ABSTRACT
Intoduction:
A severe infection such as COVID-19 may trigger a stroke. The imaging and clinical features of patients with COVID-19 are not well-defined. We aimed to analyze neuroimaging and clinical features of stroke patients with COVID-19.
Methods:
The demographic and clinical data of 21 stroke cases with confirmed COVID-19 (StrokeCov) between April 2020-May 2021 were collected prospectively. An experienced stroke neurologist evaluated neuroimaging findings. A control group of gender, age, and risk factors adjusted 104 stroke patients were included.
Results:
Mean age was 66.3 (±13.3) and 66.2 (±13) years in the StrokeCov group and control group (CG), with similar male-to-female ratios (85%) and without significant difference regarding diabetes, hypertension, hyperlipidemia, and atrial fibrillation between groups (p>0.05). Infarcts were most frequently seen in the territory of middle cerebral artery (8 patients; 40%), followed by multiple arterial territories (6 patients; 30%). Ischemic lesions were more frequently localized in both anterior and posterior vascular systems in StrokeCov group (3 patients; 15%) in comparison to CG (2 patients; 2%; p=0.02). Although, hemorrhagic transformation was observed more frequently in StrokeCov group (6 patients; 30%) than CG (11 patients; 10%; p=0.02); statistically significant difference was not seen in terms of acute and preventive treatments given to both groups. The mRS scores on discharge were worse in the StrokeCov group (p<0.00).
Conclusion:
Ischemic stroke lesions in StrokeCov group are more likely to be localized on multiple arterial territories and develop hemorrhagic transformation. Poor clinical outcome and in-hospital death are more common in stroke due to COVID-19.
Keywords: COVID-19, imaging, intracerebral hemorrhagei ischemic stroke, stroke treatment
INTRODUCTION
Coronavirus Disease-19 (COVID-19) led to the pandemic and caused high rates of mortality and morbidity due to multisystem involvement triggered mainly by systemic inflammation and hypercoagulability. Neurologic manifestations of COVID-19 infection, especially cerebrovascular diseases are well established (1–4). Acute ischemic stroke (AIS), intracerebral hemorrhage, subarachnoid hemorrhage, and cerebral venous thrombosis have been reported in association with COVID-19 (5,6). Several studies evaluated the clinical characteristics of stroke patients with concomitant COVID-19 and most of these studies used retrospective databases (7–9). According to their findings, acute stroke patients with COVID-19 tended to be younger, have more frequently large vessel occlusion and present with a higher National Institute of Health Stroke Scale (NIHSS) score (10). History of cardiovascular disease and diabetes, which were among traditional risk factors for stroke, were also shown to be risk factors for severe COVID-19 (11). However radiological findings were not evaluated in detail and therefore there were limited data on the imaging characteristics of COVID-19 associated stroke (12). In this study, we aimed to investigate primarily imaging features as well as the clinical characteristics and prognosis of patients diagnosed with stroke and COVID-19, prospectively, and also their differentiating features from stroke cases without COVID-19.
Highlights
Poor clinical outcome was more common in ischemic stroke patients with COVID-19.
Hemorrhagic transformation was more frequently seen in patients with COVID-19.
Stroke patients with COVID-19 were more likely to have multiple, scattered infarcts.
METHOD
In this case-control study, we included patients admitted between April 2020 and May 2021 diagnosed with acute stroke confirmed by either computed tomography or magnetic resonance and COVID-19 (the StrokeCov Group). The diagnosis of COVID-19 was confirmed by PCR test or anti-SARS-CoV-2 IgM positivity along with clinical features (1). Patients were recruited from Neurology Emergency Clinic, COVID-19 Clinics and intensive care unit via consultation at Istanbul University Faculty of Medicine. Acute onset neurological deficits with evidence of ischemic lesion or hemorrhage on brain Magnetic Resonance Imaging (MRI) or brain Computed Tomography (CT) were defined as acute stroke. Cervical and cerebral vasculatures were visualized with MR angiography and/or CT angiography in all patients. The clinical, radiological and laboratory data of the StrokeCov group were collected prospectively. Clinical localization of stroke was classified according to the Oxfordshire Community Stroke Project (OCSP), neuroimaging findings were evaluated by an experienced vascular neurologist (OC) and etiological subtypes were denoted using the TOAST (Trial of Org 10172 in Acute Stroke Treatment) classification system (13). We compared data of the StrokeCov group with those of gender and age matched, risk factors adjusted control group composed of patients with acute stroke retrieved from the İstanbul Medical School-Stroke Registry (14). We defined samples sizes with a ratio of 1:5 (StrokeCov: Control group) for group comparisons. CHA2DS2–VASc scoring system which included vascular risk factors such as hypertension, diabetes, congestive heart failure, and peripheral vascular disease was used to determine a matched control group (15). For each StrokeCOV patient, a list of patients meeting the criteria of the control group was created. We have selected five patients using randomization with excel commands for every StrokeCov patient. In this way, 104 patients were selected from the stroke registry as the control group.
Clinical data including initial neurological symptoms, NIHSS score, worsening of clinical course, history of previous stroke, cancer and smoking or alcohol use were collected for both groups. Blood pressure, body temperature and laboratory findings such as blood glucose levels, blood count parameters, C-reactive protein (CRP), D-dimer, INR, and prothrombin time on admission were recorded. Lipid parameters and HbA1 c levels were also measured following 12-hours of fasting.
Acute treatments and preventive therapies as well as supportive management, the requirement of oxygen treatment, and ICU admission were recorded. Finally, modified Rankin Scale (mRS) scores on discharge and the rate of in-hospital death were collected.
The local ethics committee of Istanbul University Faculty of Medicine approved the study (protocol number 2020/517). All the participants or their legal representatives read and signed the informed consent form.
Statistical Analysis
Descriptive analyses were applied and normality was checked with Shapiro-Wilks test. Mean and median with standard deviation (SD) and interquartile range (IQR) values of continuous data were reported accordingly. Categorical data were given with counts and percentages. Group comparisons were performed using Chi-square analysis, parametric student’s t-test or non-parametric Mann Whitney U test according to the data characteristics and distribution of data. Statistical analyses were performed with IBM Statistical Package for Social Sciences (SPSS) program version 22 and p<0.05 was considered as statistically significant.
Study Outcome
The primary outcome of this study was to analyze and compare neuroimaging features and mRS score on discharge. As a secondary outcome, we aimed to assess initial NIHSS score, clinical and laboratory parameters, underlying stroke etiology, acute stroke treatment, and secondary prophylaxis.
RESULTS
We recruited 21 stroke patients with COVID-19 (the StokeCov group) and age-matched 104 control stroke patients between April 2020 and May 2021, with a mean age of 66.2±13 years. Only one patient had hemorrhagic stroke and remaining patients were diagnosed with AIS. The clinical and demographical data are shown in Table 1. Gender difference in the StrokeCov group was striking with 85% male dominance. The youngest patient was a 36-year-old male patient with a history of diabetes. Previous use of antiplatelet and anticoagulant drugs in both groups was similar without statistical significance. The rates of cancer requiring active treatment were also similar in both groups (p=0.67).
Table 1.
Clinical and demographical characteristics of patients
| Stroke cases with Covid-19 (n=21)(n, percent) | Control stroke cases n=104(n, percent) | p | |
|---|---|---|---|
| Gender (M) | 18 (85%) | 89 (86%) | 1 |
| Age | 66.38 (70,36–81) | 66.24 (70.3–84) | 0.93 |
| Hypertension | 13 (62%) | 77 (74%) | 0.25 |
| Diabetes | 11 (52%) | 42 (40%) | 0.31 |
| Hyperlipidemia | 5 (23%) | 36 (34%) | 0.059 |
| History of stroke | 3 (14%) | 31 (29%) | 0.18 |
| Smoking | 5 (23%) | 33 (31%) | <0.0000 |
| Alcohol consumption | 0 | 14 (1%.4) | <0.0000 |
| Antiplatalet drug use | 7 (33%) | 41 (39%) | 0.75 |
| Anticoagulant drug use | 4 (19%) | 12 (11%) | 0.47 |
| Cancer (active) | 2 (9%) | 8 (7%) | 0.67 |
| Clinical worsening | 10 (47%) | 16 (15%) | 0.002 |
| Atrial fibrilation | 5 (23%) | 29 (27%) | 0.79 |
| NIHSS score | 10 (2–14.5) | 6 (3–10) | 0.27 |
| Treatment | |||
| Thrombolysis | 2 (9%) | 15 (14%) | 0.73 |
| Thrombectomy | 3 (14%) | 8 (7%) | 0.4 |
| Antiaggregant* | 15 (71%) | 55 (52%) | 0.3 |
| Anticoagulant* | 11 (52%) | 50 (48) | 0.47 |
| Intubation | 6 (28%) | 4(3%) | 0.002 |
| Oxygen treatment | 13 (61%) | 8 (7%) | <0.0000 |
| Blood Tests | |||
| Hemoglobin (median, IQR) | 13 (9.8–13.6) | 13 (12–14) | 0.27 |
| Hematocrit (median, IQR) | 38.6 (30.3–41) | 39.7 (36–43) | 0.33 |
| WBC (median, IQR) | 9.6 (7.7–12.1) | 8.4 (6.9–10.2) | 0.22 |
| Platelets (median, IQR) | 254.000 (194000–399000) | 229500 (185000–285000) | 0.54 |
| CRP (mean, SD) | 67.7 (89.1) | 17.3 (24.82) | 0.008 |
| Sedimentation (median, IQR) | 13 (6.5–27) | 22 (9–39) | 0.6 |
| HbA1c (median, IQR) | 7.3 (6–8) | 6 (5.5–7.9) | 0.23 |
| Total cholesterol (mean, SD) | 163.4 (±57.4) | 193.4 (±54.2) | 0.05 |
| LDL (mean, SD) | 102.69 (±45.5) | 133 (±57.9) | 0.04 |
| HDL (median, IQR) | 31 (24–52) | 38 (31–44) | 0.52 |
| APTT (median, IQR) | 25.8 (22.3–33) | 25 (22–27) | 0.23 |
| INR (median, IQR) | 1.1 (1–1.3) | 1 (0.9–1.07) | 0.006 |
APTT: activated partial thromboplastin time; CRP: C-reactive protein; HDL: high-density lipoprotein; INR: international normalized ratio; IQR: interquartile range; LDL: low-density lipoprotein; M: male; NIHSS: National Institute of Health Stroke Score; n: number; SD: standart deviation; WBC: White blood cells.
Blood glucose and CRP levels on admission were higher in the StrokeCov group than the Control group (p=0.01 and p=0.008, respectively), whereas total cholesterol and LDL levels were lower in the StrokeCov group (p=0.05 and p=0.04, respectively). Initial hemoglobin level, white blood cells and platelet counts did not show statistical difference between the groups. International normalized ratio (INR) levels were higher in the StrokeCov group (p=0.006). D-Dimer was not screened routinely for every stroke patient in the pre-pandemic period in our stroke unit, unless there was a specific additional diagnosis such as pulmonary thromboembolism, deep venous thrombosis, etc., therefore, we could not compare serum D-Dimer levels between groups. Initial D-Dimer levels (median 1170 ug/L, IQI 825–3170) were elevated in StrokeCov group as expected. Assessment of lymphopenia in StrokeCov group revealed that 52% of the patients had lymphopenia.
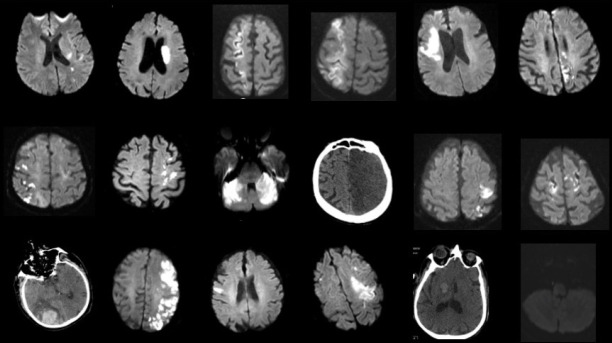
Although median NIHSS score was higher in the StrokeCov group, this difference was not statistically significant (p=0.27). Clinical syndrome according to the OCSP and radiologic evaluation including infarct localization, asymptomatic infarction, and hemorrhagic transformation are presented in Table 2. Only 2 patients (9.5%) presented with a lacunar syndrome (p=0.21). One-third of patients in the StrokeCov group had multiple infarctions in multiple arterial regions (p=0.33) Hemorrhagic transformation was seen in approximately one-third of StrokeCov patients (p=0.02). Two of 6 patients in StrokeCov group and 1 of 11 patients in the control group with hemorrhagic transformation were intubated. A sub-group analysis was performed for the intubation rate in patients with hemorrhagic transformation and revealed no significant relations between these two entities (p=0, 21). We also conducted another analysis to demonstrate a possible relationship between intubation and hemorrhagic transformation in all patients independent from the study groups. However, this analysis did not reveal a statistically significant result (p=0, 101). Multiple infarctions were seen in anterior and posterior circulation regions in the StrokeCov group (p=0.02). Brain MRI and brain CT imaging studies of the StrokeCov patients (18 of 21) are shown in Figure 1.
Table 2.
Clinical syndrome, neuroradiologic features and etiology of patients
| Stroke cases with Covid-19 (N=21)(n, percent) | Control stroke cases N=104(n, percent) | p | |
|---|---|---|---|
| Clinical syndrome | p>0.05 for all subgroups | ||
| LACS | 2 (9%.5) | 22 (21%.5) | |
| PACS | 10 (47%.6) | 37 (35%.8) | |
| POCS | 4 (19%.4) | 25 (24%) | |
| TACS | 5 (23%.8.6) | 20 (19%.2) | |
| Infarct localisation | p>0.05 for all subgroups | ||
| ACA | 2 (10%) | 3 (3%) | |
| MCA | 8 (40%) | 45 (45%) | |
| VA-PICA | 1 (5%) | 8 (8%.2) | |
| Basilar | 0 | 5 (5%.1) | |
| PCA | 1 (5%) | 15 (15%.3) | |
| Watershed | 1 (5%) | 1 (1%) | |
| Multiple-artery | 6 (30%) | 20 (20%.4) | |
| Not well-defined | 1 (5%) | 1 (1%) | |
| Etiology | p>0.05 for all subgroups | ||
| LAA | 8 (40%) | 23 (23%.5) | |
| Cardioembolism | 4 (20%) | 36 (36%.7) | |
| Small vessel disease | 0 | 1 (1%) | |
| Unknown | 5 (25%) | 17 (17%.3) | |
| Multiple | 1 (5%) | 15 (15%.3) | |
| Rare etiologies | 2 (6%) | 6 (6%.1) | |
| Asymptomatic infarction | 5 (23%.8) | 19 (18%.6) | 0.58 |
| Asymptomatic vascular lesion | 4 (19%) | 13 (13%.5) | 0.51 |
| Hemorrhagic transformation | 6 (30%) | 11 (10%) | 0.02 |
| Anterior-posterior vascular system | 0.02 | ||
| Anterior | 12 (60%) | 59 (60%.2) | |
| Posterior | 5 (25%) | 37 (37%.8) | |
| Anterior and posterior* | 3 (15%) | 2 (2%) |
ACA: anterior cerebral artery; LAA: large artery atherosclerosis; LACS: lacunar syndrome; MCA: middle cerebral artery; PACS: partial anterior circulation syndrome; PCA: posterior cerebral artery; POCS: posterior circulation syndrome; TACS: total anterior circulation syndrome; VA-PICA: vertebral artery and posterior inferior cerebellar artery.
Figure 1.
Brain MR and Brain CT imagings of stroke patients with COVID-19. Brain MRI and Brain CT imaging of 18 patients from stroke StrokeCOV group StrokeCOV: stroke cases with confirmed COVID-19
Distribution of stroke etiology according to TOAST classification, is demonstrated in Table 2. Only four patients had a cardioembolic stroke in the StrokeCov group. Large artery atherosclerosis (LAA) was found in 40% of the cases comprising the largest group in stroke etiology.
Prognosis
Of the patients in the StrokeCoV group, 61% received oxygen treatment and 28% of them were intubated (p=0.0002 and p<0.0000) while only 3% of patients were intubated in the Control group. The mRS scores at discharge are shown on Figure 2. Three patients in StrokeCoV group died in the intensive care unit. Half of the patients were discharged with mRS 4 and 5 in StrokeCov group in comparison to only 19.2% patients discharged with mRS 4 and 5 in the control group (p=0.009). Also, there was no in-hospital death in the control group.
Figure 2.
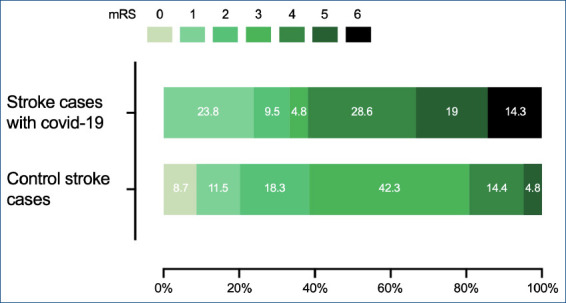
The distribution of mRS of patients at discharge mRS: modified Rankin scale
DISCUSSION
In this study, we found that hemorrhagic transformation occurred in one third of the ischemic stroke lesions in patients with COVID-19, regardless of stroke management. Infarcts localized on multiple arterial territories were more frequently seen in the group with COVID-19 in comparison to gender, age and risk factors adjusted controls. Moreover, even lesions in the same arterial territory showed multiple and scattered pattern. Poor clinical outcome and in-hospital death were also more common in stroke patients with COVID-19.
In this study, ischemic lesions did not show a specific artery distribution or anatomic localization, a finding which might be related to the modest size of our study sample. Multiple ischemic lesions were more frequently observed in the StrokeCov group similar to some previous studies (16,17). Multiple infarcts can be seen in various etiologies such as cardioembolic stroke, hypercoagulability, and cancer. Besides, only two of six patients in the StrokeCov group had atrial fibrillation, and all of the patients in the control group with multiple infarcts had atrial fibrillation. The intracranial and extracranial vessel imaging of all participants were also examined meticulously in our study. In addition, brain MRI was performed in 86% of the stroke patients with COVID-19. Another study performed with a limited number of patients reported that infarcts in the stroke patients with COVID-19 were localized more frequently than ours in the posterior circulation in 6 patients of 17 (35%) stroke patients (18). It was noteworthy that the brain MRI was performed on a very restricted number of patients in that study (18).
Hemorrhagic transformation is not a rare complication of ischemic stroke. Reperfusion therapies, hypertension and hyperglycemia are well known risk factors for this undesirable entity. Another potential underlying mechanism is systemic or local inflammation that may alter blood-brain barrier permeability with proinflammatory cytokines (19). Stroke patients with COVID-19 infection were exposed to local inflammation due to ischemic stroke, and systemic and regional inflammation due to the effects of COVID-19 infection. Another interesting finding was the higher hemorrhagic transformation rates in the StrokeCov group which might be associated with the poor clinical outcome in this group. Although we did not record the exact time of starting anticoagulant therapy in the StrokeCov group to enable a comparison between the groups, the “1–3–6–12 day” rule according to infarct size was applied routinely in all admitted patients whenever possible (20). Baudin et al. did not find a statistically significant difference regarding hemorrhagic transformation between stroke patients with and without COVID-19. However, in that study, the underlying etiology was not similar between the groups. Proximal artery occlusion was found more often in stroke patients without COVID-19, which might be a reason for those findings in that study (21).
Besides COVID-19, all of our patients had similar risk factors including cancer which were associated with poor prognosis. Moreover, there was no difference regarding acute stroke treatments, namely thrombolysis and thrombectomy between both groups. Another study also reported that stroke patients with COVID-19 received acute stroke treatments similar to stroke patients without COVID-19 akin to our cohort (10). Calmette et al. found that stroke patients with COVID-19 had poor clinical outcomes, consistent with our findings. The mortality rate in their COVID-19 stroke cohort was 13% (16). Based on the prevalence of similar risk factors and recipience of similar treatment for AIS, the poor prognosis may be attributable to COVID-19 (11,17,22,23). Elevated D-dimer and CRP levels were associated with poor prognosis in acute ischemic stroke and COVID-19 in the literature (24–26). Hypercoagulation and inflammation triggered by COVID-19 might be the underlying cause of the poor outcome. Increased inflammatory response, particularly increased IL-6 levels were associated with increased infarct volume and poor outcomes in patients with stroke (21). Endothelial cells may be affected by COVID-19 directly, which lead to endothelial damage and inflammation (22). These inflammatory responses trigger cytokines and activate platelets and microthrombi formation. As a result, D-dimer levels increase, and hypercoagulability occurs. Acute myocardial injury may be another cause of embolic-like stroke in stroke patients with COVID-19. Cerebral autoregulation may be altered by COVID-19 induced hypoxia and result in cerebral vasodilation (3). In addition, traditional vascular risk factors such as hypertension, diabetes and hyperlipidemia detected in the StrokeCov group might be contributing factors for stroke pathogenesis (5,22).
Initial NIHSS scores were higher in the StrokeCoV group, paralleling this only 2 patients presented with a lacunar syndrome, and none of the patients had small vessel disease as a stroke etiology except for one patient with putaminal hemorrhage due to hypertensive vasculopathy. A recent study also reported that small vessel disease was not a common stroke etiology for stroke patients with COVID-19 (27).
The male dominance in StrokeCov patients was expected regarding the recent literature, still the male to female ratio was striking with 5.7 fold (8). Smoking and alcohol consumption were found less in the StrokeCov group in our study. Smoking was reported 16% -38.5% in acute ischemic stroke patients with COVID-19 in different studies however there was no comparative evaluation with control group (2,7).
Secondary prophylaxis for stroke with COVID-19 cases especially presenting with elevated D– Dimer levels is challenging. Antiaggregant or anticoagulant therapy decision was made for each case taking into account other comorbidities and local hospital infection committee’s suggestions. Although cardioembolic etiology was found in 20% of patients, 52% of patients received anticoagulant therapy for the reasons listed above.
Our study was distinguishable with comprehensive imaging evaluation and prospective design in the literature. The number of patients in control group was defined as fivefold higher than the StrokeCov group and conventional vascular risk factors were also adjusted in addition to age and gender in both groups to uncover more precisely the differences between imaging features and outcomes of the StrokeCov and control groups. Another strength of our study was that neuroradiologic evaluation was performed by experienced stroke neurologist who evaluated stroke patients and recorded them to the IMSS DataBase. A limitation of our study was the modest sample size of the StrokeCov group. However neurological evaluation was conducted in detail in all of the patients.
As a result; poor clinical outcome and increased rate of in-hospital deaths were more common in stroke patients with COVID-19, which might be related to hypercoagulability and inflammation. Our findings showed that hemorrhagic transformation and scattered infarcts and multiple infarcts were more frequently seen in ischemic stroke with COVID-19. These neuroimaging findings may be used as a clue to do further investigation of COVID-19 in patients with negative initial COVID-19 PCR test. Further studies with larger samples are needed to support our findings.
Footnotes
Ethics Committee Approval: The local ethic committee of Istanbul University Faculty of Medicine approved the study (protocol number 2020/517).
Informed Consent: All the participants have read and signed the informed consent form.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept- MS, OÇ; Design- MS, NY; Supervision- NY, EE; Resource- EE; Materials- MS; Data Collection and/or Processing- MS, NY; Analysis and/or Interpretation- MS, OÇ, NY; Literature Search- EE, MS; Writing- MS, NY; Critical Reviews- OÇ.
Conflict of Interest: The authors declared that there is no conflict of interest.
Financial Disclosure: This work was supported by Scientific Research Projects Coordination Unit of Istanbul University (Project number GAP-36802).
REFERENCES
- 1.Asadi-Pooya AA, Simani L. Central nervous system manifestations of COVID-19:a systematic review [Internet. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19:a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL. Acute cerebrovascular events in hospitalized COVID-19 patients. Stroke. 2020;51(9):219–222. doi: 10.1161/STROKEAHA.120.030995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrogate S, Mortimer A, Burrows L, Fiddes B, Thomas I, Rice CM. Non-aneurysmal subarachnoid haemorrhage in COVID-19. Neuroradiolgy. 2021;63(1):149–152. doi: 10.1007/s00234-020-02535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahjouei S, Tsivgoulis G, Farahmand G, Koza E, Mowla A, Sadr AV, et al. SARS-CoV-2 and stroke characteristics:a report from the multinational COVID-19 stroke study group. Stroke. 2021;52(5):1–14. doi: 10.1161/STROKEAHA.120.032927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidale S. Risk factors, and clinical and etiological characteristics of ischemic strokes in COVID-19-infected patients:a systematic review of literature. Cerebrovasc Dis. 2021;50(4):7–10. doi: 10.1159/000514267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majidi S, Fifi JT, Ladner TR, Lara-Reyna J, Yaeger KA, Yim B, et al. Emergent large vessel occlusion stroke during New York City's COVID-19 outbreak:clinical characteristics and paraclinical findings. Stroke. 2020;51(9):2656–2663. doi: 10.1161/STROKEAHA.120.030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava PK, Zhang S, Xian Y, Xu H, Rutan C, Alger HM, et al. Acute ischemic stroke in patients with COVID-19:an analysis from get with the guidelines-stroke. Stroke. 2021;52(5):1826–1829. doi: 10.1161/STROKEAHA.121.034301. [DOI] [PubMed] [Google Scholar]
- 11.Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. Coronavirus disease 2019 and the cerebrovascular-cardiovascular systems:what do we know so far? J Am Heart Assoc. 2020;9(13):e016793. doi: 10.1161/JAHA.120.016793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, et al. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297(1):E232–E235. doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams HPJ, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Yeşilot N, Koyuncu B, Çoban O, Tuncay R, Bahar SZ. Gender differences in acute stroke:Istanbul medical school stroke registry. Neurol India. 2011;59(2):174–9. doi: 10.4103/0028-3886.79130. [DOI] [PubMed] [Google Scholar]
- 15.Lip GYH, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation:a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 16.Calmettes J, Peres R, Goncalves B, Varlan D, Turc G, Obadia M, et al. Clinical outcome of acute ischemic strokes in patients with COVID-19. Cerebrovasc Dis. 2021;50(4):412–419. doi: 10.1159/000514562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweid A, Hammoud B, Bekelis K, Missios S, Tjoumakaris SI, Gooch MR, et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15(7):733–742. doi: 10.1177/1747493020937189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martin O, Barrena C, et al. Cerebrovascular disease in patients with COVID-19:neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spronk E, Sykes G, Falcione S, Munsterman D, Joy T, Kamtchum-Tatuene J, et al. Hemorrhagic transformation in ischemic stroke and the role of inflammation. Front Neurol. 2021;12:597. doi: 10.3389/fneur.2021.661955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 21.Naval-Baudin P, Rodriguez Caamaño I, Rubio-Maicas C, Pons-Escoda A, Fernández Viñas MM, Nuñez A, et al. COVID-19 and ischemic stroke:clinical and neuroimaging findings. J Neuroimaging. 2021;31(1):62–66. doi: 10.1111/jon.12790. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, Raju M, et al. Acute ischemic stroke and COVID-19:an analysis of 27.676 patients. Stroke. 2021;52(3):905–912. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, et al. COVID-19 related neuroimaging findings:a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wijeratne T, Sales C, Karimi L, Crewther SG. Acute ischemic stroke in COVID-19:a case-based systematic review. Front Neurol. 2020;11:1031. doi: 10.3389/fneur.2020.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wool GD, Miller JL. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88(1):15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang S, Zhang J, Wang C, Chen X, Zhao X, Jing H, et al. COVID-19 and ischemic stroke:mechanisms of hypercoagulability (review) Int J Mol Med. 2021;47(3):1–13. doi: 10.3892/ijmm.2021.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogrig A, Gigli GL, Bnà C, Morassi M. Stroke in patients with COVID-19:clinical and neuroimaging characteristics. Neurosci Lett. 2021;743:135564. doi: 10.1016/j.neulet.2020.135564. [DOI] [PMC free article] [PubMed] [Google Scholar]