Abstract
Background
The TRACE (Targeted Research for Addictive and Compulsive Eating) intervention was evaluated in a 3-month randomized controlled trial which demonstrated significant improvement in Yale Food Addiction Scale scores favoring dietitian-led telehealth (active intervention) compared with passive and control groups. This study aimed to determine intervention costs and cost-utility.
Methods
Costs of each intervention (2021$AUD) and incremental net monetary benefit (iNMB; incremental benefit, defined as Quality-Adjusted Life Years (QALY) gained, multiplied by willingness to pay threshold minus incremental cost) were calculated to estimate differences between groups.
Results
The active intervention (n = 38) cost $294 (95% UI: $266, $316) per person compared to $47 (95% UI: $40, $54) in the passive intervention (n = 24), and $26 in the control group (n = 37). At a cost-effectiveness threshold of $50 000 per QALY score gained, the active intervention iNMB was -$186 (95% UI: -$1137, $834) and the passive group $127 (95% UI: -$1137, $834). Compared to the control group, estimates indicate a 30% chance of the active intervention, and a 60% chance of the passive intervention being cost effective.
Conclusion
Although the overall cost of the active intervention was low, this was not considered cost-effective in comparison to the passive intervention, given small QALY score gains.
Trial registration
Australia New Zealand Clinical Trial Registry ACTRN12621001079831.
Keywords: addictive eating, economic evaluation, food addiction, mental health, randomized controlled trial
Introduction
Addictive eating behavior, termed ‘food addiction’, refers to a pattern of compulsive and dysregulated overeating.1 This is characterized by diminished control over food consumption, strong cravings for specific foods and continued overeating despite negative consequences.2,3 Observational and cross-sectional studies show addictive eating is highly correlated with risk of obesity and mental ill-health, particularly depression, anxiety, and binge eating disorder.4,5 At present, treatment interventions for individuals meeting criteria for addictive eating are limited.6 This prompted the TRACE (Targeted Research on Addictive and Compulsive Eating) program, a dietitian-led telehealth intervention focused on reducing symptoms of addictive eating and improving mental health.7,8 This was the first trial to evaluate efficacy of a personalized behavioral intervention in Australian adults.9 The primary results from the TRACE trial demonstrated significant improvements in the primary outcome for symptoms of addictive eating as well as decreases in scores for anxiety, depression and stress.10
While dietary counselling is efficacious11 and cost-effective12 for management of obesity, few dietary interventions have conducted economic evaluations in relation to nutrition and mental health, as confirmed in a recent systematic review that included only eight studies.13 Those included studies largely focused on severe mental illness or depression and were heterogeneous in terms of type of cost analyses conducted. Therefore, limited comparisons could be made across studies. Cost-utility (n = 6; cost per healthy year equivalents, expressed as quality-adjusted life-years i.e. QALYs) and cost-effectiveness (n = 4; cost per unit change in weight, fasting glucose, depression status or healthcare utilization costs) were the most commonly reported economic evaluation methods. In comparison to the number of published dietary intervention studies in mental health, there are relatively few studies reporting economic evaluations. Therefore, it is important to investigate the costings of newly developed interventions in mental health for government investment.
The current study aimed to evaluate the cost consequences of the TRACE randomized controlled trial (RCT) to determine intervention costs alongside change in primary outcome measures (Quality-Adjusted Life Years [QALY] scores, addictive eating symptom scores and number of healthcare appointments). The analysis was conducted from a healthcare sector perspective considering the costs of implementing the intervention because if translated into routine practice, these costs would fall on public health services.
Methods
Study design and setting
The current economic evaluation utilized data from the TRACE RCT. Details of the trial protocol9 and main results10 have been published elsewhere. The trial was prospectively registered with the Australian New Zealand Clinical Trials Register ACTRN12621001079831, and approval was obtained from the University of Newcastle Human Ethics Committee (H2021–0100). This study adheres to the economic evaluation according to the Consolidated Health Economic Evaluation Reporting Standards statement.14 All participants provided written informed consent prior to randomization. Data collection occurred from August 2021 to October 2022.
Participants
Eligible participants included adults aged 18–85 years, endorsing ≥3 symptoms of addictive eating, with a body mass index (BMI) ≥ 18.5 kg/m2. Exclusion criteria included pregnancy/lactating, existing health condition/s that necessitated taking medications which affect dietary intake or weight status, and severe mental illness, purging behaviors as identified by the Eating Disorder Examination Questionnaire—Short form.15
Intervention
Eligible participants were randomly allocated to one of three study arms:
1) Active intervention: participants attended five telehealth sessions with an Accredited Practising Dietitian and were provided with the program workbook and website access. Participants received personalized feedback on addictive eating symptoms, personality, diet, physical activity and sleep. During telehealth sessions, individuals set goals around their eating behaviors.
2) Passive intervention: a self-guided approach with the program workbook and website access, personalized feedback via e-mail at baseline only.
3) Control: participants were provided with dietary feedback, via paper-based report, at baseline and followed their usual dietary pattern.
Economic study
A trial-based economic evaluation of the interventions was conducted over a 3-month time horizon, consistent with the length of the primary endpoint of the trial.
Effect measures
The primary outcome measure was utilities based on a health-related quality of life instrument. The mean change in QALY score was estimated using the generic preference-based EuroQoL EQ-5D-5L.16 The EQ-5D-5L was chosen given its consistently demonstrated psychometric properties across a broad range of population groups, conditions and settings, as well as the low respondent burden.17,18 Other outcomes included addictive eating symptom scores assessed using the Yale Food Addiction Scale 2.0 (YFAS 2.0),3 and the number of health care appointments assessed using the Consumer Services Receipt Inventory (CSRI).19 Participants completed these measures at baseline (pre-intervention) and at 3-months (immediate post-intervention). Quality of life: The EQ-5D-5L determines participant’s health-related quality-of-life based on five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has five response levels: no problems (Level 1); slight; moderate; severe; and extreme problems (Level 5). The health states are defined by combining one level from each dimension, ranging from 11 111 (full health) to 55 555 (worst health). EQ-5D-5L health states are then converted into a single index ‘utility’ score using a scoring algorithm.20 Utility values were generated using a published mapping formula21 via the EQ-5D-5L Index Value Calculator Version 2.22 QALYs were derived by ‘weighing’ the length life spent in a particular health state by the utility or value of that health state. Utility values (or weights), derived from an international sample representing a broad spectrum of health states, are constrained between 0 and 1, where 0 refers to death and one refers to perfect health with values in between denoting less than perfect health states. Addictive eating: The YFAS 2.03 is a validated, psychometrically sound, self-report 35-item tool that provides an addictive eating symptom score ranging from 0 to 11. Healthcare service utilization: The CSRI,19 an adaptable tool to ensure it is context specific, captured the number of appointments and type of healthcare services used by participants over the past 3 months. This included a multiple-choice question that allowed participants to choose from a list of 12 healthcare services they had accessed (i.e. General Practitioner, Medical Specialist, Psychiatrist, Psychologist, Psychotherapist, Counsellor, Dietitian, Exercise Physiologist, Occupational Therapist, Social Worker, hospital admission or ‘other’), with an open-ended response if ‘other’ was chosen. Following the responses individuals were prompted with a further open response question that allowed them to indicate how many visits they had with each health service selected within the timeframe.
Resource use and valuation
The cost-consequence analysis included calculating the cost of each intervention and reporting intervention costs alongside mean change outcomes. Cost data relating to the delivery of the intervention was prospectively collected using a time-driven activity-based cost-capture resource use tool developed in Microsoft Excel (Version 2303). The tool enabled documentation of the health sector costs in the following categories: (i) labor (including overheads to allow for additional costs of employment), (ii) materials (non-labor cost items such as education materials, electronic hardware, or software), and (iii) miscellaneous costs (which include costs not easily classified into the other categories e.g. telehealth delivery platform cost). Resource use valuation was based on the concept of opportunity cost, that is, the value of the benefit forgone in not employing a resource for a different use. Where available, market prices were used as a proxy for the ‘value of benefit’ forgone.23 Development and research related costs were excluded. Costs to patients and private care providers (including opportunity costs), were not assessed. Details of the approach to valuation (Table S1) and a summary of resources (Table S2) are shown in supplementary material.
Costing and cost-utility analyses
The trial-based cost analysis used measures of arithmetic means, between-group differences, and variability of differences.24,25 Component costs are reported to provide insight into the cost of active and passive forms of the intervention and specific drivers of total cost. Costs are reported in 2021 Australian dollars (AUD). The economic evaluation was performed as a within-trial analysis, meaning that only costs and effects that occur within the trial duration were included. To maintain a conservative approach to cost estimation, the implementation costs were not amortized. As the trial period was less than 12 months no discounting of costs was applied.
To evaluate cost-utility, the total and mean cost of each intervention (i.e. active, passive and control) and economic summary metrics were calculated, including the mean incremental cost per QALY as measured by patient reported EQ-5D-5L, mean incremental cost per unit change in YFAS, and mean change in the number of health care appointments in the past 3-months.
The change in QALYs was calculated by the average of baseline and 3-month EQ-5D-5L scores multiplied by 0.25 as the primary end point was 3 months (i.e. one quarter of a year). The incremental cost-effectiveness ratios (ICERs) were generated by calculating the difference in cost divided by the difference in benefit, defined by QALY scores. The ICERs were interpreted as the cost per QALY gained. Cost effectiveness was assessed against a willingness to pay threshold of $50 000. Commonly, $50 000/QALY is the decision rule used to denote value-for-money.26 To mitigate issues with interpreting negative ICERs we then calculated incremental net monetary benefit (iNMB).25,27 iNMB is expressed as monetary value and was calculated as the incremental benefit (defined in the current analysis as QALYs gained) multiplied by the willingness to pay threshold minus the incremental cost. iNMB can be easier to interpret than ICERs because results of a positive iNMB means value is generated by the intervention. A negative value means the intervention costs more money without any significant change in QALY score. Thus, the economic summary measure is iNMB at a willingness to pay threshold of $50 000. A cost effectiveness plane was created to graphically present the incremental costs and outcomes across four quadrants.
Statistical analyses
A complete case analysis was conducted utilizing only cases in the trial data set for which there were no missing pre- or post-intervention values for the YFAS, CSRI and EQ-5D-5L variables. Descriptive statistics were generated, using IBM SPSS Statistics (Version 26), to describe the sample characteristics and healthcare service utilization of complete cases included in the current economic evaluation. Between group differences at baseline for demographics, addictive eating and health profile data were assessed using chi squared (i.e. sex and EQ-5D-5L dimensions: mobility, usual activity, pain/discomfort, anxiety/depression), Fisher’s exact test (i.e. EQ-5D-5L dimension: self-care) or analysis of variance (ANOVA; i.e. age, BMI, decile, YFAS scores, QALY scores). Statistical significance was set at 0.05. Costing and cost-utility analyses were performed as outlined above using Microsoft Excel software. Uncertainty analyses: non-parametric bootstrapping with 1000 replications was used to generate uncertainty intervals around the costs, QALY scores, YFAS scores and number of healthcare appointments, and were calculated by treatment group to enable reporting of the variation in costs and effect sizes between the three groups.
Results
A total of 175 participants were recruited to the trial (active intervention n = 58, passive intervention n = 60, and control group n = 57). This economic evaluation is based on 99 participants with complete baseline and 3-month outcome data (n = 99). Baseline characteristics of the participants are summarized in Table 1.
Table 1.
Baseline characteristics of complete cases from the TRACE RCT (n = 99)
| Total Sample n = 99 |
Active Intervention n = 38 |
Passive Intervention n = 24 |
Control n = 37 |
Test statistic (F or χ2) |
p-value | |
|---|---|---|---|---|---|---|
| Mean ± SD (range) or n (%) | ||||||
| Demographics | ||||||
| Age (years) | 50.7 ± 13.4 (23–75) |
47.4 ± 14.3 (23–74) |
52.0 ± 13.8 (35–75) |
53.1 ± 11.7 (29–73) |
1.867 | 0.160 |
| Sex | 0.540 | 0.785 | ||||
| Male | 16 (16.2) | 5 (13.2) | 4 (16.7) | 7 (18.9) | ||
| Female | 83 (83.8) | 33 (86.8) | 20 (83.3) | 30 (81.1) | ||
| BMI (kg/m2) | 35.6 ± 7.1 (21.0–66.8) |
36.0 ± 6.3 (25.1–48.1) |
35.4 ± 7.4 (25.7–50.2) |
35.3 ± 8.0 (32.6–37.9) |
0.116 | 0.891 |
| Decile (postcode)a | 5.8 ± 2.5 (1–10) |
5.9 ± 2.6 (1–10) |
6.0 ± 2.5 (1–10) |
5.6 ± 2.6 (1–9) |
0.193 | 0.825 |
| Addictive eating | ||||||
| YFAS symptom scoreb | 7.5 ± 2.8 (3–11) |
8.0 ± 2.6 (3–11) |
6.7 ± 3.2 (3–11) |
7.6 ± 2.8 (3–11) |
1.514 | 0.225 |
|
Health profile Mobilityc |
1.886 | 0.389 | ||||
| No problems | 62 (62.6) | 27 (71.1) | 14 (58.3) | 21 (56.8) | ||
| Problems | 37 (37.4) | 11 (28.9) | 10 (41.7) | 16 (43.2) | ||
| Self-carec | 2.828 | 0.216 | ||||
| No problems | 85 (85.9) | 34 (89.5) | 18 (75.0) | 33 (89.2) | ||
| Problems | 14 (14.1) | 4 (10.5) | 6 (25.0) | 4 (10.8) | ||
| Usual activityc | 0.772 | 0.680 | ||||
| No problems | 26 (26.3) | 21 (55.3) | 11 (45.8) | 21 (56.8) | ||
| Problems | 46 (46.5) | 17 (44.7) | 13 (54.2) | 43.2 (37) | ||
| Pain/Discomfortc | 0.031 | 0.985 | ||||
| No problems | 26 (26.3) | 10 (26.3) | 6 (25.0) | 10 (27.0) | ||
| Problems | 73 (73.7) | 28 (73.3) | 18 (75.0) | 27 (73.0) | ||
| Anxiety/Depressionc | 1.463 | 0.494 | ||||
| No problems | 19 (19.2) | 8 (21.1) | 6 (25.0) | 5 (13.5) | ||
| Problems | 80 (80.8) | 30 (78.9) | 18 (75.0) | 32 (86.5) | ||
| QALY scored | 0.713 ± 0.175 (0.003–1.00) |
0.723 ± 0.187 (0.003–1.00) |
0.705 ± 0.158 (0.333–1.00) |
0.709 ± 0.176 (0.224–1.00) |
0.099 | 0.906 |
Chi squared, Fisher’s Exact test or ANOVA; *P < 0.05. aIndex of Relative Socio-Economic Disadvantage: this index ranks areas within Australia on a continuum from 1 to 10 (most disadvantaged to least disadvantaged). bYale Food Addiction Scale 2.0; symptom score out of 11. cFrequencies of reported health-related quality-of-life problems: for each EQ-5D-5L dimension, the five levels are dichotomized as ‘no problems’ = level 1 and ‘problems’ = levels 2 to 5. dQuality Adjusted Life Years, QALY scores generated from EQ-5D-5L scores.
Costs
The total cost of the three interventions as delivered was $13 303 (Table 2). The majority of the cost was for the active intervention group, with a total of $11 176. The passive and control interventions cost $1142 and $983 respectively. Labor contributed most to total costs ($10 323), followed by materials (e.g. workbooks and questionaries) at $2010, and then miscellaneous costs (e.g. hosting costs such as telehealth delivery platform and website) totaling $970.
Table 2.
Intervention costs and cost-consequences by intervention group from baseline to 3-months
| Total | Active | Passive | Control | ||
|---|---|---|---|---|---|
| Cases, N | 99 | 38 | 24 | 37 | |
| Total intervention costs | $13 303 | $11 176 | $1142 | $983 | |
| Costs by resource use category | |||||
| Labor | $10 323 | $9383 | $470 | $469 | |
| Materials | $2010 | $957 | $538 | $514 | |
| Miscellaneous | $970 | $836 | $133 | $0 | |
| Costs per person | $294 | $47 | $26 | ||
| Consequences | Mean ± SD (95% UI) | ||||
| Mean change in QALYs | 0.177 ± 0.04 (0.17, 0.19) |
0.179 ± 0.03 (0.17, 0.19) |
0.176 ± 0.04 (0.16, 0.19) |
||
| Mean change in YFAS score | −4.87 ± 3.61 (−6.03, −3.74) |
−3.21 ± 3.51 (−4.62, −1.83) |
−1.35 ± 3.10 (−2.38, −0.33) |
||
| Mean change in healthcare appt | −0.76 ± 2.92 (−1.68, 0.27) |
−1.04 ± 2.91 (−2.29, 0.0) |
0.66 ± 4.36 (−0.63, 2.21) |
||
QALYs, Quality-adjusted life years; UI, Uncertainty Interval; YFAS, Yale Food Addiction Scale; appt, appointment.
Cost-consequence
The active intervention cost an average of $294 (95% UI: $266, $316) per person and resulted in an average increase of 0.177 (95% UI: 0.17, 0.19) in QALY score at 3-months and an average decrease of 4.87 (95% UI: (−6.03, −3.74) in addictive eating symptoms (~$60 per reduction of one symptom) and an average decrease of 0.76 visits to healthcare services (Table 2). The passive intervention cost an average of $47 (95% UI: $40, $54) per person and resulted in an average increase of 0.179 (95% UI: 0.17, 0.19) in QALY score, and an average decrease of 3.21 in addictive eating symptoms (~$15 per reduction of one symptom) and an average decrease of 1.04 visits to healthcare services. The control cost an average of $26 per person and resulted in an average increase of 0.176 (95% UI: 0.16, 0.19) in QALY score, and an average decrease of 1.35 in addictive eating symptoms and an average increase of 0.66 visits to healthcare services. For a detailed description of changes in addictive eating behaviors in the TRACE RCT see previously published outcomes by Skinner et al.10
At baseline, the most frequently consulted health professionals over the preceding 3-months were General Practitioners (66% in the active intervention, 71% in the passive intervention and 70% in the control group) and mental healthcare professionals i.e. psychiatrists, psychologists, psychotherapists and counsellors (47% in the active intervention, 25% in the passive intervention and 27% in the control group). At 3-month follow-up (Table 3), the number of participants reporting access to General Practitioners was lower in all groups, while the number of participants reporting access to mental healthcare professionals was lower in the active intervention group only, with no change in the passive intervention or control groups. Only a small number of participants (n = 7) reported hospital admissions and this was similar for all groups at both timepoints.
Table 3.
Healthcare services accessed and the number of visits made by participants (n = 99) from baseline to 3-months
| Active Intervention | Passive Intervention | Control | ||||
|---|---|---|---|---|---|---|
| Base | 3-month | Base | 3-month | Base | 3-month | |
| n (%) or Mean ± SD (range) | ||||||
| Number of participants accessing healthcare services | ||||||
| No | 9 (23.7) | 13 (34.2) | 5 (20.8) | 7 (29.2) | 11 (29.7) | 9 (24.3) |
| Yes | 29 (76.3) | 25 (65.8) | 19 (79.2) | 17 (70.8) | 26 (70.3) | 28 (75.7) |
| Type of healthcare services accessed | ||||||
| General Practitioner | 25 (65.8) | 24 (63.2) | 17 (70.8) | 16 (66.7) | 26 (70.3) | 25 (67.6) |
| Mental healthcare professionala | 18 (47.4) | 12 (31.6) | 6 (25.0) | 6 (25.0) | 10 (27.0) | 10 (27.0) |
| Medical Specialistb | 7 (18.4) | 5 (13.2) | 6 (25.0) | 1 (4.2) | 6 (25.0) | 3 (8.1) |
| Dietitian | 2 (5.3) | 2 (5.3) | 0 (0.0) | 3 (12.5) | 4 (10.8) | 6 (16.2) |
| Exercise physiologist | 5 (13.2) | 0 (0.0) | 3 (12.5) | 2 (12.5) | 4 (10.8) | 3 (8.1) |
| Otherc | 3 (7.9) | 6 (15.8) | 2 (8.3) | 4 (16.7) | 3 (8.1) | 5 (13.5) |
| Number of visits by participants accessing healthcare services | ||||||
| General Practitioner | 2.4 ± 1.2 (1–5) |
2.5 ± 2.1 (1–10) |
1.7 ± 0.9 (1–4) |
2.0 ± 1.3 (1–5) |
2.4 ± 1.2 (1–6) |
2.0 ± 0.8 (1–3) |
| Mental healthcare professionala | 3.1 ± 2.1 (1–9) |
2.5 ± 1.5 (1–6) |
3.3 ± 3.4 (1–10) |
2.0 ± 0.6 (1–3) |
2.4 ± 1.1 (1–4) |
2.6 ± 1.0 (2–5) |
| Medical Specialistb | 1.1 ± 0.4 (1–2) |
1.4 ± 0.5 (1–2) |
1.3 ± 0.8 (1–3) |
1.0 (1) |
1.8 ± 1.0 (1–3) |
1.0 (1) |
| Dietitian | 1.5 ± 0.7 (1–2) |
3.5 ± 2.1 (2–5) |
– | 1.7 ± 0.6 (1–2) |
1.5 ± 1.3 (1–3) |
1.3 ± 0.5 (1–2) |
| Exercise physiologist | 3.0 ± 1.6 (1–5) |
– | 3.3 ± 0.6 (3–4) |
1.5 ± 0.7 (1–2) |
1.5 ± 0.6 (1–2) |
2.0 ± 1.0 (1–3) |
| Otherc | 1.7 ± 1.2 (1–3) |
1.7 ± 0.8 (1–3) |
9.0 ± 7.1 (4–14) |
1.8 ± 1.5 (1–4) |
2.0 ± 1.7 (1–4) |
6.6 ± 9.8 (1–24) |
| Hospital admissions | ||||||
| No | 37 (97.4) | 37 (97.4) | 23 (95.8) | 24 (100.0) | 35 (94.6) | 35 (94.6) |
| Yes | 1 (2.6) | 1 (2.6) | 1 (4.2) | 0 (0.0) | 2 (5.4) | 2 (5.4) |
| Number of admissions | 1.0 (1) |
1.0 (1) |
1.0 (1) |
- | 1.0 (1) |
1.5 ± 0.7 (1–2) |
Mental healthcare professionals included psychiatrist psychologist psychotherapist and counsellors. bMedical Specialists included cardiologist, endocrinologist, gastroenterologist, immunologist, rheumatologist, urologist, sports physician, colorectal surgeon and orthopedic surgeon (n = 12 not specified). c‘Other’ included physiotherapist, chiropractor, osteopath, radiologist, sleep/CPAP specialist (n = 9 not specified).
Cost-utility
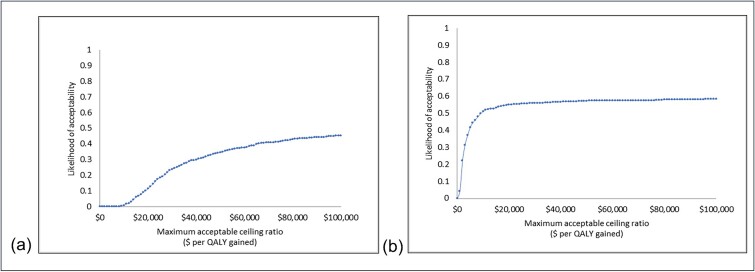
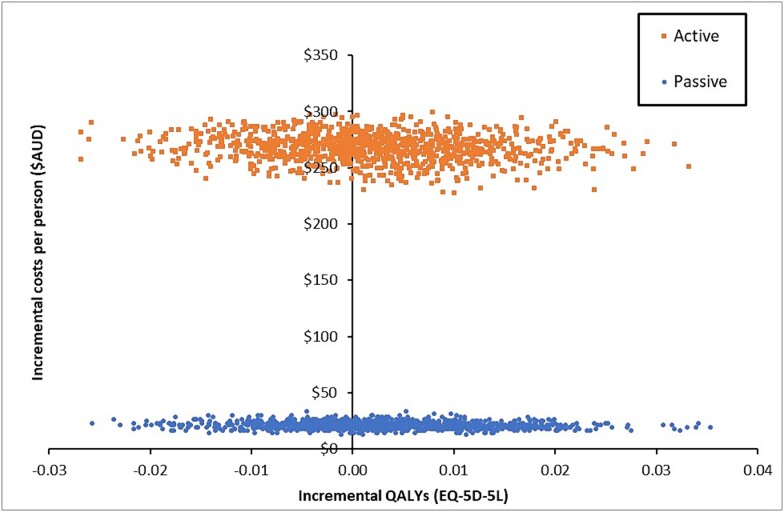
For the active intervention, one additional QALY gained would cost $164 003 more than the control version. For passive intervention, one additional QALY would cost $7084 more than the control version. At a cost-effectiveness threshold of $50 000 per QALY gained, the iNMB of the active intervention was -$186 (95% UI -$1137-$834). While the iNMB of the passive intervention was $127 (95%UI -$1137-$834). Non-parametric bootstrapping estimates indicated a less than 30% (0.296) chance of the active intervention being cost-effective (Fig. 1a). The passive intervention shows a 60% (0.59) chance of being cost effective (Fig. 1b) at a cost-effectiveness threshold of $50 000. Results from the uncertainty analyses are visually presented by group on a cost-effectiveness plane (Fig. 2) where the joint distribution of incremental costs are plotted against the incremental change in effect size between baseline and follow-up. Both groups are in the two northern quadrants of the plane, indicating those interventions cost more than the control condition. Note the scale of the x-axis which shows that there is minimal difference in QALY scores across the two groups.
Fig. 1.
Cost-effectiveness acceptability curve showing change in the probability of cost-effectiveness as the value of a QALY change for (a) active intervention compared to the control group; and (b) passive intervention compared to the control group.
Fig. 2.
Cost-effectiveness plane for active and passive intervention groups compared to the control group. The top right quadrant represents the intervention costing more as well as conferring greater benefits than the comparator. The bottom right quadrant shows where the intervention costs less but incurs greater benefits than the comparator, the top left quadrant shows the proportion of iterations where the intervention incurs a cost but fewer benefits than the comparator and the bottom left show the proportion of the iterations where the intervention costs less and has fewer benefits than the comparator group.
Discussion
Main finding of this study
This economic evaluation outlined the costs, cost consequences and cost-utility associated with the TRACE RCT that examined the effectiveness and efficiency of delivering a personality-based intervention targeting addictive eating to improve symptoms and associated dietary behaviors. While the active and passive interventions cost more than the control condition, both were inexpensive at AUD $294 and $47 per person, respectively. The iNMB for the active intervention was a negative dollar number indicating that at the $50 000 willingness to pay threshold, it is not cost-effective. The iNMB for the passive intervention was positive suggesting that the passive intervention has a higher chance of being cost-effective compared to the control. However, given the wide uncertainty intervals in the iNMB data, it is difficult to determine the absolute difference in cost effectiveness between the groups. The largely overlapping uncertainty intervals of the QALY scores in the three groups seem to show negligible QoL differences between the intervention and control groups. This indicates that cost of the intervention rather than QALYs is driving the cost-utility results. While the active intervention did not generate sufficient additional QALY gains compared to the control group in the short term at 3 months, overall the active intervention was considered very low cost, being a brief intervention comprising of five sessions, in comparison to other health interventions.28,29 These preliminary findings suggest that a stepped care model of service delivery may be an appropriate approach for the management of addictive eating, with the passive intervention provided initially followed by the more intensive active intervention for non-responders. This would align with current clinical guidelines for mental health.30,31
What is already known on this topic
Very few dietary interventions for mental health have had economic evaluations reported.13 The current study demonstrates similar change in QALYs, but a lower cost-effectiveness ratio. For example, Holt et al.32 examined the efficacy of an intensive 12-month weight loss intervention that targeted dietary change in individuals with severe mental illness (n = 414). The intervention consisted of four × 2.5-h weekly group sessions, followed by 2-weekly maintenance contact and group sessions at 4, 7 and 10 months. Holt et al.32 reported an ICER of £246 921 (~AUD $480 000) per QALY gained. In comparison, the current study using a brief intervention format (five sessions) found an increase of one additional QALY would cost AUD $164 003. Further, the current study demonstrated a marginally higher incremental gain in QALY in the intervention group over a shorter timeframe compared to that reported by Holt et al. (0.01777 gain in QALY at 3 months vs. 0.0035 gain in QALY at 12 months).
Previous dietary interventions that include a cost evaluation have largely involved interventions regarding the Mediterranean diet. For example, a 3-month study by Segal et al.33 for those with major depression (n = 152), the HELFIMED intervention, examined a group-based Mediterranean-style diet (MedDiet) intervention (including cooking workshops) compared against a social group-program. This intervention was more expensive costing >AUD $30 000 compared with only $11 173 in the current study. While not directly comparable, as another QoL tool was used, after the 3-month intervention the improvement in QALY compared to control was 0.1106. Another study, the SMILES trial34 in Australian adults with major depression (n = 67), implemented a more intensive intervention than the current intervention with seven dietitian-led sessions for dietary support focused on the Mediterranean diet over 3 months compared to an intensity matched social support (befriending) control condition. The QALYs derived from the AQoL-8D, were not significantly different between groups, similar to the current study. The SMILES trial that is reported to cost more than the current addictive eating intervention evaluated costs from both healthcare and societal perspectives. In addition to health sector costs, costs incurred by the patient, including food, medications, health professional visits and effects on productivity were evaluated. These societal costs were not included in the current study.
What this study adds
This is the first study to perform an economic evaluation selectively including participants with addictive eating. The strengths of the current study included the RCT design, the comparators chosen, i.e. passive intervention and control group, given there is no usual care arrangements for those with addictive eating.
It is important to note that in all intervention groups participants had improvements in YFAS scores and health utility. The cost per reduction in one YFAS symptom for the active intervention was ~AUD $60. However, this is a novel program in addictive eating and there are no comparable studies with cost data. It is possible that in this population 3 months was too short to measure change in healthcare usage and a longer time is needed to see improvements in health utility (and therefore QALYs) as a result of improvements in addictive eating behaviors. Sustained change in YFAS symptom scores over a longer time period may lead to a more substantial change in QALYs. More data are needed on the relationship between sustained changes in YFAS scores and longer-term health utility in order to assess the longer-term cost-effectiveness of this intervention. Further, the standard practice of economic evaluation assumes the value of being healthy is the same across ages and does not discriminate between a QALY score to a young adult and one to an older adult. Although the mean QALY score of the current sample is comparable to the population norm,35 given the broad age range in the current sample, future studies need to consider that the value of health may vary by age.
The current intervention, which was extended from three to five sessions following process evaluation of the pilot study,7,8 was designed as a brief intervention. The TRACE intervention could be implemented into existing healthcare services for those seeking treatment for addictive eating behaviors, particularly individuals who would have otherwise been turned away or for those who have previously sought help but not offered appropriate care. If the intervention were to be implemented into existing services, delivery could be modified to fit existing healthcare pathways, such as delivery of content over more or less sessions or complimented with other therapies. Further, trialing the intervention in clinical settings, such as those treating individuals with additional co-morbidities or individuals with complex needs would be worthwhile. The costs of the three interventions have been included to provide to a broad overview of the costs incurred to deliver the intervention. This may be of interest to health care providers. For example, if the intervention were to be translated into routine practice, the passive intervention may be beneficial in stepped care arrangements or the active intervention may be beneficial for individuals seeking support that are ready for behavior change.
Limitations of this study
Limitations to study include the small sample size, which was based on sample size calculation for the RCT analysis and not on the cost-utility analysis, which may limit the generalizability of the findings. Although complete case analysis is a popular approach in healthcare economics and its use has increased with time, use of this approach may have led to biased estimates. The EQ-5D-5L was chosen to measure QoL. It is possible that the use of another tool (e.g. AQoL-8D) may have resulted in other outcomes. While the generic preference-based EQ-5D-5L can be used in the economic evaluation of interventions for common mental health conditions with some confidence,36 the measurement of QoL may be improved by developing a condition-specific preference-based measure for addictive eating. Further, there has recently been EQ-5D-5L Australian population norms published.37,38 While this information was not available at the time of the current study, these values will be useful for future economic evaluations in Australian population groups. The major limitation is the measurement timeframe of 3 months. Longer-term outcome data, such as 12-month follow-up data, should be included in future iterations. While the of number of participants may affect intervention costs (for example in the active intervention arm, the cost of the trainer and web hosting would get cheaper per person with an increase in participants), it should be noted that the largest cost contributor was the delivery of the telehealth sessions in the active intervention. Therefore, the influence of the fixed costs is small relative to the variable cost of labor. Given the costs to deliver the intervention are upfront costs, these may benefit the cost effectiveness analysis. The current analysis was conducted from a healthcare sector perspective, however the costs of healthcare visits (i.e. payments made for health care services by participants) were not included in the current analysis. These costs, and out-of-pocket expenses, may be highly varied depending on the type of healthcare service received, how the service is accessed (e.g. through public or private systems; attendance via face-to-face or telehealth) and where a person lives (urban, rural or remote). Given the clustering co-morbidities of individuals with addictive eating, for example the overlap between weight management and disordered eating, future economic evaluations regarding addictive eating may benefit from using more established methods to provide an accurate representation of costs and consequences of interventions. For example, linking of a healthcare database (e.g. Medicare, Australia’s universally funded public health care system) to survey measures so that cumulative healthcare claims/costs can be captured.39,40 Lastly, costs to participants, such as food acquisition changes as a result of the intervention and medications, and costs to private care providers (including opportunity costs), were not assessed.
Conclusion
This is the first study to report the costs and cost consequence of an intervention for addictive eating. Overall, both the active and passive interventions were associated with small QALY gains in participants. Compared to the control, the passive intervention was likely to be cost-effective, and the active intervention not cost-effective. However, when comparing the active intervention to the passive intervention, the largest difference in costs were in the delivery of the content by trained dietitians via telehealth sessions. As this pertains to a large cost compared with small difference in QoL over a short 3-month time frame, the cost of the active intervention rather than QALYs is driving the cost-effectiveness results. Given the positive outcomes demonstrated by the TRACE RCT the intervention could be adopted in existing health service settings or trialed in other population groups who experience addictive eating. Although more data are needed to determine how sustained changes in addictive eating scores affect longer term health utility, and therefore QALYs, in order to assess the longer-term cost-effectiveness of this novel intervention.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Victoria McCreanor (Senior Health Research Economist, Hunter Medical Research Institute) for their assistance with the statistical analysis, and the participants who completed the study. Tracy Burrows is supported by the National Health and Medical Research Council (NHMRC) grant number [G1801414].
Janelle A Skinner, Postdoctoral Research Fellow and Accredited Practising Dietitian
Mark Leary, Research Assistant and Accredited Practising Dietitian
Olivia Wynne, Health Economist
Phillipa J Hay, Professor and Chair of Mental Health, Academic Psychiatrist
Clare E Collins, NHMRC Investigator Fellow and Accredited Practising Dietitia
Tracy L Burrows, NHMRC Research Fellow and Accredited Practising Dietitian
Contributor Information
Janelle A Skinner, School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, University Drive, Callaghan NSW 2308, Australia; Hunter Medical Research Institute, University of Newcastle, Lot 1 Kookaburra Cct, New Lambton Heights, NSW 2305, Australia.
Mark Leary, School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, University Drive, Callaghan NSW 2308, Australia; Hunter Medical Research Institute, University of Newcastle, Lot 1 Kookaburra Cct, New Lambton Heights, NSW 2305, Australia.
Olivia Wynne, Hunter Medical Research Institute, University of Newcastle, Lot 1 Kookaburra Cct, New Lambton Heights, NSW 2305, Australia.
Phillipa J Hay, Translational Health Research Institute, Western Sydney University, Building 3, David Pilgrim Avenue, Campbelltown, NSW 2751, Australia; Mental Health Services, Camden and Campbelltown Hospitals, SWSLHD, Campbelltown, NSW 2751, Australia.
Clare E Collins, School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, University Drive, Callaghan NSW 2308, Australia; Hunter Medical Research Institute, University of Newcastle, Lot 1 Kookaburra Cct, New Lambton Heights, NSW 2305, Australia.
Tracy L Burrows, School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, University Drive, Callaghan NSW 2308, Australia; Hunter Medical Research Institute, University of Newcastle, Lot 1 Kookaburra Cct, New Lambton Heights, NSW 2305, Australia.
Authors' contributions
TLB conceptualized the study, and TLB, JAS, ML, PJH and CEC designed the study. TLB, JAS, ML, PJH and CEC contributed to the intervention development and design, intervention resources and assessment methodology. TLB, JAS and ML were responsible for recruitment, data collection and intervention delivery. OW completed statistical analysis, and JAS assisted with data cleaning, and statistical analysis. JAS and TLB drafted the manuscript with all other co-authors contributing to drafts of the paper. All authors accept full responsibility for, and have read and approved the final manuscript.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC) grant number [G1801414]. The trial was conducted and evaluated independent of the study funder.
Conflict of interest
none.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale food addiction scale. Appetite 2009;52(2):430–6. [DOI] [PubMed] [Google Scholar]
- 2. Gearhardt AN, Davis C, Kuschner R et al. The addiction potential of Hyperpalatable foods. Curr Drug Abuse Rev 2011;4:140–5. [DOI] [PubMed] [Google Scholar]
- 3. Gearhardt AN, Corbin WR, Brownell KD. Development of the Yale food addiction scale version 2.0. Psychol Addict Behav 2016;30(1):113–21. [DOI] [PubMed] [Google Scholar]
- 4. Burrows T, Kay-Lambkin F, Pursey K et al. Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J Hum Nutr Diet 2018;31(4):544–72. [DOI] [PubMed] [Google Scholar]
- 5. Burrows T, Skinner J, McKenna R et al. Food addiction, binge eating disorder, and obesity: is there a relationship? Behavioral Sciences 2017;7:E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leary M, Pursey KM, Verdejo-Garcia A et al. Current intervention treatments for food addiction: a systematic review. Behavioral Sciences 2021;11(6):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burrows T, Collins R, Rollo M et al. The feasibility of a personality targeted intervention for addictive overeating: FoodFix. Appetite 2021;156(104974):104974. [DOI] [PubMed] [Google Scholar]
- 8. Leary M, Pursey KM, Verdejo-Garcia A et al. Designing an online intervention for adults with addictive eating: a qualitative integrated knowledge translation approach. BMJ Open 2022;12(6):e060196. 10.1136/bmjopen-2021-060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skinner JA, Whatnall M, Leary M et al. Examining the efficacy of a telehealth intervention targeting addictive eating in Australian adults (the TRACE programme): a randomised controlled trial protocol. BMJ Open 2023;13(6):e064151, 1–15. 10.1136/bmjopen-2022-064151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skinner JA, Leary M, Whatnall M et al. A three-arm randomised controlled trial of a telehealth intervention targeting improvement in addictive eating for Australian adults (the TRACE program). Appetite 2023;195:107211, 1–13. 10.1016/j.appet.2024.107211. [DOI] [PubMed] [Google Scholar]
- 11. Williams LT, Barnes K, Ball L et al. How effective are dietitians in weight management? A systematic review and meta-analysis of randomized controlled trials. Healthcare 2019;7(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rollo ME, Burrows T, Vincze LJ et al. Cost evaluation of providing evidence-based dietetic services for weight management in adults: In-person versus eHealth delivery. Nutrition & dietetics: the journal of the Dietitians Association of Australia 2018;75(1):35–43. [DOI] [PubMed] [Google Scholar]
- 13. Burrows T, Teasdale S, Rocks T et al. Cost effectiveness of dietary interventions for individuals with mental disorders: a scoping review of experimental studies. Nutrition & dietetics: the journal of the Dietitians Association of Australia 2022;79(3):291–302. [DOI] [PubMed] [Google Scholar]
- 14. Husereau D, Drummond M, Augustovski F. et al. CHEERS 2022 ISPOR Good Research Practices Task Force, Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations. Value Health 2022;25(1):3–9. 10.1016/j.jval.2021.11.1351. [DOI] [PubMed] [Google Scholar]
- 15. Prnjak K, Mitchison D, Griffiths S et al. Further development of the 12-item EDE-QS: identifying a cut-off for screening purposes. BMC Psychiatry 2020;20(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol group. Ann Med 2001;33(5):337–43. [DOI] [PubMed] [Google Scholar]
- 17. Martí-Pastor M, Pont A, Ávila M et al. Head-to-head comparison between the EQ-5D-5L and the EQ-5D-3L in general population health surveys. Popul Health Metr 2018;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng YS, Kohlmann T, Janssen MF et al. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res 2021;30(3):647–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beecham J, Knapp M. Costing psychiatric interventions. In: Thornicroft G (ed). Measuring Mental Health Needs, 2nd edn. London: Gaskell, 2001, 200–24. [Google Scholar]
- 20. Herdman M, Gudex C, Lloyd A et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20(10):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Hout B, Janssen MF, Feng YS et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15(5):708–15. [DOI] [PubMed] [Google Scholar]
- 22. EuroQol Research Foundation . EuroQol [homepage]. Rotterdam, The Netherlands: EuroQol. www.euroqol.org (date last accessed, July 29, 2023).
- 23. Drummond MF, Sculpher MJ, Claxton K et al. Methods for the Economic Evaluation of Health Care Programs, 3rd edn. Oxford: Oxford University Press, 2005. [Google Scholar]
- 24. Ramsey SD, Willke RJ, Glick H et al. Cost-effectiveness analysis alongside clinical trials II—an ISPOR good research practices task force report. Value Health 2015;18(2):161–72. [DOI] [PubMed] [Google Scholar]
- 25. Glick HA, Doshi JA, Sonnad SS et al. Economic Evaluation in Clinical Trials, 2nd edn. Oxford: Oxford University Press, 2014. [Google Scholar]
- 26. Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50 000 per QALY threshold. Expert Review of Pharmacoeconomics and Outcomes Research 2008;8(2):165–78. [DOI] [PubMed] [Google Scholar]
- 27. Paulden M. Why it’s time to abandon the ICER. Pharmacoeconomics 2020;38(8):781–4. [DOI] [PubMed] [Google Scholar]
- 28. Dalziel K, Segal L, Mortimer D. Review of Australian health economic evaluation—245 interventions: what can we say about cost effectiveness? Cost effectiveness and resource allocation : C/E. 2008;6(9):1–12. 10.1186/1478-7547-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. MMJ G, Uyl-de Groot CA, Redekop WK. A systematic review of cost-effectiveness studies of interventions with a personalized nutrition component in adults. Value Health 2021;24(3):325–35. [DOI] [PubMed] [Google Scholar]
- 30. National Institute for Health and Care Excellence (NICE) . Common mental health problems: identification and pathways to care. Manchester, UK: NICE, 2011. www.nice.org.uk/guidance/cg123.
- 31. Australian Government Department of Health . PHN Mental Health Flexible Funding Pool Programme Guidance: Stepped Care. Canberra: Australian Government Department of Health. 2019. https://www.health.gov.au/resources/publications/primary-health-networks-phn-primary-mental-health-care-guidance-stepped-care. [Google Scholar]
- 32. Holt RI, Hind D, Gossage-Worrall R, Bradburn MJ, Saxon D, McCrone P, et al. Structured lifestyle education to support weight loss for people with schizophrenia, schizoaffective disorder and first episode psychosis: the STEPWISE RCT. Health Technology Assessment.22(65):1–160. 10.3310/hta22650. [DOI] [PMC free article] [PubMed]
- 33. Segal L, Twizeyemariya A, Zarnowiecki D et al. Cost effectiveness and cost-utility analysis of a group-based diet intervention for treating major depression - the HELFIMED trial. Nutr Neurosci 2020;23(10):770–8. [DOI] [PubMed] [Google Scholar]
- 34. Chatterton ML, Mihalopoulos C, O'Neil A et al. Economic evaluation of a dietary intervention for adults with major depression (the "SMILES" trial). BMC Public Health 2018;18(1):599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palmer AJ, Campbell JA, de Graaff B et al. Population norms for quality adjusted life years for the United States of America, China, the United Kingdom and Australia. Health Econ 2021;30(8):1950–77. Erratum in: Health Economics. 2022;31(9):2090-2105. 10.1002/hec.4549. [DOI] [PubMed] [Google Scholar]
- 36. Mulhern B, Mukuria C, Barkham M et al. Using generic preference-based measures in mental health: psychometric validity of the EQ-5D and SF-6D. Br J Psychiatry 2014;205(3):236–43. [DOI] [PubMed] [Google Scholar]
- 37. Norman R, Mulhern B, Lancsar E et al. The use of a discrete choice experiment including both duration and dead for the development of an EQ-5D-5L value set for Australia. Pharmacoeconomics 2023;41(4):427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Redwood L, Currow D, Kochovska S et al. Australian population norms for health-related quality of life measured using the EQ-5D-5L, and relationships with sociodemographic characteristics. Qual Life Res 2024;33(3):721–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Collins CE, Patterson A, Fitzgerald D. Higher diet quality does not predict lower Medicare costs but does predict number of claims in mid-aged Australian women. Nutrients 2011;3(1):40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baldwin JN, Forder PM, Haslam R et al. Lower vegetable variety and worsening diet quality over time are associated with higher 15-year health care claims and costs among Australian women. J Acad Nutr Diet 2021;121(4):655–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.