | 19 (6.6) | 57 (11.9) | 0.018 a |
| Rheumatoid arthritis | 217 (28.4) | 86 (30.1) | 131 (27.5) | 0.440 |
| Sjögren disease | 51 (6.7) | 21 (7.3) | 30 (6.3) | 0.573 |
| Spinal stenosis | 128 (16.8) | 47 (16.4) | 81 (17.0) | 0.845 |
| Ulcerative colitis–associated arthritis | 18 (2.4) | 7 (2.5) | 11 (2.3) | 0.901 |
| Other condition | 99 (13.0) | 38 (13.3) | 61 (12.8) | 0.843 |
| Clinical symptoms | ||||
| 2011 FM survey score, mean (SD) | 12.41 (6.61) | 11.72 (6.43) | 12.83 (6.68) | 0.026 a |
| Neuropathic pain, mean (SD) | 13.79 (8.39) | 12.94 (8.32) | 14.30 (8.40) | 0.030 a |
| Physical function, mean (SD) | 39.97 (7.20) | 40.10 (6.65) | 39.90 (7.51) | 0.711 |
| Mental health, mean (SD) | 42.47 (8.39) | 42.97 (8.14) | 42.17 (8.54) | 0.203 |
The chi‐square test and independent samples t‐test were used for categorical and continuous variables, respectively. FM, fibromyalgia.
Tests are two‐tailed, with P < 0.05 considered significant.
Overall, 477 (62.5%) reported substituting MC for medications and 286 (37.5%) reported no substitution. A greater proportion of participants in the substitution subgroup reported fibromyalgia (OR 1.44; 95% CI 1.06–1.95; P = 0.021), chronic upper back pain (OR 1.67; 95% CI 1.11–2.53; P = 0.014), chronic neck pain (OR 1.59; 95% CI 1.14–2.23; P = 0.007), Ehlers Danlos syndrome (OR 4.61; 95% CI 1.05–20.31; P = 0.043), and Raynaud's disease (OR 1.91; 95% CI 1.11–3.28; P = 0.019). Participants in the substitution group reported significantly higher 2011 fibromyalgia survey criteria (d = 0.085; 95% CI −0.09 to 0.26; P = 0.026) and painDETECT scores (d = 0.026; 95% CI −0.15 to 0.20; P = 0.030), but there were no differences in PROMIS mental and physical health scores between groups.
Concomitant medications
Most study participants (90.8%) reported taking medication concomitant to MC (Table 2). The most commonly used medications in both groups were NSAIDs and DMARDs. Other than significantly higher use of muscle relaxants in the substitution group, there were no significant differences between substitution subgroups in any other medication class taken (all P values ≥ 0.118).
Table 2.
Medications taken concomitant with medical cannabis*
| Total, n (%) | No substitution, n (%) | Substitution, n (%) | P | |
|---|---|---|---|---|
| Opioid | 122 (16.1) | 38 (13.3) | 84 (17.7) | 0.118 |
| NSAID | 411 (54.2) | 144 (50.3) | 267 (56.2) | 0.141 |
| DMARD | 231 (30.4) | 89 (31.1) | 142 (29.9) | 0.676 |
| SNRI | 105 (13.8) | 34 (11.9) | 71 (14.9) | 0.251 |
| SSRI | 126 (16.6) | 46 (16.1) | 80 (16.8) | 0.817 |
| Gabapentinoid | 127 (16.7) | 47 (16.4) | 80 (16.8) | 0.917 |
| Benzodiazepine | 68 (8.9) | 21 (7.3) | 47 (9.9) | 0.243 |
| Muscle relaxant | 131 (17.2) | 39 (13.6) | 92 (19.4) | 0.047 a |
| Sleeping medication | 65 (8.6) | 22 (7.7) | 43 (9.1) | 0.534 |
| Other | 186 (24.5) | 66 (23.1) | 120 (25.3) | 0.530 |
| No medications | 70 (9.2) | 28 (9.8) | 42 (8.8) | 0.639 |
| Missing | 4 (0.5) | 2 (0.7) | 2 (0.4) |
Chi‐square tests were used for categorical variables. DMARD, disease‐modifying antirheumatic drug; NSAID, nonsteroidal anti‐inflammatory drug; SNRI, serotonin norepinephrine uptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Tests are two‐tailed, with P < 0.05 considered significant.
Substitution of MC for medication classes
Overall, 62.5% (n = 477) of participants reported substituting MC for at least one medication. Participants most often used MC as a substitute for NSAIDs (n = 261, 54.7%), opioids (n = 232, 48.6%), sleep medication (n = 141, 29.6%), muscle relaxants (n = 120, 25.2%), benzodiazepines (n = 74, 15.5%), and gabapentinoids (n = 50, 10.5%). Generally, ≥80% of the participants reported substituting, decreasing, or stopping their other medication class, with very few increasing medication use (Figure 1). The reasons for medication substitution included fewer side effects with MC compared with medication (39%), better symptom management (27%), fewer adverse effects (12%), other (9%), ability to obtain (8%), and greater social acceptance (5%). Fewer side effects, better symptom management, and fewer adverse effects were the highest reported reasons for all medication classes.
Figure 1.
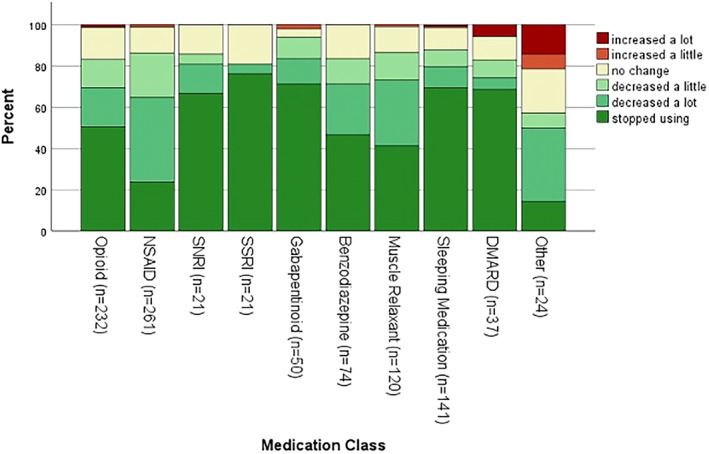
Change in medication use since starting medical cannabis. DMARD, disease‐modifying antirheumatic drug; NSAID, nonsteroidal anti‐inflammatory drug; SNRI, serotonin norepinephrine uptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
MC use characteristics
More than half (n = 436, 57.3%) of the participants used MC daily (Table 3). Compared with those who did not substitute MC for medications, those who substituted used MC more frequently, both daily (w = 0.23, P < 0.001) and weekly (d = 0.36; 95% CI 0.21–0.51; P < 0.001). The primary administration route differed significantly between substitution subgroups (w = 0.18; P < 0.001). Specifically, those who substituted were more likely to smoke (OR 1.88; 95% CI 1.29–2.73; P = 0.001), vaporize flower (OR 2.20; 95% CI 1.45–3.34; P < 0.001), vaporize concentrates (OR 4.10; 95% CI 2.13–7.89; P < 0.001), or use edibles (OR 1.80; 95% CI 1.34–2.43; P < 0.001). The type of cannabis used most often differed between substitution subgroups (w = 0.15, P = 0.002), with higher use of THC products among those who substituted.
Table 3.
Cannabis‐related characteristics*
| Total | No substitution | Substitution | P | |
|---|---|---|---|---|
| Started using medical cannabis, n (%) | ||||
| Less than 1 month ago | 38 (5.0) | 13 (4.5) | 25 (5.2) | <0.001 a |
| 1–6 months ago | 115 (15.1) | 47 (16.4) | 68 (14.3) | |
| 7–12 months | 89 (11.7) | 45 (15.7) | 44 (9.2) | |
| 1–3 years | 308 (40.4) | 125 (43.7) | 183 (38.4) | |
| More than 3 years ago | 212 (27.8) | 55 (19.2) | 157 (32.9) | |
| Missing | 1 (0.1) | 1 (0.3) | 0 (0.0) | |
| Planned duration of medical cannabis use, n (%) | ||||
| Less than 6 months | 10 (1.3) | 3 (1.0) | 7 (1.5) | 0.013 a |
| 6 months to 1 year | 8 (1.0) | 5 (1.7) | 3 (0.6) | |
| More than 1 year | 12 (1.6) | 7 (2.4) | 5 (1.0) | |
| Until symptoms are under control | 191 (25.0) | 82 (28.7) | 109 (22.9) | |
| Rest of life | 337 (44.2) | 104 (36.4) | 233 (48.8) | |
| Do not know | 204 (26.7) | 84 (29.4) | 120 (25.2) | |
| Missing | 1 (0.1) | 1 (0.3) | 0 (0.0) | |
| Medical cannabis product used most often, n (%) | ||||
| CBD | 332 (43.5) | 148 (51.7) | 184 (38.6) | 0.002 a |
| Balanced CBD and THC | 159 (20.8) | 53 (18.5) | 106 (22.2) | |
| THC | 203 (26.6) | 60 (21.0) | 143 (30.0) | |
| Other | 37 (4.8) | 8 (5.9) | 24 (4.2) | |
| Do not know | 32 (4.2) | 17 (2.8) | 20 (5.0) | |
| Frequency of use (weekly) | ||||
| Mean (SD) | 5.00 (2.08) | 4.99 (2.22) | 5.74 (1.94) | <0.001 a |
| Missing, n (%) | 2 (0.3) | 1 (0.3) | 1 (0.2) | |
| Frequency of use (daily), n (%) | ||||
| Once a day | 343 (45.0) | 160 (55.9) | 183 (38.4) | <0.001 a |
| Twice | 224 (29.4) | 84 (29.4) | 140 (29.4) | |
| Three | 99 (13.0) | 21 (7.3) | 78 (16.4) | |
| Four | 37 (4.8) | 8 (2.8) | 29 (6.1) | |
| Five or more | 51 (6.7) | 9 (3.1) | 42 (8.8) | |
| Missing | 9 (1.2) | 4 (1.4) | 5 (1.0) | |
| Administration route(s) used, n (%) | ||||
| Smoking | 172 (60.1) | 46 (16.1) | 126 (26.5) | <0.001 a |
| Vaporizing flower | 143 (18.8) | 34 (11.9) | 109 (22.9) | <0.001 a |
| Vaporizing concentrate | 78 (10.2) | 11 (3.8) | 67 (14.1) | <0.001 a |
| Edible | 373 (49.0) | 114 (39.9) | 259 (54.4) | <0.001 a |
| Topical application | 309 (40.6) | 117 (40.9) | 192 (40.3) | 0.876 |
| Tincture/oil | 361 (47.4) | 129 (45.1) | 232 (48.7) | 0.331 |
| Other | 8 (1.0) | 2 (0.7) | 6 (1.3) | 0.462 |
| Missing | 1 (0.1) | 0 (0.0) | 1 (0.2) | |
| Primary administration route used, n (%) | ||||
| Smoking | 105 (13.8) | 31 (10.8) | 74 (15.5) | <0.001 a |
| Vaporizing flower | 62 (8.1) | 19 (6.6) | 43 (9.0) | |
| Vaporizing concentrate | 37 (4.8) | 5 (1.7) | 32 (6.7) | |
| Edible | 197 (25.8) | 74 (25.9) | 123 (25.8) | |
| Topical application | 106 (13.9) | 56 (19.6) | 50 (10.5) | |
| Tincture/oil | 242 (31.7) | 96 (33.6) | 146 (30.6) | |
| Missing | 14 (1.8) | 5 (1.7) | 9 (1.9) | |
| Number of administration routes used | ||||
| Mean (SD) | 1.89 (1.03) | 1.58 (0.81) | 2.08 (1.11) | <0.001 a |
| Missing, n (%) | 1 (0.1) | 0 (0.0) | 1 (0.2) |
Chi‐square test and independent samples t‐tests were used for categorical and continuous variables, respectively. CBD, cannabidiol; THC, tetrahydrocannabinol.
Tests are two‐tailed, with P < 0.05 considered significant.
Effect of MC substitution on symptoms
Overall, participants who substituted MC for medications generally used MC for more symptom domains. Substitution was also associated with higher reported improvement across many symptoms. (Table 4). These differences were statistically significant for pain (d = 0.32; 95% CI 0.16–0.47; P = 0.018), sleep (d = 0.21; 95% CI 0.02–0.40; P = 0.034), joint stiffness (d = 0.31; 95% CI 0.12–0.50; P = 0.040), muscle spasm (d = 0.31; 95% CI 0.03–0.59; P = 0.047), inflammation (d = 0.23; 95% CI 0.04–0.42; P = 0.022), and overall health (d = 0.32; 95% CI 0.17–0.47; P < 0.001).
Table 4.
Symptoms medical cannabis is used for and change in severity of symptoms since starting medical cannabis*
| Total | No substitution | Substitution | P | |
|---|---|---|---|---|
| Pain, n (%) | 733 (93.1) | 268 (93.7) | 465 (97.5) | 0.009 a |
| Sleep, n (%) | 494 (64.7) | 154 (53.8) | 340 (71.3) | <0.001 a |
| Anxiety, n (%) | 301 (39.4) | 81 (28.3) | 220 (46.1) | <0.001 a |
| Fatigue, n (%) | 594 (22.1) | 44 (15.4) | 125 (26.2) | <0.001 a |
| Depression, n (%) | 602 (21.1) | 50 17.5) | 111 (23.3) | 0.058 |
| Memory, n (%) | 703 (7.9) | 16 (5.6) | 44 (9.2) | 0.071 |
| Joint stiffness, n (%) | 274 (64.1) | 161 (56.3) | 328 (68.8) | <0.001 a |
| Muscle spasm, n (%) | 508 (33.4) | 69 (24.1) | 186 (39.0) | <0.001 a |
| Inflammation, n (%) | 274 (64.1) | 158 (55.2) | 331 (69.4) | <0.001 a |
| Other, n (%) | 50 (6.6) | 12 (4.2) | 38 (8.0) | 0.042 a |
| Pain (n = 728), mean (SD) | 1.65 (0.93) | 1.47 (0.99) | 1.76 (0.87) | <0.001 a |
| Sleep (n = 491), mean (SD) | 1.90 (0.96) | 1.76 (0.98) | 1.96 (0.95) | 0.034 a |
| Anxiety (n = 300), mean (SD) | 1.73 (0.97) | 1.57 (0.92) | 1.78 (0.98) | 0.086 |
| Fatigue (n = 165), mean (SD) | 1.21 (1.02) | 1.12 (1.00) | 1.25 (1.03) | 0.476 |
| Depression (n = 160), mean (SD) | 1.57 (0.94) | 1.47 (0.89) | 1.61 (0.96) | 0.356 |
| Memory (n = 59), mean (SD) | 1.30 (1.07) | 1.23 (1.00) | 1.33 (1.11) | 0.730 |
| Joint stiffness (n = 479), mean (SD) | 1.47 (0.90) | 1.28 (0.90) | 1.56 (0.89) | <0.001 a |
| Muscle spasm (n = 247), mean (SD) | 1.55 (0.90) | 1.35 (1.00) | 1.62 (0.85) | 0.047 a |
| Inflammation (n = 480), mean (SD) | 1.40 (0.94) | 1.25 (0.97) | 1.47 (0.92) | 0.022 a |
| Health (n = 732), mean (SD) | 1.21 (0.96) | 1.02 (0.93) | 1.32 (0.95) | <0.001 a |
The chi‐square test or independent samples t‐test were used for categorical and continuous data, respectively. Change in symptoms was measured on a −3 to 3 scale, where positive values indicate improvement in symptoms.
Tests are two‐tailed, with P < 0.05 considered significant.
DISCUSSION
In this survey of people in the United States and Canada with a rheumatic condition who use MC, nearly two‐thirds reported substituting MC for medications associated with rheumatic disease symptom control, including NSAIDs, opioids, antidepressants, gabapentinoids, and benzodiazepines. Reasons for substitution were better symptom management and harm reduction, such as fewer adverse effects. Those who substituted reported a longer duration of use, had a higher frequency of use (both daily and weekly), were more likely to inhale MC, and used THC‐dominant products. Those who substituted used MC to treat more symptoms, reported a higher use of smoking and vaporizing compared with nonsubstitutors, and reported a higher number of administration routes used. Compared with those who did not substitute MC for medications, those who substituted used MC for more symptoms and reported larger improvements in pain, sleep, joint stiffness, muscle spasm, inflammation, and global health.
Consistent with previous studies, experience with recreational cannabis use was prevalent and reported by three‐quarters of all study participants. In addition, there were several MC use characteristics that were worth noting. First, inhalation was the most common method of administration, with all the attendant risks of respiratory disease and aggravation of an inflammatory condition. However, given the immediate pharmacokinetic effect of inhaled MC, this administration method may be most satisfactory for people seeking rapid symptom relief, especially for pain. Second, MC products containing THC were most used, especially for those reporting daily use (although dosing was not specified). This raises concerns for cognitive impairments related to THC as well as the potential for tolerance and physiologic dependence caused by prolonged use. 25 It is also plausible that some individuals may require cannabis products containing at least some THC for effective pain management, a point that should be explored in future studies. Third, it is noteworthy that more than half of participants in this survey were using MC at least daily, with those substituting more likely to be using regularly. This pattern of use supports the notion of daily continuous symptoms that need continuous management. Finally, this survey reports persistence in the use of MC, with 520 (68%) of the whole cohort reporting use for at least 1 year, a finding that suggests satisfaction with use and can be seen as a surrogate for efficacy.
Two‐thirds of MC users in this survey reported a diagnosis of an inflammatory rheumatic disease, and a similar number reported concomitant conditions, such as fibromyalgia, osteoarthritis, and mechanical spinal pain. Therefore, overlapping rheumatic complaints may represent an increased burden of disease, increased number of prescription medications, and increased risks of medication‐associated side effects. Furthermore, more than half of those substituting reported substituting MC for more than one medication. With polypharmacy an increasing problem, especially in the older adult population, the substitution of numerous medications with MC as a single product may be seen as advantageous. Poor adherence to prescribed pharmacotherapies for chronic pain has been associated with the complexity of a treatment regimen, multiple medications, and medication‐associated side effects. 26 , 27 , 28 , 29 A small number of individuals (n = 37) reported substituting MC for DMARDs, a potential cause for concern given that discontinuation of these medications without careful consultation with and oversight from one's clinician may result in symptom flares.
When considering our results as part of the broader literature, the evidence consistently demonstrates a correlation between MC use and reducing the use of prescription/over‐the‐counter medication, especially opioids. 10 , 11 , 14 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 For example, almost half of 2,697 Canadian participants in an online survey receiving legal MC reported that cannabis had enabled substitution for other substances (alcohol 18%, tobacco 8%, opioids 18%, and other prescription medications 18%). 36 Similarly, an observational study of 757 patients observed at community‐based cannabis clinics in Ontario, Canada, reported that the proportion of those using opioids decreased by half from 41% to 24% at 12 months. 14 In an online survey of more than 800 people with fibromyalgia, 53% reported that CBD products allowed them to reduce or even discontinue opioid use. 37 , 40 These findings are mirrored in prospective studies as well. In a prospective open‐label study of patients with chronic pain observed at a pain clinic in Israel, 32 of 73 participants receiving opioid therapy at baseline had discontinued treatment at 6‐month follow up. 41 When MC was added to standard treatments for 102 patients with fibromyalgia with a 64% retention rate, almost half reduced or discontinued analgesic treatment and reported improved sleep parameters and quality of life. 31 , 40 , 42 Additional research is needed to investigate the temporal and causal nature of this relationship.
However, these findings have not been replicated in clinical trials. Recent systematic reviews and meta‐analyses, including clinical trials of patients with cancer‐related pain, show little evidence of opioid reduction following cannabinoid use. 43 As noted by the authors, one key challenge of these clinical trials is the frequent requirement to maintain a stable opioid dose, thus confounding any possible substitution effects in these trials. 33 , 44 With the lack of clinical trial data and expanding legality of and access to MC, there have been modified Delphi panel studies as well as proposed clinical practice guidelines for the use of cannabis and cannabinoids for chronic pain, 45 , 46 , 47 including potential methodologies for the safe introduction and titration of cannabinoids in concert with opioid tapering. 47 These proposed approaches cite more evidence for the analgesic effect of THC compared with CBD but also more safety issues with THC, highlighting the importance of graduated dose progression according to symptom response, a recommendation for vaporizing over smoking, and consideration of attenuation of THC‐related side effects by the coadministration of CBD.
The limitations of this study included a lack of determination of causality because of its cross‐sectional design. Further, the nature of survey collection at a single timepoint allowed for potential recall bias in answering survey questions. These results are not generalizable to all demographic groups because of the sample makeup of mostly older, White females, with 40% reporting a university degree. Recruiting for this study occurred during the COVID‐19 pandemic, which may have affected our recruitment pool and potentially how participants were using MC because of changes in daily habits or routines. Additionally, many participants in this study had used cannabis for >6 months, thus our results may be biased toward those who found it more effective for symptom management. Our results may also be limited because we did not account for multiple comparisons in our statistical analyses. Finally, policy efforts to decrease opioid prescribing may have contributed to the discontinuation of opioids or increased attempts to substitute MC for opioids.
The acceptance of MC as a treatment strategy for rheumatic conditions is evolving. The changing legal status of cannabis has allowed a greater openness with more people willing to try cannabis for symptom relief. These encouraging results of medication reduction and favorable effect of MC require confirmation with more rigorous methods. At this time, survey information may be seen as a signal for effect, rather than sound evidence that could be applicable to those with musculoskeletal complaints in general. Comparative effective clinical trials of MC versus other pain treatments are needed, as are more prospective studies investigating the effects of MC on the use of medications and other substances in rheumatic populations.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr Boehnke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Boehnke, Martel, Williams, Fitzcharles.
Acquisition of data
Boehnke, Martel, Williams, Fitzcharles.
Analysis and interpretation of data
Boehnke, Scott, Martel, Smith, Bergmans, Kruger, Williams, Fitzcharles.
Supporting information
Disclosure form
Appendix S1: Supplementary Information
Dr Boehnke's work was supported by the National Institute on Drug Abuse of the NIH (award K01‐DA‐049219). Dr. Bergmans’ work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH (award T32‐AR‐07080).
Additional supplementary information cited in this article can be found online in the Supporting Information section (http://onlinelibrary.wiley.com/doi/10.1002/acr2.11717).
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11717.
REFERENCES
- 1. Statistics Canada. Arthritis, by age group . Updated November 6, 2023. Accessed March 10, 2024. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009606
- 2. Barbour KE, Helmick CG, Boring M, et al. Vital signs: prevalence of doctor‐diagnosed arthritis and arthritis‐attributable activity limitation ‐ United States, 2013‐2015. MMWR Morb Mortal Wkly Rep 2017;66(9):246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths — United States, 2013–2019. MMWR Morb Mortal Wkly Rep 2021;70(6):202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boehnke KF, Dean O, Haffajee RL, et al. U.S. trends in registration for medical cannabis and reasons for use from 2016 to 2020: an observational study. Ann Intern Med 2022;175(7):945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malfait AM, Gallily R, Sumariwalla PF, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti‐arthritic therapeutic in murine collagen‐induced arthritis. Proc Natl Acad Sci U S A 2000;97(17):9561–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hammell DC, Zhang LP, Ma F, et al. Transdermal cannabidiol reduces inflammation and pain‐related behaviours in a rat model of arthritis. Eur J Pain 2016;20(6):936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finn DP, Haroutounian S, Hohmann AG, et al. Cannabinoids, the endocannabinoid system, and pain: a review of preclinical studies. Pain 2021;162(suppl 1):S5–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corroon JM Jr, Mischley LK, Sexton M. Cannabis as a substitute for prescription drugs ‐ a cross‐sectional study. J Pain Res 2017;10:989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piper BJ, DeKeuster RM, Beals ML, et al. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J Psychopharmacol 2017;31(5):569–575. [DOI] [PubMed] [Google Scholar]
- 10. Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross‐sectional survey of patients with chronic pain. J Pain 2016;17(6):739–744. [DOI] [PubMed] [Google Scholar]
- 11. Boehnke KF, Scott JR, Litinas E, et al. Pills to pot: observational analyses of cannabis substitution among medical cannabis users with chronic pain. J Pain 2019;20(7):830–841. [DOI] [PubMed] [Google Scholar]
- 12. Boehnke KF, Gagnier JJ, Matallana L, et al. Substituting cannabidiol for opioids and pain medications among individuals with fibromyalgia: a large online survey. J Pain 2021;22(11):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aviram J, Pud D, Gershoni T, et al. Medical cannabis treatment for chronic pain: outcomes and prediction of response. Eur J Pain 2021;25(2):359–374. [DOI] [PubMed] [Google Scholar]
- 14. Meng H, Page MG, Ajrawat P, et al. Patient‐reported outcomes in those consuming medical cannabis: a prospective longitudinal observational study in chronic pain patients. Can J Anaesth 2021;68(5):633–644. [DOI] [PubMed] [Google Scholar]
- 15. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med 2014;370(23):2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cerdá M, Wall M, Keyes KM, et al. Medical marijuana laws in 50 states: investigating the relationship between state legalization of medical marijuana and marijuana use, abuse and dependence. Drug Alcohol Depend 2012;120(1‐3):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hasin DS, Sarvet AL, Cerdá M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991‐1992 to 2012‐2013. JAMA Psychiatry 2017;74(6):579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Academies of Sciences, Engineering, and Medicine . The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press; 2017. [PubMed] [Google Scholar]
- 19. Fitzcharles MA, Ste‐Marie PA, Häuser W, et al. Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Care Res (Hoboken) 2016;68(5):681–688. [DOI] [PubMed] [Google Scholar]
- 20. Boehnke KF, Martel MO, Smith T, et al. Medicinal cannabis use for rheumatic conditions in the US versus canada: rationale for use and patient‐health care provider interactions. ACR Open Rheumatol 2023;5(9):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46(3):319–329. [DOI] [PubMed] [Google Scholar]
- 22. Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient‐reported outcomes measurement information system (PROMIS) global items. Qual Life Res 2009;18(7):873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22(10):1911–1920. [DOI] [PubMed] [Google Scholar]
- 24. IBM SPSS Statistics . Version 28.0. IBM Corporation; 2021.
- 25. Coughlin LN, Ilgen MA, Jannausch M, et al. Progression of cannabis withdrawal symptoms in people using medical cannabis for chronic pain. Addiction 2021;116(8):2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timmerman L, Stronks DL, Groeneweg JG, et al. Prevalence and determinants of medication non‐adherence in chronic pain patients: a systematic review. Acta Anaesthesiol Scand 2016;60(4):416–431. [DOI] [PubMed] [Google Scholar]
- 27. Broekmans S, Dobbels F, Milisen K, et al. Determinants of medication underuse and medication overuse in patients with chronic non‐malignant pain: a multicenter study. Int J Nurs Stud 2010;47(11):1408–1417. [DOI] [PubMed] [Google Scholar]
- 28. Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23(8):1296–1310. [DOI] [PubMed] [Google Scholar]
- 29. Burkhart PV, Sabaté E. Adherence to long‐term therapies: evidence for action. J Nurs Scholarsh 2003;35(3):207. [PubMed] [Google Scholar]
- 30. Baron EP, Lucas P, Eades J, et al. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain 2018;19(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giorgi V, Bongiovanni S, Atzeni F, et al. Adding medical cannabis to standard analgesic treatment for fibromyalgia: a prospective observational study. Clin Exp Rheumatol 2020;38 Suppl 123(1):53–59. [PubMed] [Google Scholar]
- 32. Sagy I, Bar‐Lev Schleider L, Abu‐Shakra M, et al. Safety and efficacy of medical cannabis in fibromyalgia. J Clin Med 2019;8(6):807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Noori A, Miroshnychenko A, Shergill Y, et al. Opioid‐sparing effects of medical cannabis or cannabinoids for chronic pain: a systematic review and meta‐analysis of randomised and observational studies. BMJ Open 2021;11(7):e047717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abuhasira R, Schleider LB, Mechoulam R, et al. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med 2018;49:44–50. [DOI] [PubMed] [Google Scholar]
- 35. Lucas P, Walsh Z. Medical cannabis access, use, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy 2017;42:30–35. [DOI] [PubMed] [Google Scholar]
- 36. Holman A, Kruger DJ, Lucas P, et al. Healthcare provider and medical cannabis patient communication regarding referral and medication substitution: the Canadian context. J Cannabis Res 2022;4(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boehnke KF, Gagnier JJ, Matallana L, et al. substituting cannabidiol for opioids and pain medications among individuals with fibromyalgia: a large online survey. J Pain 2021;22(11):1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality‐of‐life outcomes in chronic pain: a prospective open‐label study. Clin J Pain 2016;32(12):1036–1043. [DOI] [PubMed] [Google Scholar]
- 39. Lynch ME, Clark AJ. Cannabis reduces opioid dose in the treatment of chronic non‐cancer pain. J Pain Symptom Manage 2003;25(6):496–498. [DOI] [PubMed] [Google Scholar]
- 40. Safakish R, Ko G, Salimpour V, et al. Medical cannabis for the management of pain and quality of life in chronic pain patients: a prospective observational study. Pain Med 2020;21(11):3073–3086. [DOI] [PubMed] [Google Scholar]
- 41. Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality‐of‐life outcomes in chronic pain: a prospective open‐label study. Clin J Pain 2016;32(12):1036–1043. [DOI] [PubMed] [Google Scholar]
- 42. Lucas P, Boyd S, Milloy MJ, et al. Cannabis significantly reduces the use of prescription opioids and improves quality of life in authorized patients: results of a large prospective study. Pain Med 2021;22(3):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nielsen S, Picco L, Murnion B, et al. Opioid‐sparing effect of cannabinoids for analgesia: an updated systematic review and meta‐analysis of preclinical and clinical studies. Neuropsychopharmacology 2022;47(7):1315–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonomo Y, Norman A, Collins L, et al. Pharmacokinetics, safety, and tolerability of a medicinal cannabis formulation in patients with chronic non‐cancer pain on long‐term high dose opioid analgesia: a pilot study. Pain Ther 2022;11(1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bell AD, MacCallum C, Margolese S, et al. Clinical practice guidelines for cannabis and cannabinoid‐based medicines in the management of chronic pain and co‐occurring conditions. Cannabis Cannabinoid Res 2024;9(2):669–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bhaskar A, Bell A, Boivin M, et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: results of a modified Delphi process. J Cannabis Res 2021;3(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sihota A, Smith BK, Ahmed SA, et al. Consensus‐based recommendations for titrating cannabinoids and tapering opioids for chronic pain control. Int J Clin Pract 2021;75(8):e13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Information


