Abstract
Background:
Little is known about long-term outcomes beyond survival following acute aortic dissection. The aim of this research was to evaluate rates of home care initiation and nursing home admission during the first year after discharge and to assess factors associated with these needs.
Methods:
All patients in Denmark with a first-time diagnosis of acute aortic dissection type A or B between 2006 and 2015 were identified using national registries. Patients discharged alive without nursing home or home care use before aortic dissection were included, along with age-matched and sex-matched population controls without aortic dissection (at a ratio of 1:5). Cause-specific multivariable Cox regression was used to derive adjusted hazard ratios.
Results:
The study population comprised 1093 patients and 5465 control individuals with a median (IQR) age of 64 (55-71) years; 70.6% were men. During their hospital stay, 2.7% of patients were registered with a first-time diagnosis of stroke, 7.1% with heart failure, and 2.2% with acute kidney failure; 5.9% of patients needed first-time dialysis. During the first year after discharge, 0.8% of patients who had had aortic dissection were admitted to a nursing home, 7.8% started home care, and 5.9% died. Among controls, these rates were 0.2%, 1.2%, and 1.2%, respectively. Patients who had had aortic dissection had significantly increased risk of initiating home care (hazard ratio, 7.47 [95% CI, 5.38-18.37]; P < .001) and of being admitted to a nursing home (hazard ratio, 4.28 [95% CI, 1.73-10.59]; P = .001). Initiation of home care and nursing home admission were related to advanced age, female sex, preexisting comorbidities, in-hospital complications, and conservative management of type A aortic dissection.
Conclusion:
Only a small proportion of patients who survived an aortic dissection needed home care or nursing home admission after hospital discharge.
Keywords: Aortic dissection, epidemiology, patient care management, rehabilitation, thoracic surgery
Key Points
Researchers aimed to determine the risk of home care initiation and nursing home admission after AAD.
During the first year after discharge, 0.8% of patients with AAD were admitted to a nursing home, and 7.8% started home care.
Risk factors for home care initiation and for nursing home admission were advanced age, female sex, comorbid COPD, in-hospital acute kidney failure, and conservative treatment of type A dissection.
Most patients surviving AAD return to life without a need for special care.
Introduction
Acute aortic dissection (AAD) is a devastating cardio-vascular condition with a high mortality rate if left un-treated.1,2 It is caused by an intimal tear that allows blood inflow between the media and intima layers of the aorta. Death and complications result from rupture of the aorta, pericardial tamponade, acute aortic valve regurgitation, and critical malperfusion of end organs because of either shock or aortic branch vessel involvement.3
Existing research has focused primarily on clinical profiles, diagnostics, surgical procedures, in-hospital mortality, and long-term survival.2,4-9 In recent decades, survival rates among patients presenting with type A AAD have improved markedly mainly because of a decline in surgical mortality; however, no statistically significant change in in-hospital mortality among patients with type B AAD has been seen.4,10 Patients with aortic dissection may be subject to many possible risks in addition to death. Patients may experience hypoxic brain injury; stroke; kidney failure; or spinal cord, limb, or mesenteric ischemia.1,10-16 These factors may have detrimental effects on patients’ quality of life and ability to manage without assisted living or nursing home care.
Little is known about long-term outcomes beyond survival following AAD. Therefore, the aim of this study was to evaluate home care initiation and nursing home admission in patients who survived AAD and to assess factors related to these needs.
Patients and Methods
Data Sources and National Registries
The health care system in Denmark, including home care and nursing home care, is financed through taxes; therefore, all Danish citizens have equal access to health care services. Every citizen receives a permanent and unique personal identification number (civil registration number) upon birth or immigration into Denmark. This system allows accurate linkage among all nationwide registries on an individual level. In the present study, 3 national registries were used as data sources. The first was the Danish Civil Registration System, which contains the unique civil registration number, sex, birth date, and vital status of each individual.17 The second was the Danish National Patient Register, which contains data on all inpatient contacts with the Danish health care system from 1977 onward as well as data on emergency department and outpatient contacts from 1995 onward. The data in this register include the date of admission; diagnostic codes, in accordance with the International Statistical Classification of Diseases (ICD); and procedures performed, in accordance with the Nordic Medico-Statistical Committee Classification of Surgical Procedures (NCSP).18 Third, the Danish National Prescription Registry, which contains individual-level data on all dispensed prescriptions from 1995 onward, was consulted.19 Data on nursing home admissions were retrieved from Statistics Denmark, a government entity that has registered the initiation of home care when the need is documented since 1994 and uses validated methods to identify residents in all types of nursing homes. Data on home care services were also retrieved from Statistics Denmark, which registers the initiation of home care when the need is documented. Finally, researchers obtained permission from the Danish Patient Safety Authority to access patient charts exclusively to review the computed tomography (CT) scans of patients with an unspecified aortic dissection diagnosis.
Study Population
All patients in Denmark with a first-time diagnosis of AAD recorded in the Danish National Patient Register between January 1, 2006, and December 31, 2015, were identified (ICD, Tenth Revision, [ICD-10] codes [Danish version: https://medinfo.dk/sks/brows.php?s_nod=13249] I71.0: Aortic dissection unspecified; I71.0A: Aortic dissection type A; and I71.0B: Aortic dissection type B). Diagnoses were recorded by hospital or emergency department physicians. Patients were excluded if they had a diagnosis of aortic dissection or an aortic procedure registered in the Danish National Patient Register before January 1, 2006 (NCSP codes KFCA: Repair of ascending aorta; KFCB: Repair of aortic arch; KFCC: Repair of descending aorta; and KFCD: Repair of thoracoabdominal aorta). Researchers accessed patient charts solely to review the CT scans of patients with an unspecified aortic dissection diagnosis, and patients were excluded if their CT scan revealed that they did not have aortic dissection or new aortic dissection. Review of CT scans and data regarding aortic surgical procedures also allowed the reclassification of unspecified aortic dissection diagnoses among many registered patients as either type A or type B AAD. Thus, the number of patients with an unspecified aortic dissection diagnosis was reduced from 1066 to 465, and these remaining patients were excluded. Finally, patients were excluded if they died during their hospital stay or if they received home care or lived in a nursing home before the AAD. To promote comparability between patients with AAD and the general population, risk-set matching was performed. Each patient who had had AAD was matched 1:5 to individuals from the background population according to sex, age, and time of discharge following AAD (year and month). All Danish citizens were potential controls, but individuals with prior aortic dissection, nursing home residents, and home care recipients at the time of inclusion were ineligible to act as controls.
Study Variables
Data on age, sex, and date of death were retrieved from the Danish Civil Registration System. Data on aortic dissection; surgery for aortic dissection; preexisting comorbidities; initiation of dialysis; and new-onset hypertension, stroke, acute kidney failure, paraplegia, heart failure, ischemic heart disease, and diabetes were retrieved from the Danish National Patient Register. Further, data on diabetes, chronic obstructive pulmonary disease (COPD), and hypertension were retrieved from the Danish National Prescription Registry using prescription medications as proxies for these diseases. Supplemental Table I shows the ICD-10 and Anatomical Therapeutic Chemical codes used.
Characteristics outlined in Table I were collected from registry data before hospital admission for AAD. Pre-existing conditions were defined as those present in the 5 years before the admission of patients for AAD. For control individuals, they were defined as conditions present in the 5 years before inclusion. For example, preexisting aortic dilation meant that the patient had a registered diagnosis of aortic dilatation or aortic aneurysm without mention of rupture in the Danish National Patient Register before admission for AAD. Surgery for aortic dissection meant that a patient had an aortic surgery (NCSP codes KFCA, KFCB, KFCC, and KFCD) registered in relation to their admission for AAD. Nursing home admission was defined as admission to a long-term care facility, with a permanent address change to the facility, excluding temporary care facility and rehabilitation center stays, as described in other studies.20,21
TABLE I.
Characteristics of Patients With AAD and Matched Controls
| Variable | Patients with AAD (n = 1093)a | Control individuals (n = 5465)a | P valueb |
|---|---|---|---|
| Age, median (IQR), y | 64 (55-71) | 64 (55-71) | .92 |
| Male sex, No. (%) | 772 (70.6) | 3860 (70.6) | .99 |
| Type A AAD, No. (%) | 712 (65.1) | – | – |
| Conservative treatment | 98 (13.8) | – | |
| Treated with surgery | 614 (86.2) | – | |
| Type B AAD, No. (%) | 381 (34.9) | – | – |
| Conservative treatment | 286 (75.1) | – | |
| Treated with TEVAR | 57 (15.0) | – | |
| Treated with surgery | 38 (10.0) | – | |
| Preexisting comorbidity, No. (%) | |||
| Hypertension | 471 (43.1) | 1653 (30.2) | <.001 |
| Diabetes | 7 (0.6) | 82 (1.5) | .036 |
| COPD | 39 (3.6) | 145 (2.7) | .12 |
| Ischemic heart disease | 42 (3.8) | 186 (3.4) | .53 |
| HF | 15 (1.4) | 59 (1.1) | .50 |
| Stroke | 22 (2.0) | 68 (1.2) | .064 |
| Chronic kidney disease | 9 (0.8) | 36 (0.7) | .69 |
| Aortic dilatation | 80 (7.3) | 19 (0.3) | <.001 |
AAD, acute aortic dissection; COPD, chronic obstructive pulmonary disease; HF, heart failure; TEVAR, thoracic endovascular aortic repair.
Because of rounding, percentages may not total 100.
P values were derived using the Mann-Whitney U test for patient age and the Pearson χ2 test for categorical variables. P < .05 was considered statistically significant.
Outcomes
All outcome data were collected from the nationwide registries as described above. The primary outcomes were 1-year risk of being admitted to a nursing home and 1-year risk of starting home care among patients discharged alive after AAD and among matched control individuals. Secondary outcomes were first-time initiation of dialysis or first-time diagnoses of hypertension, ischemic heart disease, heart failure (HF), stroke, acute kidney failure, or chronic kidney disease being registered during the hospital stay for AAD as well as the relation of these factors with the primary outcome. Finally, rates of death at 30 days and at 1 year after discharge were evaluated in patients and control individuals.
Ethics
In Denmark, register-based studies conducted for the sole purpose of statistics generation and scientific research do not legally require ethical approval or informed consent. The present study, however, was approved by the Capital Region of Denmark's data responsible unit (approval No. P-2019-404) according to the General Data Protection Regulation. Access to CT scans was granted by the Danish Patient Safety Authority.
Statistical Analysis
Continuous variables are reported as median and IQR; categorical variables are presented as number and percentage. Mann-Whitney U test was used to assess any differences between patients and control individuals for age, and the Pearson χ2 test evaluated differences between patients and control individuals for categorical variables. For age differences between patients and control individuals stratified by sex, the Kruskal-Wallis test was used to derive P values. Cumulative incidences of nursing home admission or home care initiation during the first year after hospital discharge are reported using the Aalen-Johansen estimator, with death as a competing risk. Multivariable cause–specific Cox regression was used to derive adjusted hazard ratios (HRs) for the outcomes of interest. Covariates were selected based on existing knowledge and literature and included age and sex as well as the presence of hypertension, diabetes, COPD, ischemic heart disease, HF, stroke, or chronic kidney disease. P < .05 was considered statistically significant. All analyses were performed using SAS, version 9.4, statistical software (SAS Institute Inc) and R, version 4.0.3, statistical software (R Foundation for Statistical Computing).
Results
Study Population and Characteristics
The final study population comprised 1093 patients discharged alive after first-time AAD (type A, 65.1%; type B, 34.9%), who neither received home care nor lived in a nursing home before hospital admission, and 5465 sex-matched and age-matched controls from the background population (Table I). A flowchart illustrating patient selection is provided in Figure 1.
Fig. 1.
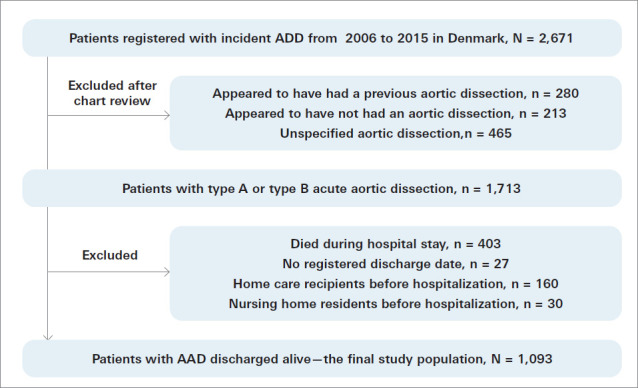
Flowchart of patient selection.
AAD, acute aortic dissection.
In the full study sample, the median (IQR) age was 64 (55-71) years, and men accounted for 71% of the study population (Table I). Among cases of type A AAD, 86% were managed surgically. Among patients with type B AAD, 75% received conservative treatment, 15% had thoracic endovascular aortic repair (TEVAR), and 10% had open aortic surgery (Table I). Forty-three percent of patients with AAD had known hypertension before admission; in control individuals, the proportion was 30% (P < .001) (Table I). Rates of registered preexisting aortic dilatation were significantly higher among patients with AAD than among control individuals (P < .001).
For the remaining comorbidities assessed, only diabetes was associated with a statistically significant difference, and this condition was observed more frequently among control individuals than among patients who had had AAD (1.5% vs 0.6%; P = .036). Supplemental Table II shows characteristics of patients with AAD and control individuals, stratified by sex.
Outcomes
During the first year after discharge, 9 patients (0.8%) were admitted to a nursing home, 85 patients (7.8%) started home care, and 65 patients (5.9%) died. Among control individuals, the numbers were 10 (0.2%), 64 (1.2%), and 66 (1.2%), respectively (Table II). One-year cumulative incidences of nursing home admission or home care initiation in patients and controls are presented in Figure 2, Figure 3, and Figure 4.
TABLE II.
Outcomes After Hospital Discharge
| Variable | Survivors of AAD, No. (%) (n = 1093) | Control individuals, No. (%) (n = 5465) | P valuea | Univariate HR (95% CI) |
|---|---|---|---|---|
| Was admitted to nursing home within 1 y | 9 (0.8) | 10 (0.2) | <.001 | 4.66 (1.89-11.46) |
| Started home care within 1 y | 85 (7.8) | 64 (1.2) | <.001 | 7.15 (5.17-9.89) |
| Was admitted to nursing home or started home care within 1 yb | 93 (8.5) | 72 (1.3) | <.001 | 6.98 (5.13-9.50) |
| Died within 30 d | 17 (1.6) | 0 (0.0) | <.001 | 13.29 (7.01-25.17) |
| Died within 1 y | 65 (5.9) | 66 (1.2) | <.001 | 5.08 (3.61-7.15) |
AAD, acute aortic dissection; HR, hazard ratio.
P values were derived from univariable Cox regression. P < .05 was considered statistically significant.
Nursing home or home care, whichever occurred first.
Fig. 2.
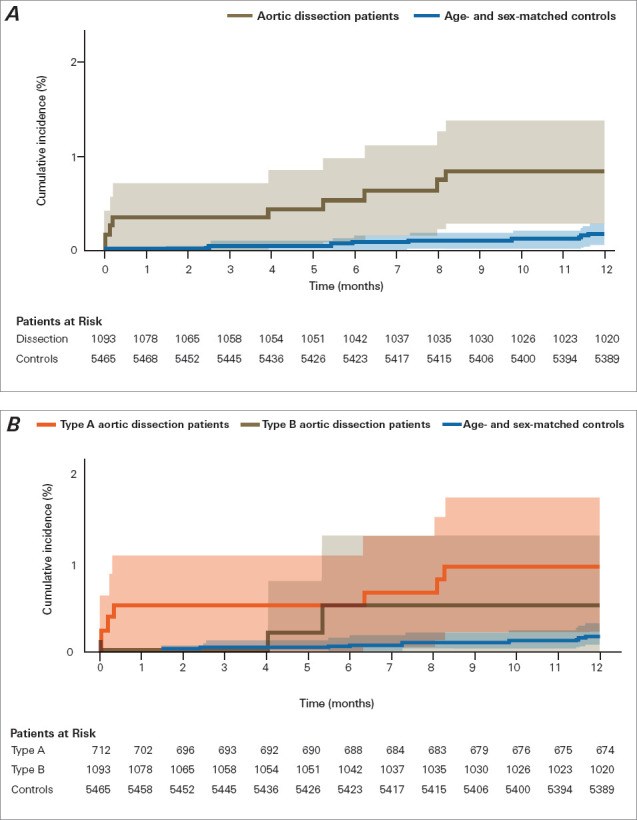
One-year cumulative incidence (Aalen-Johansen estimates, with death as competing risk) of nursing home admission in patients after aortic dissection. A) Risk for patients with aortic dissection and sex-matched and age-matched control individuals (P = .004 for difference). B) Patient risk, stratified by type of aortic dissection (P =.43 for type A vs type B). Shading indicates 95% CI. P < .05 was considered statistically significant.
Fig. 3.
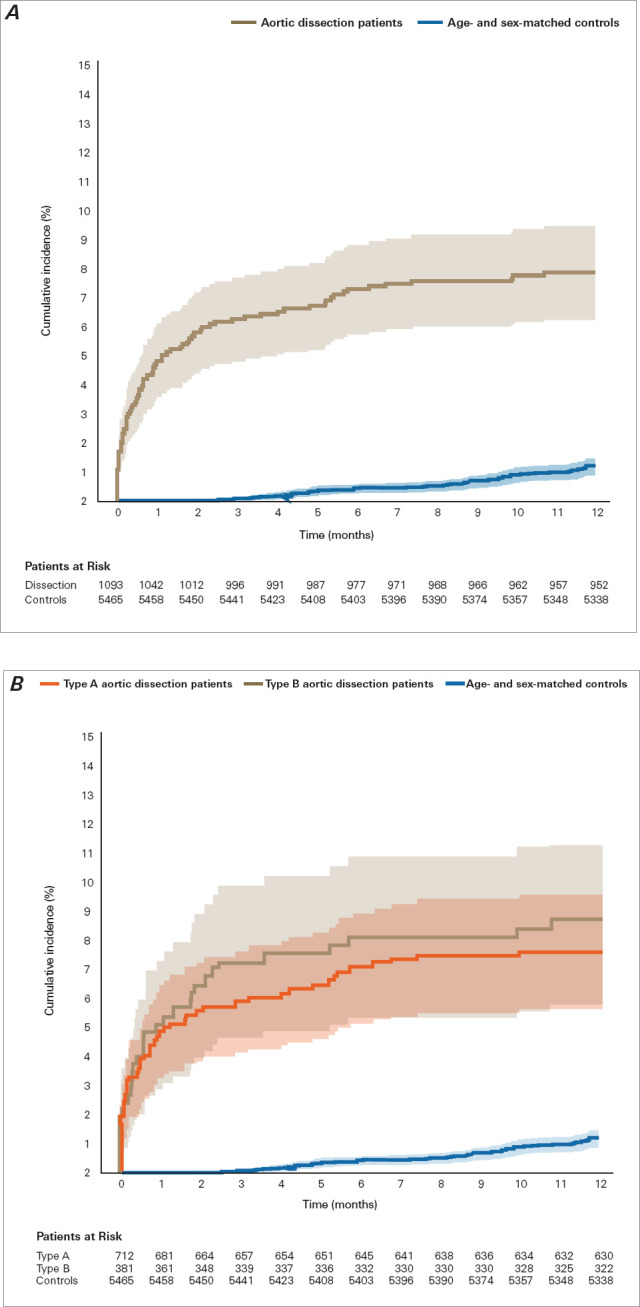
One-year cumulative incidence (Aalen-Johansen estimates, with death as competing risk) of starting home care in patients after aortic dissection. A) Risk for patients with aortic dissection and sex-matched and age-matched control individuals (P < .001 for difference). B) Patient risk, stratified by type of aortic dissection (P = .58 for type A vs type B). Shading indicates 95% CI. P < .05 was considered statistically significant.
Fig. 4.
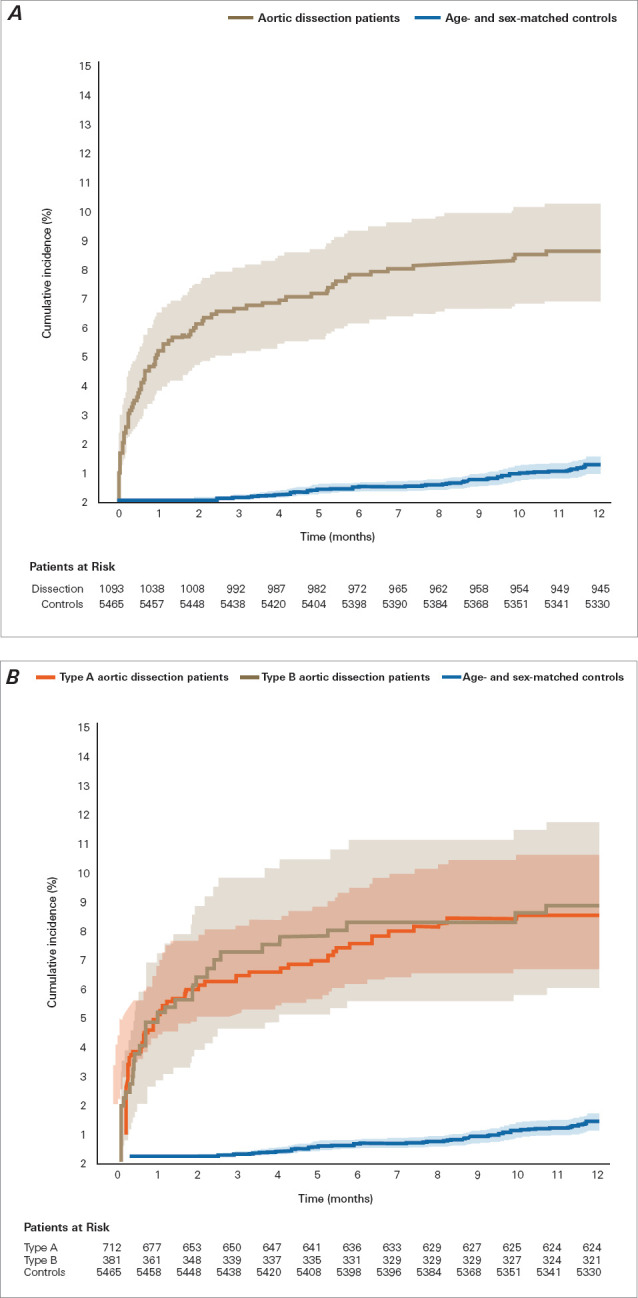
One-year cumulative incidence (Aalen-Johansen estimates, with death as competing risk) of being admitted to a nursing home or starting home care combined in patients after aortic dissection. A) Risk for patients with aortic dissection and sex-matched and age-matched control individuals (P < .001 for difference). B) Patient risk, stratified by type of aortic dissection (P = .90 for type A vs type B). Shading indicates 95% CI. P < .05 was considered statistically significant.
Compared with control individuals, patients with AAD had significantly increased 1-year risk of starting home care (adjusted HR, 7.47 [95% CI, 5.38-10.37]; P < .001) and 1-year risk of being admitted to a nursing home (adjusted HR, 4.28 [95% CI, 1.73-10.59]; P = .001) (Fig. 5). There was no statistically significant difference in 1-year cumulative incidence of nursing home admission and home care initiation combined between patients with type A AAD and patients with type B AAD (Fig. 4) or in the incidence of nursing home admission and of home care initiation separately (Fig. 2 and Fig. 3). Adjusted HR for death at 1 year in patients with AAD compared with controls was 5.22 (95% CI, 3.69-7.38; P < .001).
Fig. 5.
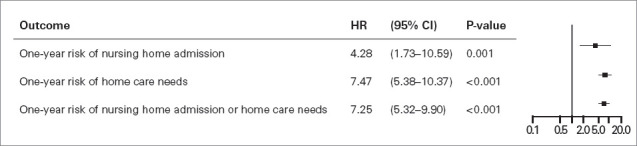
Forest plot showing adjusted hazard ratios (HRs) in patients after acute aortic dissection vs sex-matched and age-matched control individuals. Results are derived from multivariable Cox modeling, adjusted for patient age; patient sex; and the presence of hypertension, diabetes, chronic obstructive pulmonary disease, ischemic heart disease, heart failure, stroke, or chronic kidney disease. P < .05 was considered statistically significant.
In-Hospital Complications
Among patients with AAD who survived to discharge, rates of first-time diagnoses registered during their hospital stay were as follows: stroke, 2.7%; ischemic heart disease, 7.1%; HF, 2.2%; chronic kidney disease, 2.5%; and acute kidney failure, 6.4%. First-time dialysis was registered in 5.9% of this cohort. No patients had a registered diagnosis of paraplegia (Table III).
TABLE III.
In-Hospital Complications and New Diagnoses During Hospital Stay
| Variable | Survivors of AAD, No. (%) (n = 1093) |
|---|---|
| Acute kidney failure | 70 (6.4) |
| Initiation of dialysis | 65 (5.9) |
| Strokea | 30 (2.7) |
| Paraplegiaa | 0 (0.0) |
| HFa | 24 (2.2) |
| Ischemic heart diseasea | 78 (7.1) |
| Hypertensiona | 182 (16.7) |
| Diabetesa | 13 (1.2) |
| Chronic kidney diseasea | 27 (2.5) |
AAD, acute aortic dissection; HF, heart failure.
This diagnosis was registered for the patient in the Danish National Patient Register for the first time during their hospital stay for AAD.
Factors Associated With Nursing Home Admission or Home Care
Table IV shows the characteristics of the patients who started home care or were admitted to a nursing home, patients who did not take these measures, and patients who died during the first year after discharge. Patients who were admitted to a nursing home or started home care or who died during 1-year follow-up were older than the remaining patients (median age, 71 vs 63 years). Table V reports factors associated with nursing home admission and home care initiation during the first year after discharge. In addition to increasing age, female sex was associated with increased risk. Moreover, preexisting COPD as well as in-hospital acute kidney failure were associated with an increased risk of nursing home admission or initiation of home care during 1-year follow-up. No statistically significant associations were observed for any other comorbidities or in-hospital complications.
TABLE IV.
Characteristics of Patients With Assessed Outcomes
| Variable | Did not start home care or get admitted to a nursing home (n = 945) | Started home care or was admitted to a nursing home (n = 93) | Died (n = 55) |
|---|---|---|---|
| Age, median (IQR), y | 63 (53-70) | 71 (65-78) | 71 (66-80) |
| Male sex, No. (%) | 686 (72.6) | 46 (49.5) | 40 (72.7) |
| Type A AAD, No. (%) | 624 (66.0) | 60 (64.5) | 28 (50.9) |
| Preexisting comorbidity, No. (%) | |||
| Hypertension | 384 (40.6) | 52 (55.9) | 35 (63.6) |
| Diabetes | 4 (0.4) | NA | NA |
| COPD | 26 (2.8) | 9 (9.7) | 4 (7.3) |
| Ischemic heart disease | 33 (3.5) | 5 (5.4) | 4 (7.3) |
| HF | 11 (1.2) | NA | NA |
| Stroke | 18 (1.9) | NA | 3 (5.5) |
| Chronic kidney disease | 8 (0.8) | NA | 0 (0.0) |
| In-hospital complications, No. (%) | |||
| Strokea | 24 (2.5) | 4 (4.3) | NA |
| HFa | 19 (2.0) | 3 (3.2) | NA |
| Ischemic heart diseasea | 66 (7.0) | 8 (8.6) | 4 (7.3) |
| Chronic kidney diseasea | 20 (2.1) | 5 (5.4) | NA |
| Acute kidney failure | 54 (5.7) | 15 (16.1) | NA |
| Started dialysis | 53 (5.6) | 11 (11.8) | NA |
AAD, acute aortic dissection; COPD, chronic obstructive pulmonary disease; HF, heart failure; NA, not available because n < 3.
This diagnosis was registered for the patient in the Danish National Patient Register for the first time during their hospital stay for AAD.
TABLE V.
Factors Associated With Patients Starting Home Care or Being Admitted to a Nursing Home
| Variable | Multivariable HR (95% CI)a |
|---|---|
| Age, y | 1.07 (1.04-1.09) |
| Male sex | 0.47 (0.31-0.71) |
| Preexisting comorbidity | |
| Hypertension | 1.12 (0.73-1.72) |
| Diabetes | 1.02 (0.13-7.75) |
| COPD | 2.55 (1.25-5.20) |
| Ischemic heart disease | 0.96 (0.37-2.47) |
| HF | 0.43 (0.06-3.22) |
| Stroke | 1.40 (0.06-2.91) |
| Chronic kidney disease | 1.42 (0.19-10.41) |
| In-hospital complication | |
| Strokeb | 1.10 (0.39-3.10) |
| HFb | 0.95 (0.29-3.12) |
| Ischemic heart diseaseb | 1.22 (0.58-2.57) |
| Chronic kidney diseaseb | 1.01 (0.34-2.99) |
| Acute kidney failure | 2.45 (1.24-4.87) |
| Started dialysis | 1.83 (0.78-4.29) |
AAD, acute aortic dissection; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio.
Each variable is adjusted for the remaining variables listed in the table. Thus, the multivariable HRs (95% CI) reflect multivariable-adjusted associations of each variable with being admitted to a nursing home or starting home care.
This diagnosis was registered for the patient in the Danish National Patient Register for the first time during their hospital stay for AAD.a
Among the 70 hospital survivors who had acute kidney failure registered during their hospital stay, fewer than 3 were admitted to a nursing home, while 77% neither accessed in-home or nursing home care nor died during the first year after hospital discharge. Among the 30 hospital survivors who had a new diagnosis of stroke registered during their hospital stay, fewer than 3 patients were admitted to a nursing home. Eighty percent neither accessed in-home or nursing home care nor died during the first year after hospital discharge.
Outcomes by Treatment Strategy
Patients in whom type A AAD was surgically managed had a lower incidence of being admitted to a nursing home or starting home care during 1-year follow-up than patients in whom type A AAD was medically managed (7.5% vs 14.3%; P = .02). Looking only at nursing home admission reveals a significant difference, as well (surgically managed vs medically managed type A AAD: 3.1% vs 0.7%; P = .038). In contrast, when looking only at home care, this difference was not statistically significant (surgically managed vs medically managed type A AAD: 11.2% vs 6.8%; P = .11). Among patients with type B AAD, there was no difference in 1-year incidence of nursing home admission or home care initiation between patients who had type B AAD managed by surgery or TEVAR and patients who had type B AAD medically managed (10.5% vs 8.0%; P = .47). Likewise, there was no difference in 1-year incidence of being admitted to a nursing home or starting home care between patients with type B AAD that was managed with TEVAR and patients who underwent non-TEVAR surgery (10.5% vs 10.5%; P = .94). Numbers were too small to look separately at nursing home admission and home care initiation with regard to patients with type B AAD.
Outcomes by Hospital Length of Stay
Hospital length of stay for the included patients was as follows: first quartile, fewer than 8 days; second quartile, 8 to 14 days; third quartile, 15 to 23 days; and fourth quartile, 24 days or more. Compared with patients in the first quartile, only patients in the fourth quartile had a significant increase in incidence of starting home care (HR, 2.36 [95% CI, 1.26-4.41]; P =.008) and of being admitted to a nursing home or starting home care (HR, 2.95 [95% CI, 1.60-5.42] P =.001). The corresponding HRs for the second vs the first quartile were 0.78 (95% CI, 0.39-1.55) and 0.83 (95% CI, 0.42-1.64), respectively, and for the third vs the first quartile, HRs were 1.45 (95% CI, 0.73-2.88) and 1.45 (95% CI, 0.73-2.87), respectively.
Discussion
This nationwide registry-based study in Denmark evaluated more than 1000 patients who were discharged alive and neither lived in nursing homes nor received home care before admission for AAD. Compared with age-matched and sex-matched controls, patients with AAD had a significantly increased risk of starting home care and of being admitted to a nursing home during the first year after discharge (adjusted HRs, >7 and >4). The absolute hazard, however, was relatively low. There was no statistically significant difference in 1-year cumulative incidence of nursing home admission or initiation of home care between patients with type A AAD and patients with type B AAD. Patients who were admitted to nursing homes or who started home care were significantly older, and women had increased risk compared with men. Moreover, COPD and acute kidney failure during the hospital stay were strongly associated with an increased risk of nursing home admission or initiation of home care. Among patients with type A AAD, conservative treatment was related to increased risk of nursing home admission or initiation of home care compared with surgical management. Among patients with type B AAD, there was no difference based on management strategy.
In the present study, diabetes was registered less frequently among patients with AAD than among control individuals. Only patients who had AAD and survived to hospital discharge, however, were included in this study. Rates of registered preexisting diabetes were significantly higher in patients with aortic dissection who died in the hospital than in patients who survived to discharge.22 Thus, the difference in diabetes occurrence between patients with AAD and control individuals in the present study does not indicate a protective effect of diabetes in the setting of AAD.
Previous research has focused mostly on clinical profiles, diagnostics, surgical procedures, in-hospital mortality, and long-term survival in patients with AAD.1,2,8,9 Patients with AAD may be affected by many risks beyond survival.
Only a few studies have investigated long-term outcomes among patients with AAD beyond survival.23 Postoperative health-related quality of life in patients following type A AAD has been reported to be lower than in the general population.24,25 Likewise, self-reported new-onset depression or anxiety has been reported to be frequent among survivors of type A and type B AAD. A recently published single-center study reported hospitalization-associated disability in 22% of patients who survived to discharge following type A AAD.26 Fichadiya et al27 found that stroke associated with type A AAD was severe at presentation and resulted in substantial residual disability 1 month after surgery. Moreover, in the Oxford Vascular Study, disability was assessed in 19 patients discharged alive after AAD, and a mean 30-day modified Rankin scale score of 2.6 was reported.5 This score corresponds to slight to moderate disability, and 72% of patients were classified as independent 6 months after the event.5 Finally, a single-center study found that 82% of patients with type A aortic dissection managed surgically were discharged directly from hospital to home; in addition, advanced age and prolonged extracorporeal circulation were independent predictive factors of difficulty in direct discharge to home.28
The present study is the first to evaluate nursing home admission and new initiation of home care following AAD in a large nationwide cohort. The risk of accessing these types of help was, as mentioned, more than 7 times greater than in the background population. The absolute hazard, however, was not as high as one might expect: Only 0.8% of patients were admitted to a nursing home, and 7.8% started home care.
Acute aortic dissection may result in several complications that have prognostic and management implications. These complications include critical malperfusion of end organs because of either shock or aortic branch vessel involvement; in the long term, survival following aortic dissection appears worse in patients with kidney failure or stroke.23 Recent studies from international registries describe stroke in approximately 9% to 16% of patients with type A AAD2,11 and less frequently in patients with type B AAD.3 Acute kidney failure is also commonly described in patients with AAD.2,29,30 In the present study, rates of complications registered at discharge—such as stroke, ischemic heart disease, heart failure, and kidney failure—tended to be lower than previously published.2,3,7,29,30 This difference is explained by the present study's exclusion of patients receiving home care or living in a nursing home before the AAD and patients who died in hospital. In-hospital mortality is directly related to age and to these types of complications,2,31 but the present study detected that in-hospital complications such as acute kidney failure were related to the risk of requiring nursing home admission or home care following hospital discharge, as well.
The findings presented here are quite reassuring. Most patients who recover from AAD and who are discharged without major complications appear to return to a life without accessing special care. To improve survival and reduce the burden of complications, the focus of future research should therefore still be early detection of AAD and rapid surgical treatment.1,2 Furthermore, previous studies found rigorous blood pressure control and close follow-up important in reducing the risk of reintervention.23,32
Study Limitations
Despite having access to an unselected national cohort of patients with AAD with several years of follow-up from high-quality registries, the registry-based design results in some limitations. First, many patients solely had an unspecified aortic dissection diagnosis registered. A recent validity study found a high (94.8%) positive predictive value for clinically documented aortic dissection when combining ICD-10 codes for aortic dissection and surgical procedure codes for aortic surgery9; however, the positive predictive value for the unspecified AAD code is not as high, at 63.5%.33 Thus, researchers were able to reclassify many cases registered with an unspecified diagnosis by combining diagnoses with surgical procedure codes and by assessing CT scans. Although the initially high number of patients with unspecified aortic dissection registered was more than halved, more than 400 cases remained unresolved, and these patients were excluded. The potential for some degree of selection bias, however, cannot be ruled out.
Second, diagnoses in the Danish National Patient Register are registered by clinicians, and research is not the sole purpose of this process. Thus, there is a risk of incomplete registration of secondary diagnoses, such as hypertension and acute kidney failure without the need for dialysis, recognized during the hospital stay for AAD. As a result, for example, acute kidney failure and hypertension diagnosed during the hospital stay may be underestimated in the present study. Moreover, because new prescriptions have not yet been dispensed at patient discharge, medication cannot be used as a proxy for newly diagnosed hypertension, as is described for preexisting hypertension.
Third, the registry-based design of the present study implies reduced potential to assess individual patient clinical data—for example, surgical details, reasons for not performing surgery, and duration of dialysis in cases of acute kidney failure. This study lacked information regarding patient disability, as evaluated by, for example, the Barthel Index,34 as well as information about whether the patients had received treatment in a rehabilitation center before hospital discharge. Unfortunately, the registry-based study design precludes access to data of this type. Moreover, the study design does not allow access to information regarding the level of home care recipients received. Because home care and nursing home care are tax financed in Denmark, lack of affordability and other financial issues are unlikely to have influenced the use of care estimated herein.
Conclusion
Only a small proportion of patients who survived AAD initiated home care or were admitted to a nursing home in the first year after hospital discharge. The need was related to age, female sex, preexisting comorbidities, inhospital complications, and conservative management of type A AAD.
Supplementary Material
Abbreviations and Acronyms
- AAD
acute aortic dissection
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- HF
heart failure
- HR
hazard ratio
- ICD-10
International Statistical Classification of Diseases, Tenth Revision
- NCSP
Nordic Medico-Statistical Committee Classification of Surgical Procedures
- TEVAR
thoracic endovascular aortic repair
Article Information
Open Access: © 2024 The Authors. Published by The Texas Heart Institute®. This is an Open Access article under the terms of the Creative Commons Attribution-NonCommercial License (CC BY-NC, https://creativecommons.org/licenses/by-nc/4.0/), which permits use and distribution in any medium, provided the original work is properly cited, and the use is noncommercial.
Author Contributions: M.W.P. was responsible for conceptualization, methodology, investigation, validation, formal analysis, project administration, and writing the original draft as well as review and editing. R.O. was involved in resources, investigation, validation, and writing–review and editing. J.E.M. contributed to resources and writing–review and editing. A.G. contributed to validation and writing–review and editing. E.F. was involved in resources and writing–review and editing. D.G.N. contributed to resources and to writing–review and editing. L.K. was involved in resources and writing–review and editing. M.P.A. worked on data curation, resources, and writing–review and editing. C.T. was responsible for data curation, methodology, resources, and writing–review and editing. P.S. worked on conceptualization, methodology, funding acquisition, resources, supervision, and writing–review and editing. N.H.A. contributed to conceptualization, methodology, supervision, and writing–review and editing. K.K. contributed to conceptualization, methodology, resources, investigation, formal analysis, supervision, project administration, writing–review and editing, and visualization.
Conflict of Interest Disclosure: J.E.M. has received research grants from Abiomed and speaker's fees from Abiomed, Boehringer Ingelheim, Orion, Abbott, and Novartis (all unrelated to the current article). J.E.M. has participated on Data Safety Monitoring Boards for DanHeart and Glorius. L.K. has received speaker's honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Novartis. C.T.P. has received study grants from Bayer and Novo Nordisk. M.W.P., R.O., A.G., E.F., D.G.N., M.P.A., P.S., N.H.A., and K.K. disclosed no potential conflicts of interest.
Funding/Support: This work was supported by internal funding (a grant for cardiovascular research).
References
- 1.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. doi: [DOI] [PubMed] [Google Scholar]
- 2.Harris KM, Nienaber CA, Peterson MD, et al. Early mortality in type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. JAMA Cardiol. 2022;7(10):1009–1015. doi: 10.1001/jamacardio.2022.2718. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. doi: [DOI] [PubMed] [Google Scholar]
- 4.Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2015;66(4):350–358. doi: 10.1016/j.jacc.2015.05.029. doi: [DOI] [PubMed] [Google Scholar]
- 5.Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013;127(20):2031–2037. doi: 10.1161/CIRCULATIONAHA.112.000483. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai TT, Fattori R, Trimarchi S, et al. Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114(21):2226–2231. doi: 10.1161/CIRCULATIONAHA.106.622340. doi: [DOI] [PubMed] [Google Scholar]
- 7.Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2006;114(1 Suppl):I350–I356. doi: 10.1161/CIRCULATIONAHA.105.000497. doi: [DOI] [PubMed] [Google Scholar]
- 8.Gudbjartsson T, Ahlsson A, Geirsson A, et al. Acute type A aortic dissection—a review. Scand Cardiovasc J. 2020;54(1):1–13. doi: 10.1080/14017431.2019.1660401. doi: [DOI] [PubMed] [Google Scholar]
- 9.Gundlund A, Køber L, Høfsten DE, et al. Rehospitalizations, repeated aortic surgery, and death in initial survivors of surgery for Stanford type A aortic dissection and the significance of age—a nationwide registry-based cohort study. Eur Heart J Qual Care Clin Outcomes. 2023;9(5):520–528. doi: 10.1093/ehjqcco/qcac061. doi: [DOI] [PubMed] [Google Scholar]
- 10.Bossone E, Corteville DC, Harris KM, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013;128(11 suppl 1):S175–S179. doi: 10.1161/CIRCULATIONAHA.112.000327. doi: [DOI] [PubMed] [Google Scholar]
- 11.Chemtob RA, Fuglsang S, Geirsson A, et al. Stroke in acute type A aortic dissection: the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD) Eur J Cardiothorac Surg. 2020;58(5):1027–1034. doi: 10.1093/ejcts/ezaa197. doi: [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Zhong G, Lv X, et al. Risk factors for acute kidney injury after Stanford type A aortic dissection repair surgery: a systematic review and meta-analysis. Ren Fail. 2022;44(1):1462–1476. doi: 10.1080/0886022X.2022.2113795. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Eusanio M, Trimarchi S, Patel HJ, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145(2):385–390.e1. doi: 10.1016/j.jtcvs.2012.01.042. doi: [DOI] [PubMed] [Google Scholar]
- 14.Jonker FH, Patel HJ, Upchurch GR, et al. Acute type B aortic dissection complicated by visceral ischemia. J Thorac Cardiovasc Surg. 2015;149(4):1081–1086.e1. doi: 10.1016/j.jtcvs.2014.11.012. doi: [DOI] [PubMed] [Google Scholar]
- 15.Henke PK, Williams DM, Upchurch GR, Jr, et al. Acute limb ischemia associated with type B aortic dissection: clinical relevance and therapy. Surgery. 2006;140(4):532–539. doi: 10.1016/j.surg.2006.06.019. doi: [DOI] [PubMed] [Google Scholar]
- 16.Zindovic I, Gudbjartsson T, Ahlsson A, et al. Malperfusion in acute type A aortic dissection: an update from the Nordic Consortium for Acute Type A Aortic Dissection. J Thorac Cardiovasc Surg. 2019;157(4):1324–1333. doi: 10.1016/j.jtcvs.2018.10.134. doi: [DOI] [PubMed] [Google Scholar]
- 17.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. doi: [DOI] [PubMed] [Google Scholar]
- 18.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 suppl):30–33. doi: 10.1177/1403494811401482. doi: [DOI] [PubMed] [Google Scholar]
- 19.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 suppl):38–41. doi: 10.1177/1403494810394717. doi: [DOI] [PubMed] [Google Scholar]
- 20.Kragholm K, Wissenberg M, Mortensen RN, et al. Bystander efforts and 1-year outcomes in out-of-hospital cardiac arrest. N Engl J Med. 2017;376(18):1737–1747. doi: 10.1056/NEJMoa1601891. doi: [DOI] [PubMed] [Google Scholar]
- 21.Thorsteinsson K, Andreasen JJ, Mortensen RN, et al. Longevity and admission to nursing home according to age after isolated coronary artery bypass surgery: a nationwide cohort study. Interact Cardiovasc Thorac Surg. 2016;22(6):792–798. doi: 10.1093/icvts/ivw045. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen MW, Kragholm K, Oksjoki R, et al. Characteristics and Outcomes in Patients With Acute Aortic Dissection: a nationwide registry study. Ann Thorac Surg. 2023;116(6):1177–1184. doi: 10.1016/j.athoracsur.2023.06.019. doi: [DOI] [PubMed] [Google Scholar]
- 23.Melby SJ, Zierer A, Damiano RJ, Jr, Moon MR. Importance of blood pressure control after repair of acute type A aortic dissection: 25-year follow-up in 252 patients. J Clin Hypertens (Greenwich) 2013;15(1):63–68. doi: 10.1111/jch.12024. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghazy T, Eraqi M, Mahlmann A, et al. Quality of life after surgery for Stanford type A aortic dissection: influences of different operative strategies. Heart Surg Forum. 2017;20(3):E102–E106. doi: 10.1532/hsf.1738. doi: [DOI] [PubMed] [Google Scholar]
- 25.Endlich M, Hamiko M, Gestrich C, et al. Long-term outcome and quality of life in aortic type A dissection survivors. Thorac Cardiovasc Surg. 2016;64(2):91–99. doi: 10.1055/s-0035-1548734. doi: [DOI] [PubMed] [Google Scholar]
- 26.Hirakawa K, Nakayama A, Saitoh M, et al. Factors related to hospitalisation-associated disability in patients after surgery for acute type A aortic dissection: a retrospective study. Int J Environ Res Public Health. 2022;19(19):12918. doi: 10.3390/ijerph191912918. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fichadiya A, Menon BK, Gregory AJ, Teleg E, Appoo JJ. Neuroanatomy and severity of stroke in patients with type A aortic dissection. J Card Surg. 2022;37(2):339–347. doi: 10.1111/jocs.16136. doi: [DOI] [PubMed] [Google Scholar]
- 28.Igarashi T, Sato Y, Satokawa H, et al. The results of an entry-oriented strategy for acute type A aortic dissection in octogenarians: an 18-year experience. Eur J Cardiothorac Surg. 2020;58(5):949–956. doi: 10.1093/ejcts/ezaa195. doi: [DOI] [PubMed] [Google Scholar]
- 29.Arnaoutakis GJ, Ogami T, Patel HJ, et al. Acute kidney injury in patients undergoing surgery for type A acute aortic dissection. Ann Thorac Surg. 2023;115(4):879–885. doi: 10.1016/j.athoracsur.2022.10.037. doi: [DOI] [PubMed] [Google Scholar]
- 30.Musajee M, Katsogridakis E, Kiberu Y, et al. Acute kidney injury in patients with acute type B aortic dissection. Eur J Vasc Endovasc Surg. 2023;65(2):256–262. doi: 10.1016/j.ejvs.2022.10.032. doi: [DOI] [PubMed] [Google Scholar]
- 31.Tolenaar JL, Froehlich W, Jonker FH, et al. Predicting inhospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;130(11 suppl 1):S45–550. doi: 10.1161/CIRCULATIONAHA.113.007117. doi: [DOI] [PubMed] [Google Scholar]
- 32.Zierer A, Voeller RK, Hill KE, Kouchoukos NT, Damiano RJ, Jr, Moon MR. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann Thorac Surg. 2007;84(2):479–486. doi: 10.1016/j.athoracsur.2007.03.084. doi: [DOI] [PubMed] [Google Scholar]
- 33.Obel LM, Lindholt JS, Lasota AN, et al. Aortic dissections in the population-based Danish National Patient Registry from 1996-2016: a validation study. Clin Epidemiol. 2022;14:51–58. doi: 10.2147/CLEP.S341806. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


