Abstract
Exercise activates the dorsal hippocampus that triggers synaptic and cellar plasticity and ultimately promotes memory formation. For decades, these benefits have been explored using demanding and stress‐response‐inducing exercise at moderate‐to‐vigorous intensities. In contrast, our translational research with animals and humans has focused on light‐intensity exercise (light exercise) below the lactate threshold (LT), which almost anyone can safely perform with minimal stress. We found that even light exercise can stimulate hippocampal activity and enhance memory performance. Although the circuit mechanism of this boost remains unclear, arousal promotion even with light exercise implies the involvement of the ascending monoaminergic system that is essential to modulate hippocampal activity and impact memory. To test this hypothesis, we employed our physiological exercise model based on the LT of rats and immunohistochemically assessed the neuronal activation of the dorsal hippocampal sub‐regions and brainstem monoaminergic neurons. Also, we monitored the extracellular concentration of monoamines in the dorsal hippocampus using in vivo microdialysis. We found that even light exercise increased neuronal activity in the dorsal hippocampal sub‐regions and elevated the extracellular concentrations of noradrenaline and dopamine. Furthermore, we found that tyrosine hydroxylase‐positive neurons in the locus coeruleus (LC) and the ventral tegmental area (VTA) were activated even by light exercise and were both positively correlated with the dorsal hippocampal activation. In conclusion, our findings demonstrate that light exercise stimulates dorsal hippocampal neurons, which are associated with LC‐noradrenergic and VTA‐dopaminergic activation. This shed light on the circuit mechanisms responsible for hippocampal neural activation during exercise, consequently enhancing memory function.
Keywords: dopamine, dorsal hippocampus, light exercise, locus coeruleus, noradrenaline, ventral tegmental area
Our previous research with animals and humans has demonstrated that even light exercise can boost neuronal activity in the dorsal hippocampus and improve memory. While the mechanism underlying this remains undetermined, recent studies suggest the involvement of the ascending monoaminergic system. Here, we examined this hypothesis and found a possible association of noradrenergic neurons in the locus coeruleus and dopaminergic neurons in the ventral tegmental area with dorsal hippocampal activation during light exercise, implying a circuit mechanism for light‐exercise‐enhanced memory.
Abbreviations
- 5‐HT
serotonin
- AAS
ascending arousal system
- ACTH
adrenocorticotropic hormone
- AD
Alzheimer's disease
- BDNF
brain‐derived neurotrophic factor
- BSA
bovine serum albumin
- CA
cornu ammonis
- DA
dopamine
- DAPI
4′,6‐diamidino‐2‐phenylindole
- DG
dentate gyrus
- DRN
dorsal raphe nucleus
- HPLC‐ECD
high‐performance liquid chromatography with an electrochemical detector
- IGF‐I
insulin‐like growth factor I
- LC
locus coeruleus
- LT
lactate threshold
- LTD
long‐term depression
- LTP
long‐term potentiation
- MCI
mild cognitive impairment
- MRN
median raphe nucleus
- NA
noradrenaline
- PB
phosphate buffer
- PBT
phosphate buffer with Triton X‐100
- PFA
paraformaldehyde
- PRP
plasticity‐related protein
- PVN
paraventricular nucleus
- RM
repeated measures
- RRF
retrorubral field
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
1. INTRODUCTION
There is accumulating evidence showing that physical exercise has beneficial effects on the brain and its functions, including hippocampus‐dependent learning and memory. 1 , 2 , 3 , 4 The hippocampus is well‐known for being involved in several processes related to emotion, stress resilience, and cognitive function. In particular, the dorsal part of the hippocampus plays a key role in the learning process and memory formation. 5 Physical exercise has been found to stimulate the activity of the dorsal hippocampal neurons. 6 , 7 , 8 This subsequently triggers the production of plasticity‐related proteins (PRPs) such as the brain‐derived neurotrophic factor (BDNF) in the hippocampus. 9 , 10 , 11 Also, this activation increases cerebral blood flow 8 , 12 and facilitates the entrance of bioactive hormones “exerkines” secreted from peripheral organs, such as the insulin‐like growth factor I (IGF‐I), cathepsin B, and irisin, across the blood–brain barrier into the hippocampus. 13 , 14 , 15 , 16 , 17 , 18 In turn, these boosts ultimately contribute to cellar and synaptic plasticity and memory enhancement. 1 , 15 , 16 , 19 , 20 , 21
To elucidate the mechanisms behind these beneficial effects of physical exercise and achieve translation to humans, it is necessary to promote animal research using an exercise model that allows precise control of multiple parameters (e.g., FITT; Frequency, Intensity, Time, and Type 22 ). Among them, exercise intensity is an important parameter for translational research, which can be typically categorized as light, moderate, and vigorous intensity according to the American College of Sports Medicine (ACSM) guidelines. 22 For decades, exercise benefits to learning and memory have been based on evidence using exercise at moderate‐to‐vigorous intensities, 2 , 3 , 4 , 23 which is generally recommended in exercise therapy. However, such demanding exercise can cause physiological stress and reduce exercise adherence. 24 , 25 , 26 , 27 , 28 Alternatively, the chronic intervention of voluntary wheel running is widely used in animal research, 1 , 15 , 16 , 18 where rodents can run voluntarily without requiring aversive and stressful stimuli to induce running. Despite such advantages, voluntary running rodents exhibit high‐intensity (average 45 m/min) and intermittent running patterns throughout the dark cycle (i.e, the active cycle of rodents) and are often recognized as a reward‐related complex behavior. 29 , 30 These may somewhat differ from the modern lifestyle of humans who engage in regular exercise and provide less information necessary for translation to humans such as intensity and duration. 31
We thus have been employing a rodent treadmill running model that allows precise control of these factors and have determined the exercise intensity based on physiological responses common to both animals and humans such as lactate threshold (LT). LT serves as the signpost for exercise‐induced stress, where blood lactate levels, as well as stress hormone levels like adrenocorticotropic hormone (ACTH), begin to increase exponentially. 24 , 26 Given that LT appears at moderate intensity and an increase in ACTH secretion is defined as a marker of physiological stress in the fields of medical physiology, 26 , 32 , 33 , 34 the LT‐based exercise model enables us to define running below the LT as light‐intensity exercise (light exercise) with minimum physiological stress, while running above the LT is classified as vigorous‐intensity exercise (vigorous exercise) with stress‐inducing stimuli. Using this model, we found that even light exercise was sufficient to acutely stimulate hippocampal activity and BDNF induction, 9 and to promote hippocampal neurogenesis and spatial memory. 35 , 36 , 37 Conversely, excessive ACTH secretion induced by vigorous exercise cancels out these beneficial effects on hippocampal plasticity and memory due to the stress‐induced hippocampal vulnerability. 35 , 36 , 37 , 38 , 39 Additionally, our research with human participants and functional magnetic resonance imaging (fMRI) found that acute light exercise increases hippocampal activity and enhances pattern separation, which is a hippocampus‐related function. 40 Taken together, our translational research demonstrates the importance of exercise intensity for determining the effect of exercise on the hippocampus and highlights the advantages of light exercise.
However, prior studies, including our own findings, have yet to address the circuit mechanism underlying dorsal hippocampal activation by light exercise and its beneficial effect on memory performance. Classically, it is known that exercise can promote arousal, 41 , 42 , 43 which is thought to originate from the activation of the ascending arousal system (AAS). 44 , 45 This is supported by our recent studies showing that arousal promotion can occur by exercise even at very light intensity accompanied by pupil dilation, 40 , 46 , 47 , 48 , 49 which reflects the activity of AAS. 50 , 51 , 52 , 53 The AAS sends out widespread projections in order to increase global brain activity, including the dorsal hippocampus, which largely overlaps the monoaminergic neuromodulatory system. Monoamines like noradrenaline (NA) (also called “norepinephrine”), dopamine (DA), and serotonin (5‐HT) are essential for multiple functions such as arousal, attention, reward, motivation, and mood regulation. 54 , 55 , 56 , 57 , 58 , 59 , 60 At the dorsal hippocampus, monoaminergic stimulation facilitates learning and memory processes by regulating the expression of PRPs and synaptic and cellar plasticity, such as long‐term potentiation (LTP) and neurogenesis. 61 , 62 , 63 , 64 , 65 , 66 The hippocampal monoamines are mainly synthesized and provided by the specific nuclei located in the pons noradrenergic locus coeruleus (LC), 67 , 68 , 69 , 70 , 71 the midbrain dopaminergic nuclei, such as the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNpc), 72 , 73 , 74 , 75 , 76 and the midbrain serotonergic raphe nuclei (the dorsal raphe: DRN and the median raphe: MRN). 77 , 78 Previous research demonstrates that single bouts of exercise activate these brainstem monoaminergic neurons 79 , 80 , 81 and affect the hippocampal monoamine transmissions, 3 , 82 , 83 thereby contributing to the enhancement of hippocampus‐related functions. 3 , 79 , 84 These findings lead us to the hypothesis that the monoamines provided by the brainstem nuclei could be involved in the light‐exercise‐induced dorsal hippocampal activation, as the neural basis of memory enhancement.
To test this hypothesis, the involvement of the brainstem monoaminergic system in dorsal hippocampal activation during light exercise was investigated using a rat LT‐based treadmill running model that can be applied to humans. We analyzed the neuronal activation of the dorsal hippocampus and of the brainstem monoaminergic neurons using immunohistochemistry. Also, we monitored extracellular monoamine concentrations in the dorsal hippocampus with in vivo microdialysis combined with the high‐performance liquid chromatography (HPLC) system.
2. MATERIALS AND METHODS
2.1. Animals
All animal care and experimental procedures were performed in accordance with protocols approved by the University of Tsukuba Animal Experiment Committee (19‐378, 20‐405, 21‐373, and 24‐362) based on the NIH Guidelines for the Care and Use of Laboratory Animals (NIH publication, 1996). Male adult Long‐Evans rats (n = 60; 360–470 g; 10–12 weeks old at the beginning of experiments) from the TH‐Cre rat colony (i.e., Cre‐negative littermate control of TH‐Cre Long‐Evans rats) or obtained from SLC Inc. (Iar: Long‐Evans, RRID:RGD_18337282, Shizuoka, Japan) were housed with 2–3 rats in each cage and singly housed after surgery. Rats were kept in a controlled environment at 22 ± 1°C with a 12/12‐h light/dark cycle (lights on 7 a.m. to 7 p.m.) and given ad libitum access to water and food (MF, Oriental Yeast Co., Ltd., Tokyo, Japan). All experiments were done during the light cycle.
2.2. Experimental design
Four experiments were performed independently: measurement of LT (Experiment 1: n = 6), validation of exercise intensity (Experiment 2: n = 5‐7/group, three groups), immunohistochemical analysis of neuronal activation in dorsal hippocampus and brainstem monoaminergic nuclei (Experiment 3: n = 6‐8/group, three groups), and analysis of dorsal hippocampal monoamine release with in vivo microdialysis (Experiment 4: n = 5/group, three groups). All rats were handled for 1 week before the start of all procedures and habituated to treadmill running (6 days, Table 1). Then, they were randomly allocated to three groups based on LT as follows: 1) sedentary on treadmill (Control: 0 m/min), 2) below‐LT light‐intensity (low speed) running (Light: 15 m/min), and 3) above‐LT vigorous‐intensity (high speed) running (Vigorous: 25 m/min). Exercise and control conditions were maintained for 30 min.
TABLE 1.
Protocol for habituation to treadmill running.
| Day | Running speed (duration) |
|---|---|
| 1 | Rest (10 min) → 5 m/min (10 min) → 10 m/min (10 min) |
| 2 | Rest (5 min) → 5 m/min (10 min) → 10 m/min (10 min) → 15 m/min (10 min) |
| 3 | Rest |
| 4 | Rest (5 min) → 10 m/min (10 min) → 15 m/min (10 min) → 20 m/min (10 min) |
| 5 | Rest (5 min) → 15 m/min (10 min) → 20 m/min (10 min) → 25 m/min (10 min) |
| 6 | Rest (5 min) → 15 m/min (10 min) → 20 m/min (10 min) → 25 m/min (10 min) |
2.3. Surgery to insert jugular catheter
In Experiments 1 and 2, 1 day after the habituation to treadmill running periods, the rats were anesthetized with isoflurane, and a silicone catheter was inserted into the jugular vein and fixed with a silk thread suture (37 mm). The external distal end of the catheter was fixed at the nape of the neck. After the surgery, the rats were injected with antibiotics (Mycillin Sol; Meiji Seika, Tokyo, Japan). Then, they were housed individually and were allowed to recover for 2 or 3 days before the treadmill running test.
2.4. Stereotaxic surgery
In Experiment 4, after a week of handling, all rats were anesthetized with isoflurane and placed in a stereotaxic frame (Model 51 900, Muromachi Kikai Co., Ltd., Tokyo, Japan). A guide cannula (AG‐6, Eicom, Kyoto, Japan) was implanted above the dorsal hippocampus (AP: −5.1, ML: 4.8, DV: −3.5 mm, from bregma). The cannula was secured to the skull with three anchoring screws and dental cement (Super‐Bond cement C&B, SunMedical, Shiga, Japan; Unifast II, GC, Tokyo, Japan). To prevent occlusion, a dummy cannula (AD‐6, Eicom) was inserted into the guide cannula. After the surgery, the rats were injected with antibiotics (Mycillin Sol; Meiji Seika, Tokyo, Japan). Then, they were housed individually and were allowed to recover for at least 5 days before habituation to treadmill running.
2.5. Habituation to treadmill running
All rats were habituated to running on a treadmill (KN‐73, Natsume Seisakusho Co, Ltd., Tokyo, Japan) for a total of five sessions over 6 days. The running duration was 30 min/day, and the running speed was gradually increased from 5 to 25 m/min with no incline. 8 , 24 Electrical shock grids were placed at the rear end of the treadmill and provided mild but aversive foot shocks (30 V) to rats to encourage running at the set treadmill speed. To minimize any stress derived from the shocks, electric shocks were limited and a gentle tail touch was given when rats did not start running again. After the habituation period, rats were able to run at the appropriate speeds even when the electric grid was turned off.
2.6. Treadmill running tests
On the test day, all rats fasted for 2 h before running to obtain stable metabolic conditions. In Experiments 1 and 2, treadmill running tests were conducted 2–3 days after the surgery for inserting the jugular catheter. In Experiment 1, an incremental exercise test was performed to assess the running speed at LT. Rats were forced to run to exhaustion; the running speed was started at 5 m/min and increased by 2.5 m/min every 3 min. The exhaustion was considered to have occurred when rats were unable to continue running on the treadmill and remained on the electrical shock grids for 30 s despite being gently pressed with a stick. 24 , 85 The speed at which the LT was reached was determined with modified regression analysis as in previous studies. 24 , 85 , 86 , 87 Briefly, we employed two linear fits with the least squares method to clearly identify the breakpoint where blood lactate concentration begins to rise sharply. In Experiment 2, a single 30‐min bout of running was conducted to verify the validation of exercise intensity based on the speed at LT as determined in Experiment 1. Rats were randomly subjected to run at 15 m/min (Light) or 25 m/min (Vigorous), or they were placed on a treadmill as the at‐rest Control for 30 min. In both experiments, serial blood samples (0.1 mL) were taken at designated time points. Blood lactate and glucose were measured using an automated glucose–lactate analyzer (2300 Stat Plus, Yellow Springs Instruments, OH, USA).
In Experiment 3, rats were returned to their home cage after the test, rested for 90 min, and then were transcardially perfused with 0.9% saline, followed by 4% paraformaldehyde (PFA) in a 0.1 M phosphate buffer (PB; pH 7.4) under deep isoflurane anesthesia. The brains were sampled for immunohistochemical analysis.
In Experiment 4, a microdialysis probe (3 mm membrane: FX‐I‐6‐03, Eicom) was inserted into the dorsal hippocampus using a guide cannula 1 day before the test. The probe was connected to the microsyringe pump (ESP‐32, Eicom) using a PEEK tubing (JT‐10, Eicom) and perfused with Ringer's solution (147 mM NaCl, 4 mM KCl, and 2.3 mM CaCl2) at 0.8 μL/min at least 2 h before the start of the running test because a stable dialysate monoamine level is usually obtained 1 h after the start of Ringer's solution perfusion. Using a fraction collector (EFC‐82, Eicom), 24 μL of dialysate was collected every 30 min and 5 μL of 0.1 M PB (pH 3.5) was added to ensure stable monoamine detection using HPLC with an electrochemical detector (HPLC‐ECD). At the end of the experiments, rats were transcardially perfused with 0.9% saline, followed by 4% PFA in a 0.1 M PB under deep isoflurane anesthesia, and the brain was collected to verify the position of the probe in coronal sections with DAPI (4′,6‐diamidino‐2‐phenylindole). Please note that, due to an extremely high baseline extracellular 5‐HT concentration (over the mean + 3 × standard deviation (SD)) in one rat, it was excluded from the statistical analysis comparing the effects of exercise on extracellular 5‐HT concentration.
2.7. Immunohistochemistry
The brain was post‐fixed for 24 h in 4% paraformaldehyde (PFA, 02890‐45, Nacalai Tesque, Tokyo, Japan) in a 0.1 M PB and cryoprotected by immersion in a 0.1 M PB containing 20% sucrose for 1 day and 30% sucrose for 2–3 days. Frozen coronal brain sections of 30 μm thickness were cut with a microtome (REM‐710, Yamato Kohki Industrial Co., Ltd., Saitama. Japan). Sections were washed with a 0.1 M PB and blocked with a 0.1 M PB containing 1% Triton X‐100 (35501‐15, Nacalai Tesque) (PBT) plus 1% bovine serum albumin (01863‐77, BSA; Nacalai Tesque). Then, slices were incubated with the designated primary antibodies in PBT‐BSA for 24 h at 4°C. The primary antibodies used in this study were rabbit polyclonal antibody against c‐Fos (1:5000; Cat# 226‐003, RRID:AB_2231974, Synaptic Systems, GAU, Germany), mouse monoclonal antibody against NeuN (1:1000; Cat# MAB377, RRID:AB_2298772, Millipore, MA, USA), mouse monoclonal antibody against tyrosine hydroxylase (1:2000; Cat# F11:sc‐25 269, RRID:AB_628422, Santa Cruz, CA, USA), and goat polyclonal antibody against 5‐HT (1:1000; Cat# 20079, RRID:AB_572262, Immunostar Inc., WI, USA). After repeated washings with PBT‐BSA, sections were incubated in a dark space with designated secondary antibodies diluted in PBT‐BSA for >16 h at 4°C. The secondary antibodies used in this study were Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (1:5000; Cat# A‐11008, RRID:AB_143165, Invitrogen; Thermo Fisher Scientific Inc., MA, USA), Alexa Fluor 488‐conjugated donkey anti‐rabbit IgG (1:5000; Cat# ab150073, RRID:AB_2636877, abcam, Cambridge, UK), Alexa Fluor 594‐conjugated donkey anti‐goat IgG (1:1000; Cat# A‐11058, RRID:AB_142540, Invitrogen; Thermo Fisher Scientific Inc., MA, USA), and AMCA AffiniPure donkey anti‐mouse IgG (1:1000; Cat# 715‐155‐150, RRID:AB_2340806, Jackson ImmunoResearch Laboratories, Inc., PA, USA). For DAPI staining to verify the location of microdialysis probes, slices were washed three times in a 0.1 M PB and then incubated with DAPI (1:1000; 28718‐90‐3, Dojindo Laboratory, Kumamoto, Japan) in PBT‐BSA for 10 min in the dark. Slices were washed three times in a 0.1 M PB, mounted on subbed slides, air dried, and coverslipped using a mounting medium.
2.8. Counts and quantification
All fluorescence z‐stacked images were examined on an all‐in‐one microscope (BZ‐X710, Keyence, Osaka, Japan) with a 20× objective lens. Manual counts of neurons were performed by an observer blinded to the group allocations. The number of c‐Fos and NeuN double‐positive neurons (c‐Fos+/NeuN+) in the dorsal hippocampal dentate gyrus (DG), cornu ammonis (CA) 1, CA2, and CA3 sub‐regions were counted hemilaterally in four coronal sections (from Bregma −3.60, −4.16, −5.20, −5.80 mm). Total neuron numbers were normalized based on NeuN+ region volume (mm3), which was calculated by the area (mm2) multiplied by the thickness of sections (30 μm) using a BZ‐X analyzer software (https://www.keyence.co.jp/products/microscope/fluorescence‐microscope/bz‐x700/models/bz‐h3a/, version 1.4.1.1, RRID:SCR_017375). The numbers of c‐Fos and TH or 5‐HT double‐positive neurons (c‐Fos+/TH+ or c‐Fos+/5‐HT+) were bilaterally counted 120 μm apart throughout the LC (around Bregma −9.16 ~ 10.52 mm, 8 coronal sections) and 360 μm apart throughout the VTA, SNpc (around Bregma −4.80 ~ −6.72 mm, 5 coronal sections), DRN, and MRN (around Bregma −7.30 ~ −8.80 mm, 5 coronal sections), all of which are known as monoaminergic nuclei projecting to the dorsal hippocampus. Total c‐Fos+/TH+ or c‐Fos+/5‐HT+ neuron numbers were normalized based on the numbers of TH+ or 5‐HT+ neurons, respectively.
2.9. HPLC‐ECD for monoamine detection in the dorsal hippocampus
Dialysate samples collected from the probe at the dorsal hippocampus were injected into an HPLC‐ECD system (HTEC‐500; Eicom) using an autosampler (M‐510; Eicom) and analyzed for NA, DA, and 5‐HT concentration using the HPLC system with an EICOMPAK CAX column (2.0 mm, i.d. × 200 mm; Eicom) and a graft electrode (WE‐3G; Eicom) set at 450 mV (vs Ag/AgCl reference electrode). The flow rate and injection volume were set to 250 μL/min and 25 μL, respectively. The mobile phase contained a 0.1 M ammonium acetate buffer (pH 6.0), 0.034 M sodium sulfate, 57.14 mg/L EDTA, and methanol (8:2, v/v). Data analysis was conducted using Eicom EPC‐710 software, where the extracellular concentrations (pg) of the three monoamines (NA, DA, and 5‐HT) were determined by comparing their chromatographic peak areas to the corresponding standards. The limit of detection was determined by evaluating the peak height with a signal‐to‐noise ratio greater than 3. As described in the previous reports, 88 , 89 , 90 for each rat, the baseline extracellular concentrations of the three monoamines were estimated from the average of two stable sample values collected during a 1 h rest period on the treadmill before the beginning of running. Data were then expressed as the percentage increase or decrease over baseline extracellular concentrations (i.e., the averages of two values before the beginning of running).
2.10. Statistical analyses
All data are expressed as mean ± standard error (SEM) and figures are generated using GraphPad Prism (https://www.graphpad.com/, version 10, RRID:SCR_002798, GraphPad Software, San Diego, CA, USA). To ensure clarity and transparency, blood lactate concentrations during a single bout of running test, as well as extracellular concentrations of monoamines in the dorsal hippocampus during in vivo microdialysis test, are also presented as mean ± SD in the supplemental data. All data were analyzed using R software (https://www.r‐project.org/, version 4.4.1, RRID:SCR_001905). Assessment of the normality (Shapiro–Wilk test) of the data, equality of variance (Levene's test), and sphericity (Mendoza's test of sphericity) for repeated measures (RM) were performed. Statistical significance for parametric data was analyzed using one‐way analysis of variance (ANOVA) followed by Shaffer's post‐hoc test, but Welch's ANOVA was performed if equality of variance was not assumed. For the analysis of non‐parametric data, the Kruskal–Wallis test with the Mann–Whitney U test adjusted by Holm's post‐hoc test was applied. For RM, two‐way ANOVA followed by Shaffer's post‐hoc test was applied and the degree of freedom was adjusted using Huynh‐Feldt‐Lecoutre's epsilon for non‐spherical data. Pearson's correlation coefficient was used for analyzing the strength of relationships. Statistical significance was assumed at p < .05. No statistical methods were used to predetermine sample size, but the sample sizes were comparable to those reported in previous studies. 7 , 24 , 80 , 81 , 82
3. RESULTS
3.1. Measurement of LT and validation of exercise intensity
Initially, we developed a different intensity treadmill running model with adult male Long‐Evans rats using LT as a signpost for exercise‐induced stress. Our analysis revealed that the running speed at the LT of rats was 21.87 m/min ± 1.02 (n = 6; Figure 1B,C). Thus, we defined 15 m/min, which is below the LT, as the speed for light‐intensity running and 25 m/min, which is above the LT, as the speed for vigorous‐intensity running. The observation of an increase in blood lactate concentration after a 30‐min session of vigorous‐intensity running (two‐way RM ANOVA with Shaffer's post‐hoc test, effect of group: F (2,15) = 65.79, p < .001; effect of time: F (1,15) = 85.00, p < .001; group and time interaction: F (2,15) = 66.04, p < .001; at the Pre: F (2,15) = 1.40, p = .278; at the Post: F (2,15) = 80.53, p < .001, t (15) = 0.45, p = .657 Control vs. Light, t (15) = 10.73, p < .001 Control vs. Vigorous, t (15) = 10.80, p < .001 Light vs. Vigorous; Figure 1E; Figure S1A) was sufficient to validate the different intensity running model based on the LT.
FIGURE 1.
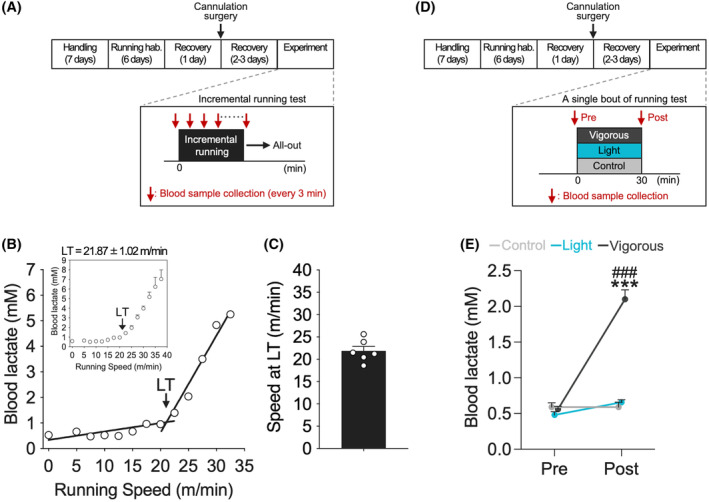
Measurement of lactate threshold and validation of exercise intensity. (A) Experimental design for incremental running test. (B) A representative LT profile from the incremental running test. (C) Speed of rats at LT was 21.87 ± 1.02 m/min (n = 6). (D) Experimental design for a single bout of running test. We defined 15 m/min for light‐intensity running and 25 m/min for vigorous‐intensity running. (E) Blood lactate concentration was increased only after a 30‐min session of vigorous‐intensity running. All data are expressed as mean ± SEM. The graph data with SD bars is shown in the supplemental data (Figure S1). Mean, SD, and SEM for each point are available in the supplemental data (Table S1). ***p < .001 vs. Control, ### p < .001 vs. Light, n = 5‐7/group. Control: Sedentary control; Light: 15 m/min running; Vigorous: 25 m/min running; hab.: Habituation.
3.2. Light‐intensity‐running‐induced neuronal activation of dorsal hippocampus
We assessed dorsal hippocampal neuronal activation during different intensities of running using c‐Fos immunostaining, as shown in the representative images in Figure 2B. We demonstrated that running increased c‐Fos expression in dorsal hippocampal sub‐regions (two‐way RM ANOVA with Shaffer's post‐hoc test, effect of group: F (2,18) = 11.02, p < .001; effect of sub‐region: F (1.59,28.62) = 127.21, p < .001; group and sub‐region interaction: F (3.18,28.62) = 6.95, p < .001), including the DG (F (2,18) = 12.88, p < .001, t (18) = 3.53, p < .01 Control versus Light, t (18) = 4.99, p < .001 Control versus Vigorous, t (18) = 1.69, p = .109 Light versus Vigorous; Figure 2C), CA1 (F (2,18) = 9.99, p < .01, t (18) = 3.44, p < .01 Control versus Light, t (18) = 4.28, p < .01 Control versus Vigorous, t (18) = 1.02, p = .323 Light versus Vigorous; Figure 2D), and CA3 (F (2,18) = 11.26, p < .001, t (18) = 3.06, p < .01 Control versus Light, t (18) = 4.71, p < .001 Control versus Vigorous, t (18) = 1.87, p = .077 Light versus Vigorous; Figure 2F), regardless of running intensity. Conversely, the CA2 sub‐region was not affected by running (F (2,18) = 0.17, p = .843; Figure 2E).
FIGURE 2.
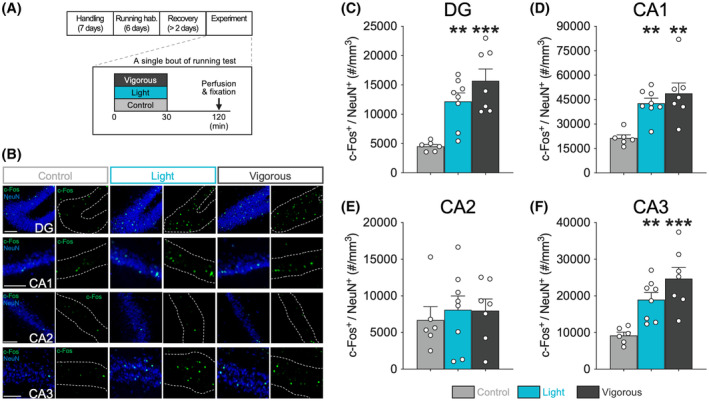
Running‐induced neuronal activation at dorsal hippocampus during different intensity treadmill running. (A) Experimental design for a single bout of running test for immunohistochemical analysis. (B) Representative double‐fluorescence images of c‐Fos (green) and NeuN (blue) in the DG, CA1, CA2, and CA3 sub‐regions of the dorsal hippocampus. Scale bars show 100 μm. c‐Fos expressions in the DG (C), CA1 (D), and CA3 (F) were increased with running exercise regardless of running speed (light or vigorous), but the CA2 sub‐region was not affected (E). All data are expressed as mean ± SEM. **p < .01, ***p < .001 vs. Control, n = 6–8 / group. Control: sedentary control; Light: 15 m/min running; Vigorous: 25 m/min running; hab.: habituation.
3.3. Light‐intensity running increased extracellular NA and DA concentrations in dorsal hippocampus
To investigate the effect of exercise on dorsal hippocampal monoamine levels, we measured extracellular monoamine concentrations in the dorsal hippocampus using in vivo microdialysis and HPLC methods. Baseline dialysate concentrations were 0.783 ± 0.069 pg. for NA, 0.156 ± 0.014 pg. for DA, and 0.461 ± 0.059 pg. for 5‐HT. We found that treadmill running induced an increase in both NA (two‐way RM ANOVA with Shaffer's post‐hoc test, effect of group: F (2,12) = 37.06, p < .001; effect of time: F (4,48) = 46.72, p < .001; group and time interaction: F (8,48) = 18.25, p < .001; at −60 ~ −30 min: F (2,12) = 1.58, p = .246; at −30 ~ 0 min: F (2,12) = 1.58, p = .246; at 0 ~ 30 min: F (2,12) = 55.86, p < .001, t (12) = 4.62, p < .001 Control vs. Light, t (12) = 10.54, p < .001 Control vs. Vigorous, t (12) = 5.92, p < .001 Light versus Vigorous; at 30 ~ 60 min: F (2,12) = 18.11, p < .001, t (12) = 3.80, p < .01 Control versus Light, t (12) = 5.94, p < .001 Control versus Vigorous, t (12) = 2.15, p = .053 Light versus Vigorous; at 60 ~ 90 min: F (2,12) = 1.71, p = .222; Figure 3E; Figure S3A) and DA levels (two‐way RM ANOVA with Shaffer's post‐hoc test, effect of group: F (2,12) = 16.12, p < .001; effect of time: F (3.75,45) = 14.83 p < .001; group and time interaction: F (7.5,45) = 7.71, p < .001; at −60 ~ −30 min: F (2,12) = 0.41, p = .671; at −30 ~ 0 min: F (2,12) = 0.41, p = .671; at 0 ~ 30 min: F (2,12) = 14.95, p < .001, t (12) = 4.06, p < .01 Control versus Light, t (12) = 5.20, p < .001 Control versus Vigorous, t (12) = 1.15, p = .273 Light versus Vigorous; at 30 ~ 60 min: F (2,12) = 15.64, p < .001, t (12) = 2.85, p < .05 Control versus Light, t (12) = 5.59, p < .001 Control versus Vigorous, t (12) = 2.75, p < .05 Light versus Vigorous; at 60 ~ 90 min: F (2,12) = 1.92, p = .189; Figure 3F; Figure S3B) in the dorsal hippocampus depending on running speed. Dorsal hippocampal 5‐HT levels, however, were not affected by running (two‐way RM ANOVA, effect of group: F (2,11) = 3.76, p = .057; effect of time: F (3.85,42.36) = 4.56, p < .01; group and time interaction: F (7.7,42.36) = 2.08, p = .062; Figure 3G; Figure S3C).
FIGURE 3.
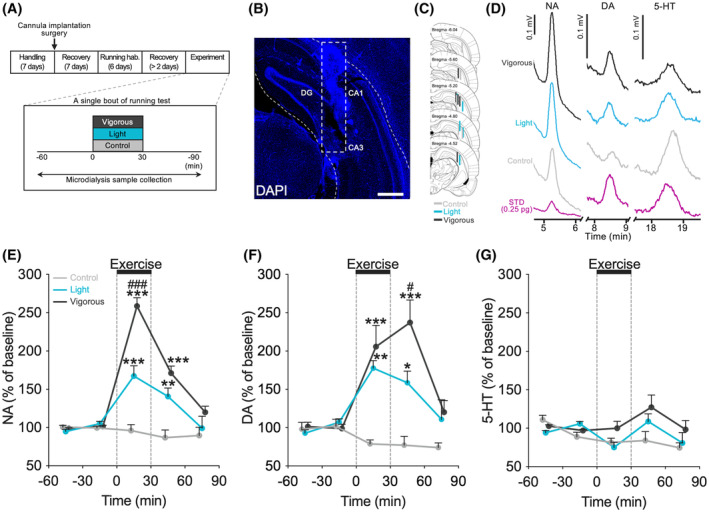
Running increases extracellular concentrations of NA and DA, but not 5‐HT, in dorsal hippocampus. (A) Experimental design for a single bout of running test for dorsal hippocampal monoamine detection with in vivo microdialysis. (B) Representative DAPI fluorescence image of microdialysis probe position in the dorsal hippocampus. Scale bars show 500 μm. (C) Schematic representations indicating the location of the microdialysis probe. Each bar represents the probe position of an individual rat, and the color of the bar corresponds to the experimental group (Control: light gray, Light: blue, and Vigorous: dark gray). (D) Representative chromatograms showing NA, DA, and 5‐HT from Control (light gray), Light (blue), Vigorous (dark gray), and 0.25 pg. standard (purple). Treadmill running induced an increase in extracellular NA (E) and DA (F) concentrations in the dorsal hippocampus, depending on running speed, but not 5‐HT (G). All data are expressed as mean ± SEM of the percentage of baseline values (i.e., the averages of two values before the beginning of running). The graph data with SD bars is shown in the supplemental data (Figure S3). Mean, SD, and SEM for each point are available in the supplemental data (Table S2). *p < .05, **p < .01, ***p < .001 vs. Sed, # p < .05, ### p < .001 vs. Light, n = 4‐5/group. Control: sedentary control; Light: 15 m/min running; Vigorous: 25 m/min running; hab.: habituation.
3.4. Running stimulated TH + neurons in LC and VTA, but not raphe 5‐HT + neurons
To examine the responsiveness of brainstem monoaminergic nuclei, we performed double‐immunostaining of c‐Fos+/TH+ neurons for the activation of NA‐ and DA‐producing neurons, and c‐Fos+/5‐HT+ neurons for the activation of serotonergic neurons, as shown in Figure 4A–C. Both light‐ and vigorous‐intensity running activated TH+ neurons in the LC (LCTH neurons; one‐way ANOVA with Shaffer's post‐hoc test, F (2,18) = 24.40, p < .001, t (18) = 4.54, p < .001 Control versus Light, t (18) = 6.94, p < .001 Control versus Vigorous, t (18) = 2.72, p < .05 Light versus. Vigorous; Figure 4D) and VTA (VTATH neurons; one‐way ANOVA with Shaffer's post‐hoc test, F (2,18) = 6.06, p < .01, t (18) = 3.21, p < .05 Control versus Light, t (18) = 2.90, p < .05 Control versus Vigorous, t (18) = 0.240, p = .813 Light versus Vigorous; Figure 4E), which are known as the main sources of dorsal hippocampal NA and DA, respectively. Running did not have any impact on dopaminergic neurons in the SNpc (SNpcTH neurons; one‐way ANOVA, F (2,18) = 2.24, p = .135; Figure 4F), or on serotonergic neurons in the DRN (DRN5‐HT neurons; one‐way ANOVA, F (2,18) = 0.58, p = .573; Figure 4G). However, vigorous‐intensity running decreased the activity of serotonergic neurons in the MRN (MRN5‐HT neurons; Welch's one‐way ANOVA with Games‐Howell post‐hoc test, F (2,10.17) = 6.50, p < .05, t (7.68) = 0.74, p = .750 Control versus Light, t (7.89) = 3.70, p < .05 Control versus Vigorous, t (10.68) = 1.25, p = .452 Light versus Vigorous; Figure 4H). Note that TH+ neurons in the retrorubral field (RRF) are also reported to have projections to the hippocampus, 91 which were not activated by running (Figure S4B). Additionally, TH+ neurons were also observed in the rostral region of the DRN (DRNTH neurons), which are reported to contribute to wakefulness by salience stimuli, opioid addiction, and pain modulation through the release of DA. 92 In this study, however, running did not affect the c‐Fos expression in DRNTH neurons (Figure S4D).
FIGURE 4.
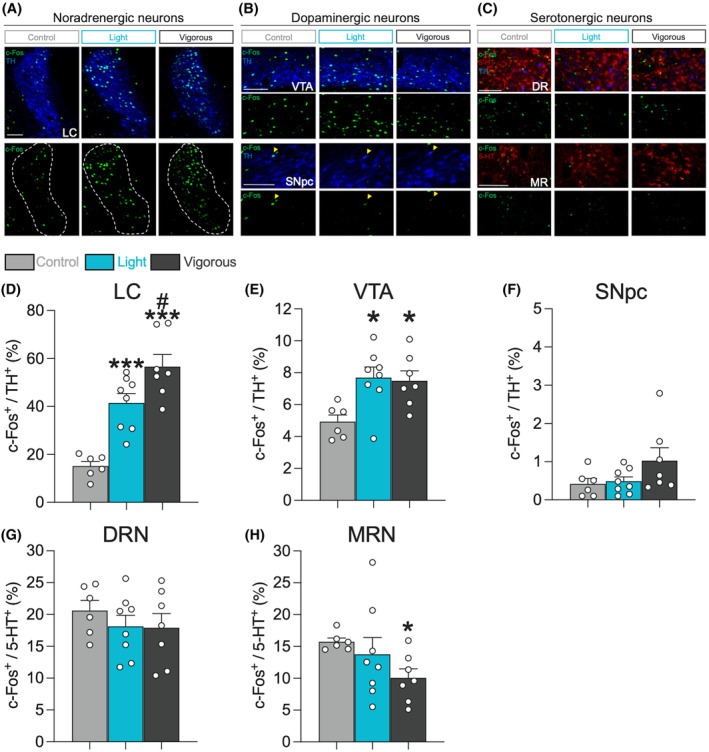
Running stimulates LCTH and VTATH neurons, but not raphe 5‐HT+ neurons. Representative double‐fluorescence images of c‐Fos (green), TH (blue), and 5‐HT (red) neurons in the LC (A); VTA and SNpc (B); and DRN and MRN (C). Scale bars show 100 μm. Yellow heads show c‐Fos+/TH+ neurons. The LCTH neurons (D) and VTATH neurons (E) were stimulated in both running groups. Running did not have an impact on the SNpcTH neurons (F) and DRN5‐HT neurons (G). However, vigorous‐intensity running decreased c‐Fos+ expression in the MRN5‐HT neurons (H). All data are expressed as mean ± SEM. *p < .05, ***p < .001 vs. Control, # p < .05 vs. Light, n = 6–8 / group. Control: sedentary control; Light: 15 m/min running; Vigorous: 25 m/min running.
3.5. Neuronal associations between dorsal hippocampus and brainstem monoaminergic nuclei
To explore the potential association between light‐exercise‐induced neuronal activities in brainstem monoaminergic nuclei and dorsal hippocampal activation, we conducted Pearson's correlation analysis using the dataset from the control group and the light‐intensity running group. This analysis assessed the correlation between c‐Fos expression in the brainstem monoaminergic nuclei and the dorsal hippocampus. A correlation matrix heatmap that summarizes the correlation results is shown in Figure 5A. c‐Fos expression in the LCTH neurons exhibited a positive correlation within the dorsal DG (r = 0.64, p < .05; Figure 5B), CA1 (r = 0.63, p < .05; Figure 5C), CA3 (r = 0.54, p < .05; Figure 5D), but not within CA2 (r = −0.11, p = .702). Additionally, positive correlations were found between c‐Fos expression in the VTATH neurons and in the dorsal DG (r = 0.54, p < .05; Figure 5E) and CA1 (r = 0.60, p < .05; Figure 5F), but not in the other sub‐regions (CA2: r = −0.04, p = .895; CA3: r = 0.46, p = .098). The higher level of c‐Fos expression in the DRN5‐HT neurons was correlated with lower levels of the dorsal CA2 (r = −0.67, p < .01; Figure 5G) and CA3 c‐Fos expression (r = −0.57, p < .05; Figure 5H), but not with the other sub‐regions (DG: r = −0.44, p = .112; CA1: r = −0.46, p = .101). No correlation was found in the c‐Fos expression between the dorsal hippocampal sub‐regions and the SNpcTH neurons (DG: r = −0.02, p = .951; CA1: r = 0.18, p = .542; CA2: r = −0.08, p = .780; CA3: r = 0.04, p = .883) or MRN5‐HT neurons (DG: r = −0.31, p = .281; CA1: r = −0.20, p = .497; CA2: r = −0.28, p = .338; CA3: r = −0.43, p = .121).
FIGURE 5.
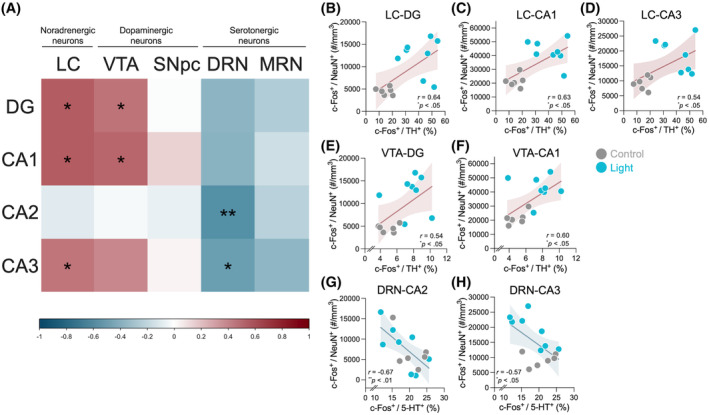
Neuronal associations between dorsal hippocampus and brainstem monoaminergic nuclei. (A) A correlation matrix heatmap is shown to summarize the correlation analysis between c‐Fos expression in dorsal‐hippocampal sub‐regions and that in the LCTH, VTATH, SNpcTH, DRN5‐HT, and MRN5‐HT neurons. Colors in the scale show correlation strength. c‐Fos expression in the LCTH neurons exhibited a positive correlation within the dorsal DG (B), CA1 (C), and CA3 (D). A positive correlation was also found between c‐Fos expression in the VTATH neurons and in the dorsal DG (E) and CA1 (F). c‐Fos expression in the DRN5‐HT neurons was negatively correlated with that in the dorsal CA2 (G) and CA3 (H). The colored line represents linear regression, and the colored band represents 95% confidence bands. *p < .05, **p < .01. n = 14. Control: Sedentary control (light gray dots); Light: 15 m/min running (blue dots).
4. DISCUSSION
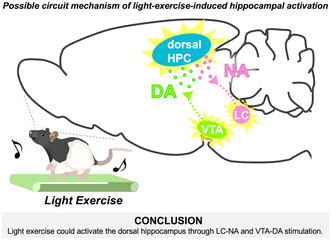
To probe the neural circuitry responsible for activating the dorsal hippocampus during light exercise and its subsequent contribution to enhanced memory functions, this study examined the involvement of the ascending monoaminergic system—a critical neuromodulatory mechanism for memory—in the activation of the dorsal hippocampus induced by light exercise. Employing a rat exercise model based on the LT, we assessed neuronal activity in the dorsal hippocampus and brainstem monoaminergic neurons and observed extracellular monoamine concentrations in the dorsal hippocampus using in vivo microdialysis. The results demonstrate that even light exercise led to increased c‐Fos expression in specific sub‐regions of the dorsal hippocampus and triggered the increase in extracellular NA and DA concentrations within this brain region. Furthermore, the activity of LCTH neurons and VTATH neurons elevated during exercise even at the light intensity, and both were positively correlated with the activation of the dorsal hippocampus. In contrast, the serotonergic system did not appear to be activated by exercise. These findings imply LC‐noradrenergic and VTA‐dopaminergic contributions to dorsal hippocampal activation during light exercise (Figure 6), offering insights into the circuitry behind lightexercise‐enhanced learning and memory.
FIGURE 6.
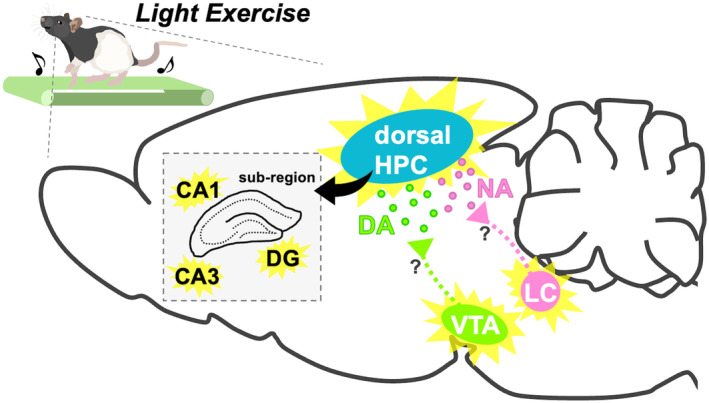
Schematic diagram showing possible circuit mechanism for light‐exercise‐induced dorsal hippocampal activation. Light exercise activates TH+ neurons at the LC and the VTA. At the dorsal hippocampus, subsequently, axon terminals originating from the LCTH and the VTATH increase the release of NA and DA, respectively. These, in turn, upregulate the neuronal activity of the dorsal hippocampal DG, CA1, and CA3 sub‐regions. DA, dopamine; DG, dentate gyrus; HPC, hippocampus; LC, locus coeruleus; NA, noradrenaline; VTA, ventral tegmental area.
Here, we first established a different intensity treadmill running model for male adult Long‐Evans rats based on LT as a physiological index of running intensity and stress. The speed at the LT was similar to that in our previous work using male adult Wistar rats (Long‐Evans: 21.87 m/min ± 1.02 vs. Wistar: 20.7 m/min ± 2.1; Figure 1B,C). 24 In addition, an increase in blood lactate concentration was observed following a single bout of vigorous‐intensity running, but not with light‐intensity running (Figure 1E; Figure S1A), which is consistent with our previous reports. 24 , 26 These results confirm the validity of our experimental model (LT‐based different intensity running) and suggest that the two running groups exhibited different metabolic changes and probably stress‐related responses.
Previous studies have reported that acute different intensity treadmill running activates the dorsal hippocampus, including the DG, CA1, and CA3 sub‐regions, even at low speeds. 6 , 7 , 9 Our current results support these findings since both light‐ and vigorous‐intensity running increased c‐Fos expression in the dorsal DG, CA1, and CA3 sub‐regions (Figure 2C,D,F). In contrast, the dorsal CA2 sub‐region was not activated by running (Figure 2E). The CA2 is known as a small hippocampal sub‐region that modulates social memory and that is stimulated by novel encounters. 93 Since all rats were well familiarized with the experimental conditions and there were no novel or social stimuli, it is reasonable that activity in the CA2 sub‐region was not affected. Additionally, we examined the activation of the ventral hippocampus following running and found an increase of c‐Fos expression across all sub‐regions of the ventral hippocampus in both running groups (Figure S2). These results suggest that light‐intensity running is sufficient to elevate the neuronal activity in the hippocampus across the dorsal‐to‐ventral axis, which may help to explain the positive effects of exercise on dorsal‐hippocampus‐related functions, such as memory, as well as ventral‐hippocampus‐related anxiolytic and antidepressant effects. 3 , 35 , 94
Our in vivo microdialysis experiment provides the first evidence that even light‐intensity running increases the extracellular concentrations of NA and DA in the dorsal hippocampus (Figure 3E,F; Figure S3A,B). Given that NA and DA both perform critical roles in dorsal hippocampal neuronal activity, plasticity, and memory process, 65 , 66 , 68 , 69 , 95 , 96 , 97 our results suggest the possibility that the dorsal hippocampal NA and DA contribute to the light exercise‐induced hippocampal activation and its associated beneficial effects, such as hippocampal neurogenesis and spatial memory performance. 9 , 35 , 36 Notably, NA and DA have been recognized as important factors in memory consolidation, 68 , 96 as shown by the synaptic tagging and capture hypothesis, 98 , 99 raising a hypothesis that a single‐bout of light‐intensity running could facilitate memory retention through noradrenergic and dopaminergic dorsal hippocampal activation. Intriguingly, prior studies demonstrate that a single session of vigorous exercise elevates NA and DA concentrations in the hippocampus and promotes novel object recognition, which is a dorsal‐hippocampus‐related function, through the β adrenergic receptor and dopamine D1 receptor, respectively. 3 , 84 Although we did not directly examine this in the present study, these findings may support our hypothesis that light‐exercise‐elevated hippocampal NA and DA contribute to memory consolidation.
We confirmed that treadmill running, even at light intensity, activates LCTH and VTATH neurons (Figure 4D,E). Furthermore, our correlation analysis observed that LCTH activation positively correlates with dorsal hippocampal activation, and the activities of VTATH were positively associated with those of the dorsal DG and CA1 sub‐region (Figure 5). Additional research is needed, but these findings suggest a possible involvement of noradrenergic projections from the LC and dopaminergic projections from the VTA for dorsal hippocampal activation during running. Although our study didn't investigate the projections from light‐exercise‐activated TH+ neurons in the LC and the VTA to the dorsal hippocampus, interestingly, a recent study with in vivo two‐photon calcium imaging provides support for our hypothesis, which demonstrates an elevation in the activities of both the LC and the VTA axons within the dorsal CA1 in head‐fixed mice during voluntary running. 100 Both nuclei are known as major sources of dorsal hippocampal NA and DA, 69 , 70 , 101 but recent studies have shown that LC terminals co‐release DA and NA to the dorsal hippocampus, and that LC‐DA, but not LC‐NA, plays a significant role in spatial memory and learning of novel context. 68 , 69 , 70 , 71 , 95 , 102 On the other hand, VTATH neurons have sparse input to the dorsal hippocampus and do not have a significant impact on spatial memory. 68 , 69 However, hippocampal DA from VTATH neurons is still reported to be involved in hippocampus‐related functions, such as spatial memory and aversive memory. 75 , 76 , 103 The significance of LCTH and VTATH neurons for the dorsal hippocampal functions remains elusive; thus, future studies using viral‐tracing techniques and chemogenetic/optogenetic manipulation are needed to investigate the noradrenergic and dopaminergic mechanisms underlying the beneficial effects of light exercise.
Unlike the noradrenergic and the dopaminergic systems, extracellular 5‐HT concentration was not increased at the dorsal hippocampus, nor were DRN5‐HT or MRN5‐HT neurons found to be stimulated by treadmill running (Figures 3G and 4G,H; Figure S3C). However, we found a vigorous‐intensity‐running‐induced decrease in c‐Fos expression in the MRN5‐HT neurons and a negative correlation between c‐Fos expression in the DG and CA2 sub‐regions and that in DRN5‐HT neurons (Figures 4H and 5G,H). Previous studies have shown that running increases hippocampal neuronal activity and also facilitates hippocampal LTP induction. 104 , 105 , 106 Conversely, the hippocampal 5‐HT can exert inhibitory effects on basal neural activity and LTP induction. 107 , 108 , 109 Considering these findings, our results imply that running enhances hippocampal neuronal activity and LTP induction through the inhibitory control of the serotonergic inhibitory projections to the dorsal hippocampus.
Recent evidence suggests that physical exercise attenuates age‐related cognitive deficits and reduces the risk of dementia, including Alzheimer's disease. 110 , 111 , 112 Additionally, some studies report that light‐exercise‐based interventions have a positive effect on cognitive decline and dementia. 113 , 114 , 115 , 116 Compared to moderate‐to‐vigorous exercise interventions, this approach may become a more accessible and feasible strategy for the elderly, including those with dementia. Although the causes of dementia are complex and involve global dysfunction in various brain regions and circuits, reduced NA and DA levels have been identified as contributing factors. 117 , 118 , 119 Further investigations are needed, but given that dopaminergic and noradrenergic drugs can rescue or mitigate neuronal plasticity deficits and cognitive impairments, 117 , 119 , 120 , 121 , 122 this study proposes the possibility of light exercise as a potential non‐pharmacologic target to exert preventive or therapeutic effects on memory decline.
In this study, we performed all experiments using only male rats. This decision was made because we were concerned about the potential influence of sex differences and the estrous cycle on running exercise performance and the associated brain monoamine activity. 31 , 123 Further studies that include female rats are needed to explore these possibilities and ultimately provide a more comprehensive understanding of the neural mechanisms behind exercise‐induced hippocampal activation.
5. CONCLUSION
Collectively, our findings using a translational exercise model revealed that even light exercise stimulates the dorsal hippocampal neuronal activity and increases extracellular concentrations of NA and DA. Additionally, light exercise activates LCTH neurons and VTATH neurons, both of which are positively correlated with the activation of the dorsal hippocampus. In contrast, exercise does not activate the serotonergic system. These results suggest that light exercise stimulates dorsal hippocampal neurons, which are associated with the activation of LC‐noradrenergic neurons and VTA‐dopaminergic neurons (Figure 6). This may provide insight into the circuit mechanism that underlies light‐exercise‐enhanced learning and memory.
AUTHOR CONTRIBUTIONS
Taichi Hiraga and Hideaki Soya conceived and designed the experiments; Joshua P. Johansen provided the experimental animals; Taichi Hiraga and Kanako Takahashi performed the treadmill running experiments for LT measurement and validation of exercise intensity; Taichi Hiraga performed the treadmill running experiments for immunohistochemistry and microdialysis; Toshiaki Hata, Shingo Soya, Ryo Shimoda, Mariko Soya, Koshiro Inoue, and Masahiro Okamoto performed experimental assistances; Taichi Hiraga and Ryo Shimoda analyzed the data; Taichi Hiraga wrote the original manuscript, and all authors were involved in reviewing and editing the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
Supporting information
Supplemental data
ACKNOWLEDGMENTS
The authors are grateful to colleagues at the Laboratory of Exercise Biochemistry and Neuroendocrinology (Faculty of Health and Sport Sciences, University of Tsukuba, Japan), particularly R. Kuwamizu, H. Koizumi, T. Ferenc, F. Grenier, and M.J. Ikemoto for insightful scientific discussions. We thank J.P. Johansen (RIKEN CBS) for providing the experimental animals (TH‐Cre Long‐Evans rats line) and discussion. We would like to express our gratitude to M. Noguchi (ELCS English Language Consultation, Japan) for helping with the manuscript. This research was supported in part by KAKENHI Grants‐in‐Aid for Scientific Research (A) (18H04081; 21H04858; 24H00670) (to H.S.); KAKENHI Grants‐in‐Aid for Scientific Research on Innovative Areas: Next Generation Exercise Program for Developing Motivation, Body and Mind Performance (16H06405) (to H.S.); Japan Science and Technology Agency (JST)‐Mirai Program (JPMJMI19D5) (to H.S.); Grant‐in‐Aid for Japan Society for the Promotion of Science Fellowships (20J20887) (to T.H.).
Hiraga T, Hata T, Soya S, et al. Light‐exercise‐induced dopaminergic and noradrenergic stimulation in the dorsal hippocampus: Using a rat physiological exercise model. The FASEB Journal. 2024;38:e70215. doi: 10.1096/fj.202400418RRR
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (H.S.) upon reasonable request.
REFERENCES
- 1. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long‐term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427‐13431. doi: 10.1073/pnas.96.23.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017‐3022. doi: 10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vargas LS, Neves BHS, Roehrs R, Izquierdo I, Mello‐Carpes PB. One‐single physical exercise session after object recognition learning promotes memory persistence through hippocampal noradrenergic mechanisms. Behav Brain Res. 2017;329:120‐126. doi: 10.1016/j.bbr.2017.04.050 [DOI] [PubMed] [Google Scholar]
- 4. van Dongen EV, Kersten IHP, Wagner IC, Morris RGM, Fernández G. Physical exercise performed four hours after learning improves memory retention and increases hippocampal pattern similarity during retrieval. Curr Biol. 2016;26(13):1722‐1727. doi: 10.1016/J.CUB.2016.04.071 [DOI] [PubMed] [Google Scholar]
- 5. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7‐19. doi: 10.1016/j.neuron.2009.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oladehin A, Waters RS. Location and distribution of Fos protein expression in rat hippocampus following acute moderate aerobic exercise. Exp Brain Res. 2001;137(1):26‐35. doi: 10.1007/s002210000634 [DOI] [PubMed] [Google Scholar]
- 7. Lee TH, Jang MH, Shin MC, et al. Dependence of rat hippocampal c‐Fos expression on intensity and duration of exercise. Life Sci. 2003;72(12):1421‐1436. doi: 10.1016/S0024-3205(02)02406-2 [DOI] [PubMed] [Google Scholar]
- 8. Nishijima T, Soya H. Evidence of functional hyperemia in the rat hippocampus during mild treadmill running. Neurosci Res. 2006;54(3):186‐191. doi: 10.1016/J.NEURES.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 9. Soya H, Nakamura T, Deocaris CC, et al. BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun. 2007;358(4):961‐967. doi: 10.1016/j.bbrc.2007.04.173 [DOI] [PubMed] [Google Scholar]
- 10. Neeper SA, Góauctemez‐Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109. doi: 10.1038/373109a0 [DOI] [PubMed] [Google Scholar]
- 11. Vaynman S, Ying Z, Gomez‐Pinilla F. Interplay between brain‐derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic‐plasticity. Neuroscience. 2003;122(3):647‐657. doi: 10.1016/J.NEUROSCIENCE.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 12. Nishijima T, Okamoto M, Matsui T, Kita I, Soya H. Hippocampal functional hyperemia mediated by NMDA receptor/NO signaling in rats during mild exercise. J Appl Physiol. 2012;112(1):197‐203. doi: 10.1152/japplphysiol.00763.2011 [DOI] [PubMed] [Google Scholar]
- 13. Trejo JL, Carro E, Torres‐Alemán I. Circulating insulin‐like growth factor I mediates exercise‐induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21(5):1628‐1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC‐1α/FNDC5 pathway. Cell Metab. 2013;18(5):649‐659. doi: 10.1016/J.CMET.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Islam MR, Valaris S, Young MF, et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab. 2021;3(8):1058‐1070. doi: 10.1038/S42255-021-00438-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise‐linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med. 2019;25(1):165‐175. doi: 10.1038/S41591-018-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishijima T, Piriz J, Duflot S, et al. Neuronal activity drives localized blood‐brain‐barrier transport of serum insulin‐like growth factor‐I into the CNS. Neuron. 2010;67(5):834‐846. doi: 10.1016/j.neuron.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 18. Moon HY, Becke A, Berron D, et al. Running‐induced systemic Cathepsin B secretion is associated with memory function. Cell Metab. 2016;24(2):332‐340. doi: 10.1016/J.CMET.2016.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaynman S, Ying Z, Gomez‐Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20(10):2580‐2590. doi: 10.1111/J.1460-9568.2004.03720.X [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Luikart BW, Birnbaum S, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to Antidepressive treatment. Neuron. 2008;59(3):399‐412. doi: 10.1016/J.NEURON.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee MC, Okamoto M, Liu YF, et al. Voluntary resistance running with short distance enhances spatial memory related to hippocampal BDNF signaling. J Appl Physiol. 2012;113(8):1260‐1266. doi: 10.1152/japplphysiol.00869.2012 [DOI] [PubMed] [Google Scholar]
- 22. American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 10th ed. Wolters Kluwer Health; 2018. [DOI] [PubMed] [Google Scholar]
- 23. Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise‐induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104(13):5638‐5643. doi: 10.1073/PNAS.0611721104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soya H, Mukai A, Deocaris CC, et al. Threshold‐like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58(4):341‐348. doi: 10.1016/j.neures.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 25. Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: to crack the 40‐year‐old nut, replace the 40‐year‐old nutcracker! Ann Behav Med. 2008;35(2):136‐149. doi: 10.1007/S12160-008-9025-Z [DOI] [PubMed] [Google Scholar]
- 26. Saito T, Soya H. Delineation of responsive AVP‐containing neurons to running stress in the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2004;286:R484‐R490. doi: 10.1152/ajpregu.00453.2003 [DOI] [PubMed] [Google Scholar]
- 27. Hill EE, Zack E, Battaglini C, Viru M, Viru A, Hackney AC. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Investig. 2008;31(7):587‐591. doi: 10.1007/BF03345606 [DOI] [PubMed] [Google Scholar]
- 28. Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21(5):452‐458. doi: 10.1037/0278-6133.21.5.452 [DOI] [PubMed] [Google Scholar]
- 29. Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol. 1989;66(3):1250‐1257. doi: 10.1152/JAPPL.1989.66.3.1250 [DOI] [PubMed] [Google Scholar]
- 30. Greenwood BN, Foley TE, Le TV, et al. Long‐term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354‐362. doi: 10.1016/J.BBR.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuller KNZ, Thyfault JP. Barriers in translating preclinical rodent exercise metabolism findings to human health. J Appl Physiol. 2021;130(1):182‐192. doi: 10.1152/JAPPLPHYSIOL.00683.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inoue K, Soya H, Murakumo K, et al. Setting treadmill intensity for rat aerobic training using lactate and gas exchange thresholds. Med Sci Sports Exerc. 2024; Published online. doi: 10.1249/MSS.0000000000003562 [DOI] [PubMed] [Google Scholar]
- 33. Ganong WF, Barrett KE. Ganong's Review of Medical Physiology. 23rd ed. McGraw‐Hill Medical; 2009. [Google Scholar]
- 34. Pacák K. Stressor‐specific activation of the hypothalamic‐pituitary‐ adrenocortical axis. Physiol Res. 2000;49(SUPPL. 1):S11‐S17. [PubMed] [Google Scholar]
- 35. Inoue K, Hanaoka Y, Nishijima T, et al. Long‐term mild exercise training enhances hippocampus‐dependent memory in rats. Int J Sports Med. 2015;36(4):280‐285. doi: 10.1055/S-0034-1390465 [DOI] [PubMed] [Google Scholar]
- 36. Inoue K, Okamoto M, Shibato J, et al. Long‐term mild, rather than intense, exercise enhances adult hippocampal neurogenesis and greatly changes the transcriptomic profile of the hippocampus. PLoS One. 2015;10(6):e0128720. doi: 10.1371/JOURNAL.PONE.0128720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Okamoto M, Hojo Y, Inoue K, et al. Mild exercise increases dihydrotestosterone in hippocampus providing evidence for androgenic mediation of neurogenesis. Proc Natl Acad Sci USA. 2012;109(32):13100‐13105. doi: 10.1073/pnas.1210023109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(5):742‐755. doi: 10.1016/J.PNPBP.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 39. Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233(1):12‐21. doi: 10.1016/J.EXPNEUROL.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suwabe K, Byun K, Hyodo K, et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc Natl Acad Sci USA. 2018;115(41):10487‐10492. doi: 10.1073/pnas.1805668115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McMorris T. Developing the catecholamines hypothesis for the acute exercise‐cognition interaction in humans: lessons from animal studies. Physiol Behav. 2016;165:291‐299. doi: 10.1016/J.PHYSBEH.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 42. Cooper CJ. Anatomical and physiological mechanisms of arousal, with special reference to the effects of exercise. Ergonomics. 1973;16(5):601‐609. doi: 10.1080/00140137308924551 [DOI] [PubMed] [Google Scholar]
- 43. Dietrich A, Audiffren M. The reticular‐activating hypofrontality (RAH) model of acute exercise. Neurosci Biobehav Rev. 2011;35(6):1305‐1325. doi: 10.1016/J.NEUBIOREV.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 44. Jones BE. Arousal systems. Front Biosci. 2003;8(SUPPL):438‐451. doi: 10.2741/1074 [DOI] [PubMed] [Google Scholar]
- 45. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726‐731. doi: 10.1016/S0166-2236(00)02002-6 [DOI] [PubMed] [Google Scholar]
- 46. Kuwamizu R, Yamazaki Y, Aoike N, Ochi G, Suwabe K, Soya H. Pupil‐linked arousal with very light exercise: pattern of pupil dilation during graded exercise. J Physiol Sci. 2022;72(1):23. doi: 10.1186/S12576-022-00849-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamazaki Y, Suwabe K, Nagano‐Saito A, et al. A possible contribution of the locus coeruleus to arousal enhancement with mild exercise: evidence from pupillometry and neuromelanin imaging. Cereb Cortex Commun. 2023;4(2):tgad010. doi: 10.1093/TEXCOM/TGAD010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kuwamizu R, Yamazaki Y, Aoike N, et al. Pupil dynamics during very light exercise predict benefits to prefrontal cognition. NeuroImage. 2023;277:120244. doi: 10.1016/J.NEUROIMAGE.2023.120244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suwabe K, Kuwamizu R, Hyodo K, et al. Improvement of mnemonic discrimination with acute light exercise is mediated by pupil‐linked arousal in healthy older adults. Neurobiol Aging. 2024;133:107‐114. doi: 10.1016/J.NEUROBIOLAGING.2023.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Reimer J, McGinley MJ, Liu Y, et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun. 2016;7:13289. doi: 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cazettes F, Reato D, Morais JP, Renart A, Mainen ZF. Phasic activation of dorsal raphe serotonergic neurons increases pupil size. Curr Biol. 2021;31(1):192‐197.e4. doi: 10.1016/J.CUB.2020.09.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zerbi V, Floriou‐Servou A, Markicevic M, et al. Rapid reconfiguration of the functional connectome after Chemogenetic locus Coeruleus activation. Neuron. 2019;103(4):702‐718.e5. doi: 10.1016/J.NEURON.2019.05.034 [DOI] [PubMed] [Google Scholar]
- 53. de Gee JW, Colizoli O, Kloosterman NA, Knapen T, Nieuwenhuis S, Donner TH. Dynamic modulation of decision biases by brainstem arousal systems. eLife. 2017;6:e23232. doi: 10.7554/ELIFE.23232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526‐1535. doi: 10.1038/nn.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bari A, Xu S, Pignatelli M, et al. Differential attentional control mechanisms by two distinct noradrenergic coeruleo‐frontal cortical pathways. Proc Natl Acad Sci USA. 2020;117(46):29080‐29089. doi: 10.1073/pnas.2015635117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saunders BT, Richard JM, Margolis EB, Janak PH. Dopamine neurons create Pavlovian conditioned stimuli with circuit‐defined motivational properties. Nat Neurosci. 2018;21(8):1072‐1083. doi: 10.1038/s41593-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhu X, Ottenheimer D, DiLeone RJ. Activity of D1/2 receptor expressing neurons in the nucleus Accumbens regulates running, locomotion, and food intake. Front Behav Neurosci. 2016;10:66. doi: 10.3389/fnbeh.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim SY, Adhikari A, Lee SY, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496(7444):219‐223. doi: 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warden MR, Selimbeyoglu A, Mirzabekov JJ, et al. A prefrontal cortex–brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428‐432. doi: 10.1038/nature11617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Teissier A, Chemiakine A, Inbar B, et al. Activity of Raphé serotonergic neurons controls emotional behaviors. Cell Rep. 2015;13(9):1965‐1976. doi: 10.1016/J.CELREP.2015.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palacios‐Filardo J, Mellor JR. Neuromodulation of hippocampal long‐term synaptic plasticity. Curr Opin Neurobiol. 2019;54:37‐43. doi: 10.1016/J.CONB.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poe GR, Foote S, Eschenko O, et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. 2020;21(11):644‐659. doi: 10.1038/s41583-020-0360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang SH, Redondo RL, Morris RGM. Relevance of synaptic tagging and capture to the persistence of long‐term potentiation and everyday spatial memory. Proc Natl Acad Sci USA. 2010;107(45):19537‐19542. doi: 10.1073/PNAS.1008638107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mahar I, Bambico FR, Mechawar N, Nobrega JN. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev. 2014;38:173‐192. doi: 10.1016/J.NEUBIOREV.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 65. Coradazzi M, Gulino R, Fieramosca F, Falzacappa LV, Riggi M, Leanza G. Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol Aging. 2016;48:93‐102. doi: 10.1016/J.NEUROBIOLAGING.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 66. Salvi R, Steigleder T, Schlachetzki JCM, et al. Distinct effects of chronic dopaminergic stimulation on hippocampal neurogenesis and striatal doublecortin expression in adult mice. Front Neurosci. 2016;10:77. doi: 10.3389/FNINS.2016.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schwarz LA, Miyamichi K, Gao XJ, et al. Viral‐genetic tracing of the input‐output organization of a central noradrenaline circuit. Nature. 2015;524(7563):88‐92. doi: 10.1038/nature14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takeuchi T, Duszkiewicz AJ, Sonneborn A, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537(7620):357‐362. doi: 10.1038/nature19325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gálvez‐Márquez DK, Salgado‐Ménez M, Moreno‐Castilla P, et al. Spatial contextual recognition memory updating is modulated by dopamine release in the dorsal hippocampus from the locus coeruleus. Proc Natl Acad Sci USA. 2022;119(49):e2208254119. doi: 10.1073/PNAS.2208254119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci USA. 2016;113(51):14835‐14840. doi: 10.1073/pnas.1616515114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wagatsuma A, Okuyama T, Sun C, Smith LM, Abe K, Tonegawa S. Locus coeruleus input to hippocampal CA3 drives single‐trial learning of a novel context. Proc Natl Acad Sci USA. 2018;115(2):E310‐E316. doi: 10.1073/pnas.1714082115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gasbarri A, Packard MG, Campana E, Pacitti C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res Bull. 1994;33(4):445‐452. doi: 10.1016/0361-9230(94)90288-7 [DOI] [PubMed] [Google Scholar]
- 73. Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C. Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res. 1994;668(1–2):71‐79. doi: 10.1016/0006-8993(94)90512-6 [DOI] [PubMed] [Google Scholar]
- 74. Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1–6):321‐353. doi: 10.1016/0361-9230(82)90145-9 [DOI] [PubMed] [Google Scholar]
- 75. Tsetsenis T, Badyna JK, Wilson JA, et al. Midbrain dopaminergic innervation of the hippocampus is sufficient to modulate formation of aversive memories. Proc Natl Acad Sci USA. 2021;118(40):e2111069118. doi: 10.1073/PNAS.2111069118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. McNamara CG, Tejero‐Cantero Á, Trouche S, Campo‐Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17(12):1658‐1660. doi: 10.1038/nn.3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Muzerelle A, Scotto‐Lomassese S, Bernard JF, Soiza‐Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct Funct. 2016;221(1):535‐561. doi: 10.1007/S00429-014-0924-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Luchetti A, Bota A, Weitemier A, et al. Two functionally distinct serotonergic projections into hippocampus. J Neurosci. 2020;40(25):4936‐4944. doi: 10.1523/jneurosci.2724-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Morikawa R, Kubota N, Amemiya S, Nishijima T, Kita I. Interaction between intensity and duration of acute exercise on neuronal activity associated with depression‐related behavior in rats. J Physiol Sci. 2021;71(1):1. doi: 10.1186/s12576-020-00788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tsai SF, Liu YW, Kuo YM. Acute and long‐term treadmill running differentially induce c‐Fos expression in region‐ and time‐dependent manners in mouse brain. Brain Struct Funct. 2019;224:2677‐2689. doi: 10.1007/s00429-019-01926-5 [DOI] [PubMed] [Google Scholar]
- 81. Rodovalho GV, Drummond LR, Coimbra CC. Involvement of brainstem noradrenergic system in cutaneous heat loss during exercise. Brain Res Bull. 2020;164:372‐379. doi: 10.1016/J.BRAINRESBULL.2020.08.029 [DOI] [PubMed] [Google Scholar]
- 82. Goekint M, Bos I, Heyman E, Meeusen R, Michotte Y, Sarre S. Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain‐derived neurotrophic factor. J Appl Physiol. 2012;112(4):535‐541. doi: 10.1152/japplphysiol.00306.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gomez‐Merino D, Béquet F, Berthelot M, Chennaoui M, Guezennec CY. Site‐dependent effects of an acute intensive exercise on extracellular 5‐HT and 5‐HIAA levels in rat brain. Neurosci Lett. 2001;301(2):143‐146. doi: 10.1016/S0304-3940(01)01626-3 [DOI] [PubMed] [Google Scholar]
- 84. Vargas LS, Ramires Lima K, Piaia Ramborger B, Roehrs R, Izquierdo I, Mello‐Carpes PB. Catecholaminergic hippocampal activation is necessary for object recognition memory persistence induced by one‐single physical exercise session. Behav Brain Res. 2020;379:112356. doi: 10.1016/j.bbr.2019.112356 [DOI] [PubMed] [Google Scholar]
- 85. Takahashi K, Shima T, Soya M, et al. Exercise‐induced adrenocorticotropic hormone response is cooperatively regulated by hypothalamic arginine vasopressin and corticotrophin‐releasing hormone. Neuroendocrinology. 2021;112:894‐903. doi: 10.1159/000521237 [DOI] [PubMed] [Google Scholar]
- 86. Beaver WL, Wasserman K, Whipp BJ. Improved detection of lactate threshold during exercise using a log‐log transformation. J Appl Physiol. 1985;59(6):1936‐1940. doi: 10.1152/jappl.1985.59.6.1936 [DOI] [PubMed] [Google Scholar]
- 87. Shima T, Takashi M, Jesmin S, et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia. 2016;60(3):597‐606. doi: 10.1007/s00125-016-4164-4 [DOI] [PubMed] [Google Scholar]
- 88. Saiki M, Matsui T, Soya M, et al. Thiamine tetrahydrofurfuryl disulfide promotes voluntary activity through dopaminergic activation in the medial prefrontal cortex. Sci Rep. 2018;8(1):10469. doi: 10.1038/s41598-018-28462-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Titulaer J, Björkholm C, Feltmann K, et al. The importance of ventral hippocampal dopamine and norepinephrine in recognition memory. Front Behav Neurosci. 2021;15:667244. doi: 10.3389/fnbeh.2021.667244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Borgkvist A, Malmlöf T, Feltmann K, Lindskog M, Schilström B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol. 2012;15(4):531‐540. doi: 10.1017/S1461145711000812 [DOI] [PubMed] [Google Scholar]
- 91. Gasbarri A, Packard M, Sulli A, Pacitti C, Innocenzi R, Perciavalle V. The projections of the retrorubral field A8 to the hippocampal formation in the rat. Exp Brain Res. 1996;112(2):244‐252. doi: 10.1007/BF00227643 [DOI] [PubMed] [Google Scholar]
- 92. Lin R, Liang J, Luo M. The raphe dopamine system: roles in salience encoding, memory expression, and addiction. Trends Neurosci. 2021;44(5):366‐377. doi: 10.1016/J.TINS.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 93. Chen S, He L, Huang AJY, et al. A hypothalamic novelty signal modulates hippocampal memory. Nature. 2020;586(7828):270‐274. doi: 10.1038/s41586-020-2771-1 [DOI] [PubMed] [Google Scholar]
- 94. Otsuka T, Nishii A, Amemiya S, Kubota N, Nishijima T, Kita I. Effects of acute treadmill running at different intensities on activities of serotonin and corticotropin‐releasing factor neurons, and anxiety‐ and depressive‐like behaviors in rats. Behav Brain Res. 2016;298:44‐51. doi: 10.1016/j.bbr.2015.10.055 [DOI] [PubMed] [Google Scholar]
- 95. Tse D, Privitera L, Norton AC, et al. Cell‐type‐specific optogenetic stimulation of the locus coeruleus induces slow‐onset potentiation and enhances everyday memory in rats. Proc Natl Acad Sci USA. 2023;120(46):e2307275120. doi: 10.1073/PNAS.2307275120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mello‐Carpes PB, da Silva de Vargas L, Gayer MC, Roehrs R, Izquierdo I. Hippocampal noradrenergic activation is necessary for object recognition memory consolidation and can promote BDNF increase and memory persistence. Neurobiol Learn Mem. 2016;127:84‐92. doi: 10.1016/J.NLM.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 97. Takamura N, Nakagawa S, Masuda T, et al. The effect of dopamine on adult hippocampal neurogenesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:116‐124. doi: 10.1016/J.PNPBP.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 98. Frey U, Morris RGM. Synaptic tagging and long‐term potentiation. Nature. 1997;385(6616):533‐536. doi: 10.1038/385533a0 [DOI] [PubMed] [Google Scholar]
- 99. Redondo RL, Morris RGM. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12(1):17‐30. doi: 10.1038/nrn2963 [DOI] [PubMed] [Google Scholar]
- 100. Heer CM, Sheffield MEJ. Distinct catecholaminergic pathways projecting to hippocampal CA1 transmit contrasting signals during behavior and learning. eLife. 2024;13:RP95213. doi: 10.7554/ELIFE.95213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Weitemier AZ, McHugh TJ. Noradrenergic modulation of evoked dopamine release and pH shift in the mouse dorsal hippocampus and ventral striatum. Brain Res. 2017;1657:74‐86. doi: 10.1016/J.BRAINRES.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 102. Chowdhury A, Luchetti A, Fernandes G, et al. A locus coeruleus‐dorsal CA1 dopaminergic circuit modulates memory linking. Neuron. 2022;110(20):3374‐3388.e8. doi: 10.1016/J.NEURON.2022.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kramar CP, Castillo‐Díaz F, Gigante ED, Medina JH, Barbano MF. The late consolidation of an aversive memory is promoted by VTA dopamine release in the dorsal hippocampus. Eur J Neurosci. 2021;53(3):841‐851. doi: 10.1111/EJN.15076 [DOI] [PubMed] [Google Scholar]
- 104. Miladi‐Gorji H, Rashidy‐Pour A, Fathollahi Y, Semnanian S, Jadidi M. Effects of voluntary exercise on hippocampal long‐term potentiation in morphine‐dependent rats. Neuroscience. 2014;256:83‐90. doi: 10.1016/J.NEUROSCIENCE.2013.09.056 [DOI] [PubMed] [Google Scholar]
- 105. Radahmadi M, Hosseini N, Alaei H. Effect of exercise, exercise withdrawal, and continued regular exercise on excitability and long‐term potentiation in the dentate gyrus of hippocampus. Brain Res. 2016;1653:8‐13. doi: 10.1016/j.brainres.2016.09.045 [DOI] [PubMed] [Google Scholar]
- 106. Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague‐dawley rats in vivo. Neuroscience. 2004;124(1):71‐79. doi: 10.1016/j.neuroscience.2003.09.029 [DOI] [PubMed] [Google Scholar]
- 107. Villani F, Johnston D. Serotonin inhibits induction of long‐term potentiation at commissural synapses in hippocampus. Brain Res. 1993;606(2):304‐308. doi: 10.1016/0006-8993(93)90998-3 [DOI] [PubMed] [Google Scholar]
- 108. Shakesby AC, Anwyl R, Rowan MJ. Overcoming the effects of stress on synaptic plasticity in the intact hippocampus: rapid actions of serotonergic and antidepressant agents. J Neurosci. 2002;22(9):3638‐3644. doi: 10.1523/JNEUROSCI.22-09-03638.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kulla A, Manahan‐Vaughan D. Modulation by serotonin 5‐HT4 receptors of long‐term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb Cortex. 2002;12(2):150‐162. doi: 10.1093/cercor/12.2.150 [DOI] [PubMed] [Google Scholar]
- 110. Voss MW, Soto C, Yoo S, Sodoma M, Vivar C, van Praag H. Exercise and hippocampal memory systems. Trends Cogn Sci. 2019;23(4):318‐333. doi: 10.1016/J.TICS.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta‐analysis. Br J Sports Med. 2018;52(3):154‐160. doi: 10.1136/BJSPORTS-2016-096587 [DOI] [PubMed] [Google Scholar]
- 112. Jia RX, Liang JH, Xu Y, Wang YQ. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: a meta‐analysis. BMC Geriatr. 2019;19(1):1‐14. doi: 10.1186/S12877-019-1175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yoon M, Yang PS, Jin MN, et al. Association of physical activity level with risk of dementia in a nationwide cohort in Korea. JAMA Netw Open. 2021;4(12):e2138526. doi: 10.1001/JAMANETWORKOPEN.2021.38526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tse ACY, Wong TWL, Lee PH. Effect of low‐intensity exercise on physical and cognitive health in older adults: a systematic review. Sport Med Open. 2015;1(1):37. doi: 10.1186/S40798-015-0034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rangasamy SB, Jana M, Dasarathi S, Kundu M, Pahan K. Treadmill workout activates PPARα in the hippocampus to upregulate ADAM10, decrease plaques and improve cognitive functions in 5XFAD mouse model of Alzheimer's disease. Brain Behav Immun. 2023;109:204‐218. doi: 10.1016/J.BBI.2023.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447‐1453. doi: 10.1001/JAMA.292.12.1447 [DOI] [PubMed] [Google Scholar]
- 117. Nobili A, Latagliata EC, Viscomi MT, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease. Nat Commun. 2017;8:14727. doi: 10.1038/ncomms14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rorabaugh JM, Chalermpalanupap T, Botz‐Zapp CA, et al. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer's disease. Brain. 2017;140(11):3023‐3038. doi: 10.1093/brain/awx232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Francis BM, Yang J, Hajderi E, et al. Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37(8):1934‐1944. doi: 10.1038/npp.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Hammerschmidt T, Kummer MP, Terwel D, et al. Selective loss of noradrenaline exacerbates early cognitive dysfunction and synaptic deficits in APP/PS1 mice. Biol Psychiatry. 2013;73(5):454‐463. doi: 10.1016/J.BIOPSYCH.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kalinin S, Polak PE, Lin SX, Sakharkar AJ, Pandey SC, Feinstein DL. The noradrenaline precursor L‐DOPS reduces pathology in a mouse model of Alzheimer's disease. Neurobiol Aging. 2012;33(8):1651‐1663. doi: 10.1016/J.NEUROBIOLAGING.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. David MCB, Del Giovane M, Liu KY, et al. Cognitive and neuropsychiatric effects of noradrenergic treatment in Alzheimer's disease: systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2022;93(10):1080‐1090. doi: 10.1136/JNNP-2022-329136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rosenfeld CS. Sex‐dependent differences in voluntary physical activity. J Neurosci Res. 2017;95(1–2):279‐290. doi: 10.1002/JNR.23896 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (H.S.) upon reasonable request.


