Abstract
Background
Acute ischemic stroke (AIS) is a major cause of morbidity and mortality, with hemorrhagic transformation (HT) further worsening outcomes. Traditional scoring systems have limited predictive accuracy for HT in AIS. Recent research has explored machine learning (ML) and deep learning (DL) algorithms for stroke management. This study evaluates and compares the effectiveness of ML and DL algorithms in predicting HT post-AIS, benchmarking them against conventional models.
Methods
A systematic search was conducted across PubMed, Embase, Web of Science, Scopus, and IEEE, initially yielding 1421 studies. After screening, 24 studies met the inclusion criteria. The Prediction Model Risk of Bias Assessment Tool (PROBAST) was used to assess the quality of these studies, and a qualitative synthesis was performed due to heterogeneity in the study design.
Results
The included studies featured diverse ML and DL algorithms, with Logistic Regression (LR), Support Vector Machine (SVM), and Random Forest (RF) being the most common. Gradient boosting (GB) showed superior performance. Median Area Under the Curve (AUC) values were 0.91 for GB, 0.83 for RF, 0.77 for LR, and 0.76 for SVM. Neural networks had a median AUC of 0.81 and convolutional neural networks (CNNs) had a median AUC of 0.91. ML techniques outperformed conventional models, particularly those integrating clinical and imaging data.
Conclusions
ML and DL models significantly surpass traditional scoring systems in predicting HT. These advanced models enhance clinical decision-making and improve patient outcomes. Future research should address data expansion, imaging protocol standardization, and model transparency to enhance stroke outcomes further.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12810-6.
Keywords: Acute ischemic stroke, Hemorrhagic transformation, Machine learning, ML, DL, Systematic review
Introduction
Stroke, particularly acute ischemic stroke (AIS), continues to be a major contributor to morbidity and mortality globally, placing a substantial burden on healthcare systems [1]. A critical complication following AIS is hemorrhagic transformation (HT), wherein ischemic brain tissue undergoes secondary bleeding. This process exacerbates neurological deficits and elevates the risk of mortality [2]. This complication typically occurs following the reperfusion of cerebral tissue, often due to thrombolytic therapies [3]. Therefore, accurate prediction of HT is crucial for optimizing therapeutic strategies and enhancing patient outcomes.
The integration of artificial intelligence (AI) into medical research holds the potential to revolutionize the prediction and management of stroke outcomes. Over the years, AI has evolved significantly, transitioning from early rule-based systems to advanced machine learning (ML) and deep learning (DL) algorithms [4]. AI encompasses the development of algorithms and computational models that mimic human cognitive functions. Within AI, ML, and DL have emerged as powerful tools in medical research, offering the capability to analyze vast amounts of data and identify patterns that traditional statistical methods may overlook [5]. Predictive models in stroke medicine are designed to estimate the risk of complications such as HT based on various patient-specific factors. Traditional models have relied on clinical, radiological, and laboratory data; however, their predictive accuracy is often limited by the complexity and heterogeneity of stroke presentations. This limitation underscores the need for more sophisticated approaches capable of handling multifaceted data and extracting meaningful patterns. Examples of such scoring systems include the Hemorrhage After Thrombolysis (HAT) score, the Safe Implementation of Treatments in Stroke Symptomatic Intracerebral Hemorrhage (SITS-SICH) risk score, and the Stroke Prognostication using Age and National Institutes of Health Stroke Scale-100 index (SPAN-100) [6].
ML, a subset of AI, focuses on developing algorithms that can learn from data and make predictions or decisions without being explicitly programmed. ML techniques, such as support vector machines (SVM), random forests (RF), and logistic regression, have been extensively applied in medical research to predict clinical outcomes. These algorithms excel in supervised learning scenarios where labeled data is available for training [5].
DL, which falls under the broader category of ML, utilizes a layered approach to analyze and learn from data [7]. This enables it to identify intricate patterns and relationships within datasets. Convolutional neural networks (CNNs) and recurrent neural networks (RNNs) are prominent DL architectures. CNN has proven to be especially effective in the analysis of medical images and shows great potential in making clinical predictions based on imaging data [8]. RNNs are adept at handling sequential data, making them suitable for time-series prediction tasks such as monitoring patient vital signs [9].
Recent studies have shown a marked increase in the application of these algorithms in areas such as stroke research [10, 11]. ML has been particularly successful in predicting HT, as evidenced by various studies [12].
Considering the life-threatening consequences of HT after AIS, and the emergence of numerous ML and DL tools designed to predict the risk of HT based on different inputs this study aims to systematically review and evaluate these predictive models. Our review will focus on comparing the efficacy of these algorithms and, where feasible, benchmark them against existing scoring systems. This comparison will not only highlight the potential of ML and DL to enhance predictive accuracy but also identify areas where these technologies could be refined for better integration into clinical workflows. Ultimately, our review endeavors to underscore the significance of leveraging cutting-edge technologies to improve patient outcomes in AIS, setting the stage for future innovations in stroke management.
Methods
Study design
This study is a systematic review to evaluate the accuracy and efficacy of ML and DL algorithms in predicting HT following AIS. The review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, detailed in Table S1 [13]. Furthermore, this study's protocol has been registered with the International Prospective Register of Systematic Reviews and assigned the identification number CRD42023492308.
Search strategy
In this systematic review, we executed an extensive and detailed search across multiple databases, including PubMed, Embase, Scopus, Web of Science (ISI), and IEEE. This search was strategically focused on keywords associated with “ischemic stroke”, “machine learning”, and “hemorrhagic transformation”, to identify pertinent literature up to May 18, 2024. For a detailed insight into the search techniques used for each database, please consult the comprehensive explanation available in Table S2.
Study selection and eligibility criteria
Initially, two reviewers independently screened the search results, examining titles and abstracts. They then conducted a detailed review of the full-text articles to determine their relevance. In instances of disagreement, a third reviewer was consulted to provide an additional opinion. Inclusion criteria were original, peer-reviewed research articles in English that developed and validated ML and/or DL models for HT risk prediction after AIS. Exclusion criteria included studies using external databases, lacking detailed methodologies, unavailable in full text, or not focusing on HT prediction. Literature types such as case reports, reviews, conference proceedings, and editorials were also excluded.
Data extraction
Two reviewers independently extracted data into a Google Sheet, consulting a third reviewer in case of disagreements. The extracted information covered various aspects, including study design, patient details, data sources, eligibility criteria, sample demographics, treatment types, definitions and assessment timings of HT, features for model training, types of algorithms, preprocessing methods, model structure, comparison models, scoring systems, and the area under the curve (AUC) as the key performance indicator, alongside principal findings, limitations, and suggestions for future research.
Risk of bias assessment
The studies were assessed using the Prediction Model Risk of Bias Assessment Tool (PROBAST), designed for bias risk evaluation in four domains and the suitability of diagnostic and prognostic models [14]. This assessment was independently conducted by two authors, with a third resolving any differences. Using PROBAST criteria, studies were categorized into low, unclear, or high bias risk, with a high-risk designation applied if significant bias was identified in any of the four domains.
Data synthesis and analysis
In this systematic review, we conducted a qualitative synthesis to compare and contrast the outcomes of various ML and DL models, alongside traditional scoring systems. Our study's broad scope, encompassing diverse ML and DL models and input variables, renders a meta-analysis impractical due to the required homogeneity in methods and metrics. Instead, our focus is on a comparative evaluation, examining the performance, adaptability, and practicality of these computational models.
Results
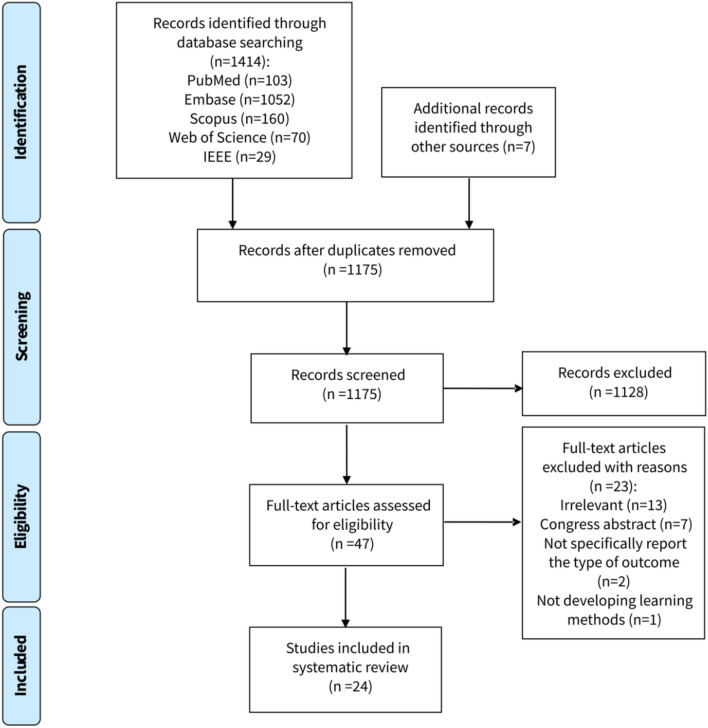
We identified 1414 studies by searching designated databases and found seven more studies through cross-referencing. Removing duplicates resulted in 1175 unique studies. Initial screening narrowed these down to 47 articles, and after a detailed review, 24 were chosen for inclusion. The selection process is depicted in Fig. 1, showcasing the PRISMA flowchart.
Fig. 1.
Study selection
Risk of bias assessment
Two independent reviewers rigorously evaluated the integrity of the included studies, resolving any discrepancies with the help of a third reviewer using the PROBAST tool. Among 24 studies examined, seven were found to have a high risk of bias, mainly due to issues in participant selection and analysis methods [15–21]. The comprehensive quality assessment results are delineated in Table S3.
Study characteristics
Among the included studies, the range of publication years extends from 2012 [18] to 2024 [22, 23]. Most of the studies, specifically 21 out of 24 (87%), were conducted starting from 2020. Methodologically, 21 (87%) studies adopted a retrospective design [10, 15–34], and only three were conducted with a prospective design [12, 35, 36]. The geographical distribution of the included studies predominantly featured research conducted in China, with 14 out of 24 studies (58%) originating from this region [10, 15–17, 21, 22, 24, 26–30, 33, 34], followed by South Korea, contributing four studies [20, 23, 25, 31]. Additional contributions came from various countries, each represented by a single study: The United Kingdom [19], Germany [12], Italy [36], Egypt [35], Taiwan [32], and Thailand [18].
Demographics
The sample sizes across the studies varied considerably, with the smallest cohort comprising 43 individuals [36] and the largest encompassing 146,062 participants [12]. The median sample size across these investigations was 350, with an interquartile range (IQR) from 129 to 1118, illustrating a significant disparity in study scales. Most studies, 19 out of 24 (79%), reported sample sizes below 2000 [10, 15–26, 28, 30, 32, 34–36].
Regarding demographic details, all studies except for two [18, 23] provided the mean age of the participant populations. The mean age spanned from 62.8 years [35] to 77 years [36], with a median age across studies being 69.22 years and an IQR from 66.6 to 71.3 years. The age distribution revealed that the majority of studies (18 studies, 82%) reported mean ages within the 65 to 75 years’ bracket. Only one study [36] reported a mean age over 75 years, while three studies [17, 26, 35] reported mean ages below 65 years.
The gender distribution across studies that reported the gender of included participants (22 studies) indicated a male predominance, with male-to-female ratios extending from 1.03 to 2.55, and a median value of 1.62 (IQR 1.28–1.95)—most of the studies, 17 out of 22 (77%), documented male-to-female ratios less than 2 [12, 15, 16, 19, 20, 22–25, 28–33, 35, 36].
Regarding treatment modalities, out of the 24 studies analyzed, 19 detailed the type of treatment administered, which included intravenous thrombolysis (IVT), endovascular thrombectomy (EVT), or both. IVT emerged as the most frequently utilized intervention in 12 studies [16, 18, 19, 21, 24, 26–30, 32, 33], followed by EVT in four studies [15, 25, 34, 36], EVT + IVT in two studies [12, 20], and EVT or IVT in one study [23]. One study investigated the risk of HT in patients who arrived late and did not undergo any treatment [22].
Further details regarding the characteristics of the included studies are summarized in Table 1.
Table 1.
Detailed characteristics of included studies on machine learning algorithms for predicting hemorrhagic transformation following acute ischemic stroke
| Study, country (year), study design | Interval time for evaluation | Treatment type | Sample size | Age (years), mean ± SD/median (IQR) | Sex (M/F) | # HT |
|---|---|---|---|---|---|---|
| Heo et al., South Korea (2024) [23], Retro | w/i 24 h | EVT or IVT | 362 | Median: 77 (IQR 69–83) | 185/177 | 218 |
| Huang et al., China (2024) [22], Retro | w/i 7 days | No treatment | 140- > train: 99, val.: 41 | 65.2 ± 12.2 | 86/54 | 59 |
| Wen et al., China (2023) [34], Retro | w/i 72 h | EVT | 105- > train: 73, int. val.: 32 |
Train: 72.0 ± 13.1 Int.val: 71.4 ± 13.9 |
66/29 | 52 |
| Ren et al., China (2023) [24], Retro | w/i 36 h | IVT | 517- > train: 355, int. val.: 90, ext. val.: 72 | 67.02 ± 12.67- > train: 67.19 ± 12.39, int. val.: 66.83 ± 12.15, ext. val.: 66.44 ± 14.70 | 333/184 | 249 |
| Ru et al., China (2023) [16], Retro | w/i 12–36 h | IVT | 828 | Non-HT: 67 (59–77), HT: 70 (62–82) | 547/281 | 69 |
| Heo et al., South Korea (2023) [25], Retro | w/i 72 h | EVT | 202 | 71.4 ± 14.5 | 103/99 | 109 |
| Li et al., China (2023) [26], Retro | NR | IVT | Cohort 1: 1182 (train and int. val.), cohort 2: 227 (ext. val.) |
Cohort 1: 62.82 ± 11.53 Cohort 2: 62.90 ± 11.43 |
Cohort 1: 835/347 Cohort 2: 165/62 |
Cohort 1: 587 Cohort 2: 112 |
| Ros et al., Italy (2023) [36], Pros | w/i 24 h | EVT | 43 | 77 (69–83) | 26/17 | 23 |
| Jiang et al., China (2023) [15], Retro | w/i 24 h | EVT | Dataset 1 (338)- > train (75%): HT: 187, non-HT: 66, test (25%): HT: 63, non-HT: 22, Dataset 2 (54) for ext. val | Dataset 1, HT: 70.9 ± 11.2, Dataset 1, non-HT: 65.7 ± 9.6, Dataset 2, HT: 71.3 ± 10.1, Dataset 2, non-HT: 64.2 ± 8.5 |
Dataset 1: 213/125 Dataset 2: 31/23 |
Dataset 1: 88 Dataset 2: 15 |
| Wen et al., China (2023) [27], Retro | w/i 36 h | IVT | Train (80%) and int. val. (20%): 6369, ext. val.: 1921 | Train and int. val.: 65 (57, 71), ext. val.: 65 (58, 72) | 5858/2429 | 121 |
| Bonkhoff et al., Germany (2022) [12], Pros | NR | IVT: 24989 / Intra-arterial thrombectomy + thrombolysis: 10706 | 146062 (dev. cohort: 74749, Val. cohort: 71,313) | 72.7 ± 13.1 | 76,828/69234 | 2580 |
| Elsaid et al., Egypt (2022) [35], Pros | w/i 7 days | NR | 354- > train: 177, test: 177 | 62.8 ± 10.5 | 199/155 | 70 |
| Xu et al., China (2022) [28], Retro | w/i 48 h | IVT |
345- > Train: 80%,val.: 20% |
70 (63–81) | 224/121 | 45 |
| Liu et al., China (2022) [29], Retro | w/i 36 h | IVT | 1738 Caucasians for training and 296 Han Chinese pts to validate | Caucasian: 68.37 ± 12.62, Chinese: 69.39 ± 13.37 | Caucasian: 1016/622, Chinese: 165/131 | 114 |
| Meng et al., China (2022) [17], Retro | w/i 72 h | NR |
71- > train: 49, val: 22 |
Non-HT: 64 (range 40–85), HT: 64 (range 41–83) | 51/20 | 11 |
| Cui et al., China (2022) [30], Retro | w/i 36 h | IVT | 517- > train: 332, int. val.: 83, ext. val.: 102 | 67.02 ± 12.67 | 333/184 | NR |
| Xie et al., South Korea (2022) [20], Retro | w/i 7 days | IVT, EVT, IVT + EVT | 118- > train: 83, test: 35 | 69.22 ± 12.33 | 63/55 | 52 |
| Wang et al., China (2022) [21], Retro | NR | IVT | 144 (288 NCCT) | 70.06 ± 11.3 | NR | 88 |
| Choi et al., South Korea (2021) [31], Retro | w/i 48 h | NR | 2028- > train: 1419, test: 609 | Total- > 69.6 ± 12.8, train: 69.7 ± 12.9, test: 69.3 ± 12.4 | 1183/845 | 318 |
| Chung et al., Taiwan (2020) [32], Retro | w/i 72 h | IVT | 331 | 69.2 ± 12.2 | 198/133 | 25 |
| Wang et al., China (2020) [33], Retro | w/i 24 h | IVT | 2237- > train and int. val.: 70:30% | Non-HT: 66.32 ± 12.67, HT: 69.54 ± 11.89 | 1438/799 | 102 |
| Yu et al., China (2018) [10], Retro | w/i 24 h | NR | 62 | HT group: 71 ± 13, non-HT group: 67 ± 13 | 42/20 | 41 |
| Bentley et al., UK (2014) [19], Retro | w/i one week | IVT | 116- > train: 106, val.: 10 | HT: 75.1 (95% CI: 69.3–80.9), non-HT: 73.2 (95% CI: 70.7–75.7) | 59/57 | 16 |
| Dharmasaroja et al., Thailand (2012) [18], Retro | W/i 36 h | IVT | 194 | NR | NR | NR |
EVT endovascular treatment, F female, h hour, HT hemorrhagic transformation, IVT intravenous thrombolysis, IQR interquartile range, M male, NR not reported, Pros prospective, Retro retrospective, SD standard deviation
Algorithms
The analysis revealed LR as the most frequently used learning algorithm, applied in 14 studies [10, 12, 20, 22–24, 26–28, 30, 31, 33–35], followed by SVM in 10 studies [10, 18, 19, 24, 27, 29–31, 33, 35], and RF in eight studies [17, 24, 26–28, 30, 33, 35]. Yu et al. utilized the broadest array of ML algorithms, encompassing six distinct models [10]. In contrast, the research conducted by Wang et al. [33], Elsaid et al. [35], Wen et al. [27], and Ren et al. [24] involved the application of five distinct algorithms. Another four studies [15, 16, 21, 25] utilized some form of CNN, either as the main component or in some part of their pipeline.
Among the studies, all conducted internal validation to assess their models' performance, but only nine also carried out external validation [15, 20, 24–27, 29, 30, 33].
Input variables
Clinical features were used as input variables in 18 studies, and imaging findings were employed in 16 studies. Twelve studies [16, 18–25, 30, 34, 36] employed computed tomography (CT) images, either as a direct input [16, 19–21, 25, 30, 36] or using extracted features [18, 22–24, 34] (e.g., radiomics). Four studies incorporated features derived from magnetic resonance imaging (MRI) in their analyses [10, 15, 17, 35]. Jiang et al. [15], Meng et al. [17], and Yu et al. [10] utilized multiparametric MRI, integrating various imaging sequences to enhance predictive capabilities. Conversely, Elsaid et al. [35] applied a comprehensive suite of conventional MRI sequences (e.g., T1, T2, FLAIR). Of the aforementioned studies, two [17, 35] utilized the extracted features from these imaging techniques, while the remaining two [10, 15] employed these imaging modalities as direct inputs.
Algorithm-level performance
We report the performance of different algorithms using AUCs. LR (without regularization) was reported 10 times with a median AUC of 0.77 [IQR 0.71, 0.81], while LR with LASSO regularization was reported twice with AUCs of 0.80 and 0.87. SVM was used 10 times across studies with a median AUC of 0.767 [IQR 0.73, 0.87]. Similarly, RF was utilized 10 times with a median AUC of 0.831 [IQR 0.76, 0.91]. Next in line, gradient boosting (GB) was used seven times across multiple studies with a median AUC of 0.91 [0.8, 0.94]. Neural networks (artificial neural network (ANN), multilayer perceptron (MLP), and probabilistic neural network (PNN)) appeared eight times throughout the studies with a median AUC of 0.81 [0.78, 0.84]. Cumulatively, CNNs were used 21 times, albeit most of them were reported in a single study [15], with a median AUC of 0.91 [IQR 0.82, 0.93].
Algorithmic performance compared to traditional scoring systems
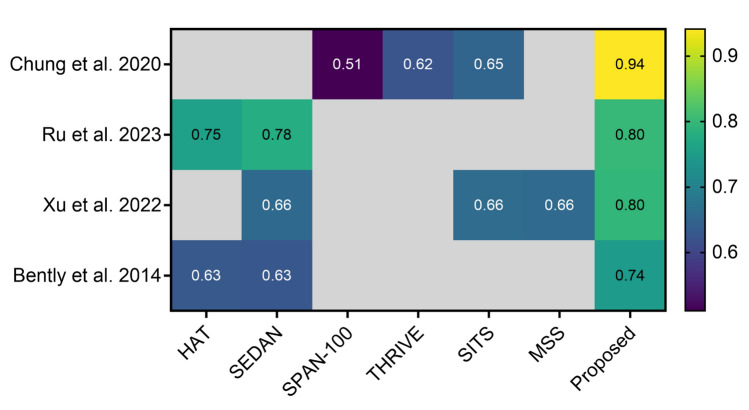
Four studies compared their proposed algorithms to traditional scoring systems [16, 19, 28, 32]. The scoring systems included HAT, SEDAN, SPAN-100, THRIVE, MSS, and SITS. All of them showed that their proposed ML model outperformed the scoring systems. Particularly notable was the study by Chung et al. [32], where their method achieved an AUC of 0.94 compared to the best-performing scoring system, SITS, which scored 0.65. Figure 2 illustrates a graphical depiction of these comparisons.
Fig. 2.
Assessment of proposed algorithm performance versus traditional scoring systems in predicting hemorrhagic transformation following acute ischemic stroke
Ru et al. [16] incorporated both non-contrast CT (NCCT) and clinical information, Chung et al. [32] and Xu et al. [28] developed their models only on clinical information, and Bentley et al. [19] solely relied on NCCT as input.
Study-level performance
Across the analyzed studies, ensemble and advanced ML techniques, particularly RF and GB, exhibited outstanding performance. In a recent study by Heo et al., the GB model utilizing radiomics features achieved an impressive AUC of 0.98 [23]. Noteworthy results were also reported by Ren et al. [24] who achieved an AUC of 0.94 with GB using a combination of clinical and radiomics features, while Li et al. [26] demonstrated an AUC of 0.95 with the same algorithm but applied to different laboratory findings. Furthermore, Cui et al. [30] identified Extreme GB (XGB) as the top-performing model, achieving an AUC of 0.91.
DL approaches, particularly those incorporating CNNs and attention mechanisms, showed promising results. Ru et al. [16] developed weakly-supervised DL (WSDL), a model integrating a pre-trained CNN with attention-based pooling, achieving an AUC of 0.8. Heo et al. [25] utilized a 3D CNN, obtaining an AUC of 0.91. Jiang et al. [15] and Wang et al. [21] explored novel neural network architectures, though with varying degrees of success, highlighting the potential and challenges of DL methods in this domain.
Studies utilizing traditional ML algorithms revealed a broad spectrum of outcomes. For instance, Wen et al. [27] and Bonkhoff et al. [12] highlighted the effectiveness of LR with L1-regularization, achieving AUCs of 0.87 and 0.80, respectively. Conversely, Xu et al. [28] found a non-significant difference between RF and LR performances. Notably, Meng et al. [17] and Elsaid et al. [35] reported high AUCs (0.91) using RF and GB models.
Ren et al. [24] and Meng et al. [17] consistently found that integrating clinical and imaging data as inputs for ML models led to improved performance.
Liu et al. [29] presented an interesting finding with their SVM model, showing a considerable disparity in performance between Caucasian and Chinese patient populations (AUC of 0.87 vs. 0.74, respectively).
Table 2 comprehensively presents the specific algorithms utilized within each study, detailing their respective inputs and various attributes.
Table 2.
In-depth overview of machine learning methodologies implemented in the included studies and their performance
| Study (year) | Algorithm | Input variables | Preprocessing methods | Missing data handling/imbalance addressing/regularization technique | Model performance, AUC (95% CI) |
|---|---|---|---|---|---|
| Heo et al. [23] (2024) | LightGBM (with all features), Extratrees (with textural features), LR (clinical variables) | Clinical variables and radiomics features from NCCT | Included data conversion, VOI definition, normalization, windowing, resampling, and feature extraction | KNN for clinical data/NR/10fold cross-validation |
LightGBM (test set) → 0.986 (0.971–1.00) Extratrees → (test set): 0.845 (0.774–0.916) LR → 0.544 (0.431–0.658) |
| Huang et al. [22] (2024) | LR | Clinical variables and radiomics features from NCCT | Normalization, segmentation, resampling, feature extraction, feature selection | NR/ Apply instance-level data augmentation techniques: flipping, scaling, rotation, cropping/3fold cross-validation |
Clinicoradiomics nomogram model: train → 0.86 (0.78–0.93), val. → 0.90 (0.80–1.00) Clinical model: train → 0.64 (0.56–0.72), val. → 0.63 (0.50–0.76) |
| Wen et al. [34] (2023) | MLRA | Clinical variables and radiomics features from NCCT | Intensity normalization, imaging interpolation, gray level discretization, manual segmentation: MCA territory regions of interest | NR/ Anonymize patient data in DICOM headers/ NR | Train → 0.781 (0.675–0.886) int.val. → 0.797 (0.642–0.951) |
| Ren et al. [24] (2023) | SGD, SVM, LR, RF, XGB | Clinical variables and radiomics features from NCCT | Normalization, resampling, standardization | Standardize DECT images: Resize to 256 × 256 × 36 with center crop or edge padding. Convert HU to voxel values; normalize using the external dataset's mean and SD/5fold cross-validation | Training cohort → SGD 0.912, SVM: 0.936, LR: 0.874, RF: 0.926, XGB: 0.953, XGB in training cohort (clinical features only): 0.996 (0.991–0.999), (radiomics only): 0.999 (0.999–1.000), Combined: 0.995 (0.991–0.999), XGB in int. val. cohort → (clinical features only): 0.898 (0.873–0.921), (radiomics only): 0.922 (0.896–0.941), (combined): 0.950 (0.925–0.967), XGB in ext. cohort → (clinical only): 0.911 (0.891–0.928), (radiomics): 0.883 (0.851–0.902), (combined): 0.942 (0.927–0.958) |
| Ru et al. [16] (2023) | WSDL | NCCT images and clinical variables | Image Preprocessing: Piecewise sampling, 256 × 256 resampling, window adjustments, channel augmentation. Clinical data: normalize, and use ImageNet pre-trained DCNN | Exclusion of tests with high missing rates and outliers. Remaining missing data handled using median, mean, or mode, depending on data characteristics. Normalization of certain data elements for consistency/NR/NR | WSDL: 0.799 (0.712–0.883) |
| Heo et al. [25] (2023) | 3D CNN (Utilized a 3D CNN w/3D ResNet structure) | DECT | NR | Multiple Imputation by Chained Equations (MICE)/SMOTE/ Backward stepwise regression for LR | Train: 0.867 (0.827–0.867), Test: 0.911 (0.774–1.000) |
| Li et al. [26] (2023) | XGB, LR, RF, DT | Laboratory results | NR | NR/ Down-sampling addresses imbalance, creating samples with 50% affected and 50% non-affected patients/NR | Int. val. → XGB: 0.95 (0.93–0.96), DT: 0.90 (0.88–0.91), RF: 0.91 (0.89–0.92), LR: 0.82 (0.80–0.85) |
| Da Ros et al. [36] (2022) | Bernoulli Naive Bayes Classifier | CB-CT | Offset correction, gain correction, scatter correction, and water beam-hardening correction | NR/NR/NR | 0.876 |
| Jiang et al. [15] (2023) | CNN models w/ both single-parameter and multi-parameter approaches | Multi-parametric MRI data (DWI, MTT, TTP, CBF, CBV) and clinical data |
Images processed w/ Pixel intensity normalization Linear compression to [0, 255] range Histogram Equalization Saved in PNG format Clinical data processing: Normalized using min–max normalization |
NR/NR/10fold cross-validation | VOI dataset = > single parameter- > clinical: 0.680, DWI: 0.830, MTT: 0.933, TTP: 0.916, CBF: 0.835, CBV: 0.878 multi-parameters model- > MT: 0.924, MTC: 0.924, DMT: 0.933, DMTC: 0.948, DMTC*: 0.939, Slice dataset = > single parameter- > clinical: 0.680, DWI: 0.609, MTT: 0.945, TTP: 0.889, CBF: 0.689, CBV: 0.702 multi-parameters model- > MT: 0.896, MTC: 0.913, DMT: 0.921, DMTC: 0.932, DMTC*: 0.927 |
| Wen et al. [27] (2023) | LR w/o regularization (reference model), LR w/ LASSO regularization, SVM, RF, GBDT, MLP | Clinical variables and therapeutic metrics | NR | 5-nearest neighbor model/SMOTE/5fold cross-validation | Ext. val. → Reference model: 0.575 (0.44–0.71), LR w/ LASSO: 0.87 (0.79–0.95), SVM: 0.582 (0.472–0.692),RF: 0.536 (0.42–0.653), GBDT: 0.436 (0.305–0.568), MLP: 0.766 (0.637–0.894) |
| Bonkhoff et al. [12] (2022) | LR, L1-regularized LR, KNN, GB | Clinical variables | Down-sampling step | NR/SMOTE/10fold cross-validation | Val. → LR: 0.79 (0.79– 0.79), L1-regularized LR: 0.80 (0.79– 0.80), KNN: 0.78 (0.78– 0.78), GB: 0.80 (0.80– 0.80) |
| Elsaid et al. [35] (2022) | LR, SVM, RF, GB, MLP | Clinical variables, laboratory findings, and MRI markers | NR | MissForest algorithm/NR/NR | Test → GB: 0.91 (0.86–0.95), RF: 0.91 (0.87–0.96), SVM: 0.90 (0.85–0.94), LR: 0.84 (0.77–0.91), MLP: 0.85 (0.78–0.92) |
| Xu et al. [28] (2022) | RF, LR | Clinical variables and laboratory results | NR | NR/NR/NR | Val. → RF: 0.795 (0.647–0.944), LR: 0.703 (0.515–0.892) |
| Liu et al. [29] (2022) | SVM | Clinical variables and laboratory results | Selecting the top-8 predictive features using RF | NR/NR/NR | Caucasian cohort: 0.87 (0.83–0.91), Chinese cohort: 0.74 (0.64–0.83) |
| Meng et al. [17] (2022) | RF | Radiomics features extracted from MRI and clinical variables | NR | NR/NR/5fold cross-validation | Clinical model: 0.556 ± 0.045, Radiomics model w/ abnormal ROI: 0.831 ± 0.006, radiomics model w/ all ROIs: 0.831 ± 0.006, combined model: 0.911 ± 0.009 |
| Cui et al. [30] (2022) | LR, RF, SVM, XGB | Clinical variables, laboratory results, and CT findings |
Normalize data: Zero mean, unit variance Feature selection: Lasso post-univariate analysis |
NR/NR/Data augmentation (rotation, shifts, zoom), mini-batch size setting for preventing overfitting, and 5fold cross validation | XGB: 0.914, LR: 0.908, RF: 0.894, SVM: 0.893 |
| Xie et al. [20] (2022) | LR | NCCT | Image normalization, lesion segmentation, ROI normalization, resampling, smoothing, and fixing bin width | NR/ Implement dynamic oversampling for the HT dataset/NR | 0.750 (0.585–0.915) |
| Wang et al. [21] (2022) | DBSE-Net | NCCT | NR | NR/random oversampling/10fold cross-validation | 0.720 |
| Choi et al. [31] (2021) | LR, SVM, XGB, ANN | Clinical variables | One-hot encoding applied. Scaling techniques used: normalization, min–max scaling, standardization, robust scaling | Use Missing-Indicator Technique for variables with > 5% missing data/ Over-sampling method and cost-sensitive adaptation/NR | ANN: 0.84, SVM: 0.73, BLR: 0.75, XGB: 0.74 |
| Chung et al. [32] (2020) | ANN | Clinical variables | NR | NR/random sampling/10fold cross-validation | Train: 0.951 ± 0.02, Val.: 0.941 ± 0.03 |
| Wang et al. [33] (2020) | RF, LR, NN, SVM, AdaBoost | Clinical variables | Imputation of missing values, normalization, and imbalance processing | NR/ensured equal counts of bleeding and non-bleeding samples/10fold cross-validation | NN: 0.82, SVM: 0.79, LR: 0.77, AdaBoost: 0.77, RF: 0.76 |
| Yu et al. [10] (2018) | SR-KDA, SR-DA, SVM, LR, DT, feedforward NN | Pre-intervention contrast time-curve from PWI, AIF, and DWI values |
Co-registration of images w/ SPM12 Automatic AIF detection via Olea Sphere Bilinear interpolation of AIF/PWI Median filter application for noise reduction |
NR/down-sampling non-sICH group/NR | SR-KDA: 0.837 ± 0.026, SVM: 0.821 ± 0.029, NN: 0.807 ± 0.043, DT: 0.798 ± 0.031, SR-DA: 0.751 ± 0.036, LR: 0.585 ± 0.075 |
| Bentley et al. [19] (2014) | SVM | NCCT |
Global mean intensity adjusted in images Excluded or replaced anomalous voxels w/ abnormal values |
NR/NR/10fold cross-validation | SVM: 0.744 (0.738–0.748) |
| Dharmasaroja et al. [18] (2012) | RBF, MLP, PNN, SVM | Clinical variables | NR | Missing data handling/imbalance addressing/3fold cross-validation | PNN: 0.787 ± 0.27, RBF: 0.686, MLP: 0.638, SVM: 0.416 ± 0.27 |
AIF arterial input function, ANN artificial neural network, AUC area under the curve, CBCT cone-beam computed tomography, CBF cerebral blood flow, CBV cerebral blood volume, CI confidence interval, CNN convolutional neural network, CP cerebral perfusion, CT computed tomography, DCNN deep convolutional neural network, DBSE dual-branch separation and enhancement, DECT dual-energy computed tomography, DMTC DWI & MTT & TTP & Clinical, DMTC* DWI & MTT & TTP & clinical of ext. val. set, DT decision tree, DWI diffusion-weighted imaging, GBDT gradient boosted decision tree, HU Hounsfield units, KNN k-nearest neighbors, LASSO least absolute shrinkage and selection operator, LR logistic regression, MCA middle cerebral artery, MRI magnetic resonance imaging, MT MTT&TTP, MTT mean transit time, MLRA multivariate logistic regression analysis, NCCT non-contrast computed tomography, NN neural network, NR not reported, PNN probability neural network, PWI perfusion weighted imaging, RBF radial basis function, RF random forest, ROI region of interest, SD standard deviation, SGD stochastic gradient descent, SR-DA spectral regression w/ discriminant analysis, SR-KDA spectral regression w/ kernel discriminant analysis, SVM support vector machine, VOI volume of interest, WSDL weekly supervised deep learning, XGB extreme gradient boosting
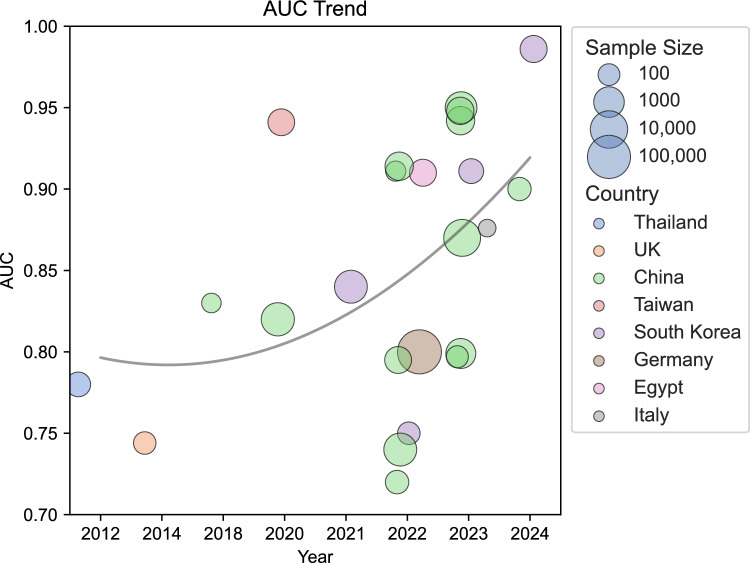
Figure 3 provides a detailed summary of the studies included in our review, highlighting key information such as sample sizes, countries of origin, publication years, best-reported AUC values, and the overall trends observed over time.
Fig. 3.
Characteristics of included studies
Table 3 summarizes the key findings, limitations, and recommendations for future research of the included studies in this systematic review.
Table 3.
Machine learning studies in acute ischemic stroke: insights, limitations, and future directions for hemorrhagic transformation prediction
| Study, (year) | Key findings | Limitations | Future suggestions |
|---|---|---|---|
| Heo et al. [23] (2024) |
The Light Gradient Boosting model using all radiomics features outperformed the ExtraTrees model with textural features and the LR model with only clinical variables Radiomics feature extraction and model execution can be performed on consumer-grade CPUs, without requiring high computational power |
Retrospective design and small sample size Differences in NCCT equipment, protocols, and parameters Lacks validation across different centers and regions Final patient groups were not perfectly matched due to exclusion of patients with image processing errors |
Conduct multi-center validation studies Develop an ML model to automate VOI drawing, ensuring consistency and reducing manual variability Explore different ML models, such as XGBoost, to potentially enhance model performance Investigate the use of different clinical cutoffs to optimize model applicability in various clinical settings |
| Huang et al. [22] (2024) | The models can help stratify the risk of HT, allowing for individualized and accurate clinical treatment plans |
Retrospective design and small sample size Focus on AIS patients with HT introduces selection bias and lowers statistical power |
Validate findings with larger, multicenter, prospective studies Develop automatic segmentation techniques for cerebral hemorrhage Incorporate detailed clinical and hematological data and a wider range of AIS patients Investigate integrating models into clinical workflows to assess real-world impact Update models with new data and advanced techniques for better accuracy Use models to stratify patients for personalized treatment |
| Wen et al. [34] (2023) | No clinical or routine radiological factors were identified as predictors of HT, in contrast to some previous studies | Retrospective design and small sample size | Conduct multicenter, large-scale, and prospective studies to further validate and refine the predictive model |
| Ren et al. [24] (2023) |
Superior accuracy of the combined clinical-radiomics model compared to models using only clinical data or radiomics Radiomics model based on NCCT image features improves HT risk prediction, linked to infarct size |
Retrospective design and small sample K-nearest Neighbor for Incomplete Data: Methodological constraint Restricted Analysis of Radiomics Features |
Explore radiomics-clinical outcomes link and expand clinical data in models Standardize CT imaging and incorporate post-thrombolysis MRI data Enhance the study of HT types and identify new risk factors Conduct multi-center studies for robust data and improve pt participation Establish collaborative networks and apply advanced AI for precise predictions |
| Ru et al. [16] (2023) |
Subgroup Analysis of the WSDL Model showed superior performance in sICH cases (AUC: 0.833); lower in asymptomatic ICH and w/o ICH Useful for diagnosis and treatment in resource-limited settings Supports f/u and treatment planning, and aids decision-making |
Risk of Overfitting: Due to a small number of positive cases Limited sICH cases HT detection flaws: HT is potentially underestimated by the NCCT method |
Add aims to better assess HT Conduct Multicenter Studies: Increases model generalizability Use More Imaging Data: Includes CTP, SWI, and reperfusion as input variables for enhanced prediction accuracy |
| Heo et al. [25] (2023) |
The developed model uses unprocessed raw DICOM images, simplifying the process DL w/ DECT shows potential for quick, automated prediction of HT post-EVT |
Single-center study Included both symptomatic and asymptomatic hemorrhage cases Differences in detecting hemorrhage w/ CT vs. MRI Many pts were excluded due to DECT constraints Lacked a calculated statistical power for determining sample size |
Intend to conduct a forward-looking, multi-institutional study to enhance the model's relevance Investigating extra elements besides DECT to better predict HT |
| Li et al. [26] (2023) |
HT-Lab10 model (using XGB) showed high accuracy in Cohort 1 (AUC: 0.95) Effective in predicting both HT occurrence and in-hospital mortality post-HT (AUC: 0.85) Cohort 2 val. confirms the model's reliability for HT predictions and post-HT mortality assessment |
Generalizability concerns due to the small, single-center sample in the Chinese population Inability to predict long-term mortality from incomplete f/u Reliance solely on lab data Technical limitations prevented the exploration of ensemble ML models |
Test results in broader, varied groups Examine long-term death risk over extended periods Use medical imaging data to increase precision Explore combining various AI models for better outcomes |
| Da Ros et al. [36] (2023) |
Deepens understanding of CB-CT images after EVT using ML Highlights standardized CB-CT analysis need for consistency and personalized care, despite early ML limitations |
Small sample size Limited Imaging f/u: Used only 24-h post-procedure NCCT Higher symptomatic hemorrhage rate (11%); bias from excluding pts w/o CB-CT |
Transition to DL for enhanced CB-CT prediction due to small sample sizes Develop robust DL tools for CB-CT analysis Standardize quantitative CB-CT analysis for improved precision in medicine |
| Jiang et al. [15] (2023) |
DMTC model, combining DWI, MTT, TTP, and clinical data, accurately predicts HT in post-EVT AIS pts Clinical, DWI, and PWI data enhance AIS management via a DL model in EVT treatment VOI and slice data sets both effectively predict HT Slice data set, selected from axial MRI images based on VOI lesions, is easier to use, highly repeatable, and provides extensive image information The slice data set could potentially replace the VOI data set for DL model training |
Small sample size, unclear optimal pts number for accuracy Data collected retro.ly, organized by admission time to resemble a pro. study Exclusion of MRI sequences T1WI, T2WI, FLAIR Included bridging therapy pts, despite similar HT rates across therapies |
Boost model accuracy by enlarging sample size Use or emulate a pro. study design for stronger data Include additional MRI sequences for a thorough analysis Investigate how various therapy methods affect HT incidence and outcomes |
| Wen et al. [27] (2023) |
MLP model suggested for post-thrombolysis hemorrhage risk prediction LR w/ lasso tops in AUC, MLP is second SVM and MLP are beneficial in Decision Curve Analysis Anticoagulation therapy is a negative predictor; rt-PA is positive |
Sample limited to Northeast China, affecting generalizability Data issues could compromise individual pts outcome accuracy ML models used few variables |
Increase sample size and diversify locations Improve data sharing and transparency Add more relevant variables to models Refine models for enhanced clinical usefulness |
| Bonkhoff et al. [12] (2022) |
Early identification of high-risk pts improves personalized care Stroke severity is the most significant predictor GB models outperform LR |
Limited data prevented DL use Basic clinical data and observational design Missing data (up to 7%) |
Expand prediction scope to include various stroke-related complications Intend regular updates to the model; essential to validate w/ independent data sets |
| Elsaid et al. [35] (2022) |
RFC and GBC models effectively forecast HT Main HT predictors: NIH stroke scale, infarction size, microbleeds |
Generalizability is limited to specific hospitals and races Small sample; rt-PA therapy pts excluded |
Conduct the study in various environments and include pts receiving rt-PA treatment Include a wider variety of biological and imaging indicators in the study |
| Xu et al. [28] (2022) |
RF outperforms other models in predicting HT SHAP values improve clinical understanding of the RF model High Accuracy: The model predicts HT in post-alteplase stroke pts w/ 66.7% sensitivity and 80.7% specificity |
Study limited to one center; broader val. needed Data set was too small for effective sICH analysis The algorithm might overlook key relationships Lack of SWI possibly undervalues HT risk |
Conduct larger, multi-center pro. studies to confirm research results Improve algorithms by including a broader set of features, beyond usual physician assessments Implement SWI in future studies for precise HT estimation |
| Liu et al. [29] (2022) |
Created a fast, efficient tool to screen pts at high risk for sICH Pinpointed major predictors of sICH in different areas The SVM model effectively predicted sICH in both Caucasian and Chinese pt groups |
VISTA trials' Caucasian sample is not widely representative Han Chinese sample small and from one hospital Missing data |
Develop clinical software Perform multi-center Chinese studies for val. And Smaller number of sICH in samples |
| Meng et al. [17] (2022) |
Multi-parametric MRI models outperform single-sequence models Single-sequence accuracy order: ADC > TTP > CBF > CBV > MTT Model accuracy peaks w/ 14 features, then declines Overfitting occurs w/ too many features Combining MRI radiomics and clinical factors yields high accuracy (AUC 0.911) |
Single-center study Complex image processing w/ basic radiomics Applicable only to non-lacunar acute cerebral infarcts |
Validate models w/ multi-center datasets Use DL for better feature extraction Study cerebral atrophy's role in AIS and HT prediction Focus on early prediction of stroke complications |
| Cui et al. [30] (2022) |
Created a practical CDSS prototype w/ Python Featuring a browser/server architecture for better integration and portability Suitable for web-based hospital systems |
Image compression in comics can lead to loss of features Research is limited by population and data size |
Utilize DL to improve predictions Broaden data and study groups for more universal applicability Apply ongoing learning for model updates Develop and evaluate the CDSS prototype consistently |
| Xie et al. [20] (2022) |
The Rad-score, based on five radiomics features, was the sole predictor for HT Model accuracy for predicting HT varied w/ infarct sizes and treatment method |
Small sample size and retro. Study design CT resolution constraints affected infarct area segmentation Excluded cerebral infarct volume on NCCT |
Validate the model in a pros. multicenter setting Use larger sample sizes for robust val Focus on the effect of infarct size in massive cerebral stroke on HT Recognize limitations in hyperacute AIS pts Recommend cerebral perfusion imaging for deeper analysis |
| Wang et al. [21] (2022) |
DBSE-Net for accurate lesion-HT prediction w/ dual-branch encoding Uses multi-scale features for NCCT-based HT prediction Algorithm addresses weak lesion features w/ key frame and adaptive encoding Efficiently extracts key information from NCCTs |
NR | NR |
| Choi et al. [31] (2021) |
ANN surpassed other ML algorithms w/ 0.844 accuracy in HT prediction post-AIS Scaling and resampling didn't improve ANN's HT prediction in AIS |
Evaluated only clinic-demographic factors and initial lab variables Did not fully assess post-stroke management and HT radiologic markers |
Incorporate post-stroke care factors in analysis Analyze radiologic indicators via DL in CT/MRI images Boost prediction accuracy w/ ensemble learning from clinical and imaging data |
| Chung et al. [32] (2020) |
ANNs match expert-level stroke diagnosis They require little statistical training and uncover complex patterns Enhance prediction of sICH and mortality in AIS after rt-PA treatment Aid in personalized AIS treatment stratification |
Retro. Design, small sample sized and single center study Potential underestimation of severity due to exclusion of pts on specific treatments |
Conduct multi-center studies for wider applicability Implement pros. studies to develop evolving AI tools Prioritize accuracy in prediction tools and decision support systems |
| Wang et al. [33] (2020) |
The best model is a three-layer neural network, achieving an AUC of 0.82 System deployment cut CT-to-treatment time from 52 to 41 min, significant at p < 0.001 Perfect identification of sICH cases as high-risk ML model reliably predicts personalized sICH risk post-stroke thrombolysis, enhancing treatment efficiency |
Sample mainly from Northeast China, reducing generalizability Predictive accuracy is potentially limited due to data scope Models built using a narrow range of variables |
Increase sample size and diversity for wider val Enhance data quality and transparency in upcoming studies Incorporate a broader range of variables in new models Address existing limitations and refine models |
| Yu et al. [10] (2018) |
Extract HT imaging markers from PWI images w/o pre-established metrics Assessed using f/u GRE for insights into pre-EVT Kernel spectral regression shows highest accuracy (83.7 ± 2.6%) |
Advanced preprocessing to address noise-related prediction errors Regularization to improve co-registration and label accuracy Multi-center evaluation for diverse recanalization cases Issue of small sample size Importance sampling or boosting over random sampling |
Create a brain region atlas for HT likelihood assessment Merge atlas w/ eloquence features for better outcome prediction Use nonlinear models for AIS HT prediction complexity Add more physiological variables for model enhancement Quantitatively study factors affecting HT for reliable predictions |
| Bentley et al. [19] (2014) |
SVM-based ML outperforms in predicting sICH ML more accurate than radiology in detecting thrombolysis-related sICH from CT scans ML via CT scans surpasses traditional methods in sICH prediction |
A small percentage (5%) of sICH cases in a single-center study Difficulties in choosing features and risk of overfitting in small datasets Time-intensive and inefficient image processing procedures |
Validate ML in thrombolysis w/ larger, diverse studies Acquire unbiased data pros.ly, including diverse pts and untreated potential cases Tackle challenges of imbalanced datasets; optimize image-space features w/caution Enhance image-processing efficiency for clinical applicability |
| Dharmasaroja et al. [18] (2012) |
AUC analysis showed no significant difference between RBF, MLP, and PNN models, while PNN outperformed SVM Three models identified stroke subtype as an important predictor for sICH, along w/ other factors like stroke location, prothrombin time, etc Using multiple ANN models showed advantages over a single model |
NR | NR |
ANN artificial neural network, ADC apparent diffusion coefficient, AUC area under the curve, AIS acute ischemic stroke, CBCT cone-beam computed tomography, CBF cerebral blood flow, CBV cerebral blood volume, CTP computed tomography perfusion, CT computed tomography, CDSS clinical decision support system, DECT dual-energy computed tomography, DL deep learning, DMTC DWI & MTT & TTP & Clinical, DWI diffusion-weighted imaging, EVT endovascular treatment, GB gradient boosting, HT hemorrhagic transformation, ICH intracerebral hemorrhage, IVT IV thrombolysis, LR logistic regression, ML machine learning, MLP multilayer perceptron, MRI magnetic resonance imaging, MT MTT&TTP, MTT mean transit time, NCCT non-contrast computed tomography, NIHSS National Institutes of Health Stroke Scale, NR not reported, PNN probability neural network, PWI perfusion weighted MRI, RBF radial basis function, RF random forest, sICH symptomatic ICH, SHAP SHapley Additive exPlanations, SVM support vector machine, SWI susceptibility weighted imaging, TT thrombin time, TTP time to peak, VOI volume of interest, WSDL weekly supervised deep learning, XGB extreme GB
Discussion
HT, a life-threatening complication after AIS, is an important contributor to morbidity and mortality [37]. Predicting the risk of HT at the time of admission can potentially assist healthcare providers in enhancing the quality of patient care. This predictive capability facilitates more informed decision-making. Currently, there are several well-established scoring systems designed to predict this complication. These systems primarily use clinical and imaging features to identify patients who are at increased risk [38–42]. However, these traditional scoring systems demonstrate only moderate performance in evaluating the risk of future HT [6, 43].
This systematic review evaluates the recently developed ML/DL models in forecasting HT after AIS. The findings from our investigation underscore the promising capability of ML and DL techniques in improving the risk estimation of HT following an AIS, surpassing conventional scoring systems in performance. Integrating clinical and imaging data can significantly improve the accuracy of HT prediction models. The model's effectiveness largely relies on the imaging methods used, with Meng et al. showing that multiparametric MRI techniques yield better predictions than single-sequence imaging methods [17].
The employment of radiomics features, which aims to extract quantitative and ideally reproducible information from diagnostic images, including intricate patterns not easily discernible or quantifiable by the human eye, holds significant importance as input factors for the development of ML algorithms in this field [44, 45]. As evidenced by various studies, the implementation of radiomics features on NCCT has shown promising performance. For instance, Heo et al. [23] demonstrated a notable improvement in model performance with a radiomics-based approach compared to models relying solely on clinical factors, with the radiomics model achieving nearly doubled performance (AUC of 0.986 vs. 0.544). Furthermore, Huang et al. [22] presented compelling results with two models: one integrating clinical and radiomics features, yielding a performance of 0.9, and another solely based on clinical features, achieving a lower performance of 0.6. These findings underscore the potential of incorporating radiomics features into ML frameworks to enhance diagnostic accuracy and prognostic capabilities in medical imaging analysis. Moreover, the observed superiority of radiomics features highlights the effectiveness of ensemble learning techniques in handling complex data patterns and optimizing model performance.
An interesting observation was that LR with the addition of LASSO (L1) regularization was able to beat other robust algorithms in some studies [12, 27]. This phenomenon signifies the fact that not always complex models outperform simpler ones. As to why LASSO regularization is so effective, it effectively shrinks the irrelevant coefficients to zero thus ‘selecting’ the most informative features [46].
In the study by Liu et al. [29], they tested their developed SVM model on two diverse populations: Caucasian and Chinese patients. They observed that the performance of the model in the Caucasian cohort was significantly higher than in the Chinese cohort. This finding highlights the necessity of adapting models to accommodate diverse genetic and environmental factors, thereby enhancing diagnostic accuracy across different ethnicities. The researchers attributed the performance disparity to factors such as small sample sizes and incomplete data, and they recommended conducting studies across multiple centers to confirm the results.
The comparative study of various ML models reveals distinct advantages and applications of each model in predicting HT risk. While simpler models like LR and DT are easier to understand, they typically underperform compared to more sophisticated methods like SVM and XGB.
Contrary to our expectations, the overall performance of DL-based methods was unexpectedly underwhelming, despite their complexity and recent successes. However, XGB and other ensemble DT-based models showcased better performance on tabular data, as demonstrated in an exploratory study by Grinsztajn et al. [47], a trend that was further supported by our review. One plausible explanation for this unexpected outcome could be the limited predictive cues visible in imaging studies of AIS patients, such as the hyperdense artery sign or ischemic changes [48]. However, models utilizing both clinical and imaging variables generally demonstrated improved performance [15, 17, 24]. In this theme, we observe that classic ML techniques fall short in terms of performance when fed high-dimensional data such as data derived from CT imaging [19], whereas CNN-based solutions shine in these scenarios [15]. As pointed out by Grinsztajn et al., ANNs are sensitive to irrelevant inputs, while tree-based solutions excel at these situations and are often used for this task (e.g., RF for feature selection) [47].
In the context of image processing, DL solutions especially CNN-based ones have dominated the field for the last 10 years [49]. It is possible to dodge using DL approaches by employing feature extraction methods such as radiomics, however, they tend to be less interpretable. The major advantage of CNNs over traditional hand-crafted methods is their ability to automatically learn relevant features directly from the data. However, this comes with its drawbacks. DL solutions, CNNs included, are data-hungry methods, requiring ample amounts of training data if one needs to train them from scratch [7]. Unfortunately, in the field of medicine, this is a scarcity. Thus, it is often seen those methods such as XGB and SVM shine the most with relatively limited data.
Over the years, several methods have been developed to increase the transparency of the ML/DL decision-making process. One of the most popular ones is Shapley values [50]. Xu et al. [28] utilized Shapley values to find the most influential factors for HT risk prediction. This method finds the most important input features using concepts from cooperative game theory. One of the most striking advantages of this method is its independence from the underlying predictive algorithm used, however, the caveat is the complexity of computing these values. Another method that is commonly used in vision models is GradCAM [51]. It uses the gradient of the classification score concerning some intermediate convolutional layers and effectively highlights the most salient regions in the input image. A significant barrier to the practical application of these solutions is their dependence on a large number of features, often ranging from dozens to hundreds, which can be impractical and expensive to supply.
The simplistic structure of medical data, such as binary indicators for diseases and basic age metrics, doesn't align well with the intricate input requirements of neural networks. This leads to a poor reflection of the actual biological diversity. Binary values for complex conditions like type 2 diabetes or hypertension reduce nuanced medical states to a mere ‘1’ or ‘0’, missing their broader biological effects. Similarly, linear age representation fails to mirror the non-linear nature of biological aging, overlooking the varied changes that occur at different life stages.
The success of DL, particularly in vision and language processing, is largely due to the vast availability of 'raw' data. Unlike traditional ML methods, DL operates in latent spaces, turning raw data into meaningful numerical forms. This shift became notable as neural networks grew more complex. In medical imaging, this success is due to the abundance of raw data available. For DL to be effectively used in healthcare, there needs to be a significant accumulation of diverse, unprocessed medical data, including images, physiological signals, and sensor data. Without such datasets, traditional ML techniques like RF and SVM will remain prevalent.
The accurate prediction of HT following AIS is paramount for guiding clinical decision-making and optimizing patient outcomes. In our study, GB demonstrated superior performance among all evaluated ML algorithms, achieving a median AUC of 0.91. This was followed by RF, LR, and SVM, which attained AUCs of 0.83, 0.77, and 0.76, respectively. Among the neural network models, CNNs had the best performance with a median AUC of 0.91, indicating comparable efficacy to GB. Furthermore, our evaluation revealed that in all studies where ML models were compared to traditional scoring systems, the ML models consistently exhibited superior performance. This suggests a significant advantage of utilizing advanced ML techniques over conventional methods.
The superior performance of certain ML and DL models, particularly those incorporating both clinical and imaging variables, underscores their potential utility as adjunctive tools for clinicians. Implementation of these advanced predictive models could facilitate early identification of patients at heightened risk of HT, enabling timely interventions and personalized treatment strategies to mitigate this critical complication and improve prognosis. Thus, this systematic review not only contributes to the scientific understanding of HT prediction models but also has tangible implications for improving patient care and outcomes in AIS management. Creating user-friendly Clinical Decision Support Systems (CDSS) and integrating ML/DL models into hospital systems could enhance the efficiency and reach of stroke care, particularly in less-equipped areas, thereby narrowing the gap in healthcare delivery. However, widespread clinical adoption faces challenges like ensuring the models' relevance for diverse populations, demystifying complex ML techniques, and avoiding overfitting through careful model selection and validation. Moreover, effectively integrating these technologies into daily medical practices is crucial, which involves simplifying data entry, continuously updating the models, and developing accessible interfaces for healthcare professionals.
In this systematic review, a major limitation was the significant diversity among the models studied and their input variables, which made a meta-analysis impractical. Nonetheless, despite this limitation, we were able to draw meaningful conclusions by comparing various models with each other and with conventional scoring systems and illustrating a clear visualization of the developed models.
The reviewed studies also had several limitations, including small sample size and retrospective design which potentially increase selection bias and affect the generalizability. Methodological issues, such as the use of specific algorithms like K-nearest Neighbor (KNN) for missing data and limited radiomics analysis, reduced the depth of the studies. Furthermore, the majority of studies were single-center studies with few HT cases introduced biases and limited the research scope. Technical and diversity limitations also hindered the models' predictive accuracy and applicability across various populations.
To enhance the precision and versatility of predictive models, future research should integrate larger, diverse sample sizes and adopt prospective, multicenter study designs to capture a wide range of clinical scenarios and patient demographics. Incorporating diverse ethnicities is essential to address population heterogeneity and reduce biases. Efforts should focus on integrating multimodal data while standardizing imaging protocols to ensure consistency and reproducibility. Enhancing model transparency through explainable AI (XAI) techniques will improve interpretability and trust. Rigorous validation using comprehensive performance metrics is also crucial. These strategies will significantly improve the performance, applicability, and reliability of ML and DL models in clinical practice.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contribution
DSL, MI, DZ, and SK conceived the project and developed the design of this study. MI, DZ and SK undertook the literature search and extracted the primary data. Quality assessment was conducted by MI, and DZ. MI and DZ performed the qualitative analysis. MI and DZ provided the first draft of the manuscript. DSL and SK provided expert review and regional context insights. All Authors approved the final version of the manuscript and agreed to the published version of the manuscript.
Funding
No funding was received to assist with the preparation of this manuscript.
Data and/or code availability
The data of the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The manuscript does not include any clinical studies or patient data. The study protocol has been registered with PROSPERO under ID: CRD42023492308.
Footnotes
Mahbod Issaiy and Diana Zarei contributed equally as the first author.
References
- 1.Katan M, Luft A (2018) Global burden of stroke. Semin Neurol 38:208–211. 10.1055/s-0038-1649503 [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Yang Y, Sun H, Xing Y (2014) Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Ann Transl Med 2:81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, Sharp FR (2014) Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab 34:185–199. 10.1038/jcbfm.2013.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA et al (2023) Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ 23:689. 10.1186/s12909-023-04698-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan MI, Mitchell TM (2015) Machine learning: trends, perspectives, and prospects. Science 349:255–260. 10.1126/science.aaa8415 [DOI] [PubMed] [Google Scholar]
- 6.Sung SF, Chen SC, Lin HJ, Chen YW, Tseng MC, Chen CH (2013) Comparison of risk-scoring systems in predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis. Stroke 44:1561–1566. 10.1161/STROKEAHA.111.000651 [DOI] [PubMed] [Google Scholar]
- 7.Taye MM (2023) Understanding of machine learning with deep learning: architectures, workflow, applications and future directions. Computers 12:91 [Google Scholar]
- 8.Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, van der Laak J, van Ginneken B, Sánchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88. 10.1016/j.media.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Hüsken M, Stagge P (2003) Recurrent neural networks for time series classification. Neurocomputing 50:223–235. 10.1016/S0925-2312(01)00706-8 [Google Scholar]
- 10.Yu Y, Guo D, Lou M, Liebeskind D, Scalzo F (2018) Prediction of hemorrhagic transformation severity in acute stroke from source perfusion MRI. IEEE Trans Biomed Eng 65:2058–2065. 10.1109/tbme.2017.2783241 [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Lee EJ, Ham S, Lee HB, Lee JS, Kwon SU, Kim JS, Kim N, Kang DW (2020) Machine learning approach to identify stroke within 4.5 hours. Stroke 51:860–866. 10.1161/strokeaha.119.027611 [DOI] [PubMed] [Google Scholar]
- 12.Bonkhoff AK, Rübsamen N, Grefkes C, Rost NS, Berger K, Karch A (2022) Development and validation of prediction models for severe complications after acute ischemic stroke: a study based on the stroke registry of Northwestern Germany. J Am Heart Assoc 11:e023175. 10.1161/jaha.121.023175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, Reitsma JB, Kleijnen J, Mallett S, Groupdagger P (2019) PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 170:51–58. 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 15.Jiang L, Zhou L, Yong W, Cui J, Geng W, Chen H, Zou J, Chen Y, Yin X, Chen YC (2023) A deep learning-based model for prediction of hemorrhagic transformation after stroke. Brain Pathol 33:e13023. 10.1111/bpa.13023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ru X, Zhao S, Chen W, Wu J, Yu R, Wang D, Dong M, Wu Q, Peng D, Song Y (2023) A weakly supervised deep learning model integrating noncontrasted computed tomography images and clinical factors facilitates haemorrhagic transformation prediction after intravenous thrombolysis in acute ischaemic stroke patients. Biomed Eng Online 22:129. 10.1186/s12938-023-01193-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng Y, Wang H, Wu C, Liu X, Qu L, Shi Y (2022) Prediction model of hemorrhage transformation in patient with acute ischemic stroke based on multiparametric MRI radiomics and machine learning. Brain Sci. 10.3390/brainsci12070858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dharmasaroja P, Dharmasaroja PA (2012) Prediction of intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke using multiple artificial neural networks. Neurol Res 34:120–128. 10.1179/1743132811y.0000000067 [DOI] [PubMed] [Google Scholar]
- 19.Bentley P, Ganesalingam J, Carlton Jones AL, Mahady K, Epton S, Rinne P, Sharma P, Halse O, Mehta A, Rueckert D (2014) Prediction of stroke thrombolysis outcome using CT brain machine learning. Neuroimage Clin 4:635–640. 10.1016/j.nicl.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie G, Li T, Ren Y, Wang D, Tang W, Li J, Li K (2022) Radiomics-based infarct features on CT predict hemorrhagic transformation in patients with acute ischemic stroke. Front Neurosci 16:1002717. 10.3389/fnins.2022.1002717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Liu Z, Li S (2022) Weak lesion feature extraction by dual-branch separation and enhancement network for safe hemorrhagic transformation prediction. Comput Med Imaging Graph 97:102038. 10.1016/j.compmedimag.2022.102038 [DOI] [PubMed] [Google Scholar]
- 22.Huang YH, Chen ZJ, Chen YF, Cai C, Lin YY, Lin ZQ, Chen CN, Yang ML, Li YZ, Wang Y (2024) The value of CT-based radiomics in predicting hemorrhagic transformation in acute ischemic stroke patients without recanalization therapy. Front Neurol 15:1255621. 10.3389/fneur.2024.1255621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo J, Sim Y, Kim BM, Kim DJ, Kim YD, Nam HS, Choi YS, Lee SK, Kim EY, Sohn B (2024) Radiomics using non-contrast CT to predict hemorrhagic transformation risk in stroke patients undergoing revascularization. Eur Radiol. 10.1007/s00330-024-10618-6 [DOI] [PubMed] [Google Scholar]
- 24.Ren H, Song H, Wang J, Xiong H, Long B, Gong M, Liu J, He Z, Liu L, Jiang X et al (2023) A clinical-radiomics model based on noncontrast computed tomography to predict hemorrhagic transformation after stroke by machine learning: a multicenter study. Insights Imaging 14:52. 10.1186/s13244-023-01399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heo J, Yoon Y, Han HJ, Kim JJ, Park KY, Kim BM, Kim DJ, Kim YD, Nam HS, Lee SK, Sohn B (2023) Prediction of cerebral hemorrhagic transformation after thrombectomy using a deep learning of dual-energy CT. Eur Radiol. 10.1007/s00330-023-10432-6 [DOI] [PubMed] [Google Scholar]
- 26.Li X, Xu C, Shang C, Wang Y, Xu J, Zhou Q (2023) Machine learning predicts the risk of hemorrhagic transformation of acute cerebral infarction and in-hospital death. Comput Methods Programs Biomed 237:107582. 10.1016/j.cmpb.2023.107582 [DOI] [PubMed] [Google Scholar]
- 27.Wen R, Wang M, Bian W, Zhu H, Xiao Y, He Q, Wang Y, Liu X, Shi Y, Hong Z, Xu B (2023) Machine learning-based prediction of symptomatic intracerebral hemorrhage after intravenous thrombolysis for stroke: a large multicenter study. Front Neurol 14:1247492. 10.3389/fneur.2023.1247492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Li X, Wu D, Zhang Z, Jiang A (2022) Machine learning-based model for prediction of hemorrhage transformation in acute ischemic stroke after alteplase. Front Neurol 13:897903. 10.3389/fneur.2022.897903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Chen X, Guo X, Xu R, Wang Y, Liu M (2022) Machine learning prediction of symptomatic intracerebral hemorrhage after stroke thrombolysis: a cross-cultural validation in Caucasian and Han Chinese cohort. Ther Adv Neurol Disord 15:17562864221129380. 10.1177/17562864221129380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui S, Song H, Ren H, Wang X, Xie Z, Wen H, Li Y (2022) Prediction of hemorrhagic complication after thrombolytic therapy based on multimodal data from multiple centers: an approach to machine learning and system implementation. J Personal Med 12:2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi JM, Seo SY, Kim PJ, Kim YS, Lee SH, Sohn JH, Kim DK, Lee JJ, Kim C (2021) Prediction of hemorrhagic transformation after ischemic stroke using machine learning. J Personal Med. 10.3390/jpm11090863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung C-C, Chan L, Bamodu OA, Hong C-T, Chiu H-W (2020) Artificial neural network based prediction of postthrombolysis intracerebral hemorrhage and death. Sci Rep 10:20501. 10.1038/s41598-020-77546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Huang Y, Xia Y, Zhang W, Fang K, Zhou X, Yu X, Cheng X, Li G, Wang X et al (2020) Personalized risk prediction of symptomatic intracerebral hemorrhage after stroke thrombolysis using a machine-learning model. Ther Adv Neurol Disord 13:1756286420902358. 10.1177/1756286420902358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen X, Xiao Y, Hu X, Chen J, Song F (2023) Prediction of hemorrhagic transformation via pre-treatment CT radiomics in acute ischemic stroke patients receiving endovascular therapy. Br J Radiol 96:20220439. 10.1259/bjr.20220439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elsaid AF, Fahmi RM, Shehta N, Ramadan BM (2022) Machine learning approach for hemorrhagic transformation prediction: capturing predictors’ interaction. Front Neurol 13:951401. 10.3389/fneur.2022.951401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Da Ros V, Duggento A, Cavallo AU, Bellini L, Pitocchi F, Toschi N, Mascolo AP, Sallustio F, Di Giuliano F, Diomedi M et al (2023) Can machine learning of post-procedural cone-beam CT images in acute ischemic stroke improve the detection of 24-h hemorrhagic transformation? A preliminary study. Neuroradiology 65:599–608. 10.1007/s00234-022-03070-0 [DOI] [PubMed] [Google Scholar]
- 37.Qureshi AI, Malik AA, Adil MM, Defillo A, Sherr GT, Suri MF (2015) Hematoma enlargement among patients with traumatic brain injury: analysis of a prospective multicenter clinical trial. J Vasc Interv Neurol 8:42–49 [PMC free article] [PubMed] [Google Scholar]
- 38.Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S (2008) A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis 17:331–333. 10.1016/j.jstrokecerebrovasdis.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 39.Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, Searls D, Selim M (2008) The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology 71:1417–1423. 10.1212/01.wnl.0000330297.58334.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menon BK, Saver JL, Prabhakaran S, Reeves M, Liang L, Olson DM, Peterson ED, Hernandez AF, Fonarow GC, Schwamm LH, Smith EE (2012) Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke 43:2293–2299. 10.1161/strokeaha.112.660415 [DOI] [PubMed] [Google Scholar]
- 41.Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, Mustanoja S, Kuzmanovic I, Sairanen T, Forss N et al (2012) Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol 71:634–641. 10.1002/ana.23546 [DOI] [PubMed] [Google Scholar]
- 42.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC (2013) Stroke Prognostication using age and NIH Stroke Scale: SPAN-100. Neurology 80:21–28. 10.1212/WNL.0b013e31827b1ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu CH, Chen CH, Lin CH, Lee CW, Lee M, Tang SC, Jeng JS (2022) Comparison of risk scores in predicting symptomatic intracerebral hemorrhage after endovascular thrombectomy. J Formos Med Assoc 121:1257–1265. 10.1016/j.jfma.2021.09.005 [DOI] [PubMed] [Google Scholar]
- 44.Mayerhoefer ME, Materka A, Langs G, Häggström I, Szczypiński P, Gibbs P, Cook G (2020) Introduction to radiomics. J Nucl Med 61:488–495. 10.2967/jnumed.118.222893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577. 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranstam J, Cook JA (2018) LASSO regression. Br J Surg 105:1348–1348. 10.1002/bjs.10895 [Google Scholar]
- 47.Grinsztajn L, Oyallon E, Varoquaux G (2022) Why do tree-based models still outperform deep learning on tabular data? arXiv:2207.08815
- 48.Elsaid N, Mustafa W, Saied A (2020) Radiological predictors of hemorrhagic transformation after acute ischemic stroke: an evidence-based analysis. Neuroradiol J 33:118–133. 10.1177/1971400919900275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alzubaidi L, Zhang J, Humaidi AJ, Al-Dujaili A, Duan Y, Al-Shamma O, Santamaría J, Fadhel MA, Al-Amidie M, Farhan L (2021) Review of deep learning: concepts, CNN architectures, challenges, applications, future directions. J Big Data 8:53. 10.1186/s40537-021-00444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Covert IC, Lundberg SM, Lee S-I (2023) Algorithms to estimate Shapley value feature attributions. Nat Mach Intell 5:590–601. 10.1038/s42256-023-00657-x [Google Scholar]
- 51.Selvaraju RR, Cogswell M, Das A et al (2020) Grad-CAM: Visual Explanations from Deep Networks via Gradient-Based Localization. Int J Comput Vis 128:336–359. 10.1007/s11263-019-01228-7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of the current study are available from the corresponding author on reasonable request.