Abstract
Objective
Group‐level analyses from the phase 3 DISCOVER‐2 trial of guselkumab demonstrated robust and durable improvements across psoriatic arthritis (PsA) domains. To specifically evaluate continuous disease control in individual patients, persistence of clinically relevant improvements was assessed, both at consecutive guselkumab dosing visits and over time.
Methods
Post hoc analyses included biologic‐naïve patients randomized to 100 mg of guselkumab at week 0, week 4, and then every 8 weeks (Q8W). Improvements in joint (minimal clinically important improvement [MCII] in Disease Activity Index for PsA [DAPSA; ≥7.25], clinical DAPSA [cDAPSA; ≥5.7]), skin (Investigator's Global Assessment [IGA] 0/1), and overall disease activity (patient global assessment of arthritis and psoriasis [PtGA Arthritis+Psoriasis; MCII ≥ 15 mm], PsA Disease Activity Score [PASDAS; MCII ≥ 0.8]) were assessed. Proportions of patients with maintenance of DAPSA and cDAPSA MCII at consecutive Q8W guselkumab dosing visits (ie, at weeks 4 and 12, weeks 12 and 20, etc through week 52) and patient‐level durability of response through week 100 (Kaplan‐Meier) were determined.
Results
Among 248 patients randomized to guselkumab Q8W, 93% to 99% maintained clinical improvement in joint disease at consecutive Q8W dosing visits through week 52 across time periods. Among guselkumab patients achieving MCII by week 24, estimated probabilities of maintenance of clinical improvement 100 weeks post achievement ranged from 68% (IGA 0/1) to 89% (PASDAS MCII). Median times to loss of improvement were not reached; estimated mean weeks of maintenance of improvement were 58.6, 52.4, 75.7, 83.6, and 76.7, respectively, for DAPSA, cDAPSA, IGA, PtGA Arthritis+Psoriasis, and PASDAS.
Conclusion
Guselkumab provided highly durable patient‐level improvements, both at consecutive Q8W dosing visits for joint disease activity and over time across PsA domains according to physician‐ and patient‐driven assessments.
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic, immune‐mediated inflammatory disease characterized by psoriasis of the skin and nails and joint disease affecting the peripheral and/or axial skeleton. 1 Most patients with PsA present with multidomain disease requiring individualized treatment. 2 Disease activity, previous therapies, prognostic factors (eg, pre‐existing structural damage), comorbid conditions, and patient characteristics may influence response to therapy. 3 Considering the chronic and heterogeneous nature of PsA and multiple factors influencing patient outcomes, 3 a consistent treatment effect at consecutive dosing visits and persistence of response over time may allow for continuous improvement and greater probability of achieving durable disease control.
Selective inhibition of the interleukin‐23 (IL‐23) p19 subunit with the fully human monoclonal antibody guselkumab has demonstrated significant multidomain efficacy in patients with active PsA. In the phase 3 DISCOVER‐1 and DISCOVER‐2 studies of patients with active PsA, guselkumab that was administered every 4 or 8 weeks (Q4W or Q8W) provided robust and durable improvements across key PsA domains, with a favorable benefit–risk profile through up to 2 years of treatment. 4 , 5 , 6 , 7 , 8 , 9 The safety profile and efficacy of guselkumab Q4W and Q8W were consistent regardless of baseline patient demographics or disease characteristics, prior tumor necrosis factor inhibitor (TNFi) exposure, or concomitant therapy with conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs). 10 , 11 , 12
The primary end point in the DISCOVER‐1 and DISCOVER‐2 studies (ie, at least 20% improvement in the American College of Rheumatology response criteria [ACR20] 13 at week 24) was achieved by significantly greater proportions of patients in the guselkumab Q4W and Q8W groups compared with placebo. 4 , 5 Response rates, conservatively estimated using nonresponder imputation, were maintained or increased over time across disease domains at 1 year in DISCOVER‐1 and 2 years in DISCOVER‐2. While informing expectations for population response rates, treatment group response rates do not reveal response patterns in individual patients (eg, group‐level rates would remain constant even if the proportions of patients losing and gaining response in a specific time interval are similar). As such, post hoc analyses of the 2‐year DISCOVER‐2 trial of biologic‐naïve patients with active PsA were conducted to evaluate individual patient‐level consistency of improvement at consecutive guselkumab Q8W dosing visits, as well as the persistence of improvement over time.
PATIENTS AND METHODS
Patients and study design
Details of the study design, inclusion and exclusion criteria, and primary efficacy and safety results at week 24, week 52, and week 100 for the DISCOVER‐2 study (ClinicalTrials.gov identifier: NCT03158285) have been reported. 5 , 7 , 8 DISCOVER‐2 was a phase 3, randomized, double‐blind, placebo‐controlled study that enrolled biologic‐naïve patients with active PsA (≥5 tender joint count [TJC 0–68] and ≥5 swollen joint count [SJC 0–66] and C‐reactive protein [CRP] level ≥0.6 mg/dL) despite standard therapies. Patients were randomized (1:1:1) to receive 100 mg of guselkumab Q4W, 100 mg of guselkumab weeks 0 and 4 and then Q8W, or placebo with crossover to 100 mg of guselkumab Q4W at week 24. Efficacy was assessed through week 100. These post hoc analyses used data only from patients randomized to the guselkumab Q8W group to evaluate maintenance of improvement from one guselkumab administration to the next and through study completion in individual patients.
The DISCOVER‐2 trial was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonization Guidelines for Good Clinical Practice. Each participating site's governing ethical body approved the study protocol, and all patients provided written informed consent.
Outcomes
Early improvements were assessed using previously reported cutoffs representing the minimum clinically important improvement (MCII) in joint, skin, and overall disease activity, as assessed by patients and physicians. The MCII has been defined as the smallest improvement in an outcome that a patient would perceive as beneficial 14 and was employed as a measure of durability of treatment effect at consecutive dosing visits, as well as over time through 2 years. Because MCII is a continuous rather than a relative measure, such as the ACR 20/50/70 criteria, it allows for an objective assessment of response and is better suited to evaluating individual patient‐level response, as compared with the ACR criteria, which are more appropriate for measuring outcomes in a large population. MCII was used for consistency across outcomes and to avoid variations in the stringency of various treatment targets, such as low disease activity and remission in the examined assessments. 15
Outcomes of interest assessed improvements in joints using the Disease Activity Index in PsA (DAPSA) and clinical DAPSA (cDAPSA), in skin with the Investigator's Global Assessment (IGA), and in overall disease activity through patient‐reported and composite measures using the patient global assessment of arthritis and psoriasis (PtGA Arthritis+Psoriasis) and the Psoriatic Arthritis Disease Activity Score (PASDAS), respectively.
The DAPSA is a validated composite measure of joint disease derived via summation of TJC, SJC, patient assessment of arthritis disease activity using a visual analog scale (VAS; 0–10 cm), patient assessment of joint pain (VAS 0–10 cm), and CRP level (mg/dL). 16 The cDAPSA, a validated variation of the DAPSA that excludes CRP, is often used in routine clinical practice. 17 , 18 The MCIIs used for DAPSA and cDAPSA were improvements (reduction) from baseline (≥7.25 19 and ≥5.7, 18 respectively), as previously reported in separate observational cohort analyses. Post baseline, DAPSA and cDAPSA scores were determined at 4‐week intervals through week 28, at 8‐week intervals through week 52, and at weeks 68, 76, 84, and 100.
The IGA is a measure of psoriatic lesion severity, graded by the investigator for induration, erythema, and scaling on a scale of 0–4: clear (0), minimal (1), mild (2), moderate (3), or severe (4). 20 Two levels of IGA response were employed in these analyses: IGA 0/1 (among patients with baseline IGA ≥2), representing an improvement from the presence of mild‐to‐severe psoriasis to no or minimal skin disease, and IGA 0 (among patients with baseline IGA ≥1), assessing the proportion of patients with any degree of psoriasis at baseline achieving complete skin clearance. Post baseline, IGA was collected at weeks 16, 24, 52, 76, and 100.
The PtGA Arthritis+Psoriasis is a patient‐reported measure of arthritis and psoriasis recorded on a VAS 21 0–100 mm. An individual response threshold of ≥15‐mm improvement (reduction) from baseline was selected as the MCII for PtGA Arthritis+Psoriasis based on a prior report that identified a value of 15% of the score range as a plausible threshold for a noticeable change in a patient‐reported outcome (PRO) rating scale such as the VAS. 22 This more conservative MCII response criterion 23 was employed for consistency with a companion study describing early achievement of MCII in clinical measures and PROs with guselkumab in patients with active PsA (Curtis JR, et al: unpublished observations). 24
The PASDAS is a multidomain measure of PsA disease activity composed of PtGA Arthritis+Psoriasis, physician global assessment of arthritis and psoriasis (VAS), TJC, SJC, Leeds Enthesitis Index score, tender dactylitis count, 36‐Item Short Form Health Survey Physical Component Summary score, and CRP level, with each component weighted before contributing to the final score (range 0–10). 25 A reduction from baseline ≥0.8 was chosen as the PASDAS MCII for these analyses. This more conservative MCII value was based on findings from previous real‐world cohort studies employing anchor‐based (minimal important change = 0.67) 26 and distribution (minimal clinically important difference = 0.76) 19 methods as well as an analysis showing that a value of 0.8 is indicative of a moderate response in most patients with PsA. 27 Following the first post baseline assessment at week 8, both PtGA Arthritis+Psoriasis and PASDAS were determined at weeks 16, 24, 52, 76, and 100.
Statistical analyses
Consistency of clinical improvement at consecutive guselkumab Q8W dosing visits through week 52 was evaluated by determining the proportion of patients who achieved the MCII at a given dosing visit and maintained this response at the following 8‐week dosing visit. Because this analysis required patient data for the outcome of interest at each pair of consecutive dosing visits (ie, at weeks 4 and 12, weeks 12 and 20, weeks 20 and 28, weeks 28 and 36, weeks 36 and 44, and weeks 44 and 52) and IGA, PtGA Arthritis+Psoriasis, and PASDAS were not assessed at all visits, only joint disease assessments, namely DAPSA and cDAPSA MCII, could be evaluated. Analyses of consistency of effect at consecutive Q8W dosing visits were restricted to 52 weeks because of longer data collection intervals (>8 weeks apart) beyond week 52 in DISCOVER‐2.
Persistence of response through week 100 at the individual patient level was assessed among patients who achieved MCII in DAPSA, cDAPSA, PtGA Arthritis+Psoriasis, and PASDAS, as well as those achieving IGA 0/1 and IGA 0, by week 24. Because the time point for achieving response may have varied among patients in the first 24 weeks, not all patients had the same initial start time for measurement of persistence duration. Time‐to‐event analyses with Kaplan‐Meier curves were used to estimate the cumulative probability of maintaining improvement from the time response was first achieved through the end of the study (week 100). An event was defined as loss of response, and patients without an event were right‐censored at their last available assessment. 28 Because Kaplan‐Meier methodology is nonparametric, the median time is typically reported. However, if the probability of maintenance of improvement exceeded 50% at the end of the observation period and the median survival time could not be computed (as was the case for each outcome assessed in these analyses), the mean and SE duration of maintenance of response were estimated as the area under the curve.
RESULTS
Patients
Detailed baseline characteristics of the overall population and patient disposition through week 100 of DISCOVER‐2 have been previously reported. 5 , 8 Disease characteristics of the 248 biologic‐naïve patients randomized to guselkumab Q8W were consistent with moderate to high levels of PsA disease activity across domains, including a mean PtGA Arthritis+Psoriasis of 67.5 (SD 20.5) and a mean PASDAS of 6.6 (SD 1.1); a majority (69%) reported csDMARD use at baseline (Table 1). Among patients randomized to receive guselkumab Q8W, approximately 90% (223 of 248) completed the study treatment through week 100, 8 resulting in minimal censoring.
Table 1.
Baseline demographics and disease characteristics*
| Guselkumab 100 mg Q8W | |
|---|---|
| Randomized and treated patients, N | 248 |
| Demographics | |
| Age, mean (SD), y | 44.9 (11.9) |
| Male, n (%) | 129 (52) |
| Bodyweight, mean (SD), kg | 83.0 (19.3) |
| BMI, mean (SD) | 28.7 (6.3) |
| Normal (<25), n (%) | 74 (30) |
| Overweight (≥25 to <30), n (%) | 82 (33) |
| Obese (≥30), n (%) | 92 (37) |
| PsA characteristics | |
| PsA disease duration, mean (SD), y | 5.1 (5.5) |
| SJC (0–66), mean (SD) | 11.7 (6.8) |
| TJC (0–68), mean (SD) | 19.8 (11.9) |
| CRP, median (IQR), mg/dL | 1.3 (0.7–2.5) |
| PtGA Arthritis+Psoriasis (VAS 0–100), mean (SD) | 67.5 (20.5) |
| DAPSA, a mean (SD) | 46.3 (19.4) |
| cDAPSA b (0–154), mean (SD) | 44.3 (18.8) |
| Psoriatic BSA (0–100), mean (SD), % | 17.0 (21.0) |
| IGA c (0–4), n (%) | |
| ≥1 | 239 (96) |
| ≥2 | 195 (79) |
| 3 or 4 | 108 (44) |
| PASI (0–72), mean (SD) | 9.7 (11.7) |
| PASDAS d (0–10), mean (SD) | 6.6 (1.1) |
| HAQ‐DI e (0–3), mean (SD) | 1.3 (0.6) |
| Concomitant medications | |
| csDMARDs, n (%) | 170 (69) |
| Methotrexate, n (%) | 141 (57) |
| Dose, mean (SD), mg/wk | 15.3 (5.2) |
| Oral corticosteroids, n (%) | 50 (20.2) |
| Dose, mean (SD), f mg/day | 6.8 (2.5) |
BMI, body mass index; BSA, body surface area; cDAPSA, clinical Disease Activity Index for Psoriatic Arthritis (excludes CRP); CRP, C‐reactive protein; csDMARD, conventional synthetic disease‐modifying antirheumatic drug; DAPSA, Disease Activity Index for Psoriatic Arthritis; HAQ‐DI, Health Assessment Questionnaire‐Disability Index; IGA, Investigator's Global Assessment; IQR, interquartile range; PASDAS, Psoriatic Arthritis Disease Activity Score; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PtGA Arthritis+Psoriasis, patient global assessment of arthritis and psoriasis; Q8W, every 8 weeks; SJC, swollen joint count; TJC, tender joint count; VAS, visual analog scale.
DAPSA disease activity states include remission (score ≤4), low disease activity (score >4 and ≤14), moderate disease activity (score >14 and ≤28), and high disease activity (score >28). 17
cDAPSA disease activity states include remission (score ≤4), low disease activity (score >4 and ≤13), moderate disease activity (score >13 and ≤27), and high disease activity (score >27). 17
IGA score of 0 indicates clear skin, 1 indicates minimal psoriasis, 2 indicates mild psoriasis, 3 indicates moderate psoriasis, and 4 indicates severe psoriasis. 20
PASDAS disease activity states include very low (score ≤1.9), low (score ≤3.2), and high disease activity (score ≥5.4). 25 , 29
HAQ‐DI score of 0–1 indicates mild difficulties to moderate disability, 1–2 moderate‐to‐severe disability, and 2–3 severe to very severe disability. 30
Prednisone or equivalent dose.
Maintenance of clinical improvement at consecutive Q8W dosing visits
Owing to the relative infrequency of skin assessments and thus global disease activity assessments through 1 year as specified in the DISCOVER‐2 protocol (eg, weeks 16, 24, and 52 for investigator skin assessment and weeks 8, 16, 24, and 52 for PtGA Arthritis+Psoriasis and PASDAS), maintenance of improvement at consecutive Q8W dosing visits could only be determined for joint outcomes, which were assessed at 4‐week intervals through week 28 and then at 8‐week intervals through week 52. Among patients who achieved MCII in cDAPSA at week 4, 94% maintained clinical improvement at week 12 (Figure 1A). High rates of cDAPSA MCII maintenance were observed at each subsequent time period assessed (ie, weeks 12 and 20, weeks 20 and 28, weeks 28 and 36, weeks 36 and 44, and weeks 44 and 52), ranging from 95% to 99%. Similar results were observed for DAPSA MCII (Figure 1B).
Figure 1.
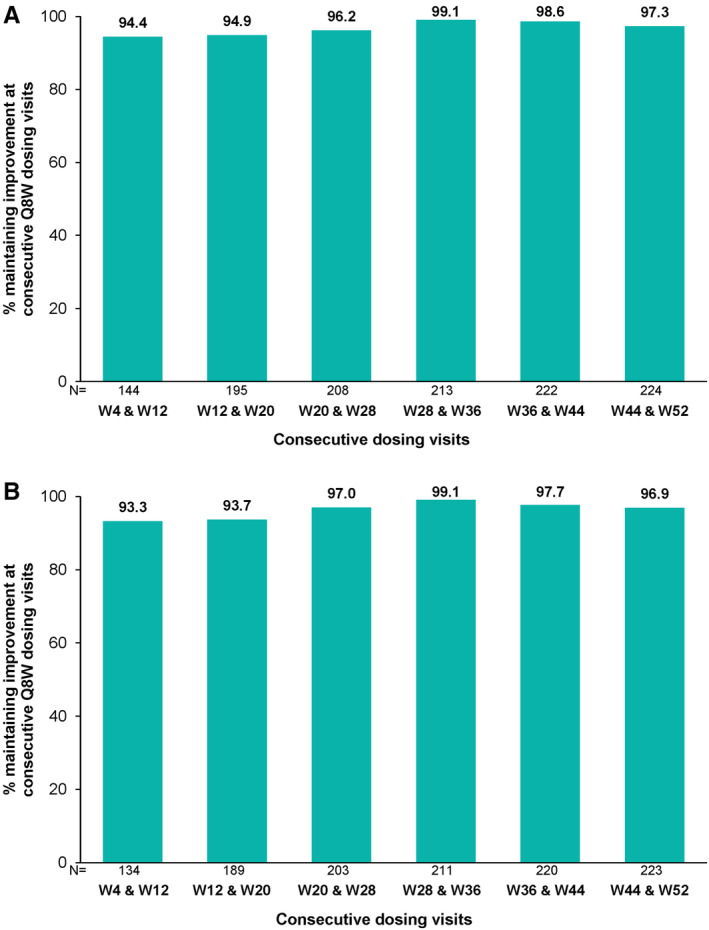
Maintenance of (A) cDAPSA MCII (≥5.7 improvement from baseline) and (B) DAPSA MCII (≥7.25 improvement from baseline) at consecutive guselkumab Q8W dosing visits. cDAPSA, clinical Disease Activity Index for Psoriatic Arthritis; DAPSA, Disease Activity Index for Psoriatic Arthritis; MCII, minimal clinically important improvement; Q8W, every 8 weeks; W, week.
Persistence of clinical improvement over time
By week 24, 96% (238 of 248) of patients treated with guselkumab Q8W achieved cDAPSA and DAPSA MCII. Among patients with MCII in joint disease activity by week 24, the probabilities of maintenance of cDAPSA and DAPSA MCII 100 weeks post achievement were 69.2% and 69.6%, respectively (Figure 2). Mean durations of maintenance were estimated to be 52.4 (SE 2.0) and 58.6 (SE 2.2) weeks, for cDAPSA and DAPSA, respectively (Figure 2).
Figure 2.
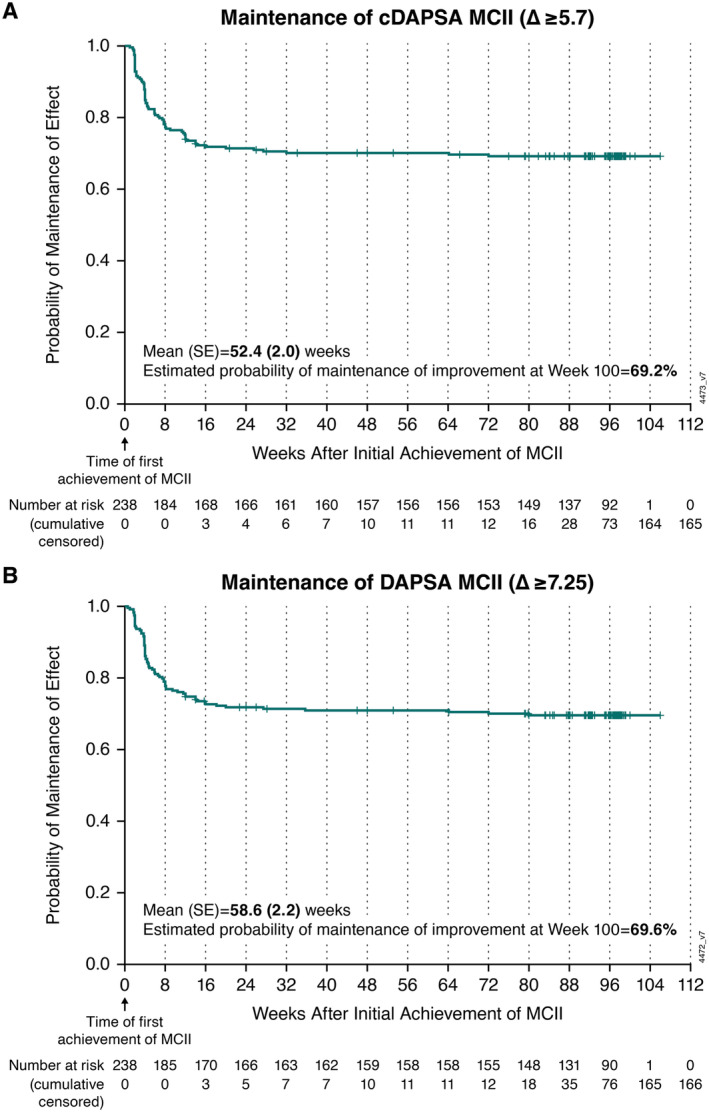
Kaplan‐Meier plot of mean duration and probability of maintenance of joint improvement with guselkumab Q8W among patients achieving (A) cDAPSA MCII and (B) DAPSA MCII by week 24. Mean (SE) durations of maintenance of MCII in cDAPSA and DAPSA were estimated using the area under the curve. cDAPSA, clinical Disease Activity Index for Psoriatic Arthritis (excludes C‐reactive protein); DAPSA, Disease Activity Index for Psoriatic Arthritis; MCII, minimal clinically important improvement; Q8W, every 8 weeks.
Among 195 patients with IGA ≥2 at baseline, 181 (93%) patients achieved IGA 0/1 (clear or minimal skin psoriasis) by week 24 of guselkumab Q8W treatment, with a 68.1% probability of maintaining this improvement 100 weeks post achievement (Figure 3A). The estimated mean duration of IGA 0/1 was 75.7 (SE 1.6) weeks (Figure 3A). By week 24 of guselkumab Q8W, 62% (147 of 239) of patients with baseline IGA ≥1 achieved IGA 0, indicative of complete clearance of psoriatic lesions. The probability of maintaining IGA 0 100 weeks post achievement was 63.7%, and the estimated mean duration of maintenance of this improvement was 65.3 (SE 2.4) weeks (Figure 3B).
Figure 3.
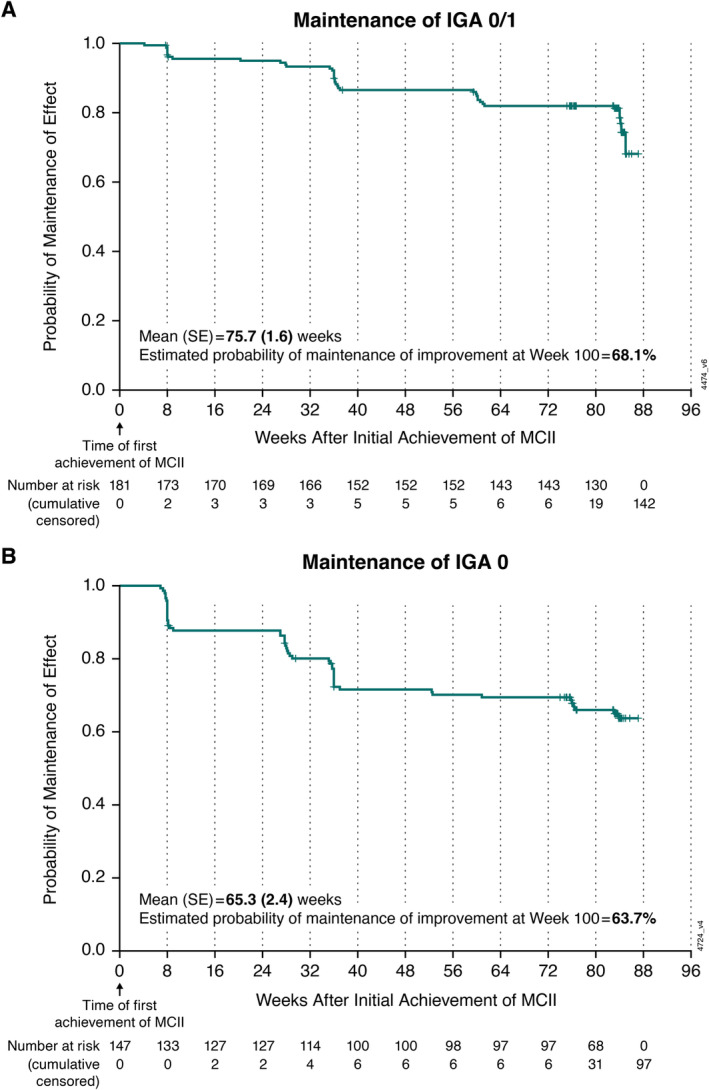
Kaplan‐Meier plot of mean duration and probability of maintenance of skin improvement with guselkumab Q8W among patients achieving (A) an IGA score of 0 (cleared) or 1 (minimal) and (B) IGA score of 0 at week 24. Mean (SE) durations of maintenance of IGA 0/1 and IGA 0 were estimated using the area under the curve. IGA, Investigator's Global Assessment; MCII, minimal clinically important improvement; Q8W, every 8 weeks.
A majority (73%, 182 of 248) of patients also achieved PtGA Arthritis+Psoriasis MCII by week 24 of guselkumab Q8W treatment, with a 74.8% probability of maintaining this improvement in overall disease activity 100 weeks post achievement (Figure 4A). The estimated mean duration of maintenance of PtGA Arthritis+Psoriasis MCII was 83.6 (SE 2.4) weeks (Figure 4A). When assessed via a composite measure of overall disease activity, 90% (224 of 248) of patients achieved PASDAS MCII by week 24 with guselkumab Q8W. The probability of maintaining PASDAS MCII 100 weeks post achievement was 89.0%, and the estimated mean duration of maintenance of this improvement was 76.7 (SE 1.4) weeks (Figure 4B).
Figure 4.
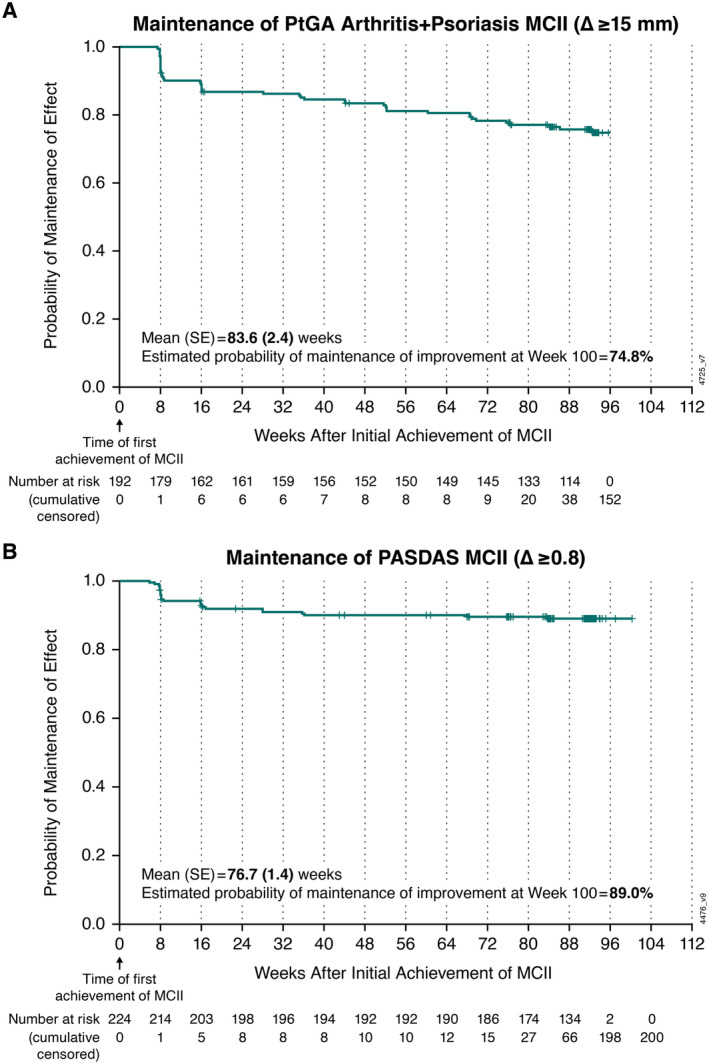
Kaplan‐Meier plot of mean duration and probability of maintenance of improvement in overall disease activity with guselkumab Q8W among patients achieving (A) PtGA Arthritis+Psoriasis MCII and (B) PASDAS MCII at week 24. Mean (SE) durations of maintenance of MCII in PtGA Arthritis+Psoriasis and PASDAS were estimated using the area under the curve. MCII, minimal clinically important improvement; PASDAS, Psoriatic Arthritis Disease Activity Score; PtGA Arthritis+Psoriasis, patient global assessment of arthritis and psoriasis; Q8W, every 8 weeks.
DISCUSSION
In the phase 3 DISCOVER‐2 trial of patients with active PsA, group‐level response rates employing nonresponder imputation showed higher ACR, IGA, and minimal disease activity response rates with guselkumab compared with placebo through the 24‐week placebo‐controlled period and durable or increased response rates among guselkumab‐treated patients through 2 years. 5 , 7 , 8 Although group‐level data are considered the gold standard for assessing comparative effectiveness and describing aggregate response to treatment over time, they may not reflect within‐patient persistence of improvement. Such patient‐level data can provide additional context for both health care providers and patients and may influence shared decision‐making by facilitating discussions related to patient expectations, clinical trial findings, and monitoring response over time. 31 To fully characterize patterns of response to guselkumab Q8W in patients with PsA, maintenance of joint response (DAPSA and cDAPSA MCII) at consecutive dosing visits was assessed only in patients who achieved response at the first of the two paired visits, and mean duration of improvement and probability of maintaining improvement at 2 years were assessed among patients who achieved this level of response by week 24.
Treatment with guselkumab Q8W was associated with high rates (94%–99%) of maintaining clinically meaningful improvement in peripheral joint activity (cDAPSA) at consecutive visits through week 52, the last time point at which cDAPSA could be assessed at 8‐week intervals. Furthermore, 96% of patients achieved cDAPSA MCII by week 24, and the estimated mean duration of joint improvement at the individual patient level was 1 year from the time of initial response, with a 69% probability of maintaining this improvement at 2 years. Similar results were observed with DAPSA, consistent with reports showing a high correlation between these indices. 32 , 33
Robust and durable efficacy has been previously demonstrated for guselkumab in psoriatic skin lesions at the group level among patients with PsA. 5 , 7 , 8 In the present analyses, durable improvements in skin psoriasis were also observed at the individual patient level. Among patients with mild‐to‐severe psoriasis at baseline, 93% achieved no or minimal skin disease or complete skin clearance by week 24, whereas 62% of patients with any degree of psoriasis at baseline achieved complete clearance of psoriatic lesions at this time point. Skin responses were maintained for an estimated 65 to 76 weeks from the time of initial response, with a 64% to 68% probability of maintaining minimal skin disease or complete clearance at 2 years. Because no longitudinal nail assessment data were collected in the DISCOVER‐2 study, nail psoriasis was not included in the present analysis. However, a post hoc analysis of data pooled from the VOYAGE‐1 and VOYAGE‐2 studies in patients with moderate‐to‐severe plaque psoriasis showed that significantly higher proportions of guselkumab‐treated patients with nail psoriasis achieved clinically meaningful improvements in nail disease as early as week 16 compared with placebo. 34 Additionally, among VOYAGE‐1 and VOYAGE‐2 participants with self‐reported PsA, response rates for achieving improvements in difficult‐to‐treat regional psoriasis of the nails, scalp, palms and/or soles were greater with guselkumab than with placebo at week 16 and numerically greater than with adalimumab at week 48 35 . These findings suggest guselkumab has the potential to benefit patients with multifaceted PsA, aligning with current Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) treatment guidelines.
Consistent with findings for improvements in the joint and skin domains, the mean durations of maintenance of clinically important improvements in measures of overall disease activity were 84 weeks for PtGA Arthritis+Psoriasis and 77 weeks for PASDAS, with respective probabilities of maintaining these improvements at 2 years of 75% and 89%. Considering treatment guidelines support selecting therapies that address all active PsA domains in individual patients, 3 persistence in improvement in PASDAS, a multidomain measure that incorporates assessment of joints, dactylitis, enthesitis, physical function, quality of life, acute‐phase response, and both patient and physician global ratings of arthritis and psoriasis, suggests a broad and durable effect of guselkumab at the individual patient level.
The enduring improvements in joint disease activity observed at consecutive Q8W dosing visits and across disease domains over time may relate to decreased production and survival of long‐lived Th17 cells or restoration of altered regulatory T cell function with guselkumab, resulting in sustained reduction in proinflammatory cytokines. 36 , 37 , 38 In addition, through its unique binding of both CD64+ myeloid cells and IL‐23, guselkumab may be enriched within the inflamed tissue microenvironment, thereby neutralizing IL‐23 at its cellular source and potentially contributing to the highly durable responses seen both at consecutive dosing visits and long term. 39
Consistency of effect at consecutive dosing visits and over time in individual patients could influence and enhance persistence on biologic therapy (time from the beginning of therapy until its discontinuation), an important determinant of patient outcomes in immune‐mediated inflammatory diseases, including PsA. 40 Clinically, efficacy and safety are contributors to persistence on medication. 41 Robust patient retention rates were reported in DISCOVER‐1 and DISCOVER‐2, with 90% to 94% of patients randomized to guselkumab Q8W completing the study treatment through 1 year and 90% doing so through 2 years 7 , 8 , 9 of DISCOVER‐2. Through the 24‐week placebo‐controlled period of DISCOVER‐2, rates of adverse events were similar between guselkumab‐ and placebo‐treated patients and across guselkumab dosing regimens. 5 The favorable benefit–risk profile of guselkumab remained consistent through up to 2 years, with no new safety concerns identified. 7 , 8
Pooled analyses of the DISCOVER studies have previously shown consistent guselkumab efficacy across clinical efficacy and PRO measures through 1 year regardless of baseline patient and disease characteristics, including concomitant csDMARD therapy, encompassing methotrexate. 10 Additional analyses using data from DISCOVER‐2 have also shown guselkumab treatment to be associated with durable achievement of stringent disease targets spanning key GRAPPA‐recognized PsA domains through up to 2 years, irrespective of baseline csDMARD and methotrexate therapy. 42 Thus, although not directly examined in the present analysis, concomitant methotrexate is not expected to influence maintenance of improvement at consecutive guselkumab dosing visits or the persistence of improvement over time.
The durable benefit–risk profile of guselkumab demonstrated in randomized controlled studies is supported by data from real‐world studies. Among patients with longstanding, treatment‐resistant active PsA enrolled in the CorEvitas PsA/Spondyloarthritis Registry, 43 nearly 80% of those who initiated on‐label guselkumab (after US Food and Drug Administration approval for active PsA and at the approved dosing regimen) had persistent treatment at 6 months. 44 Importantly, 6 months of on‐label guselkumab therapy in this treatment‐resistant population was associated with significant improvements in joint and skin symptoms. 44 High levels of treatment persistence with guselkumab were also shown in a large prospective cohort study of patients with psoriasis and concurrent rheumatologist‐confirmed PsA from the British Association of Dermatologists Biologics and Immunomodulators Register. 45 The 1‐year drug survival rate for guselkumab in patients with psoriasis, including those with comorbid PsA, was 89%, 46 and in patients with a diagnosis of both psoriasis and PsA, adherence rates through 9 months were numerically highest for those treated with guselkumab or ustekinumab, as compared with TNF and IL‐17A inhibitors. 47 More recently, real‐world claims data showed that patients with PsA receiving guselkumab Q8W were three times more likely to remain on treatment at 1 year, with 72% of patients persisting on guselkumab compared with 44% of those treated with an initial subcutaneous TNFi. 48 Similarly, in a study employing primarily commercial US health plan claims data, patients initiating guselkumab had a significantly greater likelihood of on‐label treatment persistence through 1 year compared with those initiating a subcutaneous IL‐17A inhibitor (ixekizumab or secukinumab). 49
The strengths of the present analyses include low rates of guselkumab discontinuation in DISCOVER‐2, resulting in minimal censoring, which allowed accurate estimates of efficacy persistence at the patient level. Validated PsA instruments were employed in the analyses, and MCII thresholds used have been estimated with established methodologies, including anchor‐based and distribution‐based methods. 50 Although the cDAPSA MCII of 5.7 was the only such reported at the time these analyses were planned and performed, 18 a cDAPSA MCII of 3.25 was recently reported. 51 Given that the value used in the current analyses represents a more conservative estimate, it is not likely that application of the newly reported MCII would alter the conclusions drawn from these analyses, although the higher cutoff employed may underestimate the level of cDAPSA MCII consistency at consecutive dosing visits as well as the persistence of this improvement over time. Because these post hoc analyses were conducted on a clinical trial population of biologic‐naïve patients, the results may not be generalizable to the broader population of patients with PsA. Furthermore, the analysis of clinical improvement was limited to efficacy assessments conducted at consecutive Q8W dosing visits; therefore, we are not able to evaluate efficacy during the time between these visits. Additionally, these analyses did not include all study outcomes because only some assessments were performed at 8‐week intervals, and because of longer data collection intervals (>8 weeks apart) beyond week 52, these analyses were restricted to 1 year of treatment.
Evaluation of individual patient improvements showed that guselkumab Q8W provided highly durable joint efficacy at consecutive Q8W dosing visits through 1 year and conferred persistent benefits across PsA domains through 2 years, consistent with previously reported continuous improvement in group‐level clinical response rates over time. These study findings may further inform physicians and patients when initiating and monitoring treatment plans.
AUTHOR CONTRIBUTIONS
All authors contributed to at least one of the following manuscript preparation roles: conceptualization AND/OR methodology, software, investigation, formal analysis, data curation, visualization, and validation AND drafting or reviewing/editing the final draft. As corresponding author, Dr Mease confirms that all authors have provided the final approval of the version to be published, and takes responsibility for the affirmations regarding article submission (eg, not under consideration by another journal), the integrity of the data presented, and the statements regarding compliance with institutional review board/Helsinki Declaration requirements.
ROLE OF STUDY SPONSOR
Employees of Janssen Research & Development, LLC, had a role in the study design and in the collection, analysis, and/or interpretation of the data, the writing of the manuscript, and the decision to submit the manuscript for publication. The corresponding author had full access to all study data and had final responsibility to submit for publication. Medical writing support was provided by Joanna Dembowy, PhD, of JSS Medical Research, under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2022;175:1298–304).
Supporting information
Disclosure form:
ClinicalTrials.gov identifier: NCT03158285.
Supported by Janssen Research & Development, LLC.
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Author disclosures are available at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11732.
REFERENCES
- 1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376(10):957–970. [DOI] [PubMed] [Google Scholar]
- 2. Ritchlin CT, Pennington SR, Reynolds NJ, et al. Moving toward precision medicine in psoriasis and psoriatic arthritis. J Rheumatol Suppl 2020;96:19–24. [DOI] [PubMed] [Google Scholar]
- 3. Coates LC, Soriano ER, Corp N, et al; GRAPPA Treatment Recommendations domain subcommittees . Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18(8):465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deodhar A, Helliwell PS, Boehncke WH, et al; DISCOVER‐1 Study Group . Guselkumab in patients with active psoriatic arthritis who were biologic‐naive or had previously received TNFα inhibitor treatment (DISCOVER‐1): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet 2020;395(10230):1115–1125. [DOI] [PubMed] [Google Scholar]
- 5. Mease PJ, Rahman P, Gottlieb AB, et al; DISCOVER‐2 Study Group . Guselkumab in biologic‐naive patients with active psoriatic arthritis (DISCOVER‐2): a double‐blind, randomised, placebo‐controlled phase 3 trial. Lancet 2020;395(10230):1126–1136. [DOI] [PubMed] [Google Scholar]
- 6. Rahman P, Ritchlin CT, Helliwell PS, et al. Pooled safety results through 1 year of 2 phase III trials of guselkumab in patients with psoriatic arthritis. J Rheumatol 2021;48(12):1815–1823. [DOI] [PubMed] [Google Scholar]
- 7. McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin‐23p19‐specific monoclonal antibody, through one year in biologic‐naive patients with psoriatic arthritis. Arthritis Rheumatol 2021;73(4):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McInnes IB, Rahman P, Gottlieb AB, et al. Long‐term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin‐23, through two years: results from a phase III, randomized, double‐blind, placebo‐controlled study conducted in biologic‐naive patients with active psoriatic arthritis. Arthritis Rheumatol 2022;74(3):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ritchlin CT, Helliwell PS, Boehncke WH, et al. Guselkumab, an inhibitor of the IL‐23p19 subunit, provides sustained improvement in signs and symptoms of active psoriatic arthritis: 1 year results of a phase III randomised study of patients who were biologic‐naïve or TNFα inhibitor‐experienced. RMD Open 2021;7(1):e001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ritchlin CT, Mease PJ, Boehncke WH, et al. Sustained and improved guselkumab response in patients with active psoriatic arthritis regardless of baseline demographic and disease characteristics: pooled results through week 52 of two phase III, randomised, placebo‐controlled studies. RMD Open 2022;8(1):e002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coates LC, Gossec L, Theander E, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIb, randomised, controlled study (COSMOS). Ann Rheum Dis 2022;81(3):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coates LC, Ritchlin CT, Gossec L, et al. Guselkumab provides sustained domain‐specific and comprehensive efficacy using composite indices in patients with active psoriatic arthritis. Rheumatology (Oxford) 2023;62(2):606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38(6):727–735. [DOI] [PubMed] [Google Scholar]
- 14. Kvien TK, Heiberg T, Hagen KB. Minimal clinically important improvement/difference (MCII/MCID) and patient acceptable symptom state (PASS): what do these concepts mean? Ann Rheum Dis 2007;66(suppl 3):iii40–iii41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagège B, Tan E, Gayraud M, et al. Remission and low disease activity in psoriatic arthritis publications: a systematic literature review with meta‐analysis. Rheumatology (Oxford) 2020;59(8):1818–1825. [DOI] [PubMed] [Google Scholar]
- 16. Schoels M, Aletaha D, Funovits J, et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69(8):1441–1447. [DOI] [PubMed] [Google Scholar]
- 17. Schoels MM, Aletaha D, Alasti F, et al. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75(5):811–818. [DOI] [PubMed] [Google Scholar]
- 18. Ogdie A, Husni ME, Scher J, et al. SAT0434 Minimal clinically important difference in outcome measures for use in clinical care and pragmatic trials in PsA. Ann Rheum Dis 2020;79(suppl 1):1173–1174. [Google Scholar]
- 19. Tillett W, Day J, McHugh NJ, et al. Oral presentation 0224 Continuous composite measures for routine care in psoriatic arthritis: thresholds of meaning and clinically important difference estimates for the 3 and 4 VAS scales from a UK multicentre study. Ann Rheum Dis 2021;80(suppl 1):135. [Google Scholar]
- 20. Langley RG, Feldman SR, Nyirady J, et al. The 5‐point Investigator's Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat 2015;26(1):23–31. [DOI] [PubMed] [Google Scholar]
- 21. Cauli A, Gladman DD, Mathieu A, et al; GRAPPA 3PPsA Study Group . Patient global assessment in psoriatic arthritis: a multicenter GRAPPA and OMERACT study. J Rheumatol 2011;38(5):898–903. [DOI] [PubMed] [Google Scholar]
- 22. Institute for Quality and Efficiency in Health Care . General methods version 6.1. January 2022. https://www.iqwig.de/methoden/general-methods_version-6-1.pdf [PubMed]
- 23. Kwok T, Pope JE. Minimally important difference for patient‐reported outcomes in psoriatic arthritis: Health Assessment Questionnaire and pain, fatigue, and global visual analog scales. J Rheumatol 2010;37(5):1024–1028. [DOI] [PubMed] [Google Scholar]
- 24. Curtis J, Deodhar A, Soriano E, et al. Early improvements with guselkumab associate with sustained control of psoriatic arthritis: post hoc analyses of two phase 3 trials. Rheumatol Ther 2024; doi: 10.1007/s40744-024-00702-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Helliwell PS, FitzGerald O, Fransen J, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72(6):986–991. [DOI] [PubMed] [Google Scholar]
- 26. Mulder MLM, Bertram AM, Wenink MH, et al. Defining the minimal important change (MIC) and meaningful change value (MCV) for the Psoriatic Arthritis Disease Activity Score (PASDAS) in a routine practice cohort of patients with psoriatic arthritis. Rheumatology (Oxford) 2022;61(10):4119–4123. [DOI] [PubMed] [Google Scholar]
- 27. Helliwell PS, FitzGerald O, Fransen J. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. J Rheumatol 2014;41(6):1212–1217. [DOI] [PubMed] [Google Scholar]
- 28. Bewick V, Cheek L, Ball J. Statistics review 12: survival analysis. Crit Care 2004;8(5):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ogdie A, Coates LC, Mease P. Measuring outcomes in psoriatic arthritis. Arthritis Care Res (Hoboken) 2020;72(suppl 10):82–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003;1:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. King MT, Dueck AC, Revicki DA. Can methods developed for interpreting group‐level patient‐reported outcome data be applied to individual patient management? Med Care 2019;57(suppl 5):S38–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonçalves RSG, de Almeida Martins LM, de Ataide Mariz H, et al. DAPSA versus cDAPSA: do we need to use CRP? Ann Rheum Dis 2020;79(11):e142. [DOI] [PubMed] [Google Scholar]
- 33. van Mens LJJ, van de Sande MGH, van Kuijk AWR, et al. Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real‐life cohort. Ann Rheum Dis 2018;77(2):251–257. [DOI] [PubMed] [Google Scholar]
- 34. Foley P, Gordon K, Griffiths CEM, et al. Efficacy of guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: a secondary analysis of 2 randomized clinical trials. JAMA Dermatol 2018;154(6):676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orbai AM, Chakravarty SD, You Y, et al. Efficacy of guselkumab in treating nails, scalp, hands, and feet in patients with psoriasis and self‐reported psoriatic arthritis. Dermatol Ther (Heidelb) 2023;13(11):2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Girolomoni G, Strohal R, Puig L, et al. The role of IL‐23 and the IL‐23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol 2017;31(10):1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gooderham MJ, Papp KA, Lynde CW. Shifting the focus ‐ the primary role of IL‐23 in psoriasis and other inflammatory disorders. J Eur Acad Dermatol Venereol 2018;32(7):1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siebert S, Schett G, Raychaudhuri SP, et al. Changes in serum cytokines by week 24 correlate with long‐term efficacy of guselkumab through 2‐years in bio‐naïve adults with PsA. Ann Rheum Dis 2023;82(suppl 1):1121–1122. [Google Scholar]
- 39. Krueger J, Eyerich K, McGonagle D, et al. Differentiation of therapeutic antibodies targeting interleukin (IL)‐23 [abstract]. Arthritis Rheumatol 2022;74(suppl 9). https://acrabstracts.org/abstract/differentiation-of-therapeutic-antibodies-targeting-interleukin-il-23/ [Google Scholar]
- 40. Gottlieb A, Gratacos J, Dikranian A, et al. Treatment patterns, unmet need, and impact on patient‐reported outcomes of psoriatic arthritis in the United States and Europe. Rheumatol Int 2019;39(1):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sumpton D, Kelly A, Craig JC, et al. Preferences for biologic treatment in patients with psoriatic arthritis: a discrete choice experiment. Arthritis Care Res (Hoboken) 2022;74(8):1234–1243. [DOI] [PubMed] [Google Scholar]
- 42. Ritchlin CT, Mease PJ, Boehncke WH, et al. Durable control of psoriatic arthritis with guselkumab across domains and patient characteristics: post hoc analysis of a phase 3 study. Clin Rheumatol 2024;43(8):2551–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mease PJ, Ogdie A, Chakravarty SD, et al. Clinical characteristics of registry participants with psoriatic arthritis initiating guselkumab: an analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry. Drugs Real World Outcomes 2022;9(4):617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mease PJ, Ogdie A, Tesser J, et al. Six‐month persistence and multi‐domain effectiveness of guselkumab in adults with psoriatic arthritis: real‐world data from the corevitas psoriatic arthritis/spondyloarthritis registry. Rheumatol Ther 2023;10(6):1479–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yiu ZZN, Becher G, Kirby B, et al; BADBIR Study Group . Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol 2022;158(10):1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elgaard CDB, Iversen L, Hjuler KF. Guselkumab, tildrakizumab, and risankizumab in a real‐world setting: drug survival and effectiveness in the treatment of psoriasis and psoriatic arthritis. J Dermatolog Treat 2023;34(1):2133531. [DOI] [PubMed] [Google Scholar]
- 47. Xu C, Teeple A, Wu B, et al. Treatment adherence and persistence of seven commonly prescribed biologics for moderate‐to‐severe psoriasis and psoriatic arthritis in a U.S. commercially insured population. J Dermatolog Treat 2022;33(4):2270–2277. [DOI] [PubMed] [Google Scholar]
- 48. Walsh JA, Lin I, Zhao R, et al. Comparison of real‐world on‐label treatment persistence in patients with psoriatic arthritis receiving guselkumab versus subcutaneous tumor necrosis factor inhibitors. Drugs Real World Outcomes Published online July 31, 2024. doi: 10.1007/s40801-024-00428-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mease PJ, Zhao R, Ferrante S, et al. Comparison of on‐label treatment persistence in real‐world patients with psoriatic arthritis receiving guselkumab versus subcutaneous IL‐17A inhibitors. Presented at: 17th Annual RWCS Symposium; February 14–17, 2024; Maui, HI. [DOI] [PubMed]
- 50. Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health‐related quality of life‐a systematic review. J Clin Epidemiol 2017;89:188–198. [DOI] [PubMed] [Google Scholar]
- 51. Karmacharya P, Stull C, Stephens‐Shields A, et al. Responsiveness and minimum clinically important difference in patient‐reported outcome measures among patients with psoriatic arthritis: a prospective cohort study. Arthritis Care Res (Hoboken) 2023;75(10):2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form:


