Abstract
Aim
This study: (1) estimated the effect of early discontinuation of medication for opioid use disorder (MOUD) on overdose probability and (2) measured the relationship between patient characteristics and early discontinuation probability for each MOUD type.
Design, setting and participants
This was a retrospective cohort using electronic health record data from the US Veterans Healthcare Administration. Participants were veterans initiating MOUD with buprenorphine (BUP), methadone (MET) or extended‐release naltrexone (XR‐NTX) from fiscal years 2012–19. A total of 39 284 veterans met eligibility with 22 721 (57.8%) initiating BUP, 12 652 (32.2%) initiating MET and 3911 (10.0%) initiating XR‐NTX.
Measurements
Measurements (1) determined whether the veteran experienced an overdose in the 365 days after MOUD initiation (primary) and (2) early discontinuation of MOUD, defined as discontinuation before 180 days (secondary). We assumed that unobserved patient characteristics would jointly influence the probability of discontinuation and overdose. and estimated the joint distribution with a bivariate probit model.
Findings
We found that 9.0% of BUP initiators who experienced an overdose above the predicted 3.9% had no veteran‐discontinued BUP early; findings for XR‐NTX were similar, with 12.2% of initiators overdosing above the predicted 4.5%, but this was statistically inconclusive. We found no relationship between early discontinuation and overdose for MET initiators, probably due to the high risk of both events. The patient characteristics included in our post‐estimation exploratory analysis of early discontinuation varied by MOUD type, with between 14 (XR‐NTX) and 25 (BUP) tested. The only characteristics with at least one level showing a statistically significant change in probability of early discontinuation for all three MOUD types were geography and prior‐year exposure to psychotherapy, although direction and magnitude varied.
Conclusion
Early discontinuation of buprenorphine, and probably extended‐release naltrexone, appears to be associated with a greater probability of experiencing a fatal or non‐fatal overdose among US veterans receiving medication for opioid use disorder (MOUD); methadone does not show the same association. There is no consistent set of characteristics among early discontinuers by MOUD type.
Keywords: Geographic disparities, medication treatment for opioid use disorder, opioid use disorder, overdose, racial disparities, veterans
INTRODUCTION
The opioid crisis is one of the most important public health emergencies of our time [1, 2, 3]. In the decade following 2010 overdose deaths in the United States increased nearly fourfold, with opioid overdoses accounting for three‐quarters of these deaths [3, 4]. Among US military veterans, drug overdose mortality rates increased 53% from 2010 to 2019 [5]. Medication for opioid use disorder (MOUD) includes maintenance treatment with methadone (MET, a full opioid agonist), buprenorphine (BUP, a partial opioid agonist) and extended‐release naltrexone (XR‐NTX, an opioid antagonist). MOUD is the standard of care because it reduces opioid‐related mortality and morbidity [6, 7, 8, 9, 10, 11, 12, 13]. However, early MOUD discontinuation can have swift and severe consequences for patient health [14]; for example, mortality increases sixfold in the 4 weeks following MOUD discontinuation [13]. Unfortunately, only 50–60% of patients remain on MOUD for the recommended minimum of 6 months, a minimum duration of treatment set by the Health Effectiveness Data and Information Set (HEDIS) and National Committee on Quality Assurance [15, 16, 17, 18].
A recent Agency for Healthcare Research and Quality review found no evidence‐based practices for mitigating early MOUD discontinuation for agonist/partial agonist therapy and only some evidence that one intervention (contingency management) may mitigate early MOUD discontinuation for antagonist therapy [19]. In addition to developing interventions, a possible approach to mitigating early MOUD discontinuation would be using patient characteristics to select the MOUD with which patients are most likely to be successful. For example, there is some evidence that patients with depression might benefit more from BUP than from MET [20]. There is also evidence that individuals who are White or used cocaine at baseline respond better to MET than to BUP [21]. Evidence from a recent randomized comparative‐effectiveness trial found that unhoused patients on XR‐NTX have lower discontinuation rates, while patients with permanent shelter had lower discontinuation rates with BUP [22]. Secondary analyses of clinical trials have attempted to identify patient characteristics associated with better treatment outcomes (e.g. retention, abstinence); however, clinical trials often exclude conditions prevalent in clinical populations, such as co‐occurring substance use disorders, that may be predictive of treatment outcomes. Therefore, scant evidence exists on which to personalize the selection of the type of MOUD to mitigate early MOUD discontinuation [23, 24, 25, 26].
Each type of MOUD provides differing advantages, disadvantages and challenges that can diminish or enhance its efficacy [27]. Qualitative data suggest that a mismatch between MOUD type initiated and patient characteristics or preferences could lead to early discontinuation. For example, withdrawal, the lack of adequate pain control or the ability to have telemedicine appointments have been reasons identified for early discontinuation that vary by MOUD type [28]. Clinicians need more information to help minimize the probability of discontinuation and overdose once initiated on a given type of MOUD. However, limited evidence exists regarding how the probability of early MOUD discontinuation could be minimized with greater personalization of MOUD following initiation on a given type of MOUD, which is vital for veterans, given the substantial increase in drug overdose mortality rates over the past decade.
Using real‐world data from the Veterans Health Administration (VHA), the largest integrated health‐care system and largest OUD treatment provider in the United States [29], this study estimates the association between early discontinuation of MOUD and the probability of experiencing a fatal or non‐fatal overdose for each of the three MOUD types: BUP, MET and XR‐NTX. We hypothesized that veterans who discontinued MOUD early would have a higher probability of experiencing a fatal or non‐fatal overdose compared to those who were retained on treatment for at least 180 days among all MOUD types; however, we also hypothesized that the magnitude of the probability would differ by MOUD type, as early discontinuation rates have been shown recently to differ by MOUD type [30]. In an exploratory analysis, we also sought to quantify the association between patient characteristics and the probability of early discontinuation by MOUD type to support future MOUD personalization efforts.
METHODS
Study design and data sources
This study was a National Drug Abuse Treatment Clinical Trials Network study (CTN‐0142) [31, 32]. The analysis plan was pre‐specified and is available at the National Drug Abuse Treatment Clinical Trials Network Dissemination Library. CTN‐0142 had two objectives; this paper reports on the findings from the first objective.
This retrospective cohort study used VHA's Corporate Data Warehouse (CDW) linked with the VHA/DoD Mortality Data Repository [33]. The primary (distal) outcome was overdose within 1 year of MOUD initiation. The exposure of interest was discontinuation of MOUD before completing 180 days of therapy (‘early discontinuation’). The exposure was also treated as a secondary (proximal) outcome of interest, as we sought to explore patient factors associated with early discontinuation. The analysis plan was pre‐specified and is available at the National Drug Abuse Treatment Clinical Trials Network Dissemination Library (CTN‐0142) [31, 32]. However, as the analysis plan was not pre‐registered, the results should be considered exploratory.
The CDW contains a wide range of abstracted electronic health record (EHR) data, together with detailed personal data collected at the time the veteran enrolled in VHA, during eligibility assessment or review, and demographic information collected or confirmed during health‐care encounters. The CDW also contains treating facility and provider characteristics from VHA administrative data. In addition to linking with the Mortality Data Repository, we linked veteran ZIP codes to Rural–Urban Commuting Area (RUCA) codes [34] and linked veteran county to the Social Vulnerability Index (SVI) from the US Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (CDC/ATSDR) [35, 36].
The study was approved by the Central Arkansas Veterans Healthcare System Institutional Review Board. We report results using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [37].
Study population and cohort identification
The study was set in all VHA facilities that were operational in the United States at any time during the study, providing geographic representation for all 50 States. We screened all VHA‐enrolled US military veterans diagnosed with OUD between 1 October 2011 and 30 September 2019 (fiscal years 2012–19) who also received any form of MOUD. OUD diagnosis was operationalized following a previously published algorithm [38]. When confirming eligibility, we restricted prescription or dispensation records only to initiations of BUP, MET or XR‐NTX, where initiation was defined as the start of a treatment episode with no exposure to MOUD in the 6 months prior to the fill or administration date. We adopted the date of MOUD initiation as the index date. Diagnosis codes for OUD and details on the creation of initiation episodes can be found in the Supporting information, Table S1.
To be eligible for the study, veterans had to (a) have a MOUD prescription or administration record after an OUD diagnosis within the study period [39, 40, 41], (b) be aged 18 years or older at initiation and (c) reside in the United States. We excluded veterans (a) for whom we could not determine the date when enrollment was completed, (b) who were not regular users of VHA or (c) who received MOUD from a non‐VHA source. We made additional exclusions of otherwise eligible veterans if the index date did not permit a full 365 days of historical observation and follow‐up. The processes for data checking and manipulation are described in the Supporting information.
We defined three cohorts based on the type of treatment initiated. We used a previously validated VHA‐specific algorithm to identify the type of MOUD used from a variety of record types [39]. Our final analytical sample was 22 721 BUP initiators, 12 652 MET initiators and 3911 XR‐NTX initiators (Supporting information, Figure S1). Table 1 provides sample characteristics for each cohort. Because all analyses are within cohort, we did not test for statistical differences in sample characteristics across cohorts.
TABLE 1.
Characteristics of veterans, providers and facilities by type of MOUD initiated.
| Type of MOUD initiated | ||||||
|---|---|---|---|---|---|---|
| BUP | MET | XR‐NTX | ||||
| N = 39 284 | n = 22 721 | n = 12 652 | n = 3911 | |||
| Discontinued MOUD before 180 days (n, %) | 11 464 | (50.5) | 10 721 | (84.7) | 3343 | (85.5) |
| Overdose in the year after initiation (n, %) | 2036 | (9.0) | 1007 | (8.0) | 477 | (12.2) |
| MOUD fiscal year (n, %) | ||||||
| 2012 | 2311 | (10.2) | 1877 | (14.8) | 78 | (2.0) |
| 2013 | 2480 | (10.9) | 1682 | (13.3) | 118 | (3.0) |
| 2014 | 2503 | (11.0) | 1511 | (11.9) | 165 | (4.2) |
| 2015 | 2698 | (11.9) | 1533 | (12.1) | 356 | (9.1) |
| 2016 | 2841 | (12.5) | 1947 | (15.4) | 599 | (15.3) |
| 2017 | 2915 | (12.8) | 1488 | (11.8) | 780 | (19.9) |
| 2018 | 3215 | (14.1) | 1396 | (11.0) | 958 | (24.5) |
| 2019 | 3758 | (16.5) | 1218 | (9.6) | 857 | (21.9) |
| Age (years; mean, SD) | 46.09 | (14.46) | 50.86 | (13.96) | 46.45 | (13.07) |
| Sex (n, %) | ||||||
| Male | 20 921 | (92.1) | 11 815 | (93.4) | 3615 | (92.4) |
| Self‐identified race (n, %) | ||||||
| American Indian or Alaska Native | 164 | (0.7) | 102 | (0.8) | 45 | (1.2) |
| Asian | 67 | (0.3) | 45 | (0.4) | 8 | (0.2) |
| Black or African American | 2680 | (11.8) | 3874 | (30.6) | 626 | (16.0) |
| Native Hawaiian or Other Pacific Islander | 124 | (0.5) | 60 | (0.5) | 15 | (0.4) |
| White | 18 332 | (80.7) | 7857 | (62.1) | 3030 | (77.5) |
| More than one race | 195 | (0.9) | 129 | (1.0) | 33 | (0.8) |
| Declined to answer | 812 | (3.6) | 373 | (2.9) | 108 | (2.8) |
| Unknown by patient | 233 | (1.0) | 136 | (1.1) | 36 | (0.9) |
| Not recorded | 114 | (0.5) | 76 | (0.6) | 10 | (0.3) |
| Self‐identified ethnicity (n, %) | ||||||
| Hispanic or Latino | 1130 | (5.0) | 732 | (5.8) | 197 | (5.0) |
| Not Hispanic or Latino | 20 744 | (91.3) | 11 483 | (90.8) | 3590 | (91.8) |
| Declined to answer | 526 | (2.3) | 253 | (2.0) | 77 | (2.0) |
| Unknown by patient | 297 | (1.3) | 162 | (1.3) | 44 | (1.1) |
| Not recorded | 24 | (0.1) | 22 | (0.2) | 3 | (0.1) |
| Marital status (n, %) | ||||||
| Never married | 5693 | (25.1) | 3439 | (27.2) | 1163 | (29.7) |
| Married | 6873 | (30.2) | 3187 | (25.2) | 900 | (23.0) |
| Separated | 1759 | (7.7) | 1142 | (9.0) | 331 | (8.5) |
| Divorced | 7659 | (33.7) | 4415 | (34.9) | 1416 | (36.2) |
| Widowed | 646 | (2.8) | 439 | (3.5) | 87 | (2.2) |
| Unknown | 91 | (0.4) | 30 | (0.2) | 14 | (0.4) |
| Employment status (n, %) | ||||||
| Employed for others | 4391 | (19.3) | 2084 | (16.5) | 706 | (18.1) |
| Self‐employed | 322 | (1.4) | 157 | (1.2) | 37 | (0.9) |
| Not employed | 14 545 | (64.0) | 8855 | (70.0) | 2703 | (69.1) |
| Retired | 1947 | (8.6) | 1037 | (8.2) | 234 | (6.0) |
| Unknown | 1516 | (6.7) | 519 | (4.1) | 231 | (5.9) |
| Priority group (n, %) | ||||||
| 1 | 9466 | (41.7) | 4355 | (34.4) | 1800 | (46.0) |
| 2–3 | 3843 | (16.9) | 1933 | (15.3) | 604 | (15.4) |
| 4 | 1024 | (4.5) | 1041 | (8.2) | 346 | (8.8) |
| 5 | 6529 | (28.7) | 4367 | (34.5) | 929 | (23.8) |
| 6 | 501 | (2.2) | 228 | (1.8) | 47 | (1.2) |
| 7A and 7C | 311 | (1.4) | 192 | (1.5) | 48 | (1.2) |
| 8A–G | 1047 | (4.6) | 536 | (4.2) | 137 | (3.5) |
| US census region residence (n, %) | ||||||
| Northeast | 4792 | (21.1) | 2601 | (20.6) | 1290 | (33.0) |
| South | 9040 | (39.8) | 4087 | (32.3) | 1258 | (32.2) |
| Midwest | 4033 | (17.8) | 3376 | (26.7) | 804 | (20.6) |
| West | 4856 | (21.4) | 2588 | (20.5) | 559 | (14.3) |
| Residential rurality (n, %) | ||||||
| Urban | 18 854 | (83.0) | 11 475 | (90.7) | 3394 | (86.8) |
| Large rural city/town | 2256 | (9.9) | 744 | (5.9) | 320 | (8.2) |
| Isolated small rural town | 1611 | (7.1) | 433 | (3.4) | 197 | (5.0) |
| SVI score (mean, SD) | ||||||
| Socio‐economic status | 0.43 | (0.24) | 0.45 | (0.23) | 0.40 | (0.24) |
| Household characteristics | 0.38 | (0.26) | 0.32 | (0.25) | 0.32 | (0.26) |
| Racial and ethnic minority status | 0.70 | (0.25) | 0.78 | (0.24) | 0.70 | (0.25) |
| Housing type and transportation | 0.62 | (0.24) | 0.68 | (0.23) | 0.64 | (0.23) |
| Composite SVI | 0.53 | (0.24) | 0.57 | (0.24) | 0.50 | (0.24) |
| Prior year social risk (n, %) | ||||||
| Justice involved | 2178 | (9.6) | 1127 | (8.9) | 835 | (21.4) |
| Unhoused | 7502 | (33.0) | 4783 | (37.8) | 2339 | (59.8) |
| Prior year health service use (n, %) | ||||||
| Emergency department visits | 2.25 | (3.81) | 2.54 | (4.23) | 3.81 | (5.67) |
| Psychiatric admissions | 0.59 | (1.09) | 0.50 | (1.20) | 1.80 | (2.05) |
| Non‐psychiatric admissions | 0.97 | (1.59) | 0.96 | (1.74) | 2.65 | (2.84) |
| Prior year overdose history (n, %) | ||||||
| Opioid | 731 | (3.2) | 321 | (2.5) | 158 | (4.0) |
| Other, non‐opioid | 1242 | (5.5) | 570 | (4.5) | 413 | (10.6) |
| Elixhauser index (mean, SD) | 3.86 | (2.29) | 4.11 | (2.39) | 5.08 | (2.51) |
| Prior year substance use history (n, %) | ||||||
| Alcohol use disorder | 8606 | (37.9) | 5253 | (41.5) | 3417 | (87.4) |
| Tobacco use disorder | 12 299 | (54.1) | 6810 | (53.8) | 2849 | (72.8) |
| Other drug use disorder | 11 783 | (51.9) | 6651 | (52.6) | 3064 | (78.3) |
| Prior year mental health history (n, %) | ||||||
| Anxiety disorder | 13 011 | (57.3) | 5631 | (44.5) | 3055 | (78.1) |
| Bipolar disorder | 2988 | (13.2) | 1569 | (12.4) | 999 | (25.5) |
| Depression | 15 153 | (66.7) | 7799 | (61.6) | 3148 | (80.5) |
| PTSD | 9752 | (42.9) | 4410 | (34.9) | 2350 | (60.1) |
| Psychotic disorder | 1541 | (6.8) | 1178 | (9.3) | 630 | (16.1) |
| Prior year medical history (n, %) | ||||||
| Chronic pain | 18 227 | (80.2) | 9990 | (79.0) | 3213 | (82.2) |
| Hepatitis C | 3873 | (17.0) | 2992 | (23.6) | 746 | (19.1) |
| Prior year treatment history (n, %) | ||||||
| Received long‐acting opioids | 3122 | (13.7) | 1594 | (12.6) | 72 | (1.8) |
| Received short‐acting opioids | 10 060 | (44.3) | 5519 | (43.6) | 939 | (24.0) |
| Prior year treatment history (mean, SD) | ||||||
| Average opioid dose (MME)k | 19.95 | (40.43) | 20.50 | (69.46) | 6.31 | (15.67) |
| Psychotherapy visits | 5.77 | (11.80) | 7.19 | (15.09) | 19.39 | (21.69) |
| Prior month prescription history (n, %) | ||||||
| Antidepressant | 11 055 | (48.7) | 5672 | (44.8) | 2706 | (69.2) |
| Benzodiazepine | 3054 | (13.4) | 1371 | (10.8) | 289 | (7.4) |
| Non‐benzodiazepine hypnotics | 2130 | (9.4) | 964 | (7.6) | 373 | (9.5) |
| Skeletal muscle relaxants | 3455 | (15.2) | 1686 | (13.3) | 484 | (12.4) |
| Provider and facility characteristics | ||||||
| Provider credential (n, %) | ||||||
| MD/DO | 21 003 | (88.0) | 1204 | (9.5) | 1856 | (47.5) |
| PA/NP | 791 | (3.5) | 224 | (1.8) | 712 | (18.2) |
| PharmD | 164 | (0.7) | 135 | (1.1) | 188 | (4.8) |
| OTP clinic | 0 | (0.0) | 10 146 | (80.2) | 0 | (0.0) |
| Other/undetermined | 763 | (3.4) | 943 | (7.5) | 1155 | (29.5) |
| Provider specialty (n, %) | ||||||
| Behavioral/mental health | 18 810 | (82.8) | 1609 | (12.7) | 2434 | (62.2) |
| Primary care/internal medicine | 1474 | (6.5) | 61 | (0.5) | 120 | (3.1) |
| Emergency medicine/hospitalist | 226 | (1.0) | 6 | (0.0) | 17 | (0.4) |
| Other | 2211 | (9.7) | 830 | (6.6) | 1340 | (34.3) |
| OTP clinic | 0 | (0.0) | 10 146 | (80.2) | 0 | (0.0) |
| Facility type (n, %) | ||||||
| VA Medical Center (VAMC) | 19 993 | (88.0) | 12 546 | (99.2) | 3600 | (92.0) |
| Outpatient health facility a | 2030 | (8.9) | 17 | (0.1) | 104 | (2.7) |
| Other outpatient services | 260 | (1.1) | 0 | (0.0) | 15 | (0.4) |
| Other | 438 | (1.9) | 89 | (0.7) | 192 | (4.9) |
Abbreviations: BUP, buprenorphine; MET, methadone; MD/DO, medical doctor or doctor of osteopathy; MME, morphine milligram equivalents; MOUD, medication treatment for opioid use Disorder; OTP, opioid treatment program; PA/NP, physician assistant or nurse practitioner; PharmD, doctor of pharmacy; PTSD, post‐traumatic stress disorder; SD, standard deviation; SVI, Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry Social Vulnerability Index; XR‐NTX, extended‐release naltrexone.
Outpatient health facilities include Veteran Health Administration health care centers (HCCs) and community‐based outpatient clinics (CBOCs).
Early discontinuation
Using the operational definition employed in other CTN research [42], we determined that a discontinuation occurred when there was a gap in treatment exceeding 28 days. Because we are relying on EHR data, ‘gap’ refers to the time between the last covered day from a previously documented fill (BUP) or administration (MET and XR‐NTX) and the next documented receipt of the same medication. Early discontinuation was defined as discontinuing the initiated type of MOUD before completing 180 days of therapy. Medication data were taken from inpatient and outpatient dispensations.
Outcomes
Our primary outcome of interest was the probability of experiencing a fatal or non‐fatal overdose in the 365 days following initiation. Of note, overdoses were not restricted to occurring only after early discontinuation. Fatal overdoses were identified using the VHA/DoD Mortality Data Repository [33] and non‐fatal overdoses were identified using VHA inpatient and outpatient visits and VHA‐paid health‐care encounters at non‐VHA facilities [43].
We also use the exposure to examine a secondary outcome, i.e. probability of early discontinuation, in an exploratory analysis of associated patient characteristics. These outcomes were predefined in our CTN‐0142 protocol [31, 32].
Other covariates
To adjust for possible confounding associated with health system and changes in the practice of care, we included derived measures of facility type where the initiation took place, credentials and specialty of the provider, and initiation fiscal year using the CDW data. Additional covariates for patient characteristics were selected based on existing literature establishing their association with overdose or MOUD retention [44, 45, 46, 47, 48, 49, 50, 51, 52].
Socio‐demographic characteristics
We extracted basic demographic information: age at initiation, sex, self‐reported race and ethnicity, employment status and marital status. To capture veteran‐level social risk factors, we constructed measures of a history of justice involvement and being unhoused following methods used by previous researchers [53]. We also included a categorical variable for the veteran's assigned VHA priority group from the most recent eligibility data prior to the index date. VHA priority status is a multi‐faceted measure of health and social need, determined by factors such as military service history, disability rating, income level and Medicaid eligibility [54]. For example, priority groups 1 and 4 have high levels of disability (the former due to service‐connected injuries and the latter otherwise having catastrophic injuries preventing work). Priority status also helps to determine the co‐payments that veterans pay.
Area characteristics
US Census regions were used to broadly capture geographic variations in care. We used RUCA Categorization B [55] to assess rurality of residence. Categorization B provides a three‐level measure of rurality: urban, large rural city/town and isolated small rural town. We used SVI measures to capture additional risk veterans may experience from their communities. The SVI has four themed measures of area risk: (1) ‘socio‐economic status’, (2) ‘household characteristics’, (3) ‘racial and ethnic minority status’ and (4) ‘housing type and transportation’.
Diagnosis and treatment characteristics
Using encounters in the 365 days before initiation, we assessed the presence of key comorbid diagnoses: tobacco use disorder, alcohol use disorder (AUD), other drug use disorder (e.g. cocaine use disorder, cannabis use disorder), major depression, psychotic disorder, post‐traumatic stress disorder, anxiety disorders, bipolar disorder, hepatitis C and chronic pain. Using the same encounters, we determined if the veteran had a history of opioid overdose, and constructed a count of Elixhauser comorbidities [56] and several measures of health service use (emergency room visits, psychotherapy visits, inpatient admissions, psychiatric admission). We included additional medication‐specific, proximal factors (receipt of prescription opioids, sedatives, benzodiazepines or antidepressants) to help capture the patient's clinical complexity. For medication‐specific factors, we captured medication receipt only in the 30 days before initiation under the assumption that recent exposure better indicates active treatment of the veteran's comorbidities.
Statistical analyses
All analyses were stratified by cohort; that is, we estimated a separate model for each MOUD type and results cannot be statistically compared across models. The initial specification for each model used all hypothesized covariates in the equations for early discontinuation and overdose but also included discontinuation as a predictor in the overdose equation. Models were refined using a process described in the Supporting information, but always included age, sex and ethnicity. We pre‐specified a 95% confidence level for tests and confidence intervals (CIs). We used SAS version 8.3 and SQL for data manipulation and Stata version 17.0 for analyses.
Statistical model
We hypothesized that there are unobserved (in the data) patient‐specific factors that determine the probability of overdose, and that these factors are correlated with similarly unobserved patient‐specific factors that are associated with the probability of early discontinuation, a phenomenon referred to in some disciplines as ‘endogeneity’. To account for this possible endogeneity, we used a bivariate probit model [49, 57, 58]. A bivariate probit model extends a conventional probit model to permit two outcomes of interest to be analyzed simultaneously, including the case where one ‘outcome’ is a covariate in the other equation. Our model estimates both the relationship between probability of overdose and early discontinuation, controlling for observed covariates, and simultaneously the relationship between probability of early discontinuation and the observed covariates. Together with the usual coefficient estimates of a probit model, this approach estimates 𝜌, the correlation between the error terms in the overdose and discontinuation equations.
Predicted probabilities and incremental effects
A probit model, like a logistic model, is used for binary outcomes to ensure that predictions of the outcome are strictly between 0 and 1. Unlike a logistic model, the coefficients from a probit model cannot be transformed into commonly presented measures; for example, odds ratios. We adopt two approaches to interpreting the results. First, for our main analysis, we report the average predicted probability for a counterfactual: given the within‐sample observed characteristics of each MOUD cohort, what probability of overdose would we expect if there had been no early discontinuations in the sample. This type of counterfactual analysis is useful for highlighting what is realistically possible to achieve taking all other existing characteristics of patients as fixed, and is different from simply computing the probability of overdose among those who do not actually discontinue within the sample.
Alternately, these models are often interpreted using the average marginal effect of each covariate or, in the case of a binary or categorical covariate, the average incremental effect (IE). In binary models, the IE measures changes directly on the probability scale. Average IEs are the sample‐average change in the outcome given a change in a predictor while holding all other covariates as observed, similar to interpretation of coefficients for linear regression. They are obtained by taking the average of the difference in predicted probabilities when all subjects in the sample have the exposure of interest (early discontinuation) compared to when no subjects have the exposure, regardless of the actual experience of early discontinuation. For the primary outcome, we computed the average IE of early discontinuation on the probability of overdose.
An alternative approach to computing IEs is to evaluate them where covariates are held to specified values, rather than averaging effects over the sample. This approach, computing the IE at a representative value, is valuable because the effect of each deviation from the representative value can be examined in isolation instead of a sample‐dependent average. We adopt this approach for our exploratory analysis of patient covariates associated with probability of early discontinuation. A detailed description of selecting the ‘representative’ patient is shown in the Supporting information.
RESULTS
Early discontinuation and probability of overdose
Table 2 presents the results from our primary analysis. The first row of panel I shows the sample average predicted probability of overdose if no veterans in the cohort discontinued early but otherwise kept all their same characteristics. Among veterans who initiated BUP, the predicted probability is 3.9% (95% CI = 3.2 to 4.6%). This can be compared to the observed rate of 9.0% from Table 1. Results for MET and XR‐NTX are interpreted analogously, with predicted probabilities of 8.4% (MET) and 4.5% (XR‐NTX), although the XR‐NTX estimate cannot be statistically differentiated from 0. The observed rates of overdose from Table 1 is 8.0% among MET initiators and 12.2% among XR‐NTX initiators.
TABLE 2.
Effect of early discontinuation on overdose by MOUD type.
| BUP | MET | XR‐NTX | |
|---|---|---|---|
| Panel I | |||
| Predicted Pr of overdose without early discontinuation | 3.9% | 8.4% | 4.5% |
| [3.2%, 4.6%] | [3.3%, 13.4%] | [−6.7%, 15.7%] | |
| IE of early discontinuation | 14.0 | −0.5 | 9.5 |
| [9.4, 18.6] | [−6.2, 5.2] | [−7.9, 27.0] | |
| Panel II | |||
| ρ | −0.350 | 0.072 | −0.217 |
| [−0.493, −0.189] | [−0.153, 0.291] | [−0.845, 0.663] | |
| χ2(1): ρ = 0 | 16.9121 | 0.3880 | 0.1798 |
| P‐value | 0.0000 | 0.5334 | 0.6716 |
| BIC | 42956.74 | 17200.32 | 6510.56 |
| Number of observations | 22 721 | 12 652 | 3911 |
Note: Items in bold type are significantly different from zero at the 5% or lower level.
Abbreviations: BUP, buprenorphine; IE, incremental effect; MET, methadone; MOUD, medication treatment for opioid use disorder; Pr, probability; XR‐NTX, extended‐release naltrexone.
A statistically significant average IE of early discontinuation was found only in the BUP cohort, with an average increased probability of 14.0 percentage points (95% CI = 9.4 to 18.6) after early discontinuation, all else being constant. The IE for XR‐NTX was consistent in size and direction with early discontinuation increasing the probability of overdose, but there is insufficient precision to distinguish it from zero [9.5 percentage points (95% CI = −7.9 to 27.0)]. The estimated IE of early discontinuation among MET initiators was −0.5 percentage points (95% CI = −6.2 to 5.2), which is not statistically significant.
Panel II shows the correlation between the two error terms. Among BUP initiators, ρ is −0.350 and significantly different from 0. This is consistent with our hypothesis, that unmeasured factors associated with early discontinuation are correlated with unmeasured factors that predict overdose. The estimate of ρ for XR‐NTX is of similar magnitude and direction but cannot be statistically distinguished from 0. The estimate for MET is smaller, positive and not statistically significant. Full final model specifications are available from the corresponding author upon request.
Patient characteristics and probability of early discontinuation
The predicted probability of early discontinuation for the representative patient was 45.2% in the BUP cohort (Figure 1), 79.7% in the MET cohort (Figure 2), and 78.7% in the XR‐NTX cohort (Figure 3). Figures 1, 2, 3 also display the IEs and 95% CIs for deviations from the representative patient's characteristics, computed using each cohort's final model (underlying numeric values in Supporting information, Tables S2–S4). Characteristics with CIs that do not cross the 0.0 line are statistically different from 0 at the 95% level, and are shown in dark blue. The IEs of dropped covariates are, by definition, 0. These, together with any non‐patient characteristics that are in the model, are excluded from the calculation of the patient characteristic IEs. Because patient characteristics were included in this exploratory analysis only if they had a statistically significant association in the cohort‐specific model, the number of characteristics varied by MOUD type, ranging from 14 (XR‐NTX) to 25 (BUP).
FIGURE 1.
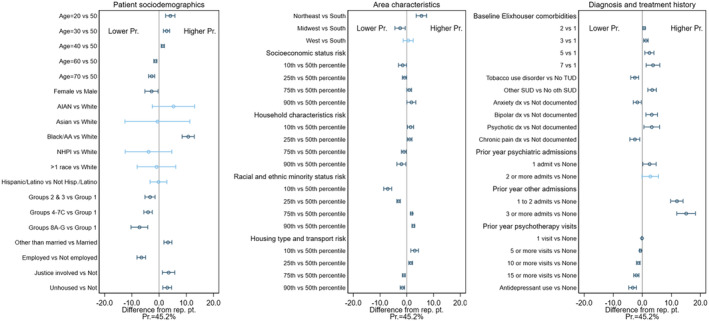
Difference from representative patient for selected patient characteristics in predicted probability of early discontinuation of buprenorphine (BUP) with 95% confidence intervals.
FIGURE 2.
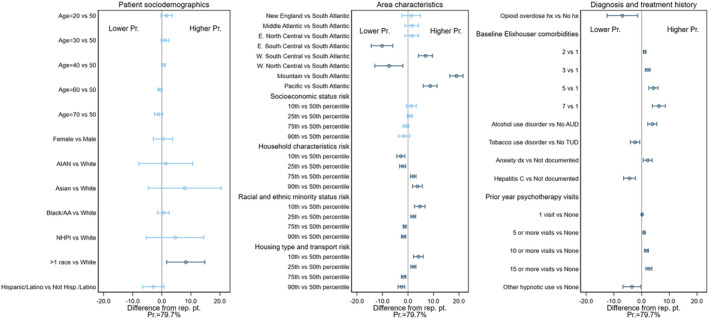
Difference from representative patient for selected patient characteristics in predicted probability of early discontinuation of met with 95% confidence intervals.
FIGURE 3.
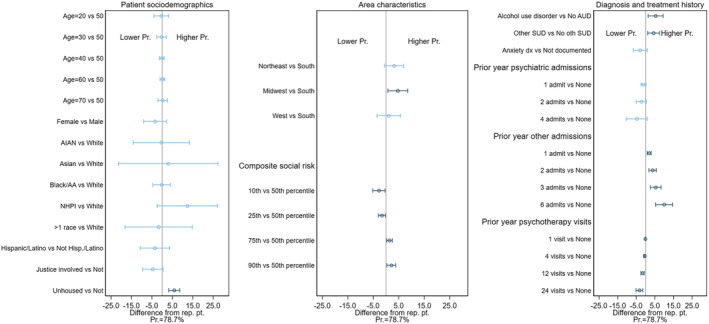
Difference in predicted probability of early discontinuation of extended‐release naltrexone (XR‐NTX) based on patient characteristics.
Each IE is the change when the listed characteristic takes the value shown instead of the value for the reference patient, with all other patient characteristics fixed at those of the reference patient. Taking the first statistically significant IE shown, if a veteran in the BUP cohort was aged 20 years instead of the 50 years specified for our reference patient yet otherwise has exactly the same characteristics as the reference patient, the predicted probability of early discontinuation is 10.7 percentage points higher (95% CI = 8.5 to 12.9). Additional examples of interpretation of these figures can be found in the Supporting information.
DISCUSSION
In a large, national sample of veterans initiating one of three types of MOUD, we evaluated the association between early discontinuation and overdose for each of three medications. We found that, given the characteristics of patients initiated on BUP within the VA, early discontinuation is associated with a statistically significant increased predicted probability of fatal or non‐fatal overdose for BUP. The direction of association was the same and the estimated magnitude was similar for XR‐NTX, but the association was not statistically significant, probably due to the much smaller sample size. By contrast, the estimated incremental effect of early discontinuation on overdose probability in the cohort that was initiated on MET was both close to zero and not statistically significant. When we examined patient characteristics linked to early discontinuation in our exploratory analysis, we found little commonality in specific predictors among medications.
Our findings regarding overdose probability in the BUP cohort are consistent with studies showing worse patient outcomes among those discontinuing BUP early [13, 59, 60] and reiterate the importance of retention among BUP initiators. Specifically, given the overdose risk profile associated with the observed characteristics of the veterans, their providers and the type of facility where they initiated, we would have expected to see a substantially lower rate of overdose (approximately 3.9%) than we actually observed (approximately 9.0%) at the current rate of early discontinuation. Interventions that aim to improve retention, such as the Stepped Care for Opioid Use Train‐the‐Trainer (SCOUTT) program [61], thus appear likely to result in long‐term improvements in the more distal outcome of overdose. Our results for the XR‐NTX cohort are also consistent with previous findings [62, 63]. Although high variation among the relatively small number of XR‐NTX initiators meant that we did not achieve sufficient precision to statistically distinguish these numbers; the alignment with BUP and prior results suggests that the same analysis in a larger sample of XR‐NTX initiators would be statistically significant.
By contrast, we found that MET initiators had the highest predicted probability of overdose if all early discontinuations were prevented (8.4%), a rate that tracked closely to the observed rate (8.0%) in our sample. We also found higher rates of MET discontinuation. This may seem to counter previous literature. For example, a 2017 meta‐analysis of cohort studies found retention on MET (and BUP) is associated with substantial reductions in risk for all cause and overdose mortality [60]. Our study is not directly comparable to such work, but rather because the use of average predicted probabilities within a cohort focuses upon the effect of an exposure in that population. The veteran population, and especially the population that is initiated on MET within VHA, differs substantially from the patient population in a typical cohort study. For example, those initiating MET, compared to those initiating BUP or XR‐NTX, were more likely to have hepatitis C, indicative of possible intravenous (IV) drug use and probably more severe OUD. Our work is better compared to other studies among veterans; for example, a recent study found higher retention rates among veterans receiving BUP compared to those receiving MET, also seemingly in contradiction to past research [30]. Our counterintuitive findings may reflect a shift in which patients are receiving different types of MOUD within VHA. As BUP has become more prevalent in more recent years VHA clinicians may be initiating patients at a higher baseline risk of overdose on MET, as treatment with MET comes with daily dosing and the structure of the opioid treatment program. In addition, subtherapeutic dosing of MET within VHA could be contributing to these findings. While VHA data do not allow for quantification of MET dosing, we have conferred with directors of OTPs in VHA who have noted that dosing is usually quite low. If that is true, subtherapeutic MET dosing could lead to continued illicit opioid use, which would contribute to both lower rates of retention and no difference in overdose risk. Our finding that MET initiators had the highest baseline risk in the absence of early discontinuation supports this supposition.
We found that patient characteristics with statistically significant changes in the predicted probability varied greatly by MOUD type. We focus our discussion on specific differences that we believe are of policy or clinical importance. We also focus here on BUP and XR‐NTX because of the lack of relationship between early discontinuation and overdose for MET in the cohort initiated on MET. Consistent with past research finding that geographic region is significantly associated with MOUD retention [64], we found that at least some geographies were associated with significantly different probabilities of retention, with the direction and magnitude of the effect varying by type of MOUD and region of the country where the veteran lives. More research is warranted into how patterns of clinical care may systematically differ within VHA across these regions or if the differences may instead be driven by societal factors, perhaps even beyond the health‐care system, that vary based on where individuals live (e.g. availability of public transportation, social support for addiction therapy, levels of stigma). The fact that each of the four domains of the SVI (i.e. socio‐economic status, household characteristics, racial and ethnic minority status and housing type and transportation) were highly significant suggests that changes at the societal or health system level (i.e. structural changes), rather than specific factors under the clinician's control, may be necessary. However, for BUP and XR‐NTX, we found a clear pattern where an increase in the number of prior year psychotherapy visits corresponded to a decrease in predicted probability of early discontinuation for the representative patient, potentially indicating the clinician's use of psychotherapy before or together with MOUD may be beneficial, providing a modifiable intervention at the clinician level.
For BUP, being employed and in the economically affluent priority groups 8A–8G was associated with a decreased predicted probability of early discontinuation compared to the reference patient, which is consistent with past research showing that employment is associated with greater BUP retention [65]. While income and employment are not modifiable through any health system intervention, it is surprising that we found effects even within VHA, where the financial barriers to obtaining care are extremely low. This suggests that the employment and income covariates may be detecting the effect of an unmeasured confounder that is associated with the likelihood of being employed and higher income. Being married was also protective for BUP initiators. Employment and being married may be proxy measures for social support, which indicates that various forms of wrap‐around social services may be beneficial. It is also worth highlighting the role of comorbid AUD within the XR‐NTX cohort, where we observed an AUD diagnosis associated with a higher predicted probability of early discontinuation compared to the reference patient. This is consistent with past research finding that patients with co‐occurring OUD and AUD have higher retention rates when prescribed BUP or MET [66]. XR‐NTX is Food and Drug Administration (FDA) approved for the treatment of AUD, and AUD was more prevalent among veterans who initiated XR‐NTX compared to the BUP or MET cohorts. Given that AUD was associated with a higher probability of early XR‐NTX discontinuation, but not with early BUP discontinuation, future research needs to explore whether BUP may be a better choice for veterans with comorbid OUD and AUD [15].
Finally, self‐identifying as Black was associated with greater predicted probability of early discontinuation among BUP initiators [43]. We observed a similar relationship when veterans initiated on BUP reside in a high‐vulnerability area for the ‘racial and ethnic minority status’ SVI theme. Past research has found that patients who are Black, compared to those who are White, are significantly less likely to receive an adequate dose of BUP (defined as at least 8 mg/day) [67], and that higher BUP doses are associated with better retention [15, 68]. Interestingly, a systematic review of MOUD retention found that, for BUP, retention of patients who are Black was significantly higher in clinical trials compared to retrospective chart review studies [69]. This finding is consistent with worse quality BUP care for patients who are Black in clinical practice [67], a bias less likely to manifest in clinical trials that use a standard dosing procedure for all participants and have research teams that are motivated to engage and retain patients. Caution is required in interpreting these findings, however, because we did not have access to dosing data, mainly for MET, and other potentially important predictors of outcomes by MOUD. Nevertheless, given the dramatic increase in overdoses in people who are Black [70, 71] and our finding that BUP retention is protective against overdose, this is a clinically important finding that warrants further evaluation.
While early [65, 66, 72, 73] MET discontinuation was not a significant factor in predicting overdose, several of our findings merit mention. First, the observed rate of early discontinuation of MET is much higher in the current study compared to previously reported rates [8, 9, 15, 74]. In addition to being indicative of a higher baseline risk for detrimental outcomes, this may reflect a tendency to use lower MET doses in the VA (personal communication with OTP clinicians). If MET doses are suboptimal, this could account for the better retention of individuals without a history of opioid overdose who may be clinically less complex or have less severe addictions. Our finding that having more than one racial identity was associated with a significantly increased predicted probability of early discontinuation for MET is an association that has not been previously reported, which may simply reflect the under‐representation of this racial identity in MOUD research [72].
Limitations
Like all studies that rely upon administrative data, our study is subject to potential measurement and classification error in key variables. We cannot accurately discern when MOUD discontinuation occurred by relying on prescription records: a greater concern with BUP, as it relies more heavily on the prescription runout date. In addition, a small number of BUP initiations made through the opioid treatment program may have been misclassified, as we assumed that all dispensations made through the opioid treatment program were MET dispensations because the data do not support distinguishing between the two [30]. Similarly, the use of clinic stop codes provides minimal information on receipt of MET (e.g. does not contain MET dose), and therefore probably introduces some degree of measurement error that is difficult to quantify. VHA CDW data is also limited to records for veterans who received care from a VHA facility or whose community‐based care was paid for by VHA. To the extent that VHA users substituted for non‐VHA care instead of discontinuing therapy, we would overcount early discontinuations. Additionally, we did not account for switching between different forms of MOUD. We focused upon discontinuation of the initiated MOUD, given its status as a probable indicator of failure of the first treatment‐attempted MOUD type. However, treating switches as discontinuations may obscure the relationship between discontinuation and overdose. Excluding individuals who switched from the analysis might have resulted in larger point estimates for the IE of early discontinuation, but would have been obtained at the cost of a smaller sample size.
Overdose deaths are undercounted to the extent that drug involvement is under‐reported on death certificates; some estimates put true rates as much as 20–35% higher than reported [75]. Similarly, non‐fatal overdoses that do not result in a health‐care visit or for which VHA does not pay for care are not captured in our data. A recent study found that the sensitivity of diagnostic codes for overdose, compared to self‐report, varied by 12.0% for opioids with diagnosis codes undercounting overdoses, which could underestimate the association between early MOUD discontinuation and overdose risk [76]. However, this is only a concern if the rate of undercounting differs by MOUD type. We are not able to capture illicit fentanyl or heroin consumption or route of drug administration with these administrative data, which have been shown to be significant predictors of relapse and overdose [77]. In addition, the role of fentanyl and its analogs in the rise of overdose rates emerged during this time‐frame, with rapid growth occurring from 2015 to 2021, encompassing the second portion of this time‐frame. Given these temporal changes, there may be substantial heterogeneity in overdose risk and predictors of discontinuation between these time‐periods [78]. Our results also may not generalize to other populations; the US veteran population differs substantially from the broader US population in terms of demographics (e.g. disproportionately male) and exposure and health history.
Caution is needed when comparing our results to existing studies that have examined the efficacy of different MOUD types. While our models adjust for a wide array of observable risks, many factors are unmeasured and unmeasurable in administrative data. For example, many individual‐level social determinants of health (e.g. social support structures, familial relationships) may be observable to the clinician and influence treatment selection but generally cannot be measured in studies such as this, which rely upon administrative data. Relatedly, dose, particularly BUP dose, has been previously linked to early MOUD discontinuation and was not included as a covariate here due to identification of MOUD type by stop codes and CPT codes [68]. Therefore, studies that assess multiple treatment options (e.g. comparing OUD treatment pathways, comparing MOUD types) but do not appropriately account for non‐random treatment assignment are probably subject to selection bias. By contrast, all analyses conducted in this study take MOUD treatment type as given, so interpretation of our results is conditional upon treatment assignment. While this approach limits the ability to make statements about which type of MOUD should be preferred for which patient, it is appropriate for our goal of highlighting differences in patient‐related risk factors across MOUD types and is not subject to selection bias.
CONCLUSIONS
Early discontinuation of BUP, and probably XR‐NTX, is associated with a greater probability of experiencing a fatal or non‐fatal overdose; we did not find the same association with MET. The lack of a consistent set of characteristics means that clinicians need to customize their focus on different characteristics to increase the probability of retention depending on the MOUD type initiated. Many influential predictive characteristics found in this study are modifiable only at the societal or health system level and thus lend themselves more to structural interventions, such as de‐stigmatizing OUD treatment or better public transportation. While few specific factors are under the clinician's control, these findings indicate that personalizing the prescribing decision to avoid medications when the patient has an attribute associated with a greater probability of early discontinuation of that medication may ultimately improve retention on MOUD therapy, and thus outcomes on key metrics such as overdose.
AUTHOR CONTRIBUTIONS
Corey J. Hayes: Conceptualization; data curation; funding acquisition; methodology; project administration; writing—original draft. Rebecca A. Raciborski: Conceptualization; statistical analysis; methodology; writing—review and editing. Matthew Nowak: Methodology; writing—original draft. Mahip Acharya: Conceptualization; methodology; writing—review and editing. Edward V. Nunes: Conceptualization; methodology; writing—review and editing. T. John Winhusen: Conceptualization; funding acquisition; methodology; project administration; supervision; writing—review and editing.
DECLARATIONS OF INTEREST
None to declare.
DISCLAIMER
The content of this work does not represent the views of the US Department of Veterans Affairs, the National Institutes of Health or the United States Government.
Supporting information
Data S1. Supporting information.
Figure S1. Derivation of the Study Cohort.
ACKNOWLEDGEMENTS
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health [UG1DA013732‐23S3] under the CTN‐0142 protocol. C.J.H. was also supported by a VA Health Services Research an Development Career Development Award‐2 (IK2HX003358).
Hayes CJ, Raciborski RA, Nowak M, Acharya M, Nunes EV Jr, Winhusen TJ. Medications for opioid use disorder: Predictors of early discontinuation and reduction of overdose risk in US military veterans by medication type. Addiction. 2025;120(1):138–151. 10.1111/add.16659
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Mancher M, Leshner AI. Medications for Opioid Use Disorder Save Lives Washington, DC: National Academies Press; 2019. [PubMed] [Google Scholar]
- 2. National Institute on Drug Abuse (NIDA) . Overdose Death Rates. Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed 12 November 2023).
- 3. Hedegaard H, Miniño AM, Spencer MR, Warner M. Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief. 2021;426:1–8. [PubMed] [Google Scholar]
- 4. National Institute on Drug Abuse (NIDA) . Drug Overdose Death Rates. Available at: https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates (accessed 12 November 2023).
- 5. Bennett AS, Guarino H, Britton PC, Cook SH, O'Brien‐Mazza D, Taveras F, et al. Military veterans and the opioid overdose crisis: a review of risk factors and prevention efforts. Ann Med. 2022;54:1826–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. David AR, Sian CR, Gebel CM, Linas BP, Samet JH, Sprague Martinez LS, et al. Barriers to accessing treatment for substance use after inpatient managed withdrawal (detox): a qualitative study. J Subst Abuse Treat. 2022;142:108870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medicaid and CHIP Payment and Access Commission (MACPAC) . Report to Congress on Medicaid and CHIP. Washington, DC: MACPAC; 2017.
- 8. Zhang P, Tossone K, Ashmead R, Bickert T, Bailey E, Doogan NJ, et al. Examining differences in retention on medication for opioid use disorder: an analysis of Ohio Medicaid data. J Subst Abuse Treat. 2022;136:108686. [DOI] [PubMed] [Google Scholar]
- 9. Biondi BE, Vander Wyk B, Schlossberg EF, Shaw A, Springer SA. Factors associated with retention on medications for opioid use disorder among a cohort of adults seeking treatment in the community. Addict Sci Clin Pract. 2022;17:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison‐Diehn C. Retention in medication‐assisted treatment for opiate dependence: a systematic review. J Addict Dis. 2016;35:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Quality Forum . NQF: Behavioral Health 2016–2017 Final Report. 2017. Available at: https://www.qualityforum.org/Publications/2017/08/Behavioral_Health_2016-2017_Final_Report.aspx (accessed 8 October 2019).
- 12. Williams AR, Nunes EV, Bisaga A, Levin FR, Olfson M. Development of a Cascade of care for responding to the opioid epidemic. Am J Drug Alcohol Abuse. 2019;45(1):1–10. 10.1080/00952990.2018.1546862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Santo T, Clark B, Hickman M, Grebely J, Campbell G, Sordo L, et al. Association of opioid agonist treatment with all‐cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta‐analysis. JAMA Psychiatry. 2021;78:979–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Connor AM, Cousins G, Durand L, Barry J, Boland F. Retention of patients in opioid substitution treatment: a systematic review. PLoS ONE. 2020;15:e0232086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi‐site trial. Addiction. 2014;109:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manhapra A, Petrakis I, Rosenheck R. Three‐year retention in buprenorphine treatment for opioid use disorder nationally in the Veterans Health Administration. Am J Addict. 2017;26:572–580. [DOI] [PubMed] [Google Scholar]
- 17. Manhapra A, Agbese E, Leslie DL, Rosenheck RA. Three‐year retention in buprenorphine treatment for opioid use disorder among privately insured adults. Psychiatr Serv. 2018;69:768–776. [DOI] [PubMed] [Google Scholar]
- 18. Weinstein LC, Iqbal Q, Cunningham A, Debates R, Landistratis G, Doggett P, et al. Retention of patients with multiple vulnerabilities in a federally qualified health center buprenorphine program: Pennsylvania, 2017–2018. Am J Public Health. 2020;110:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan B, Gean E, Arkhipova‐Jenkins I, Gilbert J, Hilgart J, Fiordalisi C, et al. Retention strategies for medications for opioid use disorder in adults: a rapid evidence review. J Addict Med. 2021;15:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dematteis M, Auriacombe M, D'Agnone O, Somaini L, Szerman N, Littlewood R, et al. Recommendations for buprenorphine and methadone therapy in opioid use disorder: a European consensus. Expert Opin Pharmacother. 2017;18:1987–1999. [DOI] [PubMed] [Google Scholar]
- 21. Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, et al. Long‐term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi‐site trial. Addiction. 2016;111:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nunes E, Scodes JM, Pavlicova M, Lee JD, Novo P, Campbell ANC, et al. Sublingual Buprenorphine‐Naloxone Compared with Injection Naltrexone for Opioid Use Disorder: Potential Utility of Patient Characteristics in Guiding Choice of Treatment. Am J Psychiatry. 2021;178:660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3:e1920622–e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Boney TY, Hoskinson RA Jr, et al. Extended‐release naltrexone to prevent opioid relapse in criminal justice offenders. N Engl J Med. 2016;374:1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwartz RP, Mitchell MM, O'Grady KE, Kelly SM, Gryczynski J, Mitchell SG, et al. Pharmacotherapy for opioid addiction in community corrections. Int Rev Psychiatry. 2018;30:117–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Degenhardt L, Clark B, Macpherson G, Leppan O, Nielsen S, Zahra E, et al. Buprenorphine versus methadone for the treatment of opioid dependence: a systematic review and meta‐analysis of randomised and observational studies. Lancet Psychiatry. 2023;10:386–402. [DOI] [PubMed] [Google Scholar]
- 27. Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poulsen MN, Asdell PB, Berrettini W, McBryan K, Rahm AK. Application of the COM‐B model to patient barriers and facilitators of retention in medication treatment for opioid use disorder in rural northeastern United States: a qualitative study. SSM—Mental Health. 2022;2:100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Price L, Shea K, Gephart S. The Veterans Affairs’ corporate data warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39:311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyse JJ, McGinnis KA, Edelman EJ, Gordon AJ, Manhapra A, Fiellin DA, et al. Twelve‐month retention in opioid agonist treatment for opioid use disorder among patients with and without HIV. AIDS Behav. 2022;26:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CTN‐0142: Towards Personalized Medicine in Medication for Opioid Use Disorder (MOUD): Analyses of Veterans Health Administration Data to Delineate Patient Characteristics Associated with Treatment Outcomes and Successful MOUD Discontinuation—CTN Dissemination Library. Available at: https://ctnlibrary.org/protocol/ctn0142/ (accessed 14 February 2024).
- 32. Towards Personalized Medicine in Medication for Opioid Use Disorder (MOUD) . : Analyses of Veterans Health Administration Data to Delineate Patient Characteristics Associated with Treatment Outcomes and Successful MOUD Discontinuation|National Institute on Drug Abuse (NIDA). Available at: https://nida.nih.gov/about-nida/organization/cctn/ctn/research-studies/towards-personalized-medicine-in-medication-opioid-use-disorder-moud-analyses-veterans-health (accessed 12 November 2023).
- 33. A Guide to Accessing and Understanding Mortality Data in the VA Mortality Data Repository. Available at: www.mentalhealth.va.gov/ (accessed 12 November 2023).
- 34. USDA ERS—Rural–Urban Commuting Area Codes. Available at: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/ (accessed 2 October 2023).
- 35. Lehnert EA, Wilt G, Flanagan B, Hallisey E. Spatial exploration of the CDC's social vulnerability index and heat‐related health outcomes in Georgia. Int J Disaster Risk Reduct. 2020;46. 10.1016/J.IJDRR.2020.101517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. CDC/ATSDR Social Vulnerability Index (SVI). Available at: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed 23 October 2023).
- 37. STROBE: Strengthening the Reporting of Observational Studies in Epidemiology. Available at: https://www.strobe-statement.org/ (accessed 12 November 2023).
- 38. Lagisetty P, Garpestad C, Larkin A, Macleod C, Antoku D, Slat S, et al. Identifying individuals with opioid use disorder: validity of international classification of diseases diagnostic codes for opioid use, dependence and abuse. Drug Alcohol Depend. 2021;221. 10.1016/J.DRUGALCDEP.2021.108583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fairley M, Humphreys K, Joyce VR, Bounthavong M, Trafton J, Combs A, et al. Cost‐effectiveness of treatments for opioid use disorder. JAMA Psychiatry. 2021;78:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Medication‐Assisted Treatment for Opioid Use Disorder (HERC)—VA Phenomics Library. Available at: https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/Medication-Assisted_Treatment_for_Opioid_Use_Disorder_(HERC) (accessed 12 November 2023).
- 41.project‐res‐opioid/_104‐pull‐mat‐20221006.sql at main · vilijajoyce/project‐res‐opioid · GitHub. Available at: https://github.com/vilijajoyce/project-res-opioid/blob/main/_104-pull-mat-20221006.sql (accessed 12 November 2023).
- 42. Optimizing retention, duration, and discontinuation strategies for opioid use disorder pharmacotherapy (NIH HEAL initiative)|National Institute on Drug Abuse (NIDA). Available at: https://nida.nih.gov/about-nida/organization/cctn/ctn/research-studies/optimizing-retention-duration-discontinuation-strategies-opioid-use-disorder-pharmacotherapy-nih (accessed 12 November 2023).
- 43. Williams AR, Samples H, Crystal S, Olfson M. Acute care, prescription opioid use, and overdose following discontinuation of long‐term buprenorphine treatment for opioid use disorder. Am J Psychiatry. 2019;177:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–1240. [DOI] [PubMed] [Google Scholar]
- 45. Cacciola JS, Alterman AI, DePhilippis D, Drapkin ML, Valadez C Jr, Fala NC, et al. Development and initial evaluation of the brief addiction monitor (BAM). J Subst Abuse Treat. 2013;44:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital signs: characteristics of drug overdose deaths involving opioids and stimulants—24 states and the District of Columbia, January–June 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiner SG, el Ibrahimi S, Hendricks MA, Hallvik SE, Hildebran C, Fischer MA, et al. Factors associated with opioid overdose after an initial opioid prescription. JAMA Netw Open. 2022;5:e2145691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lyons RM, Yule AM, Schiff D, Bagley SM, Wilens TE. Risk factors for drug overdose in young people: a systematic review of the literature. J Child Adolesc Psychopharmacol. 2019;29:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duca JV, Rosenthal SS. Borrowing constraints and access to owner‐occupied housing. Reg Sci Urban Econ. 1994;24:301–322. [Google Scholar]
- 50. Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, et al. Collaborative care of opioid‐addicted patients in primary care using buprenorphine. Arch Intern Med. 2011;171:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cousins SJ, Radfar SR, Crèvecoeur‐MacPhail D, Ang A, Darfler K, Rawson RA. Predictors of continued use of extended‐released naltrexone (XR‐NTX) for opioid‐dependence: an analysis of heroin and non‐heroin opioid users in Los Angeles County. J Subst Abuse Treat. 2016;63:66–71. [DOI] [PubMed] [Google Scholar]
- 52. Sullivan MA, Rothenberg JL, Vosburg SK, Church SH, Feldman SJ, Epstein EM, et al. Predictors of retention in naltrexone maintenance for opioid dependence: analysis of a stage I trial. Am J Addict. 2006;15:150–159. [DOI] [PubMed] [Google Scholar]
- 53. Finlay AK, Harris AHS, Timko C, Yu M, Smelson D, Stimmel M, et al. Disparities in access to medications for opioid use disorder in the Veterans Health Administration. J Addict Med. 2021;15:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. VA Priority Groups . Available at: https://veteran.com/va-priority-groups/ (accessed 27 November 2023).
- 55. WWAMI Rural Health Research Center . Rural–urban commuting area codes data. 2005. Available at: http://depts.washington.edu/uwruca/ruca-uses.php (accessed 5 December 2017).
- 56. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 57. Kaplan D, Venezky RL. Literacy and voting behavior: a bivariate probit model with sample selection. Soc Sci Res. 1994;23:350–367. [Google Scholar]
- 58. Cameron AC, Trivedi PK. Microeconometrics: Methods and Applications 1st ed. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 59. Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT, O'Connor PG. Primary care‐based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174:1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta‐analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. The SCOUTT Initiative . Increasing Medication Treatment for Veterans with Opioid Use Disorder. Available at: https://www.queri.research.va.gov/qnews/oct22/default.cfm?QnewsMenu=article1 (accessed 18 April 2024).
- 62. Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality. Ann Intern Med. 2018;169:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended‐release naltrexone versus buprenorphine‐naloxone for opioid relapse prevention (X:BOT): a multicentre, open‐label, randomised controlled trial. Lancet. 2018;391:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stafford C, Marrero WJ, Naumann RB, Lich KH, Wakeman S, Jalali MS. Identifying key risk factors for premature discontinuation of opioid use disorder treatment in the United States: a predictive modeling study. Drug Alcohol Depend. 2022;237:109507. [DOI] [PubMed] [Google Scholar]
- 65. Weinstein ZM, Kim HW, Cheng DM, Quinn E, Hui D, Labelle CT, et al. Long‐term retention in office based opioid treatment with buprenorphine. J Subst Abuse Treat. 2017;74:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mintz CM, Presnall NJ, Xu KY, Hartz SM, Sahrmann JM, Bierut LJ, et al. An examination between treatment type and treatment retention in persons with opioid and co‐occurring alcohol use disorders. Drug Alcohol Depend. 2021;226:108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Landis RK, Levin JS, Saloner B, Gordon AJ, Dick AW, Sherry TB, et al. Sociodemographic differences in quality of treatment to Medicaid enrollees receiving buprenorphine. Subst Abuse. 2022;43:1057–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chambers LC, Hllowell BD, Zullo AR, Paiva TJ, Berk J, Gaither R, et al. Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl. JAMA Netw Open. 2023;6:e2334540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hochheimer M, Unick GJ. Systematic review and meta‐analysis of retention in treatment using medications for opioid use disorder by medication, race/ethnicity, and gender in the United States. Addict Behav. 2022;124:107113. [DOI] [PubMed] [Google Scholar]
- 70. Friedman JR, Hansen H. Evaluation of increases in drug overdose mortality rates in the US by race and ethnicity before and during the COVID‐19 pandemic. JAMA Psychiatry. 2022;79:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Han B, Einstein EB, Jones CM, Cotto J, Compton WM, Volkow ND. Racial and ethnic disparities in drug overdose deaths in the US during the COVID‐19 pandemic. JAMA Netw Open. 2022;5:e2232314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nalven T, Spillane NS, Schick MR, Weyandt LL. Diversity inclusion in United States opioid pharmacological treatment trials: a systematic review. Exp Clin Psychopharmacol. 2021;29:524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krawczyk N, Williams AR, Saloner B, Cerdá M. Who stays in medication treatment for opioid use disorder? A national study of outpatient specialty treatment settings. J Subst Abuse Treat. 2021;126:108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gomes T, McCormack D, Bozinoff N, Tadrous M, Antoniou T, Munro C, et al. Duration of use and outcomes among people with opioid use disorder initiating methadone and buprenorphine in Ontario: a population‐based propensity‐score matched cohort study. Addiction. 2022;117:1972–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ruhm CJ. Corrected US opioid‐involved drug poisoning deaths and mortality rates, 1999–2015. Addiction. 2018;113:1339–1344. [DOI] [PubMed] [Google Scholar]
- 76. Riggs KR, DeRussy AJ, Leisch L, Shover CL, Bohnert ASB, Hoge AE, et al. Sensitivity of health records for self‐reported nonfatal drug and alcohol overdose. Am J Addict. 2022;31:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Luo SX, Feaster DJ, Liu Y, Balise RR, Hu MC, Bouzoubaa L, et al. Individual‐level risk prediction of return to use during opioid use disorder treatment. JAMA Psychiatry. 2024;81:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Spencer MR, Warner M, Cisewski JA, et al. Estimates of Drug Overdose Deaths involving Fentanyl, Methamphetamine, Cocaine, Heroin, and Oxycodone: United States, 2021. Vital Statistics Rapid Release no. 27. Hyattsville, MD: National Center for Health Statistics 2023. 10.15620/CDC:125504 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Figure S1. Derivation of the Study Cohort.
Data Availability Statement
Research data are not shared.


