Abstract
Background and objectives
The impact of viral infections on disease susceptibility and progression has predominantly been studied in patients with relapse-onset MS (RMS). Here, we determined immune responses to ubiquitous viruses in patients with primary progressive MS (PPMS).
Methods
Antibody responses to Epstein–Barr virus (EBV), specifically to the latent EBV nuclear antigen 1 and the lytic viral capsid antigen VCA, human herpesvirus 6 (HHV-6), human cytomegalovirus (HCMV), and measles virus were determined in a cohort of 68 PPMS patients with a mean follow-up of 8 years and compared with 66 healthy controls matched for sex and age.
Results
Compared with controls, PPMS patients showed increased humoral immune responses to the EBV-encoded nuclear antigen-1 (EBNA1), but not to the lytic EBV capsid antigen (VCA) or to other viral antigens. Seroprevalence rates for HCMV were significantly higher in PPMS. Antiviral immune responses at baseline did not correlate with disability progression over time.
Discussion
Elevated immune responses toward EBNA1 are selectively increased in people with primary progressive disease, indicating a link between EBNA1-targeting immune responses and the development of both RMS and PPMS. Our data also suggest that chronic HCMV infection is associated with progressive MS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12763-w.
Keywords: Multiple sclerosis, Primary progressive, Virus, Epstein–Barr virus, Human cytomegalovirus
Introduction
Genetic and environmental factors determine susceptibility to the development of multiple sclerosis (MS) and contribute to the progression of the disease [1]. Epidemiological studies provided strong evidence for consistent environmental risk-associations such as the increased susceptibility for MS following Epstein–Barr virus (EBV) infection and a protective role for human cytomegalovirus (HCMV) [2, 3]. These associations have primarily been studied in patients with relapse-onset MS (RMS), as this is the most prevalent manifestation and course of the disease. Only about 15% of the patients develop a progressive disease course from onset, termed primary progressive multiple sclerosis (PPMS). Given major epidemiological and clinical differences between RMS and PPMS in terms of sex predominance, age at onset, initial clinical presentation, rate of disability progression and response to immunotherapy, it has long been debated whether PPMS is a distinct disease entity or whether it just represents part of the heterogeneous clinical disease spectrum [4]. Epidemiological and clinical differences between RMS and PPMS have also led to the question whether the disease courses have distinctive risk factors. Monozygous twins can be concordant or discordant for disease courses and no clear genetic differences have been found between RMS and PPMS, indicating that the disease course is not predominantly genetically determined [5].
Methods
In the present study, we determined immune responses to ubiquitous viruses, as potential environmental trigger of the disease, in a large cohort of 68 patients with PPMS recruited from 12 European MS centers, compared to demographically matched healthy control donors (HD) (Suppl. Table 1). All patients fulfilled the 2017 revisions of the McDonald criteria [6]. Median (interquartile range—IQR) follow-up time for patients from baseline was 8.0 (7.0–10.7) years. Only one patient (1.5%) was treated during follow-up. EDSS scores were recorded at baseline (i.e. sampling time point), 2 and 6 years, and at the time of last visit. Short-term disability progression was defined as an increase of at least 1 point in the EDSS if baseline EDSS ≤ 5.0 and 0.5 points if baseline EDSS ≥ 5.5 during the first 2 years. Taking into account that most of the patients would fulfill this progression criterion at medium and long term, to assess disability progression at these time points, progression rates were computed by dividing EDSS changes by the time on follow-up between baseline and 6 years for medium-term disability progression and between baseline and the time of the last visit for long-term disability progression. Then, medium- and long-term progressors were defined as those patients displaying progression rates above the 75th percentile of disability progression. Virus antigen-specific IgG responses were assessed in sera using commercially available ELISA kits according to the manufacturers’ recommendations. The following kits were used: EBNA1 (# RE58741, Tecan IBL International GmbH, Hamburg, Germany), EBV-CA (#El 2791-9601G, Euroimmun, Lübeck, Germany), CMV (#El 2570-9601G, Euroimmun, Lübeck, Germany), HHV6 (#KA1457, Abnova, Taoyuan City, Taiwan), Anti-Measles Virus (#El 2610-9601G, Euroimmun, Lübeck, Germany). All samples were frozen upon venipuncture, not previously thawed and analyzed together at one time point. Seroprevalence rates were compared between PPMS and HD by a chi-square test, antibody-responses were analyzed using the non-parametric Mann–Whitney U test. Univariable logistic regressions were performed to assess the association between antiviral immune responses at baseline and disability progression at short term (2 years), medium term (4 years), and long term (at the time of last follow-up). Given the exploratory nature of our study, we did not apply correction algorithms for multiple testing. Anonymized data will be shared upon reasonable request. The study was approved by the corresponding Hospital Ethics Committee according to the ethical standards laid down in the 1964 Declaration of Helsinki, and participants gave written informed consent. Anonymized data will be shared upon reasonable request.
Results
Seroprevalence rates for HCMV were significantly higher in PPMS patients compared to HDs matched for sex and age (76.5% for PPMS vs. 50% for HD; p = 0.001) (Table 1). Although not statistically significant, trends towards higher seroprevalence rates for the EBV-encoded antigens EBNA1 and VCA were observed in PPMS patients. Seroprevalence rates against HHV-6 and measles virus were similar between both groups.
Table 1.
Seroprevalence rates against viruses in patients with PPMS and healthy donors
| Viruses | PPMS | Healthy donors | p-value |
|---|---|---|---|
| EBV-VCA | 68 (100%) | 63 (95.5%) | 0.075 |
| EBV-EBNA1 | 67 (98.5%) | 61 (92.4%) | 0.088 |
| HCMV | 52 (76.5%) | 33 (50.0%) | 0.001 |
| HHV-6 | 38 (55.9%) | 42 (63.6%) | 0.360 |
| Measles | 66 (97.1%) | 61 (92.4%) | 0.228 |
EBV Epstein–Barr virus, EBNA1 Epstein–Barr nuclear antigen 1, HCMV human cytomegalovirus, HHV-6 human herpesvirus 6, VCA viral capsid antigen
Significant p-values are shown in bold
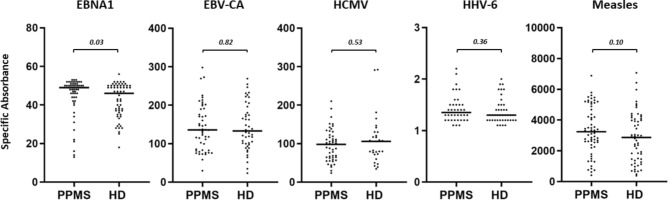
Seropositive PPMS patients showed a selective and significant increase of IgG responses towards EBNA1 compared to healthy EBV carriers (Fig. 1). Antibody responses to the lytic EBV-encoded capsid antigen as well as to proteins derived from other viruses were similar in patients and controls (Fig. 1). Taking advantage of the long follow-up of the PPMS cohort, we next investigated whether antiviral immune responses at baseline were associated with disability accrual over time. As shown in Table 2, antiviral antibody responses at baseline were not associated with disability progression at short term (2 years), medium term (6 years), or long term (at last follow-up).
Fig. 1.
Selective increase of EBNA1-specific IgG response in PPMS patients graphs show the distribution of IgG immune responses against ubiquitous viruses in patients with PPMS compared to healthy donors (HD) matched by sex and age. The non-parametric Mann–Whitney U test was used to compare antibody responses between the 2 groups. EBV Epstein–Barr virus, EBNA1 Epstein–Barr nuclear antigen 1, HCMV human cytomegalovirus, HHV-6 human herpesvirus 6, VCA viral capsid antigen
Table 2.
Association between antiviral immune responses and disability progression at short, medium, and long term in patients with PPMS
| Viruses | Short term | Medium term | Long term |
|---|---|---|---|
| EBV-VCA | HR = 0.997 (0.991–1.003); p = 0.350 | HR = 0.999 (0.992–1.006); p = 0.802 | HR = 1.001 (0.995–1.007); p = 0.729 |
| EBV-EBNA1 | HR = 1.072 (0.981–1.172); p = 0.124 | HR = 1.015 (0.945–1.089); p = 0.687 | HR = 1.032 (0.959–1.112); p = 0.401 |
| HCMV | HR = 1.002 (0.993–1.011); p = 0.671 | HR = 1.008 (0.998–1.018); p = 0.131 | HR = 1.000 (0.990–1.011); p = 0.946 |
| HHV-6 | HR = 2.043 (0.154–27.022); p = 0.588 | HR = 2.830 (0.179–44.770); p = 0.460 | HR = 0.623 (0.045–8.580); p = 0.723 |
| Measles | HR = 1.000 (1.000–1.000); p = 0.570 | HR = 1.000 (1.000–1.000); p = 0.592 | HR = 1.000 (1.000–1.000); p = 0.749 |
Data are expressed as hazard ratios (HR) and 95% confidence intervals after univariable logistic regression analysis
EBV Epstein–Barr virus, EBNA1 Epstein–Barr nuclear antigen 1, HCMV human cytomegalovirus, HHV-6 human herpesvirus 6, VCA viral capsid antigen
Discussion
The selective increase of EBNA1-specific antibody responses in PPMS patients without a concomitant increase of immune responses to other EBV-VCA and to proteins derived from other ubiquitous viruses is consistent with previous studies performed in patients with clinically isolated syndromes (CIS), in patients with RMS and in healthy individuals who will develop MS [7–10]. Suggesting specificity for MS, EBNA1-targeting antibody responses are reported to be unchanged in patients with myelin oligodendrocyte glycoprotein-antibody associated disease (MOGAD) [11] and a broader anti-EBV T cell receptor repertoire has recently been described to be specifically associated with MS but absent in aquaporin 4-antibody positive neuromyelitis optica spectrum disorder (NMOSD), MOGAD, or in Susac’s syndrome [12].
Only a few studies have investigated EBV- and antiviral immune responses as a potential risk factor for MS by disease course. Farrell et al. reported higher EBNA-1 but lower EBV-VCA IgG titers in RMS (n = 25) versus PPMS (n = 25) patients, HD sera or immune responses to viruses other than EBV were not investigated [13]. Ingram et al. found that EBNA1-specifc IgG responses are not significantly increased in neither PPMS (n = 25) nor active (n = 25) and stable (n = 25) RMS compared to HD (n = 25) but appreciated that the study was likely underpowered to detect significant differences [14]. In a population-based case–control study, comprising 7520 RMS cases, 540 PPMS cases and 11,386 HDs matched by age, sex and residential area, Hedström et al. [13] reported that EBNA1-specific IgG responses are increased in both patients RMS and PPMS compared to HD. Immune responses to viral antigens other than EBNA1 were not investigated in the latter two studies and none of aforementioned investigations included longitudinal data on disability progression.
Our data show that EBNA1-specific immune responses are increased not only in RMS but also in PPMS. The increase appears to be predominantly associated with EBNA1 as antibody responses to other viral antigens, including the lytic EBV capsid antigen, were similar in PPMS and HD. As reported for RMS [15], increased immune responses to EBNA1 at baseline were not correlated with disability progression in PPMS. These data support the notion that EBNA1-specific immune responses potentially contribute to the development of both RMS and PPMS, but do not predict disease progression after onset. This does not exclude the possibility that changes in antiviral immune responses over time may reflect clinical disease activity, severity or progression in PPMS.
The finding of higher seroprevalence rates of HCMV in PPMS compared to demographically matched HD appears surprising since HCMV seropositivity and increased antibody responses to HCMV are associated with protection from the development and progression of RMS [2, 3]. Aging is a biological factor strongly associated with both HCMV seropositivity and progressive MS. Chronic HCMV infection is believed to contribute significantly to immunosenescence, an age-related loss of innate and adaptive immune system proficiencies, thought to accelerate MS progression [16]. Our study, therefore, provides incentive to conduct larger studies on the potential role of EBV and HCMV infection as risk factor specifically related to primary progressive MS.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
M.C. and J.D.L. contributed to the conception and design of the study. J.D.L., H.H., L.M.V., K.R., A.S.A., M.B., P.C.M., J.S.G., N.M., K.B., S.M.Y., F.P.M., A.A., F.B., H.T., J.L., I.R., R.A.L., T.C.T., D.O., S.L., Y.B., A.J.S.L., A.G.M., N.F., L.G., J.V., E.M., H.W., A.V.C, X.M., and M.C. acquired and analyzed the data. M.C. and J.D.L. drafted a significant portion of the manuscript or figures.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors thank Kerstin Stein (University Hospital of Münster, Department of Neurology with Institute of Translational Neurology) for expert technical assistance. This project has received funding from the European Union’s Horizon Europe Research and Innovation Actions under grant no. 101137235 (BEHIND-MS).
Declarations
Conflicts of interest
The authors declare that they have no competing interests to declare that are relevant to the content of this article.
References
- 1.International Multiple Sclerosis Genetics Consortium, and MultipleMS Consortium (2023) Locus for severity implicates CNS resilience in progression of multiple sclerosis. Nature 619:323–331. 10.1038/s41586-023-06250-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A (2022) Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science (New York, N.Y.) 375:296–301. 10.1126/science.abj8222 [DOI] [PubMed] [Google Scholar]
- 3.Comabella M, Tintore M, Sao Avilés A, Carbonell-Mirabent P, Malhotra S, Rovira A, Fissolo N, Lünemann JD, Montalban X (2023) Increased cytomegalovirus immune responses at disease onset are protective in the long-term prognosis of patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 94:173–180. 10.1136/jnnp-2022-330205 [DOI] [PubMed] [Google Scholar]
- 4.Antel J, Antel S, Caramanos Z, Arnold DL, Kuhlmann T (2012) Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity? Acta Neuropathol 123:627–638. 10.1007/s00401-012-0953-0 [DOI] [PubMed] [Google Scholar]
- 5.Moutsianas L, Jostins L, Beecham AH, Dilthey AT, Xifara DK, Ban M, Shah TS, Patsopoulos NA, Alfredsson L, Anderson CA, Attfield KE, Baranzini SE, Barrett J, Binder TMC, Booth D, Buck D, Celius EG, Cotsapas C, D’Alfonso S, Dendrou CA, Donnelly P, Dubois B, Fontaine B, Fugger L, Goris A, Gourraud P-A, Graetz C, Hemmer B, Hillert J, Kockum I, Leslie S, Lill CM, Martinelli-Boneschi F, Oksenberg JR, Olsson T, Oturai A, Saarela J, Søndergaard HB, Spurkland A, Taylor B, Winkelmann J, Zipp F, Haines JL, Pericak-Vance MA, Spencer CCA, Stewart G, Hafler DA, Ivinson AJ, Harbo HF, Hauser SL, de Jager PL, Compston A, McCauley JL, Sawcer S, McVean G (2015) Class II HLA interactions modulate genetic risk for multiple sclerosis. Nat Genet 47:1107–1113. 10.1038/ng.3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173 [DOI] [PubMed] [Google Scholar]
- 7.Levin LI, Munger KL, Rubertone MV, Peck CA, Lennette ET, Spiegelman D, Ascherio A (2005) Temporal relationship between elevation of Epstein–Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 293:2496–2500. 10.1001/jama.293.20.2496 [DOI] [PubMed] [Google Scholar]
- 8.Lünemann JD, Tintoré M, Messmer B, Strowig T, Rovira A, Perkal H, Caballero E, Münz C, Montalban X, Comabella M (2010) Elevated Epstein–Barr virus-encoded nuclear antigen-1 immune responses predict conversion to multiple sclerosis. Ann Neurol 67:159–169. 10.1002/ana.21886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascherio A, Munger KL, Lennette ET, Spiegelman D, Hernán MA, Olek MJ, Hankinson SE, Hunter DJ (2001) Epstein–Barr virus antibodies and risk of multiple sclerosis: a prospective study. JAMA 286:3083–3088. 10.1001/jama.286.24.3083 [DOI] [PubMed] [Google Scholar]
- 10.Cortese M, Leng Y, Bjornevik K, Mitchell M, Healy BC, Mina MJ, Mancuso JD, Niebuhr DW, Munger KL, Elledge SJ, Ascherio A (2024) Serologic response to the Epstein–Barr virus peptidome and the risk for multiple sclerosis. JAMA Neurol 81:515–524. 10.1001/jamaneurol.2024.0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selter RC, Brilot F, Grummel V, Kraus V, Cepok S, Dale RC, Hemmer B (2010) Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74:1711–1715. 10.1212/WNL.0b013e3181e04096 [DOI] [PubMed] [Google Scholar]
- 12.Schneider-Hohendorf T, Wünsch C, Falk S, Raposo C, Rubelt F, Mirebrahim H, Asgharian H, Schlecht U, Mattox D, Zhou W, Dawin E, Pawlitzki M, Lauks S, Jarius S, Wildemann B, Havla J, Kümpfel T, Schrot M-C, Ringelstein M, Kraemer M, Schwake C, Schmitter T, Ayzenberg I, Fischer K, Meuth SG, Aktas O, Hümmert MW, Kretschmer JR, Trebst C, Kleffner I, Massey J, Muraro PA, Chen-Harris H, Gross CC, Klotz L, Wiendl H, Schwab N (2024) Broader anti-EBV TCR repertoire in multiple sclerosis: disease specificity and treatment modulation. Brain. 10.1093/brain/awae244 [DOI] [PubMed] [Google Scholar]
- 13.Farrell RA, Antony D, Wall GR, Clark DA, Fisniku L, Swanton J, Khaleeli Z, Schmierer K, Miller DH, Giovannoni G (2009) Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 73:32–38. 10.1212/WNL.0b013e3181aa29fe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingram G, Bugert JJ, Loveless S, Robertson NP (2010) Anti-EBNA-1 IgG is not a reliable marker of multiple sclerosis clinical disease activity. Eur J Neurol 17:1386–1389. 10.1111/j.1468-1331.2010.03083.x [DOI] [PubMed] [Google Scholar]
- 15.Cortese M, Munger KL, Martínez-Lapiscina EH, Barro C, Edan G, Freedman MS, Hartung H-P, Montalbán X, Foley FW, Penner IK, Hemmer B, Fox EJ, Schippling S, Wicklein E-M, Kappos L, Kuhle J, Ascherio A (2020) Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology 94:e1950–e1960. 10.1212/WNL.0000000000009371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanheusden M, Stinissen P, ‘t Hart BA, Hellings N (2015) Cytomegalovirus: a culprit or protector in multiple sclerosis? Trends Mol Med 21:16–23. 10.1016/j.molmed.2014.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.