Abstract
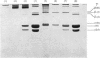
Guinea-pig dermal scar was shown to contain type III collagen, and, from densitometric analysis of gel electrophoretograms, it was shown to have a higher concentration than the surrounding dermis. This finding is consistent with the 'embryonic' nature of newly formed dermal wound tissue, reflected in increased hydroxylation of collagen lysine and the presence of dihydroxylysinonorleucine (after reduction) as the major cross-link.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Bazin S., Delaunay A. Changes in the nature of the collagen during development and resorption of granulatin tissue. Biochim Biophys Acta. 1973 Dec 6;328(2):383–390. doi: 10.1016/0005-2795(73)90272-9. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Bazin S., Sims T. J., Le Lous M., Nicoletis C., Delaunay A. Characterization of the collagen of human hypertrophic and normal scars. Biochim Biophys Acta. 1975 Oct 20;405(2):412–421. doi: 10.1016/0005-2795(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Robins S. P. Embryonic skin collagen. Replacement of the type of aldimine crosslinks during the early growth period. FEBS Lett. 1972 Apr 1;21(3):330–334. doi: 10.1016/0014-5793(72)80195-9. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J. Chemistry of the collagen cross-links. Nature of the cross-links in the polymorphic forms of dermal collagen during development. Biochem J. 1976 Feb 1;153(2):211–215. doi: 10.1042/bj1530211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Sims T. J., Le Lous, bazin S. Collagen polymorphism in experimental granulation tissue. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1160–1165. doi: 10.1016/0006-291x(75)90480-5. [DOI] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Hydroxylysine in the N-terminal regions of the 1 - and 2 -chains of various collagens. Biochem J. 1971 Nov;125(2):433–437. doi: 10.1042/bj1250433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Hydroxylysine in the N-terminal telopeptides of skin collagen from chick embryo and newborn rat. Biochem J. 1971 Dec;125(3):925–928. doi: 10.1042/bj1250925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Kodicek E. Studies in vivo on the biosynthesis of collagen and elastin in ascorbic acid-deficient guinea pigs. Evidence for the formation and degradation of a partially hydroxylated collagen. Biochem J. 1970 Sep;119(3):575–585. doi: 10.1042/bj1190575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes M. J., Constable B. J., Morton L. F., Royce P. M. Age-related variations in hydroxylation of lysine and proline in collagen. Biochem J. 1974 May;139(2):461–468. doi: 10.1042/bj1390461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P., Berger K., Golditch M., Schneir M. Recommendations concerning the use of bacterial collagenase for isolating collagen-associated molecules from tissues. Anal Biochem. 1973 May;53(1):313–316. doi: 10.1016/0003-2697(73)90438-7. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Forrest L., Shuttleworth A., Jackson D. S., Mechanic G. L. A comparison between the reducible intermolecular crosslinks of the collagens from mature dermis and young dermal scar tissue of the guinea pig. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1776–1781. doi: 10.1016/0006-291x(72)90050-2. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Epstein E. H., Jr, Piez K. A. Identification of three genetically distinct collagens by cyanogen bromide cleavage of insoluble human skin and cartilage collagen. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1024–1029. doi: 10.1016/0006-291x(71)90006-4. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Changes in guinea-pig dermal collagen during development. Eur J Biochem. 1975 Jul 1;55(2):391–395. doi: 10.1111/j.1432-1033.1975.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L., Jackson D. S. Comparison of the cyanogen bromide peptides of insoluble guinea-pig skin and scar collagen. Biochim Biophys Acta. 1975 Jan 30;379(1):207–216. doi: 10.1016/0005-2795(75)90024-0. [DOI] [PubMed] [Google Scholar]
- Shuttleworth C. A., Forrest L. Pepsin-solubilized collagens of guinea-pig dermis and dermal scar. Biochim Biophys Acta. 1974 Oct 9;365(2):454–457. doi: 10.1016/0005-2795(74)90023-3. [DOI] [PubMed] [Google Scholar]
- Sykes B. C., Bailey A. J. Molecular weight heterogeneity of the alpha-chain sub-units of collagen. Biochem Biophys Res Commun. 1971 Apr 16;43(2):340–345. doi: 10.1016/0006-291x(71)90758-3. [DOI] [PubMed] [Google Scholar]