Abstract
Background
Iron deficiency is prevalent among heart failure patients and is associated with worse clinical outcomes, including decreased quality of life and functional capacity. This condition often results in a higher incidence of hospitalization and mortality. Iron supplementation, particularly with intravenous ferric carboxymaltose (FCM), has shown potential benefits as an adjunct therapy in heart failure management. This study aims to evaluate the efficacy of FCM in the treatment of patients with heart failure and iron deficiency anemia, with a focus on its impact on mortality and hospitalization rates.
Methods
A comprehensive search was conducted in PubMed, Web of Science, and Scopus databases from their inception until 1st December 2023. Meta-analysis was performed using RevMan 5.4, employing a random-model effect. The results were reported as risk ratios (RRs), standard mean differences (SMDs), and 95 % confidence intervals (CIs).
Results
The meta-analysis included 13 studies with a total of 6271 patients. Ferric carboxymaltose administration resulted in a significant improvement in the 6-minute walk distance (SMD: 1.45; 95 % CI: 0.55, 2.36; p = 0.002), quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) (SMD: 1.49; 95 % CI: 0.87, 2.11; p < 0.00001), the rate of first hospitalization for heart failure or cardiovascular death (RR: 0.91; 95 % CI: 0.84, 0.98; p = 0.02). However, FCM did not show a significant impact on the risk of cardiovascular death (RR: 0.90; 95 % CI: 0.77, 1.05; p = 0.17), the need for intervention due to worsening heart failure (RR: 0.41; 95 % CI: 0.04, 4.51; p = 0.47), or all-cause mortality rates (RR: 0.89; 95 % CI: 0.69, 1.16; p = 0.28).
Conclusion
While FCM treatment in patients with heart failure and iron deficiency anemia significantly improves functional capacity and quality of life, it has no notable effect on mortality rates or the likelihood of hospitalization. These findings highlight the need for further research to explore comprehensive treatment strategies that address both the symptomatic and survival aspects of heart failure management in this patient population.
Key Points
| Treatment of iron deficiency anemia in heart failure patients with intravenous ferric carboxymaltose (FCM) improves quality of life and functional capacity. |
| Intravenous FCM does not notably reduce mortality or hospitalization rates in patients with heart failure and iron deficiency anemia. |
| Intravenous FCM is beneficial for alleviating symptom, physical, and social limitations of heart failure in iron deficiency anemia patients. |
Introduction
Iron deficiency is a common co-morbidity in patients with heart failure (HF) and has been associated with worse clinical outcomes and reduced quality of life and functional capacity. Up to 50% of people with HF suffer from iron deficiency, and they exhibit higher rates of hospitalization and mortality [1, 2].
Intravenous iron supplementation has shown promise in effectively restoring iron levels in various HF patients due to its vital role in oxygen metabolism and cellular immune response, particularly in cardiac myocytes. It may offer incremental benefits alongside beta-blockade and renin-angiotensin-aldosterone system inhibition [3].
According to the guidelines from the European Society of Cardiology (ESC) and the American Heart Association/American College of Cardiology, routine screening for iron deficiency is recommended for patients with HF [4]. Furthermore, both sets of guidelines provide a class II recommendation for intravenous iron replacement therapy, with a particular emphasis on ferric carboxymaltose (FCM) in the ESC guidelines. This therapy is recommended for patients with iron deficiency anemia and HF with reduced ejection fraction to improve symptoms, exercise capacity, and quality of life. In the ESC guidelines, there is also a recommendation that intravenous iron therapy may help reduce HF hospitalizations [4, 5].
Several studies have investigated the impact of intravenous FCM on patients with HF and iron deficiency anemia. These studies have consistently demonstrated that FCM effectively enhances functional capacity and improves quality of life [6]. However, its influence on hospitalizations and mortality has shown less conclusive results [6, 7].
This meta-analysis aims to evaluate the efficacy of FCM specifically for the treatment of patients with HF and iron deficiency anemia. By pooling data from relevant studies comparing intravenous FCM with placebo, this article aims to provide a comprehensive assessment of the impact of intravenous FCM on important clinical outcomes, including treatment effects on mortality and hospitalization.
Methods
This study follows the Preferred Reporting Items for Systematic Review and Meta-Analysis Statement standards (PRISMA 2020) for conducting our systematic literature review and meta-analysis [8].
Search Strategy
A thorough literature search using PubMed, Web of Science, and Scopus was conducted with last search on 1st December, 2023. There were no limitations regarding language or date of publication.
The pre-made Medical Subject Headings (MeSH) phrases were combined with the Boolean operators “AND” and “OR”. The terms (“Ferric Carboxymaltose” OR “FCM” OR “Injectafer” OR “Ferinject”) AND (“Iron Deficiency Anemia” OR “IDA” OR “anemia” OR “iron deficiency”) AND (“Heart Failure” OR “cardiac failure” OR “heart insufficiency” OR “congestive heart failure” OR “CHF”) were included in the search strategy.
Selection of Studies and Eligibility Assessment
Inclusion criteria: (i) randomized controlled trials (RCTs) or observational studies; (ii) clinically diagnosed (acute or chronic) HF patients regardless of left ventricular ejection fraction; (iii) patients with iron deficiency anemia (Hb less than 7.7 mmol/l [13 g/dL] in men and 7.4 mmol/l [12 g/dL] in women) along with HF.
Exclusion criteria: (i) letters to editors/commentaries, case reports, literature reviews, systematic reviews, and meta-analyses; (ii) research carried out using animal models; (iii) studies where the clinical data needed to understand the outcomes under study was insufficient.
Authors S.A.N. and S.S. independently evaluated the eligibility of the abstracts and titles, removing duplicates and papers that did not meet the inclusion criteria. Following the initial screening, the full texts of the references were examined to determine if they met the established inclusion criteria. In cases of disagreement, a consensus was reached to resolve any differences.
Data Extraction and Quality Assessment
Authors S.A.N. and S.S. extracted data from the selected papers and used it in a pre-piloted Google Sheet. Each reviewer verified the retrieved data twice. Any discrepancy in the data extraction was addressed by the perspective of an independent author. Data gathered from the eligible studies included the first author’s name, the year of publication, the country, the study design, the sample size, the intervention details, the baseline characteristics, and the stated results of the study. The quality of the included studies was appraised separately by two authors, and any discrepancies were handled through consensus. The updated Cochrane risk of bias tool for randomized trials (ROB-II) was used to assess the risk of bias in the RCT studies [9]. The risk of bias table addressed biases associated with randomization, deviations from intended interventions, missing data, outcome assessment, and the selection of reported outcomes. The assessment determined if each study was high risk, had some issues, or was low risk. Furthermore, the risk bias of the included cohort studies was assessed using the Newcastle-Ottawa Scale (NOS) [10], a star system consisting of nine questions, with one point awarded for each answer marked with an asterisk. It focuses on three main perspectives: the selection of the study groups, the comparability of the groups, and the assessment of the outcome of interest.
Study Endpoints
The primary outcomes were 6-min walk test distance (this is a one-item objective test that evaluates submaximal aerobic/functional walking capacity) and EQ-5D visual analog scale (VAS; a quantitative measure of health outcome and its effect on life quality that represents the patient's subjective judgment on a vertical VAS).
Secondary outcomes included Kansas City Cardiomyopathy Questionnaire (KCCQ) (a 23-item self-administered questionnaire intended to assess the patient's perception of their health state, including HF symptoms, physical and social function, and how their HF affects their quality of life), differences in Hb, all-cause mortality, all-cause mortality at 1 year, first hospitalization for HF or cardiovascular death, first hospitalization for HF during follow-up, cardiovascular death, and cardiovascular death or intervention for worsening HF during follow-up.
Data Synthesis
Using Review Manager Version 5.4 (Cochrane Collaboration), one author (A.A.) combined the retrieved data for the meta-analysis. The net change scores, which indicate the least square mean (LSM) percent change in cardiovascular health from the baseline, were combined for the continuous outcomes that were used to evaluate the effectiveness of FCM. The standardized mean difference (SMD) of the net percent change with a 95% confidence interval (CI) served as the effect measure’s representation. The effect magnitude was described by the risk ratio (RR) with a 95% CI for dichotomous outcomes that were used to determine the clinical endpoints of FCM. With a 95% CI, p < 0.05 was considered statistically significant. All results underwent Der-Simonian and Laird random-effects meta-analyses due to potential heterogeneity among individual study estimates that may arise from clinical or methodological diversity (due to different design and risk of bias). In the included studies statistical heterogeneity was evaluated using the chi-squared and Higgins I2 statistics. Heterogeneity is considered statistically significant if p < 0.05. The grade of heterogeneity was interpreted as following:
0 to 40%: absent or non-significant
30 to 60%: moderate heterogeneity
50 to 90%: substantial heterogeneity
75 to 100%: considerably high heterogeneity [11].
Results
Literature Search and Study Selection
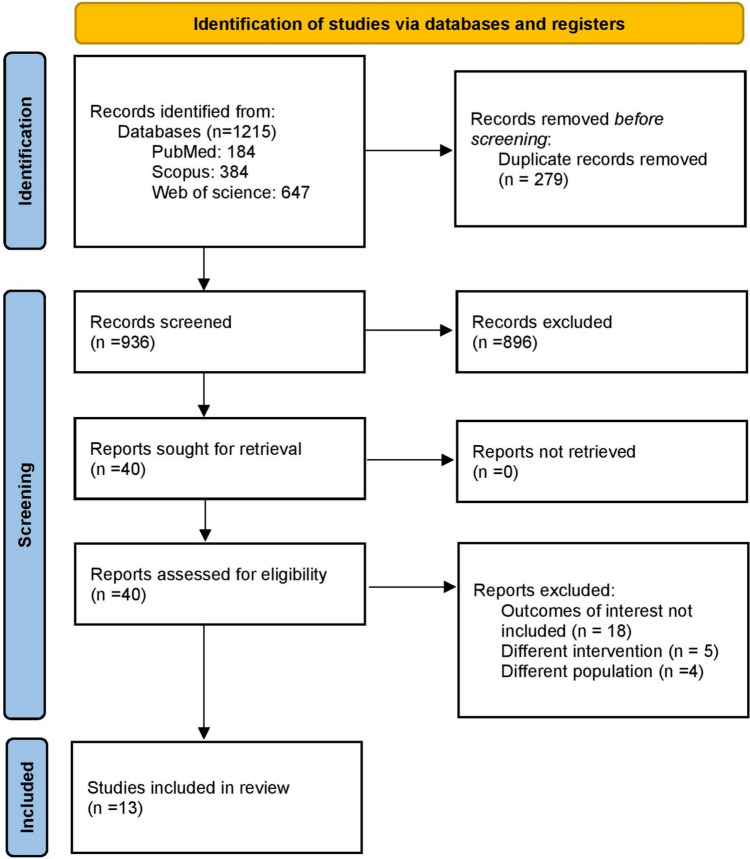
The literature search identified 1215 records from the electronic databases. Duplicates were removed then the titles and abstracts of these articles were checked independently by two co-authors S.A.N. and S.S. When checking the eligibility of 40 chosen articles, a thorough screening of the entire text was carried out. Finally, we incorporated 13 studies, including 10 RCTs [7, 12–20] and 3 observational studies [21–23]. The search results are demonstrated and the reasons for exclusion are explained via the PRISMA flow diagram in Fig. 1.
Fig. 1.
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram
Study Baseline Characteristics
From 13 included studies, this study reviewed the data of 6271 patients with HF and iron-deficiency anemia with mean age ranging from 51 and 83 years and 59.9% were male patients. Table 1 summarizes baseline information along with key findings extracted from selected literature with more details about comorbidities and concomitant treatment.
Table 1.
Summary of general and baseline characteristics of included studies
| Study | Group | Sample size | Study design | Age (y) | Male n (%) | BMI (kg/m2) | NYHA (%) | Concomitant treatment n (%) | Comorbidities n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Capone et al. 2023 [22] | FCM group | 55 | Retrospective cohort | 83.0 (8.4) | 44 (60.3) | 27.3 ± 6.0 |
NYHA I = 1 (1.9) NYHA II = 9 (16.7) NYHA III = 24 (44.4) NYHA IV = 20 (37.0) |
ARNI or SGLT2 inhibitor = 0 (0.0) ACEI or ARB or ARNI = 26 (47.3) β-blocker = 37 (67.3) Aldosterone antagonists = 16 (29.1) Digoxin = 3 (6.0) Loop diuretics = 42 (76.4) |
DM = 23 (41.8) HTN = 43 (78.2) AF = 14 (25.5) |
| Control group | 18 | 82.6 (10.7) | 13 (72.2) | 25.9 ± 4.9 |
NYHA I = 1 (5.9) NYHA II = 3 (17.7) NYHA III = 7 (41.2) NYHA IV = 6 (35.3) |
ARNI or SGLT2 inhibitor = 0 (0.0) ACEI or ARB or ARNI = 8 (44.4) β-blocker = 9 (50.0) Aldosterone antagonists = 5 (27.8) Digoxin = 0 (0.0) Loop diuretics = 10 (55.6) |
DM = 5 (27.8) HTN = 14 (77.8) AF = 3 (16.7) |
||
| Yeo et al. 2018 [12] | FCM group | 24 | RCT | 61.1 (10.8) | 18 (75) | NA | NA |
ACEI = 11 (45.8) ARB = 8 (33.3) β-blocker = 24 (100) Aldosterone antagonists = 7 (29.2) |
DM = 15 (62.5) HTN = 21 (87.5) MI = 12 (50) |
| Control group | 25 | 64 (10) | 20 (80) | NA | NA |
ACEI = 8 (32) ARB = 5 (20) β-blocker = 20 (80) Aldosterone antagonists = 10 (40) |
DM = 15 (60) HTN = 18 (72) MI = 13 (52) |
||
| Van et al. 2017 [7] | FCM group | 86 | RCT | 63 (12) | 60 (70) | 27.5 (5.0) |
NYHA II = 61 (71) NYHA III = 25 (29) |
ACEI = 81 (94) β-blocker = 84 (98) Aldosterone antagonists = 58 (67) Diuretics = 80 (93) |
DM = 26 (30) HTN = 62 (72) AF = 35 (41) MI = 58 (67) Stroke = 12 (14) |
| Control group | 86 | 64 (11) | 69 (80) | 26.9 (4.4) |
NYHA II = 54 (63) NYHA III = 32 (37) |
ACEI = 77 (90) β-blocker = 85 (98) Aldosterone antagonists = 62 (72) Diuretics = 82 (95) |
DM = 32 (37) HTN = 56 (65) AF = 41 (48) MI = 55 (64) Stroke = 13 (15) |
||
| Anker et al. 2022 [20] | FCM group | 454 | RCT | 67.8 (10.1) | 228 (50.2) | 28.1 (4.7) | NYHA III = 321 (70.7) |
ACEI = 423 (93.2) β-blocker = 393 (86.6) Triple therapy = 194 (42.7) Aldosterone antagonists = 237 (52.2) |
DM = 131 (28.9) HTN = 373 (82.2) AF = 493 (53.9) MI = 500 (54.7) Stroke = 99 (10.8) |
| Control group | 306 | 68.2 (10.4) | 147 (48) | 28.6 (5.4) | NYHA III = 186 (60.8) |
ACEI = 283 (92.5) β-blocker = 267 (87.3) Triple therapy = 122 (39.9) Aldosterone antagonists = 147 (48.0) |
DM = 82 (26.8) HTN = 259 (84.6) AF = 431 (57.7) MI = 395 (52.9) Stroke = 103 (13.8) |
||
| Mollace et al. 2022 [13] | FCM group | 26 | RCT | 61.9 (8.9) | 13 (26) | 26.8 (5.1) | NA | NA | NA |
| Control group | 38 | 63.2 (9.6) | 21 (38) | 27.2 (4.6) | NA | NA | NA | ||
| Macdougall et al. 2023 [19] | FCM group | 292 | RCT | 75 (9) | 158 (54) | NA |
NYHA class I = 6 (2) NYHA class II = 129 (44) NYHA class III = 149 (51) NYHA class IV = 8 (3) |
ACEI = 209 (72) β-blocker = 244 (84) Triple therapy = 121 (41) Aldosterone antagonists = 177 (61) |
DM = 119 (41) |
| Control group | 288 | 74 (9) | 148 (51) | NA |
NYHA class I = 3 (1) NYHA class II = 116 (40) NYHA class III = 159 (55) NYHA class IV = 9 (3) |
ACEI = 207 (72) β-blocker = 235 (82) Triple therapy = 100 (35) Aldosterone antagonists = 162 (56) |
DM = 140 (49) | ||
| Anker et al. 2009 [14] | FCM group | 304 | RCT | 67.8 (10.3) | 145 (47.6) | NA |
NYHA class II = 53 (17.4) NYHA class III = 251 (82.6) |
ACEI = 281 (92.4) β-blocker = 262 (86.2) Diuretics = 280 (92.1) |
DM = 93 (30.6) HTN = 243 (79.9) AF = 94 (30.9) MI = 168 (55.3) Stroke = 24 (7.9) |
| Control group | 155 | 67.4 (11.1) | 70 (37.8) | NA |
NYHA class II = 29 (18.7) NYHA class III = 126 (81.3) |
ACEI = 141 (91.0) β-blocker = 129 (83.2) Diuretics = 140 (90.3) |
DM = 37 (23.9) HTN = 128 (82.6) AF = 44 (28.4) MI = 90 (58.1) Stroke = 9 (5.8) |
||
| López-Vilella et al. 2023 [23] | FCM group | 196 | Retrospective cohort | 71 (14) | 82 (42) | 31 (16) |
NYHA class I = 0 (0) NYHA class II = 58 (30) NYHA class III = 29 (14) NYHA class IV = 2 (1) |
ACEI = 123 (37) β-blocker = 123 (63) Loop diuretics = 145 (74) Thiazides = 33 (17) |
DM = 84 (43) AF = 139 (71) |
| Control group | 288 | 65 (13) | 204 (71) | 35 (12) |
NYHA class I = 0 (0) NYHA class II = 99 (34 NYHA class III = 61 (20) NYHA class IV = 2 (1) |
ACEI = 199 (69) β-blocker = 187 (65) Loop diuretics = 198 (69) Thiazides = 35 (12) |
DM = 135 (47) AF = 139 (71) |
||
| Ponikowski et al. 2014 [15] | FCM group | 150 | RCT | 68.8 (9.5) | 55 | 28.3 (4.6) |
NYHA class II = 80 (53) NYHA class III = 70 (47) |
ACEI = 116 (77) ARB = 34 (23) β-blocker = 133 (89) Diuretics = 132 (88) |
DM = 38 (25) HTN = 129 (86) AF = 66 (44) MI = 90 (60) |
| Control group | 151 | 69.5 (9.3) | 80 | 29.1 (5.7) |
NYHA class II = 91 (60) NYHA class III = 60 (40) |
ACEI = 118 (78) ARB = 37 (25) β-blocker = 139 (92) Diuretics = 139 (92) |
DM = 45 (30) HTN = 130 (86) AF = 73 (48) MI = 90 (60) |
||
| Dhoot et al. 2022 [16] | FCM group | 35 | RCT | 51.0 (11.6) | 57.1% | NA |
NYHA Class II 26 (74.3) NYHA Class III 9 (25.7) |
NA | NA |
| Control group | 35 | 54.8 (9.0) | 60% |
NYHA Class II 30 (85.7) NYHA Class III 5 (14.3) |
NA | NA | |||
| Martens et al. 2021[17] | FCM | 37 | RCT | 72 (12) | 26 (70) | 27 ( 5) |
NYHA Class II 22 (59) NYHA Class III 15 (41) |
ACEI = 34 (92) β-blocker = 37 (100) loop diuretics = 20 (54) Aldosterone antagonists = 30 (81) |
DM = 17 (46) HTN = 32 (87) Stroke = 1 (3) |
| Control group | 38 | 73 (9) | 25 (66) | 27 ( 5) |
NYHA Class II 19 (50) NYHA Class III 19 (50) |
ACEI = 33 (87) β-blocker = 37 (97) loop Diuretics = 21 (55) Aldosterone antagonists = 29 (76) |
DM = 19 (50) HTN = 37 (97) Stroke = 4 (11) |
||
| Mentz et al. 2023 [18] | FCM group | 1532 | RCT | 68.6 (10.9) | 1026 (66.9) | NA |
NYHA class II = 797/1532 (52.0) NYHA class III = 711/1532 (46.4) NYHA class IV = 22/1532 (1.4) |
ACEI = 901/1532 (58.8) β-blocker = 1415/1532 (92.4) Aldosterone antagonists = 858/1532 (56.0) |
NA |
| Control group | 1533 | 68.6 (11.2) | 1002 (65.3) | NA |
NYHA class II = 820/1532 (53.5) NYHA class III = 692/1532 (45.2) NYHA class IV = 19/1532 (1.2) |
ACEI = 923/1530 (60.3) β-blocker = 1418/1532 (92.6) Aldosterone antagonists = 847/1532 (55.3) |
NA | ||
| Arias-Barrera et al. 2019 [21] | FCM group | 69 | Retrospective cohort | 67 (13) | 37 (53.6) | NA |
NYHA class II = 28 (40.6) NYHA class III = 40 (58.0) NYHA class IV = 1 (1.40) |
NA |
DM = 40 (58.0) HTN = 32 (46.4) Ischemic etiology = 29 (42.0) Dyslipidemia = 22 (31.9) COPD = 21 (30.4) |
| Control group | 50 | 67.5 (17.75) | 26 (52) | NA |
NYHA class II = 14 (28.0) NYHA class III = 31 (62.0) NYHA class IV = 5 (10.0) |
NA |
DM = 34 (68.0) HTN = 25 (50.0) Ischemic etiology = 17 (34.0) Dyslipidemia = 23 (46.0) COPD = 14 (28.0) |
ACEI angiotensin-converting enzyme inhibitor, AF atrial fibrillation, ARB angiotensin receptor blocker, ARNI angiotensin receptor/neprilysin inhibitor, COPD chronic obstructive pulmonary disease, DM diabetes mellitus, FCM ferric carboxymaltose, HTN hypertension, MI myocardial infarction, NYHA New York Heart Association classification, RCT randomized controlled trial, SGLT-2 sodium glucose transporter-2
Risk of Bias Assessment
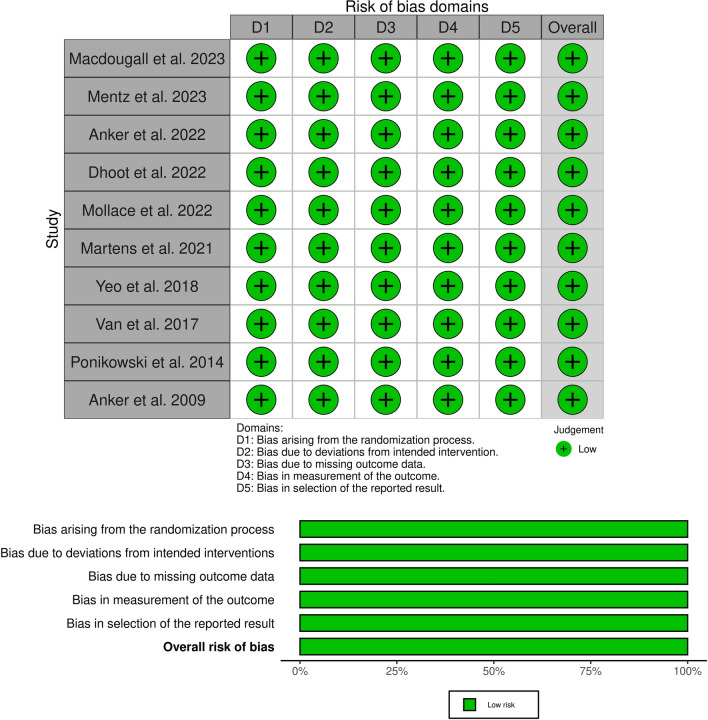
The Revised Cochrane risk-of-bias tool (ROB-II) was used to assess the quality of RCTs. All studies showed a low risk of bias in random sequence generation, blinding participants and personnel risk, selection bias, and outcome data reporting (Fig. 2).
Fig. 2.
Cochrane risk-of-bias quality assessment of randomized clinical trials (ROB-II)
The Newcastle Ottawa Scale was employed to assess the quality of the included observational studies, which rated as having overall good quality with three stars in the selection domain, two stars in the comparability domain, and three stars in the outcome/exposure area (Table 2).
Table 2.
Newcastle Ottawa Scale for quality assessment of included cohort studies
| Study ID | Arias-Barrera et al. 2019 [21] | Capone et al. 2023 [22] | López-Vilella et al. 2023 [23] | |
|---|---|---|---|---|
| Selection | Representativeness of the exposed cohort | – | – | – |
| Selection of the non-exposed cohort | * | * | * | |
| Ascertainment of exposure | * | * | * | |
| Demonstration that outcome of interest was not present at start of study | * | * | * | |
| Comparability | Comparability of cohorts on the basis of the design or analysis | ** | ** | ** |
| Outcome | Assessment of outcome | * | * | * |
| Was follow-up long enough for outcomes to occur | * | * | * | |
| Adequacy of follow up of cohorts | * | * | * | |
| Overall quality | Good quality | Good quality | Good quality | |
*High quality answers are marked by an asterisk with a maximum of one asterisk for each item within ‘Selection’ and ‘Outcome’ categories compared to a maximum of two asterisks in ‘Comparability’ category
Outcomes
The 6-Min Walk Distance
Four studies [12, 14, 16, 20] reported 6-min walk distance at baseline and 4, 12, and 24 weeks and the meta-analysis showed a statistically significant enhancement in the 6-min walk distance for the FCM group [SMD: 1.45; 95% CI: 0.55, 2.36; p = 0.002 (I2 = 99%; p < 0.00001)]. However, the high heterogeneity (I2 = 99%) implies that patient experiences varied significantly across the studies (Fig. 3).
Fig. 3.
Forest plot of the effect of FCM on the 6-min walk distance. 6-MWT 6-minute walk test, CI confidence interval, df degree of freedom, FCM ferric carboxymaltose, IV inverse variance, SD standard deviation
Quality of Life in Heart Failure Patients
Quality of life assessed via the EQ-5D VAS in 2 of the included studies [12, 14] at baseline and at 4 and 12 weeks shows that FCM was associated with non-significant perceived health status improvement (SMD: 1.56; 95% CI: −0.33, 3.45; p = 0.11 [I2 = 99%; p < 0.00001]) (Fig. 4A). Assessing the KCCQ at 4, 12, and 24 weeks showed that FCM was associated with statistically significant improvement in health status quality of life (SMD: 1.49; 95% CI: 0.87, 2.11; p < 0.00001 [I2 = 98 %; p < 0.00001]) (Fig. 4B) with the high heterogeneity reflecting a considerable variability in the quality of life measured by KCCQ among the studies.
Fig. 4.
A Forest plot of the effect of FCM on EQ-5D visual analog scale, and B Kansas City Cardiomyopathy Questionnaire (KCCQ) at 4, 12, and 24 weeks. 6-MWT 6-minute walk test, CI confidence interval, df degree of freedom, FCM ferric carboxymaltose, IV inverse variance, SD standard deviation
Hospitalization for Heart Failure
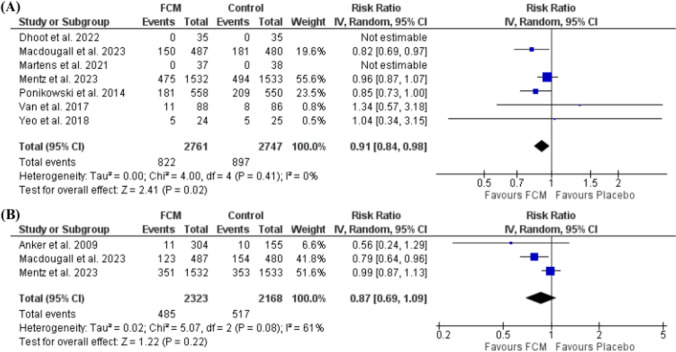
The pooled data from seven included studies observed that FCM significantly reduced the incidence of first hospitalization for HF or cardiovascular death (RR: 0.91; 95% CI: 0.84, 0.98; p = 0.02) (I2 = 0%, p = 0.41) (Fig. 5A). While the incidence of first hospitalization due to HF during follow-up indicated no significant difference from the control ([RR: 0.87; 95% CI: 0.69, 1.09; p = 0.22] [I2 = 61%, p = 0.08]) (Fig. 5B).
Fig. 5.
A Forest plot of the effect of FCM on the incidence of first hospitalization for heart failure or cardiovascular death, and B the incidence of first hospitalization due to heart failure. CI confidence interval, df degree of freedom, FCM ferric carboxymaltose, IV inverse variance
Cardiovascular Death or Intervention for Worsening Heart Failure
Treatment with FCM did not significantly reduce the risk of cardiovascular death (RR: 0.90; 95% CI: 0.77, 1.05; p = 0.17 [I2 = 0%, p = 0.45]), the consistency among study results is evidenced by non-existing heterogeneity (Fig. 6A) [7, 12, 14–18]. Similarly, when considering the outcome of death or the need for intervention due to worsening HF, the effect of FCM did not reach statistical significance (RR: 0.41; 95% CI: 0.04, 4.51; p = 0.47 [I2 = 66%, p = 0.09]) (Fig. 6B) [14, 18].
Fig. 6.
A Forest plot of the effect of FCM on cardiovascular death, and B death or the need for intervention due to worsening heart failure. CI confidence interval, df degree of freedom, FCM ferric carboxymaltose, IV inverse variance
All-Cause Mortality
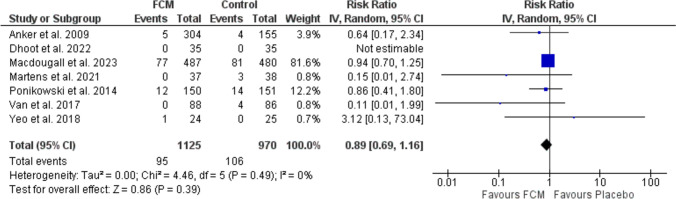
Mentz et al. 2023 reported the one-year mortality rates for patients treated with FCM compared to a control group and revealed no difference between either group (RR: 0.83; 95% CI: 0.67, 1.03; p = 0.1) [18]. Similarly, studies [7, 12, 14, 15, 17, 19], which examined the all-cause mortality rates, declared that FCM did not significantly alter mortality risk (RR: 0.89; 95% CI: 0.69, 1.16; p = 0.28 [I2 = 0 %, p = 0.49]). No existing heterogeneity observed (I2 = 0 %) suggests that there was consistency among study outcomes (Fig. 7).
Fig. 7.
Forest plot of the effect of FCM on all-cause death. CI confidence interval, df degree of freedom, FCM ferric carboxymaltose, IV inverse variance
Discussion
This meta-analysis offers a thorough and detailed assessment of the effect of FCM on a range of clinical outcomes in patients with iron deficiency anemia and HF. Our meta-analysis indicated a significant enhancement in the 6-minute walk distance for patients receiving FCM [SMD: 1.45; 95% CI: 0.55, 2.36; p = 0.002; I2 = 99 %]. However, the high heterogeneity observed suggests substantial variation in patient experiences across the studies. Variations in baseline characteristics, treatment regimens, or other factors impacting the ability of FCM to improve physical capacity may have an impact on this. A statistically significant improvement was observed when the KCCQ was used to analyze symptoms, social and physical limitations, and overall quality of life (SMD: 1.49; 95% CI: 0.87, 2.11; p < 0.00001; I2 = 98 %). The variability in quality-of-life improvements across the studies is highlighted by the observed heterogeneity, which calls for additional investigation into the contributing factors.
Ferric carboxymaltose had a consistent and non-heterogeneous effect on the risk of cardiovascular death (RR: 0.90; 95% CI: 0.77, 1.05; p = 0.17). With high heterogeneity (I2 = 66 %), the combined outcome of cardiovascular death or intervention for worsening HF did not demonstrate a significant effect (RR: 0.41; 95% CI: 0.04, 4.51; p = 0.47). There was no discernible difference between the FCM group and the control group in the analysis of the impact of FCM on the incidence of first hospitalization for HF. Ferric carboxymaltose did not significantly change the risk of all-cause mortality. The lack of heterogeneity indicates consistency between study results – bolstering the validity of the conclusions.
Iron deficiency anemia is a common occurrence (over 50% of patients) in HF [24]. Iron deficiency serves as a reliable indicator of decreased ability to exercise and decreased survival, in a patient with HF [25]. Unlike chronic inflammatory conditions, HF has different diagnostic criteria for iron deficiency. Specifically, a ferritin level of less than 100 µg/L or a ferritin range of 100–299 µg/L in conjunction with a transferrin saturation of less than 20% are required [26].
Regarding the primary objective (change in peak oxygen uptake) of iron deficiency anemia (IDA) treatment in HF patients, there was no appreciable difference seen between the oral iron therapy (iron polysaccharide, 150 mg twice daily for 16 weeks) and placebo in the 225 patients with HF who were enrolled in the IRONOUT HF study. Both the N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels and the 6-min walk test, two secondary outcomes, did not exhibit any appreciable alterations. Even though oral iron therapy was linked to a modest increase in serum ferritin and transferrin saturation, the authors concluded that oral iron supplementation is not advised for HF patients with a reduced ejection fraction [27]. Thus, for patients with HF, intravenous iron is currently the recommended treatment. Iron dextran, iron gluconate, iron sucrose, or FCM are among the intravenous iron agents that are available [28].
The 6-min walk test distance change from baseline was measured in a clinical trial. The mean (SD) change for participants receiving FCM was 31.8 (79.2) meter at Week 24 and 31.1 (62.3) meter at Week 12, respectively, compared to −4.8 (84.4) m and 0.2 (77.1) m with placebo. Significant gains were shown by the fixed-effects model with a mean difference of 26.8 (95% CI: 16.6, 37.0) at Week 12 and 34.2 (95% CI: 22.0–46.4) at Week 24 after FCM [20].
In another study, FCM showed consistent efficacy across varying levels of kidney function in patients with acute HF who had an ejection fraction of less than 50% and an iron deficiency. The results indicate that there is no discernible correlation between the efficacy of FCM and kidney function. This implies that patients with left ventricular ejection fraction should receive prompt diagnosis and treatment for iron deficiency, irrespective of their kidney function [19].
In a study by Anker et al., significant improvements were observed in the quality of life, as assessed by both the EQ-5D visual assessment score and the overall Kansas City Cardiomyopathy score, at Weeks 4, 12, and 24 (p < 0.001) [14].
Among the 3065 patients in a study, 1532 received FCM, and 1533 received a placebo; at 12 months, there was no statistically significant difference between FCM and placebo in overall death (8.6% vs 10.3%), hospitalizations due to HF (297 vs 332), or 6-minute walk distance (8 ± 60 m vs 4 ± 59 m) [18].
In a meta-analysis by Anker et al., FCM significantly decreased rates of recurrent hospitalizations due to HF and cardiovascular mortality (rate ratio 0.53, 95% CI: 0.33, 0.86; p = 0.011) in comparison to a placebo. Additionally, FCM significantly decreased rates of recurrent cardiovascular hospitalizations and cardiovascular mortality (RR 0.59; 95% CI: 0.40, 0.88; p = 0.009). Moreover, FCM showed significant decreases in all-cause mortality and recurrent hospitalizations for cardiovascular (RR 0.60, 95% CI: 0.41, 0.88; p = 0.009) and recurrent hospitalizations for HF (RR 0.54; 95% CI: 0.34, 0.87; p = 0.011) [29].
In another meta-analysis by Khan et al., FCM treatment significantly decreased the composite endpoint of cardiovascular death or HF hospitalization in a meta-analysis of three trials involving 1868 patients (OR = 0.68; 95% CI: 0.54, 0.84; p = 0.0004; I2 = 69%). Furthermore, hospitalizations for HF were significantly reduced in the FCM arms during follow-up (OR = 0.61; 95% CI: 0.47, 0.79; p = 0.0002; I2 = 72%). Treatment with FCM did not, however, significantly lower the risk of cardiovascular mortality (OR = 0.93; 95% CI: 0.69, 1.27; I2 = 0%) or all-cause mortality (OR = 0.97; 95% CI: 0.73, 1.28; I2 = 0%) [30].
A meta-analysis found that patients receiving ferric carboxymaltose (FCM) had significantly lower rates of hospitalization for worsening HF (HF), with a RR of 0.34 (95% CI: 0.19, 0.59, p = 0.0001), and for any cardiovascular (CV) hospitalizations, with an RR of 0.49 (95% CI: 0.35, 0.70, p < 0.0001). However, FCM showed no significant effect on mortality outcomes, including death due to worsening HF (RR 0.41, 95% CI: 0.02, 7.36, p = 0.55) or any CV death (RR 0.80, 95% CI: 0.40, 1.57, p = 0.51) [31]. In contrast to our meta-analysis, which demonstrated that FCM significantly enhanced exercise capacity, quality of life, and lowered the combined risk of first hospitalization for HF or CV death (RR 0.91, 95% CI: 0.84, 0.98, p = 0.02), the other study focused mainly on the reduction in hospitalization rates without showing a notable effect on mortality outcomes [31].
Ferric carboxymaltose therapy, when given to patients with HF with reduced ejection fraction and iron deficiency, significantly lowered the risk of recurrent HF hospitalizations and cardiovascular death, according to another analysis of four RCTs involving over 3000 patients. However, subgroup analyses showed that the FCM and placebo groups had consistent treatment effects across several variables, such as age, sex, HF etiology, transferrin saturation, estimated glomerular filtration rate, hemoglobin, ferritin, and New York Heart Association class [32].
Given the high population-level burden of hospitalizations in HF patients, treating iron deficiency with IV FCM has the potential to significantly reduce healthcare costs while also improving patient outcomes.
Cost-offset modeling revealed that IV FCM is expected to be a cost-saving intervention in the UK, Switzerland, and Italy over a five-year time horizon following an episode of acute HF. Savings were mainly caused by fewer hospitalizations and prevented cardiovascular mortality [33]. Furthermore, Hofmarcher et al. [34] demonstrated that IV FCM use in chronic HF patients with iron deficiency anemia reduced the burden on both primary care and hospital treatment equally, but within hospitals, there is a significant shift in expenses from inpatient to outpatient care.
Limitations and Recommendations
This is the first meta-analysis to focus on the effect of IV FCM on the quality of life in HF patients with iron deficiency anemia. It also provides evaluation of a range of clinical outcomes, which offers insightful information about the possible advantages of FCM in patients with HF with iron deficiency anemia.
The observed heterogeneity in some outcomes, which may limit the generalizability of results, even though our meta-analysis suggests potential benefits of FCM in improving physical capacity, quality of life, and CV outcomes in HF patients with iron deficiency anemia. Healthcare providers should consider the unique characteristics of each patient. More robust randomized control trials are needed to provide stronger evidence on the benefit of IV FCM to in HF patients with iron deficiency anemia
Conclusion
The study shows FCM significantly improves quality of life and functional capacity in HF patients with iron deficiency, but it does not notably reduce mortality or hospitalization rates. This indicates that while FCM is beneficial for symptom management, it does not address all aspects of HF care. These findings highlight the need for more comprehensive treatment approaches that focus on both symptom relief and long-term patient outcomes.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Author Contributions
A.M.T. conceived the idea. A.M.T. designed the research workflow. S.A.N., S.S., M.M.G., A.A., M.R.M. and K.A. searched the databases. S.A.N., S.S., M.M.G., A.A., M.R.M. and K.A. screened the retrieved records, extracted relevant data, assessed the quality of evidence, and A.M.T. and A.S.E. resolved the conflicts. A.A. performed the analysis. A.S.E., S.A.N., S.S., M.M.G., A.A., M.R.M., and K.A. wrote the final manuscript. A.M.T. and A.S.E. supervised the project. All authors have read and agreed to the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding
We received no funding for this study.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
Not applicable.
Code Availability
Not applicable.
References
- 1.Lam CSP, Doehner W, Comin-Colet J, IRON CORE Group. Iron deficiency in chronic heart failure: case-based practical guidance. ESC Heart Fail. 2018;5(5):764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand IS, Gupta P. Anemia and iron deficiency in heart failure. Circulation. 2018;138(1):80–98. [DOI] [PubMed] [Google Scholar]
- 3.Ebner N, von Haehling S. Why is iron deficiency recognised as an important comorbidity in heart failure? Card Fail Rev. 2019;5(3):173–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-1032. [DOI] [PubMed] [Google Scholar]
- 6.von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, et al. Improving exercise capacity and quality of life using non-invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. 2021;23(1):92–113. [DOI] [PubMed] [Google Scholar]
- 7.van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation. 2017;136(15):1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372): n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen L, Paludan-Müller AS, Laursen DRT, Savović J, Boutron I, Sterne JAC, et al. Evaluation of the cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in cochrane and non-cochrane reviews. Syst Rev. 2016;10(5):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;1(14):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deeks J, Higgins J, Altman D, editors. Chapter 10: Analysing data and undertaking meta-analyses. In: Cochrane Handbook for Systematic Reviews of Interventions. version 6.4 (updated August 2023). [cited 2024 Sep 28]. Available from: https://training.cochrane.org/handbook
- 12.Yeo TJ, Yeo PSD, Hadi FA, Cushway T, Lee KY, Yin FF, et al. Single-dose intravenous iron in Southeast Asian heart failure patients: a pilot randomized placebo-controlled study (PRACTICE-ASIA-HF). ESC Heart Fail. 2018;5(2):344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollace A, Macrì R, Mollace R, Tavernese A, Gliozzi M, Musolino V, et al. Effect of ferric carboxymaltose supplementation in patients with heart failure with preserved ejection fraction: role of attenuated oxidative stress and improved endothelial function. Nutrients. 2022;14(23):5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–48. [DOI] [PubMed] [Google Scholar]
- 15.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36(11):657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhoot S, Mittal S, Singh SP, Patel V, Kasliwal RR, Mehta V. Effect of ferric-carboxy maltose on oxygen kinetics and functional status in heart failure patients with iron deficiency. Future Sci OA. 2020;6(5): FSO467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martens P, Dupont M, Dauw J, Nijst P, Herbots L, Dendale P, et al. The effect of intravenous ferric carboxymaltose on cardiac reverse remodelling following cardiac resynchronization therapy-the IRON-CRT trial. Eur Heart J. 2021;42(48):4905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentz RJ, Garg J, Rockhold FW, Butler J, De Pasquale CG, Ezekowitz JA, et al. Ferric Carboxymaltose in heart failure with iron deficiency. N Engl J Med. 2023;389(11):975–86. [DOI] [PubMed] [Google Scholar]
- 19.Macdougall IC, Ponikowski P, Stack AG, Wheeler DC, Anker SD, Butler J, et al. Ferric Carboxymaltose in iron-deficient patients with hospitalized heart failure and reduced kidney function. Clin J Am Soc Nephrol CJASN. 2023;18(9):1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anker SD, Ponikowski P, Khan MS, Friede T, Jankowska EA, Fabien V, et al. Responder analysis for improvement in 6-min walk test with ferric carboxymaltose in patients with heart failure with reduced ejection fraction and iron deficiency. Eur J Heart Fail. 2022;24(5):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias-Barrera C, Palacios-Ariza MA, Pradilla I, Alvarez-Moreno C. A cohort study of two intravenous treatments for iron deficiency in patients with heart failure. Acta Cardiol. 2020;75(7):605–12. [DOI] [PubMed] [Google Scholar]
- 22.Capone F, Cipriani A, Molinari L, Noale M, Gusella B, Lucente F, et al. Ferric carboxymaltose in patients with acute decompensated heart failure and iron deficiency: a real-life study. J Pers Med. 2023;13(8):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Vilella R, Lozano-Edo S, Arenas Martín P, Jover-Pastor P, Ezzitouny M, Sorolla Romero J, et al. Impact of intravenous ferric carboxymaltose on heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2022;9(1):133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caminiti G, Sposato B, Volterrani M. The role of iron deficiency in heart failure. Eur Heart J Suppl J Eur Soc Cardiol. 2023;25(Suppl C):C306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. 2013;165(4):575-582.e3. [DOI] [PubMed] [Google Scholar]
- 26.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. [DOI] [PubMed] [Google Scholar]
- 27.Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. 2017;317(19):1958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebner N, von Haehling S. Iron deficiency in heart failure: a practical guide. Nutrients. 2013;5(9):3730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail. 2018;20(1):125–33. [DOI] [PubMed] [Google Scholar]
- 30.Khan MS, Usman MS, von Haehling S, Doehner W, Stewart Coats AJ. Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: a systematic review and meta-analysis. ESC Heart Fail. 2020;7(6):3392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalal J, Katekhaye V, Jain R. Effect of ferric carboxymaltose on hospitalization and mortality outcomes in chronic heart failure: a meta-analysis. Indian Heart J. 2017;69(6):736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anker SD, Khan MS, Butler J, von Haehling S, Jankowska EA, Ponikowski P, et al. Effect of intravenous iron replacement on recurrent heart failure hospitalizations and cardiovascular mortality in patients with heart failure and iron deficiency: a Bayesian meta-analysis. Eur J Heart Fail. 2023;25(7):1080–90. [DOI] [PubMed] [Google Scholar]
- 33.McEwan P, Ponikowski P, Davis JA, Rosano G, Coats AJS, Dorigotti F, et al. Ferric carboxymaltose for the treatment of iron deficiency in heart failure: a multinational cost-effectiveness analysis utilising AFFIRM-AHF. Eur J Heart Fail. 2021;23(10):1687–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmarcher T, Borg S. Cost-effectiveness analysis of ferric carboxymaltose in iron-deficient patients with chronic heart failure in Sweden. J Med Econ. 2015;18(7):492–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.