Abstract
Introduction
While ≥ 40 CAG repeat expansions in HTT present a well-established cause of Huntington’s disease (HD), an enrichment of HTT repeat expansions was recently reported also in patients with amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), including FTD/ALS patients with additional HD neuropathology. This raises the question whether the phenotypic spectrum of HTT expansions can be extended to ALS and FTD, and whether HTT should be considered as a new causative gene of FTD/ALS. If HTT repeat expansions were indeed systematically related to FTD/ALS, one would expect an increased frequency of HTT carriers in FTD/ALS, who can clinically/neuropathologically not be explained better than by the presence of the HTT repeat expansions.
Methods
Screening of HTT repeat expansions in 249 consecutive patients with ALS or FTD by short-read genome sequencing took place. The post-mortem neuropathological examination was performed in the identified HTT repeat expansion carrier.
Results
One HTT repeat expansion [40/22 repeats (± 1)] was identified in an ALS patient, giving a frequency of 0.4% (1/249) (frequency in the general population: 0.03–0.18%). This patient showed a classic ALS phenotype, but no clinical or imaging signs of HD. Post-mortem brain examination revealed—in addition to ALS-typical degeneration of upper and lower motor neurons with TDP-43 inclusions—HD-typical polyQ-aggregates in gyrus cinguli, striatum and frontal lobe, yet without evidence of striatal degeneration.
Conclusions
Our study does not support the notion of an increased frequency of HTT repeat expansions in FTD/ALS. Moreover, the phenotype of the HTT carrier identified can be better explained by two co-existent, but independent diseases: (i) ALS and (ii) presymptomatic HD, which—given the low repeat number—is likely to become manifest only later in life. These findings corroborate the concept that HTT repeat expansions are likely co-existent/coincidental, but not causative in FTD/ALS.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12822-2.
Keywords: Huntington, ALS, Amyotrophic lateral sclerosis, Frontotemporal dementia, HTT, MAPT
Introduction
While ≥ 40 CAG repeat expansions in HTT present a well-established cause of Huntington`s disease (HD), an enrichment of HTT repeat expansions was recently reported also in patients with amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD), including FTD/ALS patients with additional HD neuropathology [1]. Specifically, Dewan and colleagues found: (i) a frequency of 0.12% (3/2442) and 0.14% (5/3674) HTT-carriers, respectively, in FTD/ALS cohorts compared to 0.03% (10/31372) in the general population; and (ii) both FTD/ALS-typical TDP-43-pathology and HD-typical polyQ-inclusions without evidence of striatal degeneration in autopsy of two HTT-carriers with an ALS-phenotype [2]. These findings raise the question whether the phenotypic spectrum of HTT expansions can be extended to ALS and FTD, and whether HTT should be considered as a new causative gene of FTD/ALS.
Given the recent controversy on this notion [3, 4] and the need for validation by independent screening studies and post-mortem studies (as emphasized by the authors themselves, [1, 5]), we here investigated the following two-fold hypothesis: if HTT repeat expansions were indeed systematically related to FTD/ALS, one would expect (1.) an increased frequency of HTT carriers in FTD/ALS, (2.) who can clinically/neuropathologically not be explained better than by the presence of the HTT repeat expansions. This hypothesis was tested by a large screening of HTT repeat expansions by short-read genome sequencing (SR-GS) of 249 patients with ALS or FTD, combined with post-mortem neuropathological examination in the HTT carrier identified by this large screening.
Methods
Genome-based HTT expansion screening of a consecutive FTD/ALS series and in-depth phenotyping
A consecutive series of 249 subjects with ALS (N = 188), FTD (N = 52) or FTD/ALS (N = 9)—each diagnosed to standard criteria (ALS: [6–8]; FTD: [9, 10]; FTD/ALS: [11])—was recruited by the FTD/ALS outpatient clinics of the Center of Neurology, Tuebingen, between 2019 and 2022 and investigated by short-read genome sequencing (SR-GS). The sequencing libraries were generated using the Illumina DNA PCR-Free protocol and sequenced on an Illumina NovaSeq 6000 sequencer with a target depth of 38x. The sequencing reads were mapped to the GRCh38 reference genome using BWA-mem2 v.2.2.1 (https://github.com/bwa-mem2/bwa-mem2) and repeat expansions were detected with ExpansionHunter v5.0.0 (https://github.com/Illumina/ExpansionHunter). In-depth phenotyping was performed in the identified patient with a pathological CAG HTT expansion by clinical, imaging, electrophysiological and fluid biomarker studies, followed by autopsy and post-mortem neuropathology examination. All subjects provided written informed consent before participation and publication according to the Declaration of Helsinki.
Neuropathological examination
The identified patient with a HTT repeat expansion, who died at age 63 years, underwent autopsy of brain and spinal cord, performed at the Brain Bank Unit Tuebingen of the DZNE Brain Bank. Neuropathological evaluation was performed on formalin-fixed paraffin embedded tissue sections from 20 standardized neuroanatomical regions (including various neocortical regions, basal ganglia, thalamus, amygdala, hippocampus, brain stem, cerebellum and spinal cord) following guidelines for the assessment and diagnosis of neurodegenerative diseases including hematoxylin and eosin staining and immunohistochemistry with antibodies against phosphorylated TDP-43 (clone 1D3, [12]), phosphorylated tau (clone AT8, Thermo Fisher), α-synuclein (clone 4D6, Origene), beta-amyloid (clone 4G8, Covance), polyQ (clone 1C2, Millipore), p62 (BD Transduction Laboratories) and GFAP (clone GA5, Diagnostic BioSystems) using the Ventana BenchMark XT automated staining system with the Optiview DAB detection kit (Ventana). The 1C2 antibody against polyQ is widely used in neuropathological evaluation of postmortem HD brains against expanded polyglutamine tracts [13, 14].
Results
Frequency of HTT repeat expansions in FTD/ALS
HTT repeat expansion screening by SR-GS in 249 FTD/ALS patients identified one ALS patient with a pathogenic HTT repeat expansion (40/22 CAG repeats (± 1) (repeat size confirmed by fragment length analysis)), giving a frequency of 0.4% (1/249) (HTT repeat expansion allele frequency in the literature: 0.03 [2]—0.18% [15]) (for cohort characteristics in terms of family history and further genetic findings, see Supplement 1). 18 patients (7.2%) had a predicted intermediate expansion within the range 27–35 CAG repeats; 1 patient a pathogenic repeat expansion with reduced penetrance (37 CAG repeats).
No other second mutation was identified in the ALS patient with a pathogenic HTT repeat expansion which might have explained either his clinical ALS phenotype or the late-onset dementia syndrome in his ancestors (see below); except a variant in microtubule associated protein tau (MAPT), NM_001123066.4: c.509del, p.(Pro170LeufsTer24), GRCh38(chr17):g.45983312del. This was formally categorized as variant of uncertain significance (VUS), but was unlikely to have a pathogenic impact as it was located only deep-intronic in the main brain expressed MAPT transcripts (ENST00000351559.10, ENST00000446361.7, ENST00000535772.6). Furthermore, in those weakly brain expressed transcripts, where it was located exonic (ENST00000262410.10, ENST00000344290.10, ENST00000415613.6, ENST00000571987.5), it predicts a premature stop – yet loss-of-function does not present a mutational mechanism known to lead to disease in MAPT. Two wildtype C9orf72 alleles were predicted by ExpansionHunter, and a C9orf72 repeat expansion was additionally also ruled out by PCR-based fragment length analysis (for further details on the sequencing statistics and coverage of genes of interest, see Supplement 2).
In-depth phenotyping
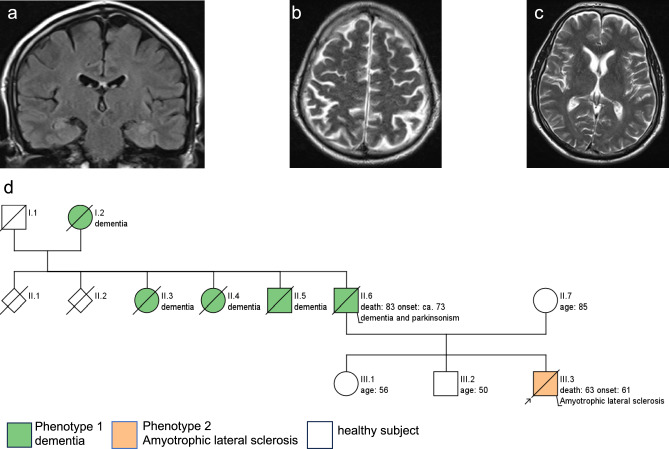
The male patient showed a classic ALS phenotype, with disease onset at age 61 years with progressive dysarthria, dysphagia, paralysis and death after 2 years due to global respiratory insufficiency. The neurological examination revealed an involvement of both upper and lower motor neuron, without any clinical signs or changes of behaviour characteristic of HD even on repeated investigations by independent movement disorders neurologists. Furthermore, the patient scored only 8 points on the Unified Huntington's Disease Rating Scale (UHDRS [16]), likely reflecting the effects of ALS rather than (even incipient) HD, as they included: gait disturbances from paresis, severe dysarthria, and difficulties with tandem walking. There were no specific clinical signs of HD, including no abnormalities of tongue protrusion, chorea or dystonia. CSF NfL levels were substantially increased to 4090 pg/mL (cut-off < 916 pg/mL). Cerebral and spinal MRI ruled out competing diagnoses like ischemic lesions, tumors and spinal stenosis. No regional atrophies including frontal lobe, insula, striatum and caudate nucleus were found (see Fig. 1a, b, c). Overall Huntington's Disease Integrated Staging System (HD-ISS [17]) disease stage was 0. Family history was positive for late-onset (> 60 years) dementia syndromes in several generations, including reported behavioural changes, progressive speech decline and parkinsonism in the index patient´s father (see Fig. 1d), indicating an autosomal-dominant family history for a, partly complex, late-onset neurodegenerative dementia syndrome.
Fig. 1.
Cerebral magnetic resonance imaging (cMRI) in the index patient. cMRI at age 61 years revealed no evidence of atrophy in the frontal lobe, insula or caudate nucleus (a: frontal T2 FLAIR image showing intact insula; b: axial T2-weighted image demonstrating absence of frontal atrophy; c axial T2-weighted image indicating no caudate nucleus atrophy). Pedigree of the index patient. Family history of the index patient (marked by an arrow) is positive for Parkinson`s disease and dementia (d). His father was diagnosed with a “Parkinsonian syndrome” at about age of 60 years, developed symptoms of a dementia with 73 years comprising language decline and changes in behaviour, and died at age 83 years. Two paternal aunts and one paternal uncle out of in total six siblings as well as their mother were also diagnosed with a late-onset neurodegenerative dementia syndrome. None of the relatives were suffering from ALS
Neuropathology
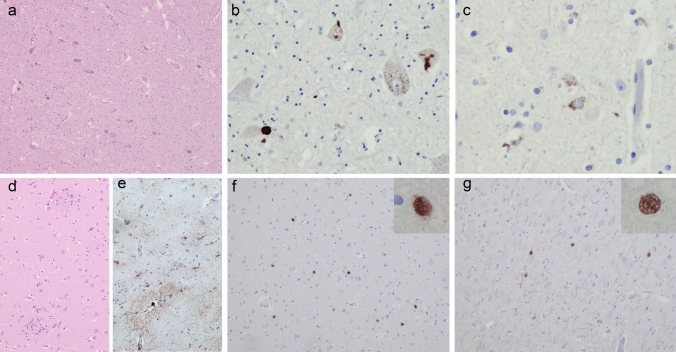
Macroscopically, the spinal cord revealed atrophic anterior roots, otherwise the CNS was unremarkable. The histological analysis showed mild to moderate loss of Betz’s cells in the precentral gyrus, as well as moderate loss of motor neurons in the hypoglossal nucleus and anterior horns of the spinal cord (Fig. 2a). TDP-43 immunoreactive inclusions, characteristic for ALS (neuronal cytoplasmic inclusions and oligodendroglial inclusions), were present in the spinal cord, brain stem and precentral gyrus (Fig. 2b, c).
Fig. 2.
Neuropathology. Typical ALS pathology with (a) loss of motor neurons in the anterior horn of the spinal cord (H&E) and TDP-43-immunoreactive neuronal cytoplasmic inclusions in the spinal cord (b) and precentral gyrus (c). (d) The caudate nucleus revealed no signs of neurodegeneration by H&E. However, mild astrogliosis was detected by GFAP immunohistochemistry (e). PolyQ-immunoreactive neuronal intranuclear inclusions were present in the striatum (f) and frontal cortex (g)
No obvious cell loss and gliosis were seen in H&E stains in HD-characteristic brain regions such as the caudate nucleus (Fig. 2d) and putamen. However, GFAP-immunohistochemistry revealed mild to moderate gliosis in the head of the caudate nucleus (Fig. 2e), in line with Vonsattel grade 1 [18]. Moderate numbers of anti-polyQ labelled neuronal nuclei as well as more compact intranuclear inclusions were found in the striatum (Fig. 2f), frontal cortex (Fig. 2g) and gyrus cinguli, as characteristic for HD.
As an additional minor comorbid finding, mild Alzheimer’s Disease associated neuropathological changes (ABC score A2, B1, C1) were present.
Discussion
Our work tested the recently reported hypothesis that the phenotypic spectrum of HTT expansions might be extended to ALS and FTD, and that, correspondingly, HTT might be considered as a new causative gene, or at least genetic risk factor, of FTD/ALS [1]. Combining a large-scale genetic screening plus in-depth phenotyping and post-mortem neuropathology investigations, we did not find an increased frequency of HTT repeat expansions in 249 WGS datasets (1/249 = 0.4%, compared to the HTT repeat expansion allele frequency of 0.03 [2]—0.18% [15] in the general population). Moreover, the phenotype and neuropathology of the only HTT repeat expansion carrier identified by this large screening was better explained by two independent diseases: (i) ALS and (ii) as of yet still presymptomatic HD stage, which, given the low repeat number, is likely to become manifest only later in life. Given that polyQ-inclusion pathology was still mild, and atrophies absent, it is more parsimonious to assume that this very mild HD neuropathology is indicative of a presymptomatic HD stage, rather than the putative cause of the full-blown, fatal ALS disease.
These results question the presumed causality of HTT repeat expansions in FTD/ALS. This notion had been especially based in a prior study on two findings: (i) a frequency of 0.12% (3/2442) and 0.14% (5/3674) HTT-carriers, respectively, in FTD/ALS cohorts compared to 0.03% (10/31372) in the general population; and (ii) both FTD/ALS-typical TDP-43-pathology and polyQ-inclusions without evidence of striatal degeneration in autopsy of two HTT-carriers with an ALS-phenotype [1]. These findings have already been criticized on conceptual grounds [3]: (i) The clinical phenotype with cognitive symptoms and language disturbance described in five out of eight presumable “FTD patients” of this series might be compatible with HD (especially in one patient with apathy and irritability) rather than with FTD, rendering it likely that the observed HTT repeat expansions simply caused HD, rather than FTD. (ii) In the three identified ALS patients with HTT repeat expansions, the CAG repeat expansions size was near the threshold of 40 repeats, rendering it possible that also in these three instances, like probably the case in the patient reported here, these respective patients were carrying two independent conditions: a manifest ALS disease, and an, as of yet presymptomatic, Huntington’s disease. This would also present the most parsimonious explanation for the neuropathology investigations performed in two of the three subjects: the presence of polyQ and p62 staining, in the absence of striatal degeneration, is best indicative of a presymptomatic HD stage [19]. Given the well-established correlation between repeat length and severity of clinical symptoms, age at onset and extent of striatal degeneration [20] as well as the small expansion of the repeat size, HD would only become manifest later in life in these subjects, allowing other concomitant diseases, such as ALS, to manifest earlier in life. Supporting this notion, in the 63-year-old subject reported here with a small expansion of the HTT repeat, we also found typical signs of full-blown ALS pathology with atrophy of first and second motor neuron, accompanied by TDP-43-positive inclusions; but with only mild levels of polyQ- aggregates without striatal atrophy, in sum indicative of concomitant HD, which was, in contrast to the ALS disease, yet a still very early stage. HD would likely have become manifest later in life, possibly with a complex late-onset neurodegenerative dementia syndrome as in the patient’s ancestral generations.
This notion proposed here would also provide the most parsimonious explanation for other recent findings. Hickman et al. found both HD and ALS neuropathology in 6 out of 751 brains from the New York Brain Bank. While exceeding the prevalence of ALS in the USA (0.8% vs. 0.0052%), all of these patients had HTT repeat expansions in the range from 41 to 44 CAG repeats [21], and detailed clinical information was available only for 2/6 subjects. In at least 1 of the 2 cases, where clinical information was available (Case #1; 41 CAG repeats; ALS disease, but only HD neuropathology grade 1), the HTT repeat expansion is likewise most parsimoniously explained as a coincidental finding of a not yet-manifest HD disease, coexistent, but not causative, to the ALS disease. Correspondingly, like our study, another study could also not confirm an increased frequency of pathogenic HTT repeat expansions in ALS, observing only 2 patients with intermediate, but no patient with pathogenic HTT repeat expansions in a screening cohort of 608 ALS patients [22]. However, our study is limited by the fact that we identified only a single ALS patient with a HTT repeat expansion, allowing no robust statistical comparison on the HTT repeat carrier frequency in our ALS cohort vs general population. To test this further, future studies of additional larger existing FTD/ALS cohorts vs general population data—ideally from the same ethnic origin and sequencing technique—are warranted.
In summary, our findings suggest a parsimonious notion to explain our and others’ observations of HTT repeat expansions in FTD/ALS patients. Specifically, they suggest that there is yet no sufficient evidence for extending the phenotypic spectrum of HTT mutations beyond HD to include also ALS and FTD phenotypes. A more likely explanation is that, in some patients, HTT repeat expansions are simply a coincidental finding, at a yet presymptomatic HD stage, coexistent, but not causative, to an ALS/ FTD disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Dr. Andreas Traschütz, Dr. Anna Hofmann and Moritz Löffler in their supporting examination and care of the patient. This work was supported by the Clinician Scientist program "PRECISE.net" funded by the Else Kröner-Fresenius-Stiftung (to D.M, and M.S.), by the EU Joint program—Neurodegenerative Disease Research GENFI-PROX grant (2019-02248; to M.S), and by Solve-RD project funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No 779257 (to M.S). D.M. is supported by the Clinician Scientist program of the Medical Faculty Tuebingen (459-0-0) and the Elite Program for Postdoctoral researchers of the Baden-Württemberg-Foundation (1.16101.21). M.Z. is supported by the Clinician Scientist program of the Medical Faculty Tuebingen (481-0-0).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The pseudonymized data of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
Dr. Milan Zimmermann reports no disclosures. Dr. David Mengel reports no disclosures. Dr. Katrin Klein reports no disclosures. Dr. Tobias Haack reports no disclosures. Dr. Manuela Neumann reports no disclosures. Dr. Matthis Synofzik has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Servier, Solaxa, Biogen, Reata, GenOrph, AviadoBio, Biohaven, Zevra,and Lilly, all unrelated to the present manuscript.
References
- 1.Dewan R, Chia R, Ding J, Hickman RA, Stein TD, Abramzon Y, Ahmed S, Sabir MS, Portley MK, Tucci A, Ibanez K, Shankaracharya FNU, Keagle P, Rossi G, Caroppo P, Tagliavini F, Waldo ML, Johansson PM, Nilsson CF, American Genome C, Rowe JB, Benussi L, Binetti G, Ghidoni R, Jabbari E, Viollet C, Glass JD, Singleton AB, Silani V, Ross OA, Ryten M, Torkamani A, Tanaka T, Ferrucci L, Resnick SM, Pickering-Brown S, Brady CB, Kowal N, Hardy JA, Van Deerlin V, Vonsattel JP, Harms MB, Morris HR, Ferrari R, Landers JE, Chio A, Gibbs JR, Dalgard CL, Scholz SW, Traynor BJ, American Genome C, Consortium FS, Genomics England Research C, International ALSFTDGC, International FTDGC, International LBDGC, Consortium NA, Consortium P (2021) Pathogenic Huntingtin repeat expansions in patients with frontotemporal dementia and amyotrophic lateral sclerosis. Neuron 109(448–460):e444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardiner SL, Boogaard MW, Trompet S, de Mutsert R, Rosendaal FR, Gussekloo J, Jukema JW, Roos RAC, Aziz NA (2019) Prevalence of carriers of intermediate and pathological polyglutamine disease-associated alleles among large population-based cohorts. JAMA Neurol 76:650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas Q, Coarelli G, Heinzmann A, Le Ber I, Amador MDM, Durr A (2021) Questioning the causality of HTT CAG-repeat expansions in FTD/ALS. Neuron 109:1945–1946 [DOI] [PubMed] [Google Scholar]
- 4.Manini A, Gagliardi D, Meneri M, Antognozzi S, Del Bo R, Scaglione C, Comi GP, Corti S, Ronchi D (2022) Analysis of HTT CAG repeat expansion in Italian patients with amyotrophic lateral sclerosis. Ann Clin Transl Neurol 9:1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewan R, Scholz SW, Chio A, Traynor BJ (2021) Highlighting the clinical potential of HTT repeat expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Neuron 109:1947–1948 [DOI] [PubMed] [Google Scholar]
- 6.Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299 [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K, Mitsumoto H, Nodera H, Shefner J, Swash M (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–503 [DOI] [PubMed] [Google Scholar]
- 8.Hannaford A, Pavey N, van den Bos M, Geevasinga N, Menon P, Shefner JM, Kiernan MC, Vucic S (2021) Diagnostic utility of gold coast criteria in amyotrophic lateral sclerosis. Ann Neurol 89:979–986 [DOI] [PubMed] [Google Scholar]
- 9.Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, Knopman D, Kertesz A, Mesulam M, Salmon DP, Galasko D, Chow TW, Decarli C, Hillis A, Josephs K, Kramer JH, Weintraub S, Grossman M, Gorno-Tempini ML, Miller BM (2007) Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 21:S14-18 [DOI] [PubMed] [Google Scholar]
- 10.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong MJ, Abrahams S, Goldstein LH, Woolley S, McLaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobaGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR (2017) Amyotrophic lateral sclerosis—frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener 18:153–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herndon ES, Hladik CL, Shang P, Burns DK, Raisanen J, White CL 3rd (2009) Neuroanatomic profile of polyglutamine immunoreactivity in Huntington disease brains. J Neuropathol Exp Neurol 68:250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hedreen JC, Berretta S, White Iii CL (2024) Postmortem neuropathology in early Huntington disease. J Neuropathol Exp Neurol 83:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thion MS, Tezenas du Montcel S, Golmard JL, Vacher S, Barjhoux L, Sornin V, Cazeneuve C, Bieche I, Sinilnikova O, Stoppa-Lyonnet D, Durr A, Humbert S (2016) CAG repeat size in Huntingtin alleles is associated with cancer prognosis. Eur J Hum Genet 24:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huntington Study Group (1996) Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 11:136–142 [DOI] [PubMed] [Google Scholar]
- 17.Tabrizi SJ, Schobel S, Gantman EC, Mansbach A, Borowsky B, Konstantinova P, Mestre TA, Panagoulias J, Ross CA, Zauderer M, Mullin AP, Romero K, Sivakumaran S, Turner EC, Long JD, Sampaio C, Huntington’s Disease Regulatory Science C (2022) A biological classification of Huntington’s disease: the integrated staging system. Lancet Neurol 21:632–644 [DOI] [PubMed] [Google Scholar]
- 18.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577 [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Tortosa E, MacDonald ME, Friend JC, Taylor SA, Weiler LJ, Cupples LA, Srinidhi J, Gusella JF, Bird ED, Vonsattel JP, Myers RH (2001) Quantitative neuropathological changes in presymptomatic Huntington’s disease. Ann Neurol 49:29–34 [PubMed] [Google Scholar]
- 20.Langbehn DR, Registry Investigators of the European Huntington Disease N (2022) Longer CAG repeat length is associated with shorter survival after disease onset in Huntington disease. Am J Hum Genet 109:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hickman RA, Dewan R, Cortes E, Traynor BJ, Marder K, Vonsattel JP (2022) Amyotrophic lateral sclerosis is over-represented in two Huntington’s disease brain bank cohorts: further evidence to support genetic pleiotropy of pathogenic HTT gene expansion. Acta Neuropathol 143:105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henden L, Fearnley LG, Grima N, McCann EP, Dobson-Stone C, Fitzpatrick L, Friend K, Hobson L, Chan Moi Fat S, Rowe DB, D’Silva S, Kwok JB, Halliday GM, Kiernan MC, Mazumder S, Timmins HC, Zoing M, Pamphlett R, Adams L, Bahlo M, Blair IP, Williams KL (2023) Short tandem repeat expansions in sporadic amyotrophic lateral sclerosis and frontotemporal dementia. Sci Adv 9:eade2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pseudonymized data of this study are available from the corresponding author upon reasonable request.