To the Editor:
Lower respiratory tract infections, including community-acquired pneumonia (CAP), are a frequent cause of death worldwide.1 A previous study2 found that 38% of patients with CAP requiring mechanical ventilation did not survive their hospital stay. Conversely, only 16% of patients with CAP dying in hospital have been mechanically ventilated.2 Predictors of disease severity are required, because they would help identify high-risk patients with CAP who may benefit from early continuous monitoring to detect complications and reduce mortality. Previous studies found that eosinophil levels < 50/μL correlate with an increased mortality in hospitalized patients with CAP in addition to acute exacerbation of COPD.3 Also, eosinopenia is associated with a higher 4-week mortality in COVID-19 pneumonia.4 However, the role of eosinopenia for predicting short-term outcome in CAP is poorly understood.
This study therefore analyzed blood eosinopenia ≤ 50/μL as a predictor of disease severity in patients with CAP.
Methods
We retrospectively reviewed ≥ 18-year-old patients hospitalized with CAP as their primary diagnosis from five university hospitals in Germany between 2009 and 2020. The diagnosis of CAP was based on the International Classification of Diseases Code J10-18 and its subgroups according to the 10th revision, German Modification.5
This study analyzed age, sex, and comorbidities including sepsis based on International Classification of Diseases, 10th Revision, German Modification5 in patients with eosinopenia ≤ 50/μL and non-eosinopenia (> 50/μL). Laboratory parameters were measured during the first 24 hours of admission. If multiple laboratory values were available for a single parameter, the first value was used for analysis. Mortality, need for both noninvasive and invasive mechanical ventilation in all patients, length of mechanical ventilation in survivors, date and time of hospital admission in all patients, and discharge in survivors or death of deceased patients were recorded based on the data in the hospital information system.
Data extraction and handling was performed under the umbrella of the Medical Informatics in Research and Care in University Medicine consortium.6 The study protocol was approved by all five local ethics committees, data privacy advocates, and the use and access committee. Data analysis was performed using DataSHIELD,7 an open-source software allowing privacy-preserving federated learning and anonymous co-analysis of individual-level data held at multiple locations. Descriptive statistics were reported as sample size-weighted average of the site-specific medians, and 25th and 75th percentiles. Percentages were used to report categorical variables distributions.
Differences in the comorbidity prevalence between the eosinopenia and non-eosinopenia groups were calculated via Fisher exact test in a univariate model. Differences in mortality and the need for mechanical ventilation between the two groups were analyzed using a multivariate general linear model that considered the following variables: age; sex; and blood values for C-reactive protein, creatinine, and hemoglobin in the eosinopenia and non-eosinopenia group.
Differences in length of stay in survivors, time to in-hospital death, and the number of hours of mechanical ventilation in survivors between the two groups were determined using Mann-Whitney U test8 in a univariate model. For reasons of data protection and technical requirements of the analysis software DataSHIELD, Mann-Whitney U test was modified as follows: Integer values were exported, decimal places were replaced by uniformly randomized numbers, and significance was subsequently calculated. This was done with N = 10,000, and the least significant P values were reported.
The statistical analysis was conducted using DataSHIELD version 6.1.1 and R versions 4.1.2 and 4.2.0. Nonadjusted P < .05 was considered statistically significant.
Results
Overall, 6,748 (4,060 eosinopenic and 2,688 non-eosinopenic) patient cases were included in the analysis. Demographic data, laboratory parameters, and comorbidities of the eosinopenia and non-eosinopenia group are shown in Table 1.
Table 1.
Baseline Characteristics in the Eosinopenia and Non-Eosinopenia Group
| Variable | Eosinopenia Group (n = 4,060) | Non-Eosinopenia Group (n = 2,688) |
|---|---|---|
| Median age in years (25th-75th percentile) | 71.08 (58.2-80.0) | 69.45 (55.78-78.88) |
| Male sex, No. (%) | 2,483 (61.2) | 1,632 (60.7) |
| C-reactive protein, No. (%) | 4,021 (99.0) | 2,637 (98.1) |
| Median (mg/L), 25th-75th percentile (mg/L) | 94.09, 39.61-175.92 | 78.92, 28.12-148.79 |
| Procalcitonin, No. (%) | 1,907 (47.0) | 1,033 (38.4) |
| Median (ng/mL), 25th-75th percentile (ng/mL) | 0.49, 0.23-1.76 | 0.27, 0.12-0.65 |
| Leukocytes, No. (%) | 4,060 (100) | 2,688 (100) |
| Median (×109/L), 25th-75th percentile (×109/L) | 9.76, 6.33-14.35 | 10.19, 7.46-13.61 |
| Hemoglobin, No. (%) | 4,059 (100) | 2,688 (100) |
| Median (g/dL), 25th-75th percentile (g/dL) | 12.43, 10.73-13.94 | 12.14, 10.5-13.7 |
| Hematocrit, No. (%) | 4,059 (100) | 2,688 (100) |
| Median (%), 25th-75th percentile (%) | 36.81, 32.31-40.87 | 36.51, 31.66-40.66 |
| Creatinine, No. (%) | 4,041 (99.5) | 2,662 (99.0) |
| Median (mg/dL), 25th-75th percentile (mg/dL) | 1.04, 0.8-1.49 | 1.02, 0.77-1.52 |
| Asthma, No. (%) | 87 (2.1) | 76 (2.8) |
| COPDa, No. (%) | 577 (14.2) | 452 (16.8) |
| Congestive heart failure, No. (%) | 801 (19.7) | 503 (18.7) |
| Myocardial infarctionb, No. (%) | 66 (1.6) | 66 (2.5) |
| Peripheral vascular diseasec, No. (%) | 292 (7.2) | 242 (9.0) |
| Cerebrovascular disease, No. (%) | 242 (6.0) | 154 (5.7) |
| Dementia, No. (%) | 340 (8.4) | 190 (7.1) |
| Diabetes mellitus, No. (%) | 966 (23.8) | 634 (23.6) |
| Liver disease, No. (%) | 146 (3.6) | 95 (3.5) |
| Renal diseased, No. (%) | 880 (21.7) | 697 (25.9) |
Significant differences between eosininopenia and non-eosinopenia group:
P = .004;
P = .019;
P = .008;
P < .0001.
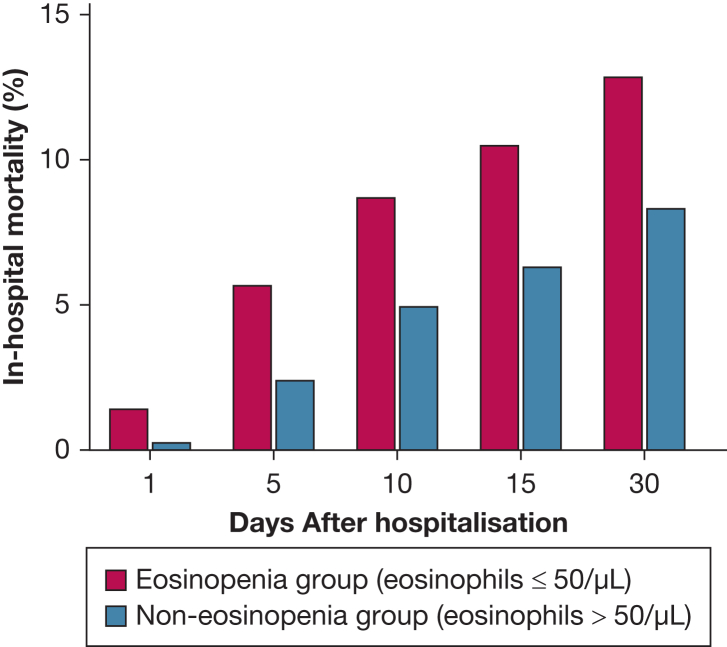
In-hospital mortality was significantly higher in the eosinopenia vs non-eosinopenia group (13.8% vs 9.1%; relative risk [RR], 1.51; 95% CI, 1.31-1.74; P < .0001). In-hospital mortality in the two groups on days 1, 5, 10, 15, and 30 after hospitalization is displayed in Figure 1. The need for mechanical ventilation was significantly elevated in the eosinopenia vs non-eosinopenia group (19.2% vs 14.3%; RR, 1.34; 95% CI, 1.20-1.50; P < .0001). Risk of sepsis was significantly elevated in the eosinopenia vs non-eosinopenia group (7.5% vs 5.0%; RR, 1.50; 95% CI, 1.23-1.83; P < .0001). Median length of stay in eosinopenic survivors was significantly prolonged compared with non-eosinopenic survivors (8.41 vs 7.64 days; P < .0001). Median time to in-hospital death was significantly reduced in the eosinopenia group (6.73 vs 8.92 days; P = .001). In survivors, median length of mechanical ventilation in the eosinopenia group was 121.93 hours vs 93.39 hours in the non-eosinopenia group. The difference was not significant (P = .152).
Figure 1.
In-hospital mortality in the eosinopenia vs non-eosinopenia group on days 1, 5, 10, 15, and 30 after hospitalization.
Discussion
The key finding of this study was an association between blood eosinopenia (≤ 50/μL) and an increase in in-hospital mortality, need for mechanical ventilation, risk of sepsis, length of stay in survivors, and reduced time to in-hospital death in a real-world patient cohort.
Because the prevalence of COPD, myocardial infarction, peripheral vascular disease, and renal disease was lower in the eosinopenia group, whereas the other comorbidities were balanced in the eosinopenia and non-eosinopenia groups, the worse outcomes in eosinopenic patients do not seem to be caused by comorbidities.
In line with our findings, eosinopenia < 50/μL correlates with an increased 18-month mortality in hospitalized patients with acute exacerbations of COPD and CAP,3 and, as part of the Dyspnoea, Eosinopenia, Consolidation, Acidaemia, and Atrial Fibrillation score,9 elevated short-term mortality in patients with acute exacerbations of COPD. Furthermore, eosinopenia is associated with a higher 4-week mortality in COVID-19 pneumonia.4
Solid data about eosinophil numbers as a predictor of short-term outcome in CAP in humans are missing. In a mouse model,10 after induction of pneumonia with Staphylococcus aureus, an IL-33-induced increase in eosinophil levels inhibited acute lung injury as indicated by reduced pulmonary edema and higher oxygen saturations. This was associated with improved survival. Eosinophil reduction prevented IL-33-mediated protection against mortality in mice. Future studies considering eosinophil and IL-33 levels, as well as downstream signaling pathways, are needed to elucidate the mechanism by which eosinophils might benefit survival after CAP.
The strengths of our study relate to the high number of real-world patient cases.
The limitations of this study involve its retrospective design; that its analysis was based on cases instead of patients; the lack of information about the immune status, microbial etiology, and clinical parameters of patients; and the technical restrictions of DataSHIELD, which did not allow for propensity score matching and analyzing eosinophil count as continuous variables.
In conclusion, blood eosinopenia ≤ 50/μL (vs non-eosinopenia) seems suitable to predict disease severity in patients with CAP. Further prospective studies are required to confirm our findings.
Funding/Support
This work was funded in part by the Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research; BMBF), MIRACUM within the Medical Informatics Funding Scheme (FKZ 01ZZ1606A-H) to H.R. and C.V.; PermedCOPD—FKZ 01EK2203A to C. V. and B. S.; CALM-QE—FKZ 01ZZ2318A to B. S. and H. R.; the Deutsche Forschungsgemeinschaft (SFB/TR-84 TP C01), and the von-Behring-Röntgen-Stiftung (66-LV07) to B. S., and the Hessisches Ministerium für Wissenschaft und Kunst (LOEWE Diffusible Signals) to A. L. J. and B. S.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: B. C. W. reports one unpaid participation in an Advisory Board with AstraZeneca. A. L. J. reports research grants from the Behring Röntgen Foundation. G. R. reports personal fees from Astra Zeneca, Atriva, Boehringer Ingelheim, GSK, Insmed, MSD, Sanofi, Novartis, and Pfizer for consultancy during advisory board meetings and personal fees from Astra Zeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Sanofi, Solvay, Takeda, Novartis, Pfizer, and Vertex for lectures including service on speakers’ bureaus. F. T. reports consulting fees from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Fisher & Paykel, GlaxoSmithKline, Janssen-Cilag, Novartis, Omron, OM-Pharma, Roche, Sanofi-Aventis, and support for attending meetings or travel from AstraZeneca, Actelion, Bayer, Berlin Chemie, Boehringer Ingelheim, Chiesi, Mundipharma, Novartis, Pfizer, TEVA. T. G. reports Grant from Grifols to institution for AATD laboratory, consulting fees from AstraZeneca, Berlin-Chemie, Boehringer-Ingelheim, Chiesi, CSL-Behring, Grifols, GSK, Mundipharma, Novartis, Takeda, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Berlin-Chemie, Boehringer-Ingelheim, Chiesi, CSL-Behring, Grifols, GSK, Mundipharma, Takeda, support for attending meetings or travel from AstraZeneca, Berlin-Chemie, Chiesi, CSL-Behring, Grifols, GSK, Novartis, participation on a Data Safety Monitoring Board or Advisory Board with AstraZeneca, Berlin-Chemie, Boehringer-Ingelheim, Chiesi, CSL-Behring, Grifols, GSK, Mundipharma, Novartis, Takeda, leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid as scientific Advisor for Alpha-1-Deutschland. C. F. V. reports grants or contracts from German Ministery of Education and Science (BMBF), AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Grifols, Novartis, consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis, Nuvaira, Sanofi, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis, Roche, Sanofi. Bernd Schmeck reports Grants or contracts from CSL-Behring. None declared (H. P., A. R., N. J., K. K., S. R., C. S., I. S., H. R., S. Fähndrich., W. B., A. M., D. Z., S. Finotto., S. S., S. A. S., B. S.).
Acknowledgments
Author contributions: B. C. W.: contribution to the conception and design of this research project, analysis and interpretation of data, drafting and reviewing the manuscript, and final approval of the version to be published. H. P., S. S., S. A. S.: contribution to reviewing the manuscript and final approval of the version to be published. A. R.: contribution to the design and analysis of the manuscript, reviewing it, and final approval of the version to be published. N. J., K. K., C. S.: contribution to the acquisition and analysis of the data, reviewing the manuscript, and final approval of the version to be published. S. R.: contribution to the acquisition, analysis, and interpretation of the data, designing the figure, reviewing the manuscript, and final approval of the version to be published. I. S., S. Fähndrich., W. B., A. M., S. Finotto., F. T.: contribution to the interpretation of the data, reviewing the manuscript, and final approval of the version to be published. H. R., A. L. J.: contribution to the funding of this research project, interpretation of the data, reviewing the manuscript, and final approval of the version to be published. D. Z.: contribution to the analysis of the data, reviewing the manuscript, and final approval of the version to be published. G. R., T. G.: contribution to the design of this research project, interpretation of the data, reviewing the manuscript, and final approval of the version to be published. C. F. V.: contribution to the design and funding of this research project, interpretation of the data, reviewing the manuscript, and final approval of the version to be published. B. S.: contribution to the conception, design, and funding of this research project, analysis and interpretation of data, reviewing the manuscript, and final approval of the version to be published.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank all patients for participation in the study and the collaborating universities for making their data available for the analysis. We also thank Miriam Rößner and Sebastian Mate from the Medical Centre for Information and Communication Technology in Erlangen in Germany, and Abishaa Vengadeswaran from the Institute of Medical Informatics of the Goethe University Frankfurt, Frankfurt am Main, Germany, for their contribution.
Human Ethics Approval Declaration: The study was approved by the ethics committees of all participating Medical Informatics in Research and Care in University Medicine sites.
Footnotes
An abstract of this manuscript was presented at the German Centre of Lung Research Annual Meeting, June 14-16, 2023, Fürstenfeldbruck, Germany, and a presentation of the data was given at the German Respiratory Society Annual Conference, March 23, 2024, Mannheim, Germany.
References
- 1.World Health Organization The Top 10 Causes of Death, Updated December 9, 2020 World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 2.Ewig S., Birkner N., Strauss R., et al. New perspectives on community-acquired pneumonia in 388 406 patients: results from a nationwide mandatory performance measurement programme in healthcare quality. Thorax. 2009;64:1062–1069. doi: 10.1136/thx.2008.109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao Y., Qian Y., Sun X., et al. Eosinopenia predicting long-term mortality in hospitalized acute exacerbation of COPD patients with community-acquired pneumonia: a retrospective analysis. Int J Chron Obstruct Pulmon Dis. 2021;16:3551–3559. doi: 10.2147/COPD.S347948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazzaniga M., Fumagalli L.A.M., D’angelo L., et al. Eosinopenia is a reliable marker of severe disease and unfavourable outcome in patients with COVID-19 pneumonia. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Federal Institute for Drugs and Medical Devices ICD-10-GM: international statistical classification of diseases, German modification. Federal Institute for Drugs and Medical Devices website. https://www.bfarm.de/EN/Code-systems/Classifications/ICD/ICD-10-GM/_node.html
- 6.Medical Informatics in Research and Care in University Medicine (MIRACUM). MIRACUM website. https://www.miracum.org/en [DOI] [PMC free article] [PubMed]
- 7.Gaye A., Marcon Y., Isaeva J., et al. DataSHIELD: taking the analysis to the data, not the data to the analysis. Int J Epidemiol. 2014;43:1929–1944. doi: 10.1093/ije/dyu188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann H.B., Whitney D.R. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 9.Steer J., Gibson J., Bourke S.C. The DECAF Score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67:970–976. doi: 10.1136/thoraxjnl-2012-202103. [DOI] [PubMed] [Google Scholar]
- 10.Krishack P.A., Hollinger M.K., Kuzel T.G., et al. IL-33-mediated eosinophilia protects against acute lung injury. Am J Respir Cell Mol Biol. 2021;64:569–578. doi: 10.1165/rcmb.2020-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]