Abstract
Purpose
Controversies exist regarding the prevailing spectrum of microorganisms in microbial ureteral stent colonization (MUSC) and their clinical significance. The aim of this comprehensive review is to determine the predominant microbial spectrum in patients with an indwelling ureteral stent in comparison to catheter-associated urinary tract infections (CAUTI) and uncomplicated urinary tract infections (UTI).
Methods
Google scholar, PubMed, Embase, Medline, and Cochrane literature databases were searched from inception to April 2022 to identify manuscripts on MUSC, uncomplicated UTI and CAUTI. A principal component analysis (PCA) was performed to identify patterns of the pathogen spectrum of the different groups.
Results
We included 29 studies on MUSC, 28 studies on uncomplicated UTI and 23 CAUTI studies. The proportion of Staphylococci, Enterococci and Candida were significantly higher in MUSC and stent associated bacteriuria compared to their proportion in uncomplicated UTIs where E. coli dominates. By comparing MUSC, CAUTI and UTI with a PCA, the detected pathogen spectrum exhibited clearly distinguishable trends in the frequency of the main isolated pathogens influencing these three groups of urinary tract infections. With respect to MUSC and UTI, their 95% confidence interval ellipse only showed minimal overlap emphasizing that the spectrum of pathogens in the two groups is clearly distinct.
Conclusions
The frequency of detection of Staphylococci, Enterococci and Candida is more common in MUSC as compared to UTI. Thus, patients with indwelling ureteral stents should undergo an antimicrobial prophylaxis targeting this microbial spectrum in case of further surgery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00345-024-05354-x.
Keywords: Urinary tract infections, Bacteriuria, Ureteral catheterization, Ureteroscopy
Introduction
Ureteral stents are an important component of urology routine practice intended to maintain ureteral patency and to avoid obstruction of the upper urinary tract. Main indications for ureteral stent placement are urolithiasis, ureteral strictures, direct invasion or external compression by pelvic, retroperitoneal or metastatic malignancies as well as upper urinary tract carcinoma [1]. Ureteral stents are also used to prevent post-surgical complications. This makes ureteral stents indispensable devices in urology practice. However, they offer an ideal surface for microbial adhesion and biofilms are prone to develop on such materials. Indeed, antibiotic prophylaxis does not prevent stent colonization, which appears in 100% of patients with a permanent ureteral stent and in 70% of those temporarily stented [2].
In the majority of cases, microbial ureteral stent colonization (MUSC) remains asymptomatic. However, MUSC can be associated with infectious complications and is a leading risk associated with ureteral stent placement [3]. Infection associated with ureteral stents can lead to significant morbidity such as acute pyelonephritis, renal failure or urosepsis [4]. Therefore, antimicrobial prophylaxis is recommended during the placement of ureteral stents [2]. Microbial ureteral stent colonization and ureteral stent associated bacteriuria have been researched in in many studies, however the isolated pathogens differed between the different studies. Still, no consensus exists regarding the prevailing spectrum of microorganisms as well as the clinical significance of MUSC and stent associated bacteriuria. Therefore, the aim of this comprehensive review is to highlight differences in the pathogen spectrum encountered in MUSC compared to uncomplicated UTI and CAUTI. This will further allow to determine the predominant microbial spectrum in patients with an indwelling ureteral stent to optimize the choice of peri-interventional antimicrobial prophylaxis in those patients.
Materials and methods
Data collection
Google scholar, PubMed, Embase, Medline, and Cochrane literature databases were searched from inception to April 2022 to identify manuscripts on ureteral stent colonization. The search terms used were “(urinary tract infection, UTI or catheter-associated urinary tract infection, CAUTI) and (stent or stenting) and (pathogen or colonization)”, “infection on ureteral stent”, “ureteral stent colonization”, “ureteral stent pathogens”. A total of 6780 manuscripts were identified. Two authors performed independent scrutiny of these manuscripts and selected manuscripts to be included and selected studies were cross-checked by the same authors. Included studies had to fulfill the following criteria: (1) pathogens isolated were identified at least down to the genus level and E. coli down to the species level; (2) numbers of isolates or percentages of pathogens were present in the main text or supplementary material and allowed further grouping and final percentages calculation if needed; (3) material and methods section was considered clear and reproducible. A second author checked the relevance of all manuscripts. Finally, 29 studies were selected for data retrieval. We selected to retrieve the percentage of microorganisms isolated from stents and catheters and grouped them by type of microorganism. This choice was dictated by the reporting of the microbial spectrum in studies and aimed to maximize the number of studies that could be included as principal component analysis requires more samples (i.e., studies) than descriptors (i.e., pathogens included). When grouping of microorganisms or numbers of isolates were available, the percentage were calculated from the raw data.
For comparison purposes, data from studies on the search terms “uncomplicated urinary tract infection” (UTI) and “nosocomial UTI” (catheter associated urinary tract infection, CAUTI) were used for further analysis. For this purpose, Google scholar, PubMed, Embase, Medline, and Cochrane literature databases were searched from January 2002 to April 2022. We used the same inclusion criteria as described above for MUSC. In addition, we tried to select papers from different geographical origins to avoid bias due to similar populations belonging to the same locations (i.e., country). Papers with limited dataset (< 20 patients were excluded) as below such size the changes induced by 1 isolates affected the final pathogen spectrum by more than 5%.
Statistical analysis
All data retrieved from the original publications were compiled using spreadsheet software (Libreoffice 6 or MS Excel). Basic descriptive statistics and principal component analysis (PCA) were performed using R [5]. PCA was chosen as it is a linear dimensionality reduction technique that transforms a set of correlated features in a high dimensional space (in our case the multidimensional pathogen spectrum where the percentage of each pathogen corresponds to a dimension) into a series of uncorrelated features in the low dimensional space (two dimensions in our case). The technique is useful to visualize data as reducing the dimensions of data to 2D allows us visualizing patterns contained in the datasets more clearly. Before performing the PCA care was taken to make sure that the final dateset used met the minimal requirements for PCA. Multi-normality was not met, however acceptable skewness and kurtosis were found for the data allowing to perform a PCA without biais to the analysis. Furthermore, the dataset contained more object (i.e., studies) than descriptors (i.e., pathogens) and contained an acceptable number of 0. Under those conditions, the dataset was thus considered acceptable for PCA.
Furthermore, using the raw data and the information gathered from PCA, boxplots focusing on the main identified pathogens were plotted with R. Because of non-normal distributions and inhomogeneous variances the differences between the observed percentages of those pathogens where analysed using the Kruskal–Wallis test followed by post-hoc pairwise comparison with the Wilcoxon test with Benjamini and Hochberg [6] correction for multiple testing.
Results
We included 29 studies on MUSC from which 2201 pathogens were isolated [3, 7–34]. Similarly, for comparison purposes, we included 28 studies on uncomplicated UTI [35–60] from which 24,885 pathogens were isolated as well as 23 CAUTI [61–83] studies with 20,887 pathogens identified. Finally, 7 studies with 215 isolates from urine of patients with indwelling ureteral stents were also included [8, 9, 22, 34, 84–86]. Baseline characteristics of the included studies can be found in Table 1.
Table 1.
Characteristics of included studies on the microbial spectrum of ureteral stent colonization (stent), catheter associated urinary tract infection (CAUTI), urinary tract infection (UTI) and urine culture on patients with indwelling ureteral stent (urine stent)
| Author, year | Reference | Datapoint in Fig. 2 | Pathogens isolated (n) | Type of sample (stent, urine) | Positive culture (%) | Type of microorganism detected (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterococcus spp. | Streptococcus spp. | Staphylococcus spp. | E. coli | Proteus spp. | Enterobacteriaceae spp. | Pseudomonas spp.. | Candida spp. | Other | ||||||
| Al-Ghazo, 2010 | [7] | 8 | 29 | Stent | 23 | 0 | 17 | 0 | 52 | 7 | 14 | 0 | 7 | 3 |
| Amine Saouli, 2021 | [8] | 28 | 38 | Stent | 100 | 18 | 0 | 13 | 47 | 0 | 21 | 0 | 0 | 0 |
| Anak Agung, 2019 | [9] | 25 | 18 | Stent | 60 | 0 | 0 | 0 | 39 | 0 | 6 | 50 | 0 | 6 |
| Aydin, 2016 | [10] | 18 | 30 | Stent | 29 | 3 | 10 | 40 | 17 | 0 | 7 | 0 | 13 | 10 |
| Ben-Meir, 2009 | [11] | 11 | 82 | Stent | 10 | 33 | 10 | 26 | 9 | 0 | 7 | 10 | 0 | 12 |
| Bonkat, 2013 | [12] | 5 | 306 | Stent | 8 | 24 | 1 | 17 | 9 | 3 | 4 | 6 | 14 | 22 |
| Bonkat, 2013 | [13] | 4 | 200 | Stent | 16 | 17 | 7 | 19 | 14 | 2 | 3 | 1 | 13 | 24 |
| Bonkat, 2011 | [14] | 1 | 224 | Stent | 23 | 18 | 8 | 18 | 12 | 1 | 4 | 2 | 10 | 27 |
| Bonkat, 2012 | [3] | 6 | 26 | Stent | 4 | 31 | 4 | 19 | 11 | 0 | 0 | 4 | 8 | 23 |
| Farsi, 1995 | [15] | 10 | 140 | Stent | 30 | 0 | 16 | 25 | 9 | 0 | 14 | 23 | 1 | 12 |
| Garcia-Aparicio, 2015 | [16] | 21 | 43 | Stent | 100 | 12 | 0 | 5 | 12 | 14 | 23 | 21 | 5 | 7 |
| Kehinde, 2004 | [17] | 2 | 80 | Stent | 7 | 14 | 0 | 24 | 29 | 0 | 0 | 20 | 13 | 0 |
| Klis, 2009 | [18] | 7 | 70 | Stent | 26 | 15 | 0 | 52 | 7 | 2 | 4 | 8 | 1 | 11 |
| Kozyrakis, 2018 | [19] | 19 | 115 | Stent | 74 | 23 | 6 | 26 | 17 | 0 | 7 | 5 | 0 | 15 |
| Lifshitz, 1999 | [20] | 3 | 100 | Stent | 15 | 31 | 0 | 14 | 31 | 0 | 3 | 14 | 7 | 0 |
| Matsukawa, 2005 | [21] | 13 | 37 | CAUTI | 30 | 14 | 3 | 43 | 16 | 0 | 13 | 8 | 8 | 3 |
| Mehmet, 2021 | [22] | 22 | 67 | Stent | 31 | 27 | 4 | 15 | 19 | 0 | 4 | 9 | 15 | 6 |
| Nevo, 2019 | [23] | 24 | 103 | Stent | 100 | 18 | 15 | 15 | 17 | 5 | 11 | 3 | 10 | 8 |
| Paick, 2003 | [24] | 12 | 47 | Stent | 21 | 24 | 8 | 8 | 20 | 4 | 8 | 4 | 4 | 20 |
| Rahman, 2010 | [25] | 14 | 45 | Stent | 21 | 0 | 2 | 7 | 36 | 18 | 21 | 16 | 0 | 0 |
| Reid, 1992 | [26] | 15 | 44 | Stent | 27 | 3 | 3 | 41 | 3 | 0 | 3 | 0 | 0 | 47 |
| Riedl, 1999 | [27] | 9 | 118 | Stent | 100 | 33 | 10 | 26 | 9 | 3 | 7 | 10 | 1 | 2 |
| Salari, 2021 | [28] | 29 | 64 | Stent | 64 | 25 | 0 | 17 | 14 | 0 | 0 | 11 | 2 | 13 |
| Sarier, 2017 | [29] | 16 | 24 | Stent | 100 | 58 | 0 | 13 | 4 | 0 | 13 | 0 | 13 | 3 |
| Sarier, 2017 | [30] | 17 | 18 | Stent | 100 | 44 | 0 | 6 | 17 | 0 | 11 | 0 | 22 | 4 |
| Shabeena, 2018 | [31] | 23 | 40 | Stent | 100 | 10 | 18 | 10 | 20 | 0 | 10 | 13 | 0 | 20 |
| Volkan Ülker, 2019 | [32] | 20 | 8 | Stent | 20 | 23 | 6 | 26 | 17 | 0 | 7 | 5 | 8 | 7 |
| Wang, 2021 | [33] | 27 | 39 | Stent | 95 | 15 | 0 | 5 | 38 | 0 | 13 | 10 | 3 | 15 |
| Zhang, 2018 | [34] | 26 | 46 | Stent | 100 | 20 | 0 | 9 | 37 | 2 | 13 | 20 | 0 | 10 |
| Ahmad, 2012 | [35] | 34 | 591 | UTI | 100 | 0 | 7 | 0 | 54 | 4 | 25 | 8 | 0 | 2 |
| Arlene Rodriguez, 2012 | [36] | 31 | 140 | UTI | 100 | 0 | 0 | 1 | 76 | 6 | 12 | 6 | 0 | 1 |
| Bahadin, 2011 | [37] | 35 | 333 | UTI | 100 | 2 | 1 | 4 | 75 | 3 | 4 | 2 | 1 | 8 |
| Batra, 2020 | [38] | 51 | 295 | UTI | 100 | 2 | 0 | 4 | 67 | 2 | 18 | 4 | 0 | 3 |
| Beyene, 2011 | [39] | 36 | 21 | UTI | 100 | 0 | 0 | 19 | 33 | 5 | 43 | 0 | 0 | 0 |
| Bouskraoui, 2010 | [40] | 37 | 121 | UTI | 100 | 2 | 1 | 1 | 72 | 6 | 16 | 2 | 0 | 0 |
| Cui, 2021 | [41] | 54 | 401 | UTI | 100 | 6 | 1 | 4 | 41 | 5 | 15 | 5 | 4 | 19 |
| Dariusz Chojeta, 2021 | [42] | 46 | 285 | UTI | 100 | 5 | 0 | 0 | 51 | 14 | 22 | 3 | 0 | 5 |
| Duicu, 2021 | [43] | 56 | 331 | UTI | 100 | 3 | 0 | 1 | 72 | 7 | 11 | 6 | 0 | 1 |
| F.M.E. Wagenlehner, 2009 | [44] | 33 | 3018 | UTI | 100 | 3 | 1 | 9 | 77 | 5 | 7 | 0 | 0 | 2 |
| Gordon, 2003 | [45] | 41 | 1466 | UTI | 100 | 16 | 0 | 6 | 43 | 6 | 20 | 7 | 0 | 2 |
| Gordon, 2003 | [45] | 42 | 783 | UTI | 100 | 13 | 0 | 3 | 46 | 10 | 16 | 9 | 0 | 3 |
| Gordon, 2003 | [45] | 43 | 531 | UTI | 100 | 4 | 0 | 3 | 60 | 7 | 18 | 6 | 0 | 2 |
| Guclu, 2021 | [46] | 48 | 241 | UTI | 100 | 0 | 0 | 0 | 69 | 3 | 19 | 5 | 0 | 5 |
| Huang, 2021 | [47] | 49 | 7646 | UTI | 100 | 16 | 4 | 6 | 43 | 4 | 9 | 5 | 0 | 13 |
| Ioannou, 2020 | [48] | 50 | 205 | UTI | 100 | 13 | 0 | 3 | 41 | 12 | 17 | 5 | 5 | 4 |
| Kolawole, 2010 | [49] | 45 | 180 | UTI | 100 | 0 | 0 | 22 | 31 | 16 | 8 | 23 | 0 | 0 |
| Samia S. Khamees, 2012 | [50] | 44 | 256 | UTI | 100 | 0 | 2 | 11 | 34 | 22 | 27 | 3 | 0 | 1 |
| Nath, 2021 | [51] | 55 | 49 | UTI | 100 | 12 | 0 | 16 | 39 | 4 | 19 | 4 | 6 | 0 |
| Onifade, 2011 | [52] | 38 | 42 | UTI | 100 | 4 | 0 | 8 | 66 | 6 | 13 | 3 | 0 | 0 |
| Oorji, 2022 | [53] | 53 | 333 | UTI | 100 | 13 | 5 | 14 | 50 | 1 | 15 | 0 | 0 | 3 |
| Rupinder Bakshi, 2021 | [54] | 52 | 1306 | UTI | 100 | 1 | 0 | 9 | 49 | 11 | 26 | 3 | 0 | 1 |
| Shamataj, 2012 | [55] | 30 | 411 | UTI | 100 | 0 | 0 | 10 | 38 | 1 | 29 | 3 | 8 | 11 |
| Shams, 2017 | [56] | 57 | 152 | UTI | 100 | 1 | 4 | 13 | 55 | 0 | 13 | 0 | 9 | 6 |
| Turnidge, 2002 | [57] | 32 | 903 | UTI | 100 | 11 | 2 | 6 | 38 | 4 | 21 | 11 | 0 | 7 |
| Wang, 2013 | [58] | 39 | 92 | UTI | 100 | 17 | 2 | 3 | 64 | 4 | 6 | 3 | 0 | 1 |
| Wariso, 2010 | [59] | 40 | 234 | UTI | 100 | 5 | 0 | 17 | 33 | 9 | 19 | 8 | 0 | 9 |
| Yi-Te, 2020 | [60] | 47 | 4519 | UTI | 100 | 15 | 5 | 9 | 30 | 2 | 15 | 7 | 0 | 18 |
| Ahmed, 2019 | [61] | 72 | 89 | CAUTI | 100 | 8 | 0 | 6 | 27 | 7 | 33 | 5 | 0 | 16 |
| Amna Butt, 2015 | [62] | 80 | 118 | CAUTI | 100 | 0 | 0 | 7 | 49 | 10 | 12 | 14 | 8 | 0 |
| Bi, 2009 | [63] | 77 | 450 | CAUTI | 100 | 11 | 0 | 28 | 39 | 4 | 10 | 0 | 0 | 9 |
| Bizuaeyhu, 2022 | [64] | 75 | 88 | CAUTI | 100 | 13 | 0 | 9 | 6 | 0 | 6 | 9 | 45 | 13 |
| Chitnis, 2012 | [65] | 64 | 5756 | CAUTI | 100 | 19 | 0 | 0 | 26 | 0 | 15 | 16 | 25 | 1 |
| Darma-Kusuma, 2012 | [66] | 68 | 36 | CAUTI | 100 | 0 | 3 | 28 | 44 | 0 | 14 | 11 | 0 | 0 |
| Duszynska, 2020 | [67] | 78 | 307 | CAUTI | 100 | 20 | 0 | 0 | 14 | 3 | 14 | 6 | 12 | 31 |
| H Guanche-Garcell, 2011 | [68] | 60 | NA | CAUTI | 100 | 0 | 0 | 23 | 54 | 0 | 15 | 7 | 0 | 1 |
| Joon, 2013 | [69] | 66 | 61 | CAUTI | 100 | 31 | 0 | 12 | 38 | 0 | 3 | 5 | 10 | 1 |
| Lai, 2017 | [70] | 73 | 66 | CAUTI | 100 | 17 | 0 | 3 | 24 | 5 | 3 | 15 | 27 | 6 |
| Lee, 2004 | [71] | 59 | 40 | CAUTI | 100 | 22 | 0 | 15 | 0 | 10 | 0 | 29 | 0 | 24 |
| Lili Tao, 2011 | [72] | 61 | 7064 | CAUTI | 100 | 22 | 1 | 4 | 19 | 1 | 8 | 4 | 35 | 6 |
| Milan, 2009 | [73] | 76 | 49 | CAUTI | 100 | 0 | 0 | 0 | 40 | 16 | 26 | 18 | 0 | 0 |
| Mladenovic, 2015 | [74] | 62 | 71 | CAUTI | 100 | 13 | 0 | 1 | 11 | 4 | 23 | 20 | 28 | 0 |
| Nirmala Poddar, 2020 | [75] | 74 | 76 | CAUTI | 100 | 22 | 0 | 4 | 25 | 11 | 22 | 8 | 0 | 8 |
| Puri, 2002 | [76] | 67 | 73 | CAUTI | 100 | 8 | 0 | 12 | 33 | 3 | 15 | 15 | 14 | 0 |
| Sabir, 2017 | [77] | 71 | 1070 | CAUTI | 100 | 11 | 0 | 0 | 52 | 0 | 23 | 5 | 0 | 8 |
| Smitha Bagali, 2021 | [78] | 79 | 50 | CAUTI | 100 | 4 | 0 | 6 | 38 | 4 | 34 | 10 | 0 | 4 |
| Taiwo, 2006 | [79] | 70 | 126 | CAUTI | 100 | 0 | 0 | 11 | 20 | 3 | 36 | 27 | 3 | 0 |
| Talaat, 2010 | [80] | 58 | 188 | CAUTI | 100 | 13 | 0 | 2 | 7 | 2 | 13 | 7 | 51 | 5 |
| Temitz, 2012 | [81] | 63 | 22 | CAUTI | 100 | 15 | 0 | 0 | 24 | 5 | 12 | 9 | 33 | 2 |
| Toshie Tsuchida, 2008 | [82] | 69 | NA | CAUTI | 100 | 32 | 0 | 0 | 20 | 0 | 0 | 13 | 13 | 22 |
| Wazait, 2003 | [83] | 65 | 5109 | CAUTI | 100 | 17 | 0 | 10 | 31 | 16 | 12 | 11 | 0 | 3 |
| Altunal, 2017 | [84] | 84 | 22 | Urine Stent | 100 | 14 | 0 | 5 | 55 | 0 | 9 | 9 | 9 | 9 |
| Amine Saouli, 2021 | [8] | 87 | 41 |
Urine Stent |
100 | 17 | 2 | 15 | 39 | 0 | 27 | 0 | 0 | 0 |
| Anak Agung, 2019 | [9] | 85 | 12 |
Urine Stent |
37 | 8 | 0 | 0 | 25 | 0 | 0 | 58 | 0 | 8 |
| He, 2021 | [85] | 82 | 21 | Urine Stent | 100 | 0 | 0 | 11 | 58 | 0 | 0 | 11 | 0 | 21 |
| Mehmet, 2021 | [22] | 81 | 22 | Urine Stent | 10 | 23 | 5 | 5 | 32 | 0 | 18 | 14 | 5 | 4 |
| Useok Choi, 2021 | [86] | 83 | 70 |
Urine Stent |
100 | 24 | 4 | 0 | 30 | 9 | 13 | 14 | 0 | 6 |
| Zhang, 2018 | [34] | 86 | 27 | Urine Stent | 100 | 30 | 0 | 7 | 33 | 0 | 11 | 11 | 4 | 8 |
In most of the MUSC studies (23 out of 29) more gram-positive microorganisms than E. coli were isolated from stents [3, 10–24, 26–32]. In contrast, in studies of UTIs associated with ureteral stents, E. coli accounted for 30–50% of the pathogens isolated in UTIs. [35–38, 40, 42–46, 52, 53, 56, 58] [39, 41, 45, 47–51, 54, 55, 57, 59, 60] with rather high variability between studies. Regarding studies on CAUTIs, we noticed that in a quarter of the studies (8 out of 24) gram-positive microorganisms were more present than E. coli [64, 67, 69, 71, 72, 74, 75, 80], while in only two studies E. coli accounted for more than 50% of the pathogens [68, 77].
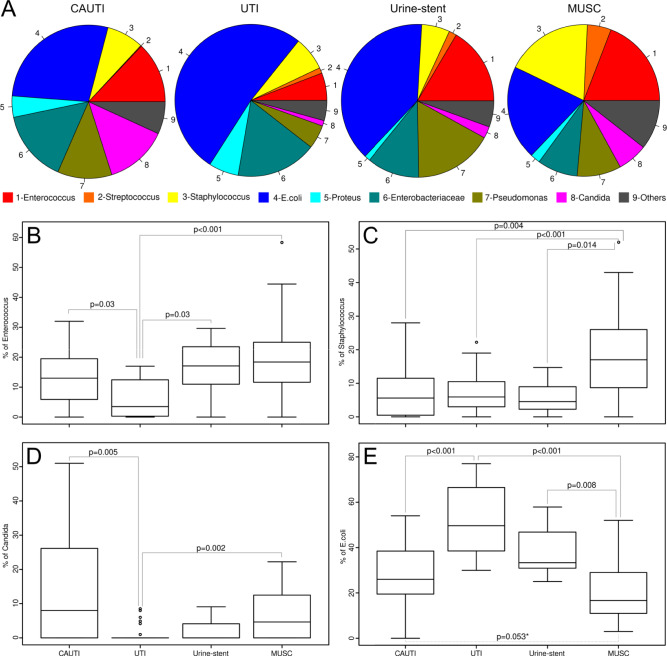
Boxplots were created using the data of four pathogens (Staphylococci Fig. 1C, Enterococci Fig. 1, Candida Fig. 1D, E. coli Fig. 1E) chosen for their relevance and isolation frequency. The most prevalent pathogens isolated from ureteral stents were Enterococci (19%), Staphylococci (19%), E. coli (20%) and Candida spp. (6%). The most prevalent pathogens detected in UTI were E. coli (52%), Enterococci (6%), Staphylococci (7%), and Candida spp. (1%). The most prevalent pathogens detected in CAUTI were E. coli (29%), Enterococci (12%), Staphylococci (8%) and Candida spp. (12%). The most prevalent pathogens isolated from urine in patients with stents were Enterococci (16%), Staphylococci (6%), E. coli (39%) and Candida spp. (2%) (Fig. 1). Significant differences were detected for most of the comparisions between the different groups analyzed (Fig. 1).
Fig. 1.
A Pie figure showing the spectrum of pathogens in the different groups CAUTI, UTI, urine from stent patients, MUSC. Boxplot showing the variation in the proportion of each pathogen in the different studies. B Enterococcus. C Staphylococcus. D Candida. E E. coli
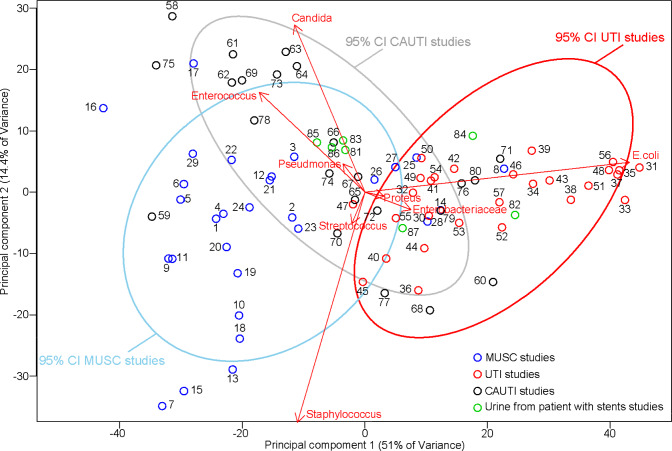
By comparing MUSC, CAUTI data and overall UTI data using a PCA (Fig. 2), we observed that the detected pathogen spectrum exhibited clearly distinguishable trends in the frequency of the main isolated pathogens influencing these 3 groups of urinary tract infections (Fig. 2). With respect to UTIs and MUSC, their 95% confidence interval ellipse only showed minimal overlap emphasizing that the spectrum of pathogens in the two groups are clearly distinct.
Fig. 2.
Principal component analysis showing the grouping of UTI, stent colonization and CAUTI studies. Each point represents a study with the specific pathogen spectrum described in this study (numbers correspond to the number of the study see supplementary material for details); red UTI studies, blue stent colonization studies, grey CAUTI studies. Arrows show the projections of the original features on to the principal components. 95% confidence interval ellipses were also drawn for each group. With the use of the ellipses, the grouping of the different samples in the PCA projection can be observed. Such ellipses allow understanding which data deviate from the groups formed in this PCA projection
Figure 2 shows that UTIs are mainly characterized by E. coli. All points are within the confidence interval ellipse, which means that the group is homogeneous. The isolates data from MUSC are driven towards gram-positive microorganisms with Enterococcus and Staphylococcus as major representative of bacterial infection, as well as Candida spp. for fungal infections.
The 95% confidence interval ellipse of CAUTIs is pointing in the direction of Staphylococcus and Candida spp. and partly intersects with the ellipse of UTIs. However, it is clearly more comparable to the ellipse of MUSC. Data from patients with ureteral stents where pathogens were collected from urine were scarce and exhibited a large variance resulting in a large 95% confidence interval ellipse not suitable for further analysis. With the current dataset, it does not seem likely that pathogens isolated are characterized by a specific spectrum. Therefore, although the data are included in the PCA, no grouping or confidence interval was drawn using those data.
Discussion
In the present literature review, we aimed to investigate differences in pathogen spectrum detected on ureteral stents compared to patients with UTI or CAUTI. We found that different pathogen spectrums are involved in MUSC (Staphylococci and Enterococci) and possibly CAUTI compared to common UTIs where E. coli dominates. Consequently, bacteriuria in stented patients will likely be comprised of different pathogens than E. coli. Such asymptomatic bacteriuria occurring due to stent colonization is usually not considered as a risk factor, unless procedures entering the urinary tract and breaching the mucosa, particularly in endoscopic urological surgery, are considered. In addition, it needs to be considered that MUSC primarily is associated with biofilm formation on ureter stents. In many cases, these bacteria show antimicrobial resistance and MUSC cannot be identified by standard urine culture techniques as MUSC does not necessarily lead to bacteriuria. Therefore, the results shown here have potential implications for guiding peri-operative antimicrobial prophylaxis for patient with indwelling ureteral stents. In particular, antimicrobial prophylaxis prior to ureteral stent placement, ureteroscopy in patients with indwelling stents as well as change of stents in patients with long-term drainage might be more appropriate if it is also targeted against gram-positive pathogens (mostly Staphylococci and Enterococci) representing an average of 36% of isolates and up to 60% in some studies. Currently, no clear recommendations from major guidelines with respect to antimicrobial prophylaxis prior to ureteroscopy exist. This is mainly due to the low certainty of evidence as well as the lack of high-quality prospective randomized studies. Some guidelines such as the AUA 2019 guideline recommend selective antimicrobial prophylaxis based on the expected spectrum in high-risk individuals such as immunocompromised patients. Given the lack of a clear recommendations for prophylaxis in patients with indwelling ureteral stents, we suggest that a prophylaxis also covering gram-positive bacteria such as Enterococci and Staphylococci (e.g. amoxicillin-clavulanate) may be appropriate. In contrast, for patients without indwelling stents antimicrobials mainly covering the gram-negative spectrum such as first- and second-generation cephalosporins, or trimethoprim/sulfamethoxazole as a single dose are considered appropriate. Fungi represent an additional challenge in the treatment of ureteral stent associated infections. A differentiation between asymptomatic colonization, symptomatic urinary tract infection and systemic infection is relevant in deciding on the individualized treatment approach.
To investigate whether studies falling outside the 95% confidence interval of MUSC focus on a specific patient population that cannot be compared to the general population, further investigation of included patients was performed. The study no. 8 is the most extreme point that lies outside the ellipse in direction of the UTI group. The data isolated in the study represented by the study no. 8 focuses on the analysis of stent pathogens in immunosuppressed patients [7]. Also the studies no. 25 and 28 are in the ellipse of UTI, even though these studies include patients with ureteral stents. The study represented by study no. 25 were patients with diabetes mellitus and chronic kidney disease (CKD) who also had immunosuppression [9]. The study represented by study no. 28 does not deal with a particular population. Interestingly, in this study 23% of patients had CKD and 19% were diabetic [8], such conditions could lead to a shift in the pathogen spectrum.
With PCA, we observed that studies investigating pathogens in the urine of patients with indwelling stents do not follow a specific pathogen pattern [8, 9, 84]. In this context, it is important that for the identification of MUSC, an analysis of the stents seems more appropriate than urine culture. Our results suggest that urine culture is not a reliable method for identifying pathogens that colonize the stent [8, 9, 84]. These data are preliminary given the low number of datapoints that we have, but they are very much scattered.
Given that an analysis of the stent prior to change or manipulation virtually is impossible, the results of the present study provide a very helpful insight to support clinical decision making in the choice of antimicrobial prophylaxis.
The present analysis has limitations, which need to be acknowledged. Due to the exploratory nature of the study, it was not possible to fully adhere to PRISMA or other guidelines that are more fitted for univariate or bi-variate analysis in the context of network meta-analyses, meta-analyses of individual participant data, systematic reviews of harms, systematic reviews of diagnostic test accuracy studies. It is likely that this resulted in some biais. With respect to the literature retrieved, the spectrum of pathogens investigated in the included studies depends on the authors' choice and varies, especially regarding less common pathogens such as Lactobacilli, Corynebacteria, Proteus and others. With respect to fungi, a comparable limitation is present. Depending on the author, fungi are researched in a group or a subcategory of Candida species is formed. This implies that in some cases it is not possible to separate candida data from the fungi data, thus leading to exclusion of the study. As a consequence, the resulting pathogen set included in the PCA indeed present a risk of biais as it is strongly influence by the requirement of the PCA itself (not too many 0 values and more samples (i.e., studies) than descriptors (i.e., pathogens included).
In addition, the methods for the detection of MUSC may differ between sonication as well as roll-out technique which may impact the detected spectrum of microorganisms. Nevertheless, we feel that despite these limitations, our study provides helpful insight into a clinically relevant topic and may help clinical decision-making.
Future studies should include demographic data and patient data to refine the findings of the present study. Also next-generation sequencing might provide valuable help in understanding some trends observed here (in particular supporting that urine from catheterized patients provides very variable results), and provide more data on the urinary microbiota that might adhere on such catheters. Still results for next-generation sequencing can be quite long to obtain and analyse, but over time the gathered data will certainly help providing a better empirical therapy as well. Moreover, we believe that adding antimicrobial resistance data to similar studies would benefit to the overall interpretation. This will potentially help overcoming some of the limitations encountered during this review.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
M. Lepori: protocol/project development, data collection or management, data analysis, manuscript writing/editing. O. Braissant: protocol/project development, data collection or management, data analysis, manuscript writing/editing. G. Bonkat: manuscript writing/editing. M. Rieken: protocol/project development, data analysis, manuscript writing/editing.
Funding
Open access funding provided by University of Basel.
Data availability
All the data used for the present study are compiled in Table 1.
Declarations
Conflict of interest
The authors confirm that there are no conflicts of interest involved with the present work.
Research involving human participants and/or animals
The present work is a literature review. No human participants or animals were involved in this work.
Informed consent
The present work is a literature review, no informed consent was required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sali GM, Joshi HB (2020) Ureteric stents: overview of current clinical applications and economic implications. Int J Urol 27(1):7–15. 10.1111/iju.14119 [DOI] [PubMed] [Google Scholar]
- 2.Bonkat G, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B (2021) EAU urological infections guidelines. In: EAU annual congress Milan Italy
- 3.Bonkat G et al (2012) Microbial ureteral stent colonization in renal transplant recipients: frequency and influence on the short-time functional outcome. Transpl Infect Dis 14(1):57–63. 10.1111/j.1399-3062.2011.00671.x [DOI] [PubMed] [Google Scholar]
- 4.Scotland KB et al (2019) Ureteral stent-associated infection and sepsis: pathogenesis and prevention: a review. Biofouling 35(1):117–127. 10.1080/08927014.2018.1562549 [DOI] [PubMed] [Google Scholar]
- 5.McVean G (2009) A genealogical interpretation of principal components analysis. PLoS Genet 5(10):e1000686. 10.1371/journal.pgen.1000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. http://www.jstor.org/stable/2346101. 10.1111/j.2517-6161.1995.tb02031.x
- 7.Al-Ghazo MA et al (2010) The risk of bacteriuria and ureteric stent colonization in immune-compromised patients with double J stent insertion. Int Urol Nephrol 42(2):343–347. 10.1007/s11255-009-9607-0 [DOI] [PubMed] [Google Scholar]
- 8.Amine Saouli TK, El Khader K, Koutani A, Andaloussi AIA (2021) Study of risk factors for urinary colonization in patients with the double J catheter. Afr J Urol. 10.1186/s12301-021-00144-y [Google Scholar]
- 9.Anak Agung Gede Oka GWKD, Wulandari SR, Mahadewa TGB, Santosa B, Yudiana W, Tirtayasa PW (2019) Characteristics of bacterial colonization after indwelling ureteral stents in urinary stone patients with diabetes mellitus and chronic kidney disease. Urol Sci 30(5):211–215. 10.4103/UROS.UROS_124_18 [Google Scholar]
- 10.Aydin HR et al (2016) Incidence of bacterial colonisation after indwelling of double-J ureteral stent. Arch Ital Urol Androl 87(4):291–294. 10.4081/aiua.2015.4.291 [DOI] [PubMed] [Google Scholar]
- 11.Ben-Meir D et al (2009) Characteristics and clinical significance of bacterial colonization of ureteral double-J stents in children. J Pediatr Urol 5(5):355–358. 10.1016/j.jpurol.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 12.Bonkat G et al (2013) Comparison of the roll-plate and sonication techniques in the diagnosis of microbial ureteral stent colonisation: results of the first prospective randomised study. World J Urol 31(3):579–584. 10.1007/s00345-012-0963-5 [DOI] [PubMed] [Google Scholar]
- 13.Bonkat G et al (2013) Microbial colonization and ureteral stent-associated storage lower urinary tract symptoms: the forgotten piece of the puzzle? World J Urol 31(3):541–546. 10.1007/s00345-012-0849-6 [DOI] [PubMed] [Google Scholar]
- 14.Bonkat G et al (2011) Improved detection of microbial ureteral stent colonisation by sonication. World J Urol 29(1):133–138. 10.1007/s00345-010-0535-5 [DOI] [PubMed] [Google Scholar]
- 15.Farsi HM et al (1995) Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol 9(6):469–472. 10.1089/end.1995.9.469 [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Aparicio L et al (2015) Bacterial characteristics and clinical significance of ureteral double-J stents in children. Actas Urol Esp 39(1):53–56. 10.1016/j.acuro.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 17.Kehinde EO et al (2004) Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol 18(9):891–896. 10.1089/end.2004.18.891 [DOI] [PubMed] [Google Scholar]
- 18.Klis R et al (2009) Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol 23(6):1015–1019. 10.1089/end.2008.0518 [DOI] [PubMed] [Google Scholar]
- 19.Kozyrakis D et al (2018) Is there a role for double J stent culture in contemporary urology? Urol Int 100(2):203–208. 10.1159/000486798 [DOI] [PubMed] [Google Scholar]
- 20.Lifshitz DA et al (1999) Predictive value of urinary cultures in assessment of microbial colonization of ureteral stents. J Endourol 13(10):735–738. 10.1089/end.1999.13.735 [DOI] [PubMed] [Google Scholar]
- 21.Matsukawa M et al (2005) Bacterial colonization on intraluminal surface of urethral catheter. Urology 65(3):440–444. 10.1016/j.urology.2004.10.065 [DOI] [PubMed] [Google Scholar]
- 22.Mehmet Çağlar Çakıcı FK, Çulpan M, Efiloğlu Ö, Miçooğulları U, Tahra A, Yıldırım A (2021) Is the clinical significance of double-J stent colonization following ureteroscopic lithotripsy ignored? Endourol Bull 13(6):47–55 [Google Scholar]
- 23.Nevo A et al (2019) Predicting the risk of sepsis and causative organisms following urinary stones removal using urinary versus stone and stent cultures. Eur J Clin Microbiol Infect Dis 38(7):1313–1318. 10.1007/s10096-019-03555-6 [DOI] [PubMed] [Google Scholar]
- 24.Paick SH et al (2003) Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology 62(2):214–217. 10.1016/s0090-4295(03)00325-x [DOI] [PubMed] [Google Scholar]
- 25.Rahman MA et al (2010) Evaluation of bacterial colonization and bacteriuria secondary to internal ureteral stent. Mymensingh Med J 19(3):366–371 [PubMed] [Google Scholar]
- 26.Reid G et al (1992) Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J Urol 148(5):1592–1594. 10.1016/s0022-5347(17)36976-8 [DOI] [PubMed] [Google Scholar]
- 27.Riedl CR et al (1999) Bacterial colonization of ureteral stents. Eur Urol 36(1):53–59. 10.1159/000019927 [DOI] [PubMed] [Google Scholar]
- 28.Salari B et al (2021) Urine versus stent cultures and clinical UTIs. Int Urol Nephrol 53(11):2237–2242. 10.1007/s11255-021-02964-x [DOI] [PubMed] [Google Scholar]
- 29.Sarier M et al (2017) Evaluation of ureteral stent colonization in live-donor renal transplant recipients. Transplant Proc 49(3):415–419. 10.1016/j.transproceed.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 30.Sarier M et al (2017) Comparision of ureteral stent colonization between deceased and live donor renal transplant recipients. Transplant Proc 49(9):2082–2085. 10.1016/j.transproceed.2017.09.028 [DOI] [PubMed] [Google Scholar]
- 31.Shabeena KS et al (2018) Characteristics of bacterial colonization after indwelling double-J ureteral stents for different time duration. Urol Ann 10(1):71–75. 10.4103/UA.UA_158_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkan Ülker NY, Ağuş N, Can E, Çakmak Ö, Yücel C, Çelik O, İlbey YÖ (2019) Bacterial colonization of ureteral double-J stents in patients with negative urine culture. J Urol Surg 6(2):125–129. 10.4274/jus.galenos.2019.2343 [Google Scholar]
- 33.Wang J et al (2021) Pathogen distribution and risk factors for urinary tract infection in infants and young children with retained double-J catheters. J Int Med Res 49(5):3000605211012379. 10.1177/03000605211012379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JM et al (2018) Observations of bacterial biofilm on ureteral stent and studies on the distribution of pathogenic bacteria and drug resistance. Urol Int 101(3):320–326. 10.1159/000490621 [DOI] [PubMed] [Google Scholar]
- 35.Ahmad S (2012) Pattern of urinary tract infection in Kashmir and antimicrobial susceptibility. Bangladesh Med Res Counc Bull 38(3):79–83. 10.3329/bmrcb.v38i3.14330 [DOI] [PubMed] [Google Scholar]
- 36.Arlene Rodriguez-Encarnacion MDCHH (2012) Cebu, pathogens causing urinary tract infection and their resistance patterns among pediatric patients in Chong Hua Hospital (January 2003 to June 2005). PIDSP J 13(1):37–43 [Google Scholar]
- 37.Bahadin J, Teo SS, Mathew S (2011) Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolated. Singap Med J 52(6):415–420 [PubMed] [Google Scholar]
- 38.Batra P et al (2020) Susceptibility pattern of oral antimicrobials in uncomplicated UTI: does fosfomycin still stand effective? J Fam Med Prim Care 9(2):850–853. 10.4103/jfmpc.jfmpc_970_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beyene G, Tsegaye W (2011) Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in jimma university specialized hospital, southwest ethiopia. Ethiop J Health Sci 21(2):141–146. 10.4314/ejhs.v21i2.69055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouskraoui M et al (2010) Epidemiology of urinary tract infection in children in Marrakech. Arch Pediatr 17(Suppl 4):S177–S178 [DOI] [PubMed] [Google Scholar]
- 41.Cui H (2021) Distribution and drug resistance of pathogens causing urinary tract infection in patients with urinary calculi. Am J Transl Res 13(9):10554–10561 [PMC free article] [PubMed] [Google Scholar]
- 42.Dariusz Chojeta IS-W, Koziol MM (2021) Pathogen profile of urinary tract infections in nephrology unit. Curr Issues Pharm Med Sci 34(4):201–205. 10.2478/cipms-2021-0036 [Google Scholar]
- 43.Duicu C et al (2021) Antibiotic resistance patterns of urinary tract pathogens in children from Central Romania. Exp Ther Med 22(1):748. 10.3892/etm.2021.10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagenlehner FME, Wagenlehner C, Savov O, Gualco L, Schito G, Naber KG (2009) Klinik und Epidemiologie der unkomplizierten Zystitis bei Frauen. Urologe 49:253–261. 10.1007/s00120-009-2145-7 [DOI] [PubMed] [Google Scholar]
- 45.Gordon KA, Jones RN, Groups SP (2003) Susceptibility patterns of orally administered antimicrobials among urinary tract infection pathogens from hospitalized patients in North America: comparison report to Europe and Latin America. Results from the SENTRY Antimicrobial Surveillance Program (2000). Diagn Microbiol Infect Dis 45(4):295–301. 10.1016/s0732-8893(02)00467-4 [DOI] [PubMed] [Google Scholar]
- 46.Guclu E et al (2021) Risk factors of multidrug-resistant bacteria in community-acquired urinary tract infections. Afr Health Sci 21(1):214–219. 10.4314/ahs.v21i1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang L et al (2021) Urinary tract infection etiological profiles and antibiotic resistance patterns varied among different age categories: a retrospective study from a tertiary general hospital during a 12-year period. Front Microbiol 12:813145. 10.3389/fmicb.2021.813145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ioannou P et al (2020) Characteristics of urinary tract infections in older patients in a tertiary hospital in Greece. Geriatr Gerontol Int 20(12):1228–1233. 10.1111/ggi.14080 [DOI] [PubMed] [Google Scholar]
- 49.Kolawole AS, Kolawole OM, Kandaki-Olukemi YT, Babatunde S, Durowade KA, Kolawole CF (2010) Prevalence of urinary tract infections (UTI) among patients attending Dalhatu Araf Specialist Hospital, Lafia, Nasarawa State, Nigeria. Int J Med Med Sci 1(5):163–167 [Google Scholar]
- 50.Khamees SS (2012) Urinary tract infection: causative agents, the relation between bacteriuria and pyuria. World Appl Sci J 20(5):683–686. 10.5829/idosi.wasj.2012.20.05.251212 [Google Scholar]
- 51.Nath T, Das SK, Hazra S (2021) Pattern of uropathogens and antibiotic sensitivity in diabetes patients attending to out—patient department and diabetes clinic of a teaching hospital: a cross-sectional study. J Fam Med Prim Care 10(10):3638–3643. 10.4103/jfmpc.jfmpc_71_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onifade AKA, Anibijuwon II, Azariah EJ (2011) Urinary tract infection in apparently healthy individuals in Ile-Ife, Nigeria: detection of predominant microorganisms and antibiotics susceptibility profile. Afr J Microbiol Res 5(20):3233–3236. 10.5897/AJMR11.279 [Google Scholar]
- 53.Orji O, Dlamini Z, Wise AJ (2022) Urinary bacterial profile and antibiotic susceptibility pattern among pregnant women in Rahima Moosa Mother and Child Hospital, Johannesburg. S Afr J Infect Dis 37(1):343. 10.4102/sajid.v37i1.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rupinder Bakshi SKAVJSG (2021) Importance of antimicrobial stewardship in the treatment of urinary tract infection. J Pure Appl Microbiol 15(5):2170–2176. 10.22207/JPAM.15.4.40 [Google Scholar]
- 55.Shamataj Kattalagere Razak VG (2012) Bacteriology of urinary tract infection and antibiotic susceptibility pattern in a tertiary care hospital in South India. Int J Med Sci Public Health 1(2):109–112. 10.5455/ijmsph.2012.1.109-112 [Google Scholar]
- 56.Shams SF et al (2017) Urinary tract infections in kidney transplant recipients 1(st) year after transplantation. J Res Med Sci 22:20. 10.4103/1735-1995.200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turnidge J et al (2002) Pathogen occurrence and antimicrobial resistance trends among urinary tract infection isolates in the Asia-Western Pacific Region: report from the SENTRY Antimicrobial Surveillance Program, 1998–1999. Int J Antimicrob Agents 20(1):10–17. 10.1016/s0924-8579(02)00050-x [DOI] [PubMed] [Google Scholar]
- 58.Wang F et al (2013) Survey on hospital-acquired urinary tract infection in neurological intensive care unit. APMIS 121(3):197–201. 10.1111/j.1600-0463.2012.02956.x [DOI] [PubMed] [Google Scholar]
- 59.Wariso KT, Siminialayi IM, Odigie JO (2010) Pattern and antibiogram of urinary tract infection at the University of Port Harcourt Teaching Hospital. Asian Pac J Trop Med 3(1):66–69. 10.1016/S1995-7645(10)60036-3 [Google Scholar]
- 60.Yi-Te C et al (2020) Urinary tract infection pathogens and antimicrobial susceptibilities in Kobe, Japan and Taipei, Taiwan: an international analysis. J Int Med Res 48(2):300060519867826. 10.1177/0300060519867826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmed SS et al (2019) Uropathogens and their antimicrobial resistance patterns: relationship with urinary tract infections. Int J Health Sci (Qassim) 13(2):48–55 [PMC free article] [PubMed] [Google Scholar]
- 62.Amna Butt SB, Shoaib H, Mukhtar H (2015) Isolation and characterization of urinary pathogens from catheterized patients in a tertiary care hospital. Pak Postgrad Med J 26(3):84–89 [Google Scholar]
- 63.Bi XC et al (2009) Pathogen incidence and antibiotic resistance patterns of catheter-associated urinary tract infection in children. J Chemother 21(6):661–665. 10.1179/joc.2009.21.6.661 [DOI] [PubMed] [Google Scholar]
- 64.Bizuayehu H et al (2022) Catheter-associated urinary tract infections in adult intensive care units at a selected tertiary hospital, Addis Ababa, Ethiopia. PLoS ONE 17(3):e0265102. 10.1371/journal.pone.0265102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chitnis AS et al (2012) Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect Control Hosp Epidemiol 33(10):993–1000. 10.1086/667745 [DOI] [PubMed] [Google Scholar]
- 66.Darma-Kusuma IG, Duarsa GWK, Golden N (2012) The effectiveness of netilmicin sulphate instilatione on the urethra catheter removal procedure in reducing the incidence of urinary tract infection. Bali Med J 1(3):112–115. 10.15562/bmj.v1i3.25 [Google Scholar]
- 67.Duszynska W et al (2020) Device associated -health care associated infections monitoring, prevention and cost assessment at intensive care unit of University Hospital in Poland (2015–2017). BMC Infect Dis 20(1):761. 10.1186/s12879-020-05482-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guanche-Garcell H, Requejo-Pino O, Rosenthal VD, Morales-Pérez C, Delgado-González O, Fernández-González D (2011) Device-associated infection rates in adult intensive care units of Cuban university hospitals: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis 15(5):357–362. 10.1016/j.ijid.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 69.Joon Ho Lee SWK, Yoon BI, Ha U-S, Sohn DW, Cho Y-H (2013) Factors that affect nosocomial catheter-associated urinary tract infection in intensive care units: 2-year experience at a single center. Korean J Urol 54(1):59–65. 10.4111/kju.2013.54.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lai CC et al (2017) Implementation of a national bundle care program to reduce catheter-associated urinary tract infection in high-risk units of hospitals in Taiwan. J Microbiol Immunol Infect 50(4):464–470. 10.1016/j.jmii.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 71.Lee SJ et al (2004) A comparative multicentre study on the incidence of catheter-associated urinary tract infection between nitrofurazone-coated and silicone catheters. Int J Antimicrob Agents 24(Suppl 1):S65–S69. 10.1016/j.ijantimicag.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 72.Lili Tao BH, Rosenthal VD, Gao X, He L (2011) Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis 15:774–780. 10.1016/j.ijid.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 73.Milan PB, Ivan IM (2009) Catheter-associated and nosocomial urinary tract infections: antibiotic resistance and influence on commonly used antimicrobial therapy. Int Urol Nephrol 41(3):461–464. 10.1007/s11255-008-9468-y [DOI] [PubMed] [Google Scholar]
- 74.Mladenovic J et al (2015) Catheter-associated urinary tract infection in a surgical intensive care unit. Vojnosanit Pregl 72(10):883–888. 10.2298/vsp140624078m [DOI] [PubMed] [Google Scholar]
- 75.Nirmala Poddar KP, Pathi B, Pattnaik D, Praharaj A, Jena J (2020) Microbiological profile of catheter associated urinary tract infection in ICUs of a tertiary care hospital Bhubaneswar, Odisha, India. IP Int J Med Microbiol Trop Dis 6(2):107–112. 10.18231/j.ijmmtd.2020.023 [Google Scholar]
- 76.Puri J et al (2002) Catheter associated urinary tract infections in neurology and neurosurgical units. J Infect 44(3):171–175. 10.1053/jinf.2002.0968 [DOI] [PubMed] [Google Scholar]
- 77.Sabir N et al (2017) Bacterial biofilm-based catheter-associated urinary tract infections: causative pathogens and antibiotic resistance. Am J Infect Control 45(10):1101–1105. 10.1016/j.ajic.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 78.Smitha Bagali PGM (2021) Bacteriological profile of catheter associated urinary tract infection and its antimicrobial susceptibility pattern in a tertiary care hospital. Int J Health Clin Res 4(22):268–271 [Google Scholar]
- 79.Aderounmu AOA, Taiwo SS (2006) Catheter associated urinary tract infection: aetiologic agents and antimicrobial susceptibility pattern in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. Afr J Biomed Res 9:141–148. 10.4314/ajbr.v9i3.48897 [Google Scholar]
- 80.Talaat M et al (2010) Surveillance of catheter-associated urinary tract infection in 4 intensive care units at Alexandria university hospitals in Egypt. Am J Infect Control 38(3):222–228. 10.1016/j.ajic.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 81.Temiz E et al (2012) Factors associated with catheter-associated urinary tract infections and the effects of other concomitant nosocomial infections in intensive care units. Scand J Infect Dis 44(5):344–349. 10.3109/00365548.2011.639031 [DOI] [PubMed] [Google Scholar]
- 82.Toshie Tsuchida KM, Ohsako S, Fujino M, Kaneda M, Miyazaki T, Fujiwara F, Sugimoto T (2008) Relationship between catheter care and catheter-associated urinary tract infection at Japanese general hospitals: a prospective observational study. Int J Nurs Stud 45(3):352–361. 10.1016/j.ijnurstu.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 83.Wazait HD et al (2003) Catheter-associated urinary tract infections: prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital (1996–2001). BJU Int 91(9):806–809. 10.1046/j.1464-410x.2003.04239.x [DOI] [PubMed] [Google Scholar]
- 84.Altunal N, Willke A, Hamzaoglu O (2017) Ureteral stent infections: a prospective study. Braz J Infect Dis 21(3):361–364. 10.1016/j.bjid.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He M et al (2021) Risk factors of urinary tract infection after ureteral stenting in patients with renal colic during pregnancy. J Endourol 35(1):91–96. 10.1089/end.2020.0618 [DOI] [PubMed] [Google Scholar]
- 86.Useok Choi, Kim EJ, Lyu DH, Park BH, Chung H, Han CH, Bae S (2021) Ureteral stent induced urinary tract infection and microbial inconsistency between bladder and renal pelvis. Urogenit Tract Infect 16(3):61–66. 10.14777/uti.2021.16.3.61 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used for the present study are compiled in Table 1.