Abstract
Cell-based therapy is a new direction of treatment of diseases such as type 1 diabetes mellitus (T1DM); but unfortunately, its severe side effects include immunogenicity and tumor development. Using Mesenchymal stem cells conditioned medium (MSCs-CM) may be an alternative therapy to avoid stem cell risks, preserving effectiveness and demonstrating noticeably increased levels of cytokines, angiogenic factors, and growth factors that encourage and support regenerative processes. In the current work, we examined the effects of MSCs-CM injected in tail vein and pancreas directly compared with the standard antidiabetic drug, glimepiride in streptozotocin-induced type 1 diabetic rats. Fifty adults Male Wistar rats were allocated equally into five groups: normal, diabetic control and three diabetic groups treated respectively with glimepiride, MSCs-CM injected daily into tail vein (MSCs-CMT) and MSCs-CM injected directly in pancreas (MSCs-CMP); all treatments continued for 28 days. The treatments produced a significant improvement in blood glucose level and glycosylated hemoglobin A1c (HbA1c), serum insulin level and lipid panel, and pancreas apoptosis-related markers including B cell lymphoma-2 (Bcl-2) and vimentin. In addition, the treatments resulted in suppression in the oxidation stress and enhancement in the antioxidant, which were manifested by the suppressed lipid peroxidation and the increased antioxidant markers (glutathione, catalase and superoxide dismutase) in the pancreas. In association with the significant decrease in tumour necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) and a significant increase in interleukin-10 (IL-10) levels, the inflammatory mediator nuclear factor-kappa B (NF-κB) expression was significantly decreased by MSCs-CMT and MSCs-CMP. The histological amelioration of the pancreatic islet cells assured our study especially in MSCs-CMP group than MSCs-CMT which supports islet regeneration and elevated circulating insulin. These results imply that MSCs-CM infusion has therapeutic benefits in T1DM rats and may be a viable novel therapeutic approach; MSCs-CMP was shown to be more effective than glimepiride and MSCs-CMT. The mechanisms of antidiabtic actions may be mediated via the antioxidant, anti-apoptotic and anti-inflammatory effects.
Keywords: Mesenchymal-stem cells conditioned media, Streptozotocin, Diabetes mellitus, Wistar rats
1. Introduction
Type 1 diabetes mellitus (T1DM) impacts millions of people worldwide and needs to be carefully managed to prevent major long-term consequences such heart and renal disease, stroke, and blindness [1]. The group of patients with T1DM is incredibly diverse; they have varying genetic origins, discrete etiologies, and appear with the illness at different stages and severities [2]. In T1DM, autoreactive T lymphocytes target pancreatic β-cells, leading to the loss of islet β-cells linked to insulitis. This complete absence of insulin secretion causes hyperglycemia, aberrant glucose metabolism, and a permanent need on external insulin [3]. Most T1DM patients have significant blood sugar fluctuations and inadequate blood glucose control. Prolonged hyperglycemia contributes to the emergence of major diabetes complications, including microvascular and macrovascular problems, which lower quality of life and place a significant financial strain on both T1DM patients and society as a whole [4].
Numerous anti-diabetic medications belonging to several groups that function through multiple mechanisms have been introduced to address DM [5]. Glimepiride (Amaryl) is a third-generation sulfonylurea derivative, and is one of the top three most commonly prescribed oral antidiabetic medications [6]. It reduces blood glucose concentration by activating intracellular insulin receptor signalling pathway and encouraging the production of insulin by pancreatic β-cells [7]. Numerous studies demonstrate that glimepiride has no negative effects on heart conditions, but it also increases the risk of stroke [8]. Also, there are many synthetic drugs available for diabetic patients but these drugs are expensive and have unavoidable side effects [9]. Therefore, we are in dire need to test natural products to find their antidiabetic effects with less adverse effects, so that they can be given to help diabetic patients Islet β-cell transplantation has demonstrated efficacy in treating T1DM [3,10]. Nonetheless, the increasing number of people with diabetes worldwide, a scarcity of donors, and the requirement for continuous immunosuppression limit the extensive application of β-cell transplantation [11]. Many treatment options have been used to find β-cell replacements; one of the most promising approaches is stem cell transplantation. Many studies have demonstrated that stem cells can successfully treat type 1 diabetes by preserving islet β-cell function and regaining immunotolerance [3,12]. Undifferentiated stem cells have the ability to self-renew and can develop into almost any type of tissue or organ [[13], [14], [15]]. Mesenchymal stem cells (MSCs) have been employed to preserve β-cells through islet protection and regeneration. Additionally, stem cells have the potent ability to reestablish peripheral tolerance toward β-cells through immune response remodeling and autoreactive T-cell function inhibition [16,17]. In general, stem cells can rebuild immunotolerance by suppressing the immunological response of T cells and T helper 1 (Th1) cells through Transforming growth factor-β (TGF-β) and inflammatory pathways, and they can increase the mass of islets by differentiating into organoids that resemble β-cells [18]. Since T1DM is characterized by an autoimmune disease that triggers immune cells to target and eliminate pancreatic β-cells, stem cell therapy for T1DM treatment should take into account the immunomodulatory characteristics of stem cells as well as their potential to differentiate into cells that produce insulin [3]. MSCs have the ability to change the tissue microenvironment and encourage the survival and regeneration of already-existing β-cells, which increases their bulk and restores normal blood glucose levels [19,20]. The researchers postulated that the cells' potential antioxidative, anti-inflammatory, and anti-apoptotic properties were responsible for their protective function [21]. However, stem cell treatments are difficult and can have major side effects, such as immunogenicity and tumor development [22,23], It has been demonstrated that Mesenchymal stem cells conditioned medium (MSCs-CM) substantially reduces stem cells' risks while sustaining stem cells' efficacy and demonstrating markedly increased levels of growth factors, angiogenic factors, and cytokines that stimulate angiogenesis and help diabetics heal their fractures [24]. In addition, MSCs-CM contain extracellular vesicles (EVs) that carry a variety of proteins, coding and non-coding ribonucleic acid (RNA), small RNAs, mitophagosomes, and autophagosomes [25]. Conditioned medium (CM) may offer the following benefits over cell-based therapies: (1) As opposed to using whole cells, CM uses proteins to promote regeneration; (2) It can be stored for an extended period of time without the need for toxic reagents like dimetylsulfoxide DMSO; (3) It is more affordable to prepare and can be produced in large quantities; and (4) It will be easier to assess CM's safety and efficacy than traditional pharmaceutical preparations [25,26].
Therefore, this research was planned to assess the therapeutic potential effect and mode of actions of MSCs-CM in tail vein and pancreas directly compared with the standard antidiabetic drug, glimepiride in streptozotocin (STZ)-induced diabetic Wistar rats.
2. Materials and methods
2.1. Experimental animals
Male Wistar strain rats weighing around 190 ± 10 g and aged 10 ± 1 weeks were employed in this investigation. They were kept at the Medical Experimental Research Centre (MERC), Mansoura, Egypt, and were obtained from Animal Facilities of VACSERA company (Helwan, Cairo, Egypt). Throughout the experiment, they had unlimited access to water and a conventional meal while being kept in a non-stressful environment for one week to help them acclimatise (temperature: 25 5 °C, humidity: 55 5 %, and light-dark cycle: 12:12 h). Beni-Suef University's IACUC (Institutional Animal Care and Use Committee) approved the study) Approval Number, 024-019).
2.2. Chemicals and drugs
STZ was obtained from Sigma Aldrich Co. MO, USA. It was dissolved in 4.5 pH citrate buffer solution and was injected into peritoneal cavity in a dose of 45 mg/kg body weight (b. wt) of animals. AMARYL® (glimepiride tablets) (Sanofi, Cairo, Egypt) was purchased from pharmacy.
2.3. Induction of diabetes mellitus
After the animals were starved for 16 h, 45 mg/kg of dissolved STZ in citrate buffer (pH 4.5) was injected by intraperitoneal injection to induce T1DM in the Wistar rats [27]. Rats were given STZ injections, and blood glucose levels were measured ten days later. Animals that had been fasting for 10–12 h were fed glucose via stomach intubation at a rate of 3 g/kg body weight. Following 2 h of oral administration, serum glucose concentration was assessed after blood samples were drawn from the lateral tail vein, allowed to clot, and centrifuged. After 2 h of glucose ingestion, rats with blood glucose concentrations between 180 and 300 mg/dl were classified as having moderate diabetes and included to the study; other rats were excluded.
2.4. Preparation of mesenchymal stem cells conditioned medium (MSCs-CM)
MSCs-CM was prepared according to the following steps:
-
1)
Dulbecco's Modified Eagle Medium (DMEM) was used as a culture medium and MSCs in culture medium was divided into 50 mL Falcon tubes each containing 45 mL under laminar air flow and preserved it at 2–8 °C.
-
2)
Antibiotic vial has been thawed in water bath for 20 min as it was preserved at −20 °C.
-
3)
Antibiotic was aliquoted at 15 mL tubes each contains 10–15 mL.
-
4)
To prepare a serum-free basal medium, 45 mL DMEM and 0.5 mL antibiotic were mixed in the 50 mL tube.
-
5)
For a duration of one day, MSCs were cultured in a whole culture medium at a density of 10,000 cells/cm2
-
6)
For 48 h, stem cells were cultured in serum-free basal medium after being stripped three times using PBS.
-
7)
After 24 h of incubation at an atmosphere of 95 % humidity with 5 % CO2 [28,29], for filtration and concentration, the supernatant was collected at 12,000 rpm [30,31].
2.5. Experimental design
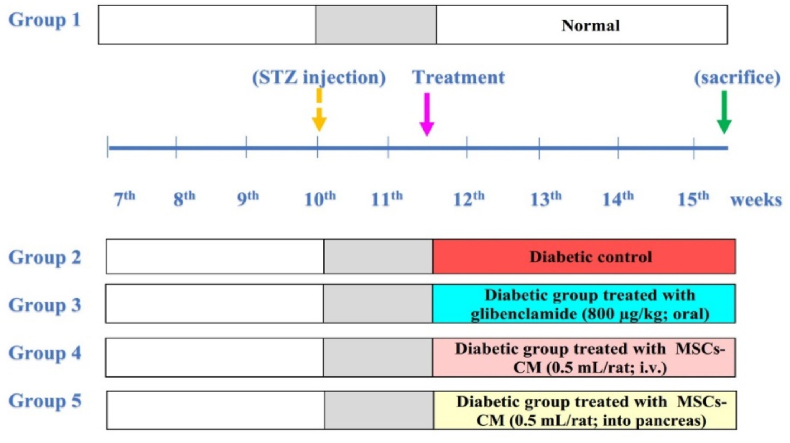
Five groups, each of 10 rats were used (Fig. 1). The first group was the control group, which received the same vehicle quantities for a period of 28 days. Rats with diabetes who were given the same amounts of the vehicles for a period of 28 days made up the second group. The third group was made up of diabetic rats that received oral glimepiride for 28 days at a dosage of 800 μg/kg body weight/day. The fourth group consisted of diabetic rats that received 0.5 mL of MSCs-CM intravenously every 28 days in their lateral tail vein. The fifth group consisted of diabetic rats that received a 28-day treatment with MSCs-CM (0.5 mL CM for each rat) administered locally into the pancreas (0.5 mL for each rat).
Fig. 1.
Experimental design. STZ, strptozotocin; MSCs-CM, mesenchymal stem cells conditioned medium.
2.6. Samples collection and preparation
At the end of the experiment, rats were anesthetized by ethyl ether inhalation anesthesia for 2–3 min. Blood samples were collected from jugular vein and were then allowed to coagulate at room temperature before being centrifuged for 30 min at 3000 round per minute (r.p.m.). After a short time, the supernatant sera were separated into three parts for each animal, and stored at −30 °C for further examination. After collection of blood samples and decapitation, rats were dissected and the pancreas was quickly excised. The pancreas of each rat was separated into three sections: one portion of pancreas was preserved in 10 % neutral buffered formalin to prepare paraffin sections and stain them for histological analysis. The second part was saved in glutaraldehyde 25 % for electron microscope examination and third part was homogenized and centrifuged at 3000 r.p.m. for 5 min to get rid of cell debris, supernatant was kept at −30 °C, and applied to biochemical examinations.
2.7. Laboratory biochemical investigations
2.7.1. Determination of glucose, glycated hemoglobin A1c (HbA1c) and insulin levels
Samples were taken for the assessment of blood biochemical parameters in ethylenediamine tetraacetic acid (EDTA) and serum-separating tubes. Blood glucose level was determined by CERA-CHECK™ 1070 glucometer, HbA1c was calculated with Bisse and Abraham's method [32] and insulin in serum was measured according to Temple et al. method [33].
2.7.2. Detection of lipid profile in serum
Serum cholesterol and triglyceride concentrations were detected according to Young, and Friedman [34]. The levels of high-density lipoprotein cholesterol (HDL-C) were detected using the method of Warnick et al. [35], whereas the levels of low-density lipoprotein cholesterol (LDL-C) and very low-density lipoprotein cholesterol (vLDL-C) were determined using the formulas of Tietz [36] and Frideewald [37], respectively.
2.7.3. Determination of oxidative stress and antioxidant parameters in pancreas
Pancreatic sample homogenates were prepared using potassium phosphate buffer (pH 6.5, 1:10) and centrifuged for 20 min at 4 °C. The supernatant for each sample was fractioned into 3 Eppendorf tubes. Malondialdehyde (MDA) was determined based on the methods of Satoh [38] and Ohkawa et al. [39]. Superoxide dismutase (SOD) activity, GSH content, and catalase (CAT) activity were measured based on the publications of Beutler et al. [40], Nishikimi et al. [41], and Aebi [42] respectively.
2.7.4. Determination of inflammatory and anti-inflammatory markers
The manufacturer's instructions were followed in measuring serum concentrations of interleukin-1beta (IL-1β), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) using the ELISA, which was based on the techniques of DeVita et al. [43], Hodge et al. [44], and Brouckaert et al. [45] respectively. The pancreatic nuclear factor kappa-B (NF-κB) was measured using quantitative real time-polymerase chain reaction (qRT-PCR). Total RNA was isolated and extracted in accordance with Qiagen, CA, USA manufacturer's instructions [46].
2.7.5. Determination of B-cell lymphoma 2 (Bcl-2) and vimentin
The mRNA expressions of Bcl-2, and Vimentin were determined in the pancreas by qRT-PCR. Total RNA was isolated from pancreas based on the manufacturer's instructions (Qiagen, CA, USA).
2.8. Histopathology and ultrastructural preparations of the pancreas
In accordance with Bancroft and Gamble, slices of pancreases were stained using hematoxylin and eosin stain [47] method. The other specimens were prepared for ultrastructural examination by the method of Bain and Mercer [48].
2.9. Statistical analyses
The data was displayed as mean ± SE, and one-way analysis of variance (ANOVA) was carried out using computer software (SPSS version 20, IBM Corp., 2011) to find any significant differences between groups. Next, the Duncan Multiple Range Test (DMRT) was run. A difference was considered significant if p-value is less than 0.05.
3. Results
3.1. Effects on glucose, glycated hemoglobin A1c (HbA1c) and insulin levels
The diabetic control rats presented a significant (p < 0.05) raise in blood glucose and HbA1c with a marked (p < 0.05) reduction in serum insulin levels contrasted with the normal control. When compared to the diabetic group, the diabetic rats were treated with glimepiride, MSCs-CMT, or MSCs-CMP and demonstrated a significant improvement in these parameters. The greatest successful recovery towards normal levels was demonstrated by MSCs-CMP therapy (Table 1). MSCs-CMP was more potent than MSCs-CMT in improving postprandial blood glucose at the end of the experiment (PPBG2) and its effect was comparable to that of glimepiride.
Table 1.
Changes in glucose, HbA1c and insulin in normal, diabetic control and diabetic treated groups.
| Parameters | Normal | Diabetic control | Diabetic treated with glimepiride | Diabetic treated with MSCs-CMT | Diabetic treated with MSCs-CMP |
|---|---|---|---|---|---|
| FBG1 (mg/dL) | 78.17 ± 4.18a | 163.00 ± 10.30b | 165.83 ± 4.75b | 174.06 ± 3.43b | 159.50 ± 6.86b |
| PPBG1 (mg/dL) | 113.67 ± 5.73a | 243.83 ± 8.02b | 222.50 ± 13.84b | 212.00 ± 6.67b | 232.17 ± 15.81b |
| FBG2 (mg/dL) | 89.17 ± 3.03a | 172.67 ± 6.31c | 104.50 ± 5.01ab | 111.83 ± 7.30b | 112.17 ± 4.41b |
| PPBG2 (mg/dL) | 124.83 ± 1.35a | 238.50 ± 15.41c | 117.50 ± 4.10a | 165.83 ± 10.16b | 123.67 ± 5.17a |
| HbA1c (%) | 2.52 ± 0.09a | 4.08 ± 0.06c | 3.67 ± 0.08b | 3.70 ± 0.09b | 3.63 ± 0.09b |
| Insulin (μIU/mL) | 3.43 ± 0.16c | 1.56 ± 0.16a | 2.40 ± 0.19b | 2.72 ± 0.16b | 2.98 ± 0.09bc |
Values are represented as Mean ± SE of 10 rats in each group. Values, which have different superscript symbols (a, b and c) for each parameter, are significantly different at p < 0.05. FBG1 and PPBG1 are fasting blood glucose and postprandial blood glucose concentrations before treatment while FBG2 and PPBG2 are fasting blood glucose and postprandial blood glucose concentrations at the end of the treatment period. HbA1c: glycosylated hemoglobin A1c. MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas.
3.2. Effects on serum lipid profile
When compared to the nondiabetic control group, the diabetic group had a substantial (p < 0.05) elevation in the lipid profile, which included blood cholesterol, triglycerides, LDL-C, and vLDL-C, as well as a significant (p < 0.05) drop in HDL-C levels. Following therapy, everyone is improved (Table 2). The most effective method of enhancing the serum lipid profile was the MSCs-CMP therapy.
Table 2.
Lipid profile parameters in normal, diabetic control and diabetic treated groups.
| Parameters | Normal | Diabetic control | Diabetic treated with glimepiride | Diabetic treated with MSCs-CMT | Diabetic treated with MSCs-CMP |
|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 102.67 ± 5.65a | 211.17 ± 5.40d | 178.33 ± 6.23c | 144.33 ± 2.95b | 105.17 ± 3.19a |
| Triglycerides (mg/dL) | 80.83 ± 2.72a | 157.60 ± 3.30d | 113.67 ± 3.45c | 102.67 ± 2.11b | 82.00 ± 2.65a |
| HDL-C (mg/dL) | 54.67 ± 0.88c | 37.33 ± 1.09a | 47.00 ± 0.63b | 52.50 ± 0.99c | 55.00 ± 0.45c |
| LDL-C (mg/dL) | 32.45 ± 1.20a | 134.97 ± 3.25d | 113.33 ± 4.77c | 70.83 ± 3.52b | 36.83 ± 1.14a |
| vLDL-C (mg/dL) | 15.55 ± 0.19a | 31.70 ± 0.96d | 22.67 ± 0.89c | 19.33 ± 0.67b | 16.32 ± 0.78a |
Values are expressed as Mean ± SE of 10 rats in each group. Values, which have different superscript symbols (a, b, c and d) for each parameter, are significantly different at p < 0.05. MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; vLDL-C, very low-density lipoprotein cholesterol.
3.3. Effect on pancreas oxidative stress and antioxidant defense system
Table 3 reveals that the pancreatic tissues of the diabetic group showed a notable drop in SOD, CAT, and GSH, as well as a significant increase of MDA when compared to the control group. On the other hand, several treatment plans demonstrated a substantial (p < 0.05) improvement, with the MSCs-CMP therapy demonstrating the greatest amelioration.
Table 3.
Oxidative stress and antioxidant parameters in normal, diabetic control and diabetic treated groups.
| Parameters | Normal | Diabetic control | Diabetic treated with glimepiride | Diabetic treated with MSCs-CMT | Diabetic treated with MSCs-CMP |
|---|---|---|---|---|---|
| MDA (nmol/g. tissue) | 39.04 ± 4.34a | 128.83 ± 2.49d | 70.00 ± 5.29c | 58.92 ± 4.05bc | 50.20 ± 2.29ab |
| GSH (mmol/g. tissue) | 89.50 ± 2.25c | 38.8 ± 1.74a | 65.13 ± 3.40b | 71.25 ± 1.74b | 83.88 ± 1.06c |
| CAT (U/gm tissue) | 47.12 ± 7.20d | 115.02 ± 2.23a | 196.65 ± 2.91b | 202.65 ± 4.63bc | 218.87 ± 2.42c |
| SOD (U/gm tissue) | 36.85 ± 1.63c | 12.03 ± 0.39a | 21.70 ± 1.55b | 20.93 ± 1.79b | 31.47 ± 1.41c |
Values are expressed as Mean ± SE of 10 rats in each group. Values, which have different superscript symbols (a, b, c and d) for each parameter, are significantly different at p < 0.05. MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas. MDA, malondialdehyde; GSH, glutathione; CAT, catalase; SOD, superoxide dismutase.
3.4. Effect on inflammatory and anti-inflammatory markers
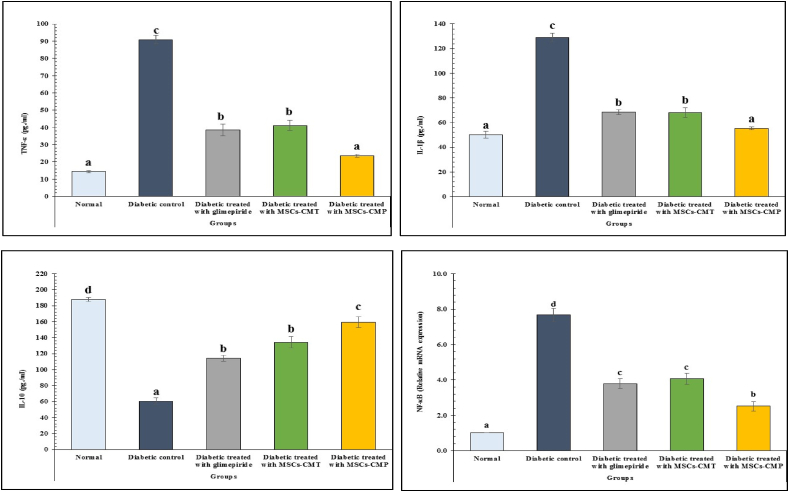
Comparing the diabetic group to the control, a substantial (p < 0.05) drop in IL-10 levels and a rise in serum TNF-α and IL-1β were noted. In comparison to the normal groups, the diabetic rats also showed a considerable increase in pancreatic NF-κB mRNA expression. On the other hand, compared to the diabetes group, all diabetic treated groups exhibited significant (p < 0.05) reductions in pancreatic NF-κB mRNA exoression and serum TNF-α and IL-1β. Furthermore, improvement in serum TNF-α, IL-1β, and IL-10 levels as well as pancreatic NF-κB mRNA expression was most successful with MSCs-CMP therapy (Fig. 2).
Fig. 2.
Changes in serum TNF-α, IL-1β, Interleukin-1β and IL-10 and pancreas mRNA expression of NF-κB in normal, diabetic control and diabetic treated groups. Values, which have different superscript symbols (a, b, c and d) for each parameter, are significantly different at p < 0.05. MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas, TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1beta; IL-10, interleukin-10; NF-κB, nuclear factor kappa-B.
3.5. Effects on pancreas Bcl2 and vimentin expression
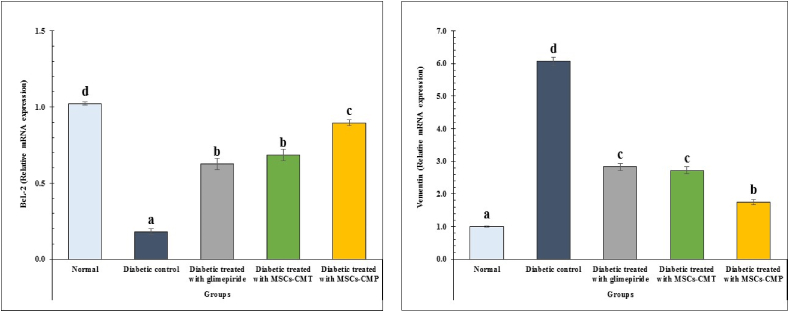
In the pancreatic tissues of diabetic rats, the expression of the anti-apoptotic gene Bcl-2 was dramatically (p < 0.05) lowered, whereas the expression of the vimentin gene was significantly (p < 0.05) enhanced. Following glimepiride therapy, diabetic rats receiving MSCs-CMT and MSCs-CMP showed a substantial (p < 0.05) increase in Bcl-2 mRNA expression and a significant (p < 0.05) decrease in vimentin gene expression as compared to the diabetic group (Fig. 3).
Fig. 3.
Changes in apoptotic markers in normal, diabetic control and diabetic treated groups. Values, which have different superscript symbols (a, b, c and d) for each parameter, are significantly different at p < 0.05. MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas; Bcl-2, B-cell lymphoma 2.
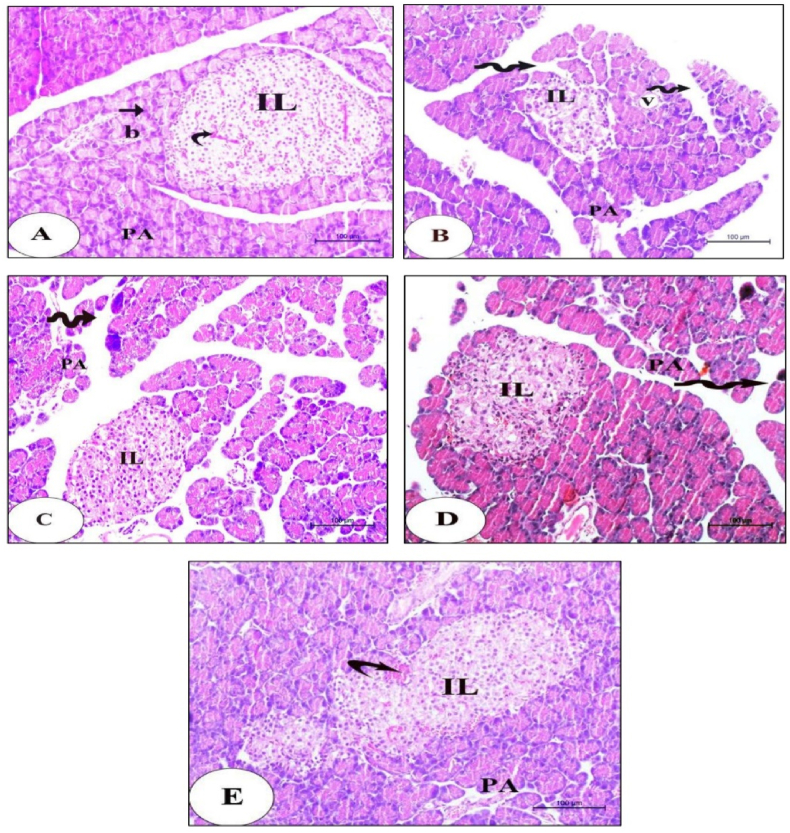
3.6. Histopathological and ultrastructural analysis
The results of histopathological investigation of pancreata from each experimental group are shown in Fig. 4. The normal group (Fig. 4A) had tissue architecture and intact pancreatic islets of Langerhans without any injuries. Pancreata of the diabetic control rats (Fig. 4B) illustrated markedly shrunken islets attained with vigorous depletion in the islet cells number. Disrupted islets with extreme necrosis and vacuolar degeneration were seen. Moreover, some damaged pancreatic acini with irregular borders, while others are vacuolated, and wide spaces between pancreatic lobules were also observed. Moderately alleviated histological architecture and integrity of islets of Langerhans with marked improvement in size and number of β-cells of vacuolar degeneration and few damaged pancreatic acini leaving empty spaces were observed in diabetic rats treated with glimepiride and MSCs-CMT group (Fig. 4C&D). The MSCs-CMP group (Fig. 4E) revealing improved islets architecture, integrity and an increase in number of β-cells. Few degenerated acini and dilated congested blood vessels could be seen.
Fig. 4.
Histological changes in normal, diabetic control and diabetic treated groups. A photomicrograph of pancreas sections of Normal group (A): revealing the endocrine portion consists of well-circumscribed islets of Langerhans (IL) with blood capillaries in-between (curved arrow). The exocrine portion of the pancreas consists of pancreatic acini (PA) with basal rounded nuclei (arrow) and notice the intralobular blood vessels (b). Scale bare = 100 μm. (B): Diabetic control group illustrating markedly shrunken islets (IL) attained with vigorous depletion in the islet cells number. Notice the disrupted islets with extreme necrosis and vacuolar degeneration. Some damaged pancreatic acini with irregular borders (PA), other acinar cells are vacuolated (v), and wide spaces between pancreatic lobules (zigzag arrows) were also observed. Scale bare = 100 μm. (C&D): Diabetic rats treated with glimepiride group or which were injected with mesenchymal stem cells conditioned media in tail vein group (MSCs-CMT) showing histological architecture and integrity of islets of Langerhans (IL) with marked improvement in size and number of β-cells of moderately alleviated. Vacuolar degeneration and few damaged pancreatic acini (PA) leaving empty spaces (zigzag arrows) were also detected. Scale bare = 100 μm. (E): Diabetic rats injected with mesenchymal stem cells conditioned medium direct in the pancreas group (MSCs-CMP) revealing improved islets architecture (IL), integrity and an increase in number of β-cells. Few degenerated acini (PA) and dilated congested blood vessels (curved arrow) could be seen. Scale bare = 100 μm.
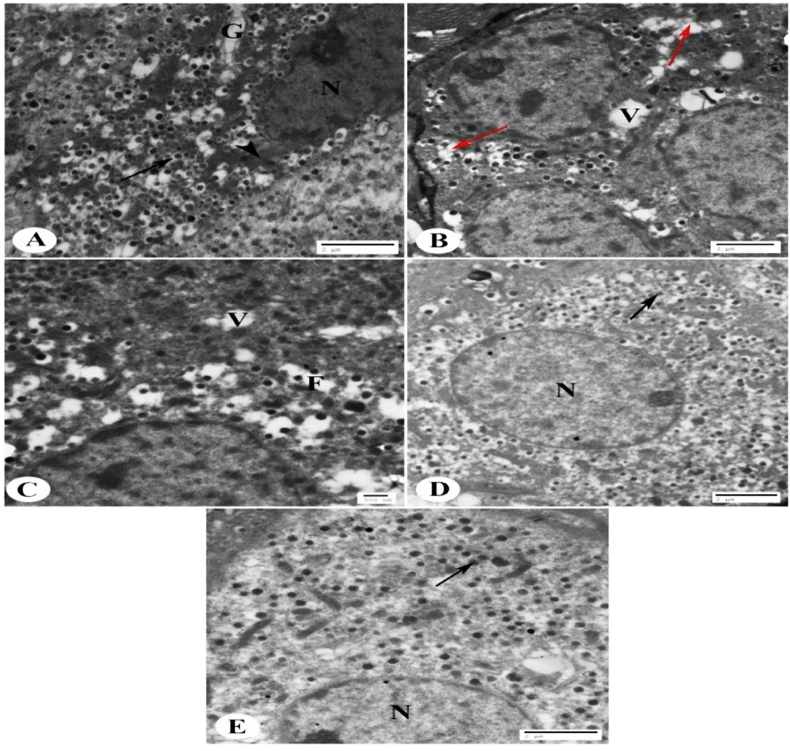
The control group of islets of Langerhans, mostly composed of β-cells, had an electron micrograph that revealed their cytoplasm included many electron-dense secretory granules encircled by a broad lucent halo, mitochondria, the Golgi apparatus, and a euchromatic nucleus (Fig. 5A). The diabetic control group displayed β-cells that were clearly vacuolated, had fewer secretory granules, and some granules had fused together (Fig. 5B). In comparison to the diabetes group, the glimepiride-treated diabetic group had β-cells with less vacuolation, fewer granule fusions, and more secretory granules (Fig. 5C). In comparison to the diabetes group, the diabetic group treated with MSCs-CMT exhibited an increased number of secretory granules, a euchromatic nucleus, and few vacuoles in β-cells (Fig. 5D). In comparison to the diabetes control group, the diabetic group treated with MSCs-CMP exhibited an increase in secretory granules, euchromatic nuclei, and few or no vacuoles in β-cells (Fig. 5E).
Fig. 5.
Electron micrograph for the control, diabetic and diabetic treated groups. Electron micrograph of islets of Langerhans showing (A): Normal β-cells. Several electron-dense secretory granules are shown in their cytoplasm, encircled by a broad lucent halo (arrow), mitochondria (arrow head), the Golgi apparatus (G), and the euchromatic nucleus (N) (Scale bar = 2 μm). (B): Diabetic control group showing β-cells with vacuolation (V), decreased numbers of secretory granules and fusion of some granules (red arrows) (Scale bar = 2 μm). (C): Diabetic rats treated with glimepiride group showing β-cells of diabetic rats with few vacuolations (V), few fusion of granules (F) and increased number of secretory granules compared to diabetic group (Scale bar = 500 nm). (D): Diabetic group injected with mesenchymal stem cells conditioned medium in the tail vein (MSCs-CMT) depicting euchromatic nuclei (N), few vacuoles in β -cells and increased number of secretory granules (arrow) compared to the diabetic group (Scale bar = 2 μm). (E): Diabetic group injected with mesenchymal stem cells conditioned medium direct in the pancreas (MSCs-CMP) depicting euchromatic nuclei (N), few or no vacuoles in β-cells and increase of secretory granules (arrow) in comparison with the diabetic group (Scale bar = 2 μm).
4. Discussion
In this study, T1DM in Wistar rats was induced by STZ, which is a cytotoxic agent to the pancreatic β-cells. This is indicated histologically by destruction and reduced number of β-cells and decreased insulin level in the diabetic rats. Therefore, they exhibited fating and postprandial hyperglycemia linked with increased glycated hemoglobin. The effect of STZ on apoptosis is indicated by decreased expression of anti-apoptotic mediator, Bcl-2, which is considered as caspase inhibitor and thus in inhibiting apoptosis [49]. The decreased level of Bcl-2 expression also explained the increased oxidation and β-cell death in diabetic rats. On the other hand, the increased vimentin may serve as an early marker of endocrine pancreas disorders [50].
The direct impact of glimepiride on reducing blood glucose by promoting the release of insulin from pancreatic β-cells indicates the improvement following therapy [51], which may also due to the raising the peripheral tissues' sensitivity to insulin [52]. Additionally, research has shown that glimepiride interacts with exchange protein activated by cyclic adenosine monophosphate (Epac3), a nucleotide exchanger that mediates the exocytosis of insulin granules [7]. These findings align with the findings of EL-Kassaby and colleagues [53] who has proven the improvement in pancreatic islets of Langerhans in group treated with glimepiride.
Therapy of diabetic rats with MSCs-CMT or MSCs-CMP causes an obvious restoration of serum insulin levels. These results are in accordance with those of Sabry et al. [54] and Elshemy et al. [24] who stated that MSCs-CM attenuated hyperglycemia by regenerating insulin-producing cells, enhancing pancreatic β-cell regeneration, boosting α cell to β cell conversion. Unique proteins found in MSC-Exosomes have been shown to have a role in gap junction construction, extracellular matrix modification, cell-matrix and cell-cell attachments, and the suppression of the intrinsic apoptotic pathway. Additionally, MSC-Exosomes have a variety of transcription factors that control metabolic pathways, differentiation, cell survival, and proliferation [55]. This explained the improved anti-apoptotic mechanism in the treated groups, indicated by increased expression Bcl-2. The improvements of pancreatic islets and serum insulin levels by treatment of diabetic rats with MSCs-CMT, MSCs-CMP and glimepiride were associated with the increase of pancreatic anti-apoptotic protein Bcl-2 and decrease in pancreatic vimentin. The increase in Bcl-2 reflects the decrease in pancreatic cells apoptosis as it inhibits Bax (Bcl-2–associated X protein), Bad (Bcl2-associated agonist of cell death) and Bak (BCL-2 homologous antagonist/killer) leading to inhibition of caspase-3 and apoptosis [56,57]. Vementin on the other hand stimulate dedifferentiation or conversion of β-cells to other pancreatic endocrine cell types that no longer contribute to insulin production in diabetic islets [58].
Histopathological amelioration revealed a decrease in the necrotic score and regeneration of β-cells with a pancreatic islet morphology close to normal. This is in line with the findings of Sabry et al. [54] and Elshemy et al. [24], who similarly reported islet cell regeneration with a rise in Langerhans islet size and number and a decrease in inflammation and fibrosis, indicating their improvement following MSCs-CM therapy. Furthermore, the diabetic dyslipidemia with its specific pattern of abnormally high level of triglycerides, cholesterol, a high proportion of LDL-C and vLDL-C with a low level of HDL-C indicated in our results, as a common complication in diabetics owing to insulin deficiency and/or insulin resistance [59,60]. Treatment of diabetic rats with glimepiride has a role in improving diabetic dyslipidemia by amelioration of the glycemic state as proven in a study of Elbably and ElNahas [61], and by increasing insulin secretion by pancreatic β-cells and therefore preventing the lipolysis [62]. Additionally, MSCs from different sources have the ability to repair cells that create insulin, which can improve the condition of hyperglycemia, according to Elshemy et al. [24]. Because of their antioxidant properties, MSCs shield tissues from damage brought on by reactive oxygen species (ROS) [63].
The present results demonstrated that STZ produced oxidative stress in pancreatic cells, leading to a significant reduction in antioxidant indicators such as SOD and CAT activities as well as low GSH content in the diabetic group's pancreatic tissues when compared to the control group. These cells also exhibit markedly higher levels of ROS and MDA, a marker of lipid peroxidation (LPO). Perhaps the reason for the high level of MDA is a result of diabetes induction which increases the oxidative stress in the tissues and the accumulation of LPO products accordingly [64]. These results agree with those of many reports that showed diabetic animals exhibited obvious imbalance in oxidation [[65], [66], [67]]. Gurgul-Convey et al. [68] revealed that pancreatic β-cells have higher vulnerability to ROS damage, as evidenced by reduced production of CAT and glutathione peroxidase 1 (GPx1). Activation of the polyol and hexosamine pathways, increased intracellular advanced glycation end product formation, protein kinase C (PKC) activation, and oxidative phosphorylation are some of the other mitochondrial pathways thought to be involved in ROS production in β-cells under hyperglycemia [69,70].
Moreover, it has been demonstrated that hyperlipidemia increases the formation of ROS via activating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), inducing matrix metalloproteinase-2 (MMP2), and promoting macrophage infiltration [71,72]. Inflammatory cytokines, like hyperlipidemia, enhanced the generation of hydrogen peroxide (H2O2) and other ROS products, which in turn regulated the activity of nitrogen oxide(s) and elevated the expression of genes that attract macrophages to β-cells [68]. One of the main cytokines that are overexpressed in the diabetic liver is TNF-α; this alteration leads to apoptosis and inflammation of the liver and is linked to oxidative stress and hyperglycemia [73].
Glimepiride significantly reduced serum MDA levels and increased SOD activity, which could be attributed to two different mechanisms for glimepiride's antioxidant potential. First, by blocking the cyclooxygenase processes within cells [74] and the second potential mechanism could be connected to glimepiride's ability to upregulate the genes of antioxidant enzymes like SOD and paraoxonase by lowering the activity of redox-sensitive NF-κB [75] perhaps it could have agonistic effects on the peroxisome proliferator-activated receptor gamma (PPARγ) [76].
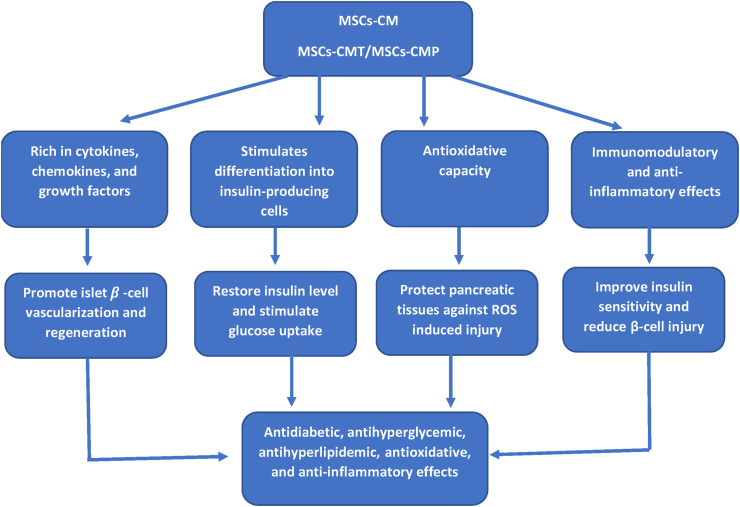
When MSCs-CMT and MSCs-CMP were administered to diabetic rats, the amount of LPO in their pancreas was significantly reduced, and their antioxidant status was improved as seen by increased CAT and SOD activity as well as GSH content. These results agreed with that of Elshemy et al. [24] and Yuan et al. [77]. It has been suggested that MSCs can lessen oxidative damage by contributing their mitochondria to repair injured cells, scavenging free radicals, boosting host antioxidant defenses, controlling the inflammatory response, and boosting cellular respiration and mitochondrial activities [78]. The administration of MSCs-CM can also lower oxidative stress in vivo, indicating a paracrine component to their mechanism. The antioxidant actions of MSCs frequently occur in a paracrine way in vitro [79]. Moreover, MSCs-CM exhibit strong antioxidant capacity (Fig. 6), suggesting that MSCs release antioxidants on an active basis [80]. Models of diabetes showed that MSCs raised GSH levels [81]. The MSCs-CM also saw these effects [79].
Fig. 6.
Diagram showing potential mechanism of action of MSCs-CM on T1DM. MSCs-CM, mesenchymal stem cells conditioned medium; MSCs-CMT, MSCs-CM injected daily into tail vein; MSCs-CMP, MSCs-CM injected directly in pancreas.
Glimepiride has anti-inflammatory effect that is proven in Matta et al. [82] study who found that glimepiride seemed to affect the expression of several cytokines that cause inflammation. All things considered, sulfonylurea can inhibit activated macrophages' ability to produce cytokines by inhibiting their K+ channels [83]. Furthermore, giving MSCs-CMT or MSCs-CMP to diabetic rats reduced TNF-α and IL-1β levels, increased IL-10 expression levels, and lowered NF-κB expression levels. Specifically, as compared to MSCs-CMT, MSCs-CMP demonstrated an extremely substantial anti-inflammatory impact. The results of Hashemi et al. [84] are consistent with our findings. They demonstrated how MSCs and MSCs-CM therapies reduced the levels of inflammatory and anti-inflammatory cytokines and improved the clinical symptoms of diabetes in a mouse model. In his work, adipose tissue-derived MSCs (AD-MSCs) and CM treatment led to a significant decrease in blood glucose levels and the repair of pancreatic islets because of the anti-inflammatory and immunomodulatory qualities of MSCs (Fig. 6) [85]. The ability of MSCs to regenerate may be connected to their anti-inflammatory characteristics [86]. MSCs regulate the inflammatory milieu and shield injured tissues from inflammatory reactions by producing trophic factors and anti-inflammatory cytokines. The protective benefits of MSCs-CM on the survival of islet beta cells seem to be mostly IL-10-dependent [87]. Also, the expression of pro-inflammatory cytokines, like IL-1β, was decreased by the MSCs-CM [88].
The M2/M1 polarization ratio is increased by MSCs-CM, which lowers inflammation and speeds up healing in diabetic ulcer wounds [89]. By enhancing the production of pentaerythritol tetranitrate (PETN), which controls the M1/M2 polarization ratio, MSCs-CM diminish the phosphorylation of AKT and prevent the activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway. This reduces the inflammatory reaction to wounds in rats with diabetes and speeds up the process of tissue regeneration after inflammation [90].
Overall, the current study's findings showed that injecting MSCs-CM into the pancreas and tail vein reduced hyperglycemia by regenerating insulin-producing cells, enhancing the regeneration of pancreatic islet β-cells, shielding pancreatic tissues from damage caused by ROS, and influencing immunity and angiogenesis through secreted paracrine factors (Fig. 6). However, MSCs-CMP seemed to have more potent hypoglycemic action compared to MSCs-CMT. This may be attributed to direct injection into pancreatic cells. Growth factors and MSCs-CM's interleukin content have been proposed to have an impact on intrapancreatic environment [91,92] and facilitate regeneration through resident/circulating stem cells and progenitor cells being activated and drawn to the injury site, where they work together to repair injured tissues [93]. MSCs-CM injected direct in pancreas is safe and biocompatible and displayed better bioavailability and efficiency against diabetes and its complications in comparing to MSCs-CM injected in tail of rats. Thus, this method could be suggested as a potential candidate for management of diabetes, also for further in vivo and clinical investigations.
5. Conclusions
The study concluded that the injection of MSCs-CM in both tail vein and pancreas alleviated hyperglycemia and the hyperlipidemia via promoting the growth of pancreatic islet β-cells, repairing insulin-producing cells, shielding pancreatic tissues from damage caused by ROS and inflammation. MSCs-CM therapy by injection directly into pancreas has the potent improvement antidiabetic effects when compared to Amaryl and MSCs-CM injected into lateral tail vein. Hence, this method could be suggested as a potential candidate for management of diabetes, also for further in vivo and clinical investigations.
Author contributions
ESA, AN, and AA proposed the research plan and managed the research work. MSS performed the experimental work and carried out the investigations and statistical analysis under supervision of ESA, TNA, LAA, AN, OMA and AA. MSS wrote the original draft of the manuscript. ESA, TNA, LAA, AN, OMA and ME revised the manuscript. All authors read and approved the final manuscript.
Ethical approval statement
The Beni-Suef University Institutional Animal Care and Use Committee (IACUC) granted approval for the experimental investigation (IACUC Approval Number: 024-019).
Data availability statement
All information is available upon reasonable request from the associated authors.
Funding
This research received no external funding.
Declaration of competing interest
All the authors declared no conflict of interest.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Bhana S., Variava E., Mhazo T.V., de Beer J.C., Naidoo P., Pillay S., et al. Healthcare resource utilization in controlled versus uncontrolled adults living with type 1 diabetes in the South African public healthcare sector. Value in Health Regional Issues. 2023;36:66–75. doi: 10.1016/j.vhri.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Akil A.A.S., Yassin E., Al-Maraghi A., Aliyev E., Al-Malki K., Fakhro K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J Transl Med. 2021;19(1):1–19. doi: 10.1186/s12967-021-02778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan X.X., Zhang D.Y., Khan M.A., Zheng S.Y., Hu X.M., Zhang Q., et al. Stem cell transplantation in the treatment of type 1 diabetes mellitus: from insulin replacement to beta-cell replacement. Front Endocrinol. 2022;13:859638. doi: 10.3389/fendo.2022.859638. 859638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pang H., Luo S., Xiao Y., Xia Y., Li X., Huang G., et al. Emerging roles of exosomes in T1DM. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.593348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrada-Soto S., Ornelas-Mendoza K., Navarrete-Vázquez G., Chávez-Silva F., Almanza-Pérez J.C., Villalobos-Molina R., et al. Insulin sensitization by PPARγ and GLUT-4 overexpression/translocation mediates the antidiabetic effect of plantago australis. Pharmaceuticals. 2023;16(4):535. doi: 10.3390/ph16040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halder M., Bhatia Y., Singh Y. Self-assembled di-and tripeptide gels for the passive entrapment and pH-responsive, sustained release of an antidiabetic drug, glimepiride. Biomater Sci. 2022;10(9):2248–2262. doi: 10.1039/d2bm00344a. [DOI] [PubMed] [Google Scholar]
- 7.Madhukar D.S., Laxman G.K., Shivaji G.P., Vasant H.M., Kailas I.S., Dnyaneshwar J.M. 2022. A review on antidiabetic drugs (allopathy) [Google Scholar]
- 8.Zhu J., Yu X., Zheng Y., Li J., Wang Y., Lin Y., et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. Lancet Diabetes Endocrinol. 2020;8(3):192–205. doi: 10.1016/S2213-8587(19)30422-X. [DOI] [PubMed] [Google Scholar]
- 9.Shahzadi I., Fazal R.M., Hussain Z., Yasin R., Aziz M.K., Samiullah K., et al. Anti-diabetic effects of aqueous plant (Citrullus colocynthis) extract in streptozotocin induced diabetic mice. Haya Saudi J Life Sci. 2022;7(6):199–205. [Google Scholar]
- 10.Puskar A., Saadah B., Rauf A., Kasperek S.R., Umair M. A primer on contrast agents for magnetic resonance imaging of post-procedural and follow-up imaging of islet cell transplant. Nano Select. 2023;4(3):181–191. [Google Scholar]
- 11.Coe T.M., Markmann J.F., Rickert C.G. Current status of porcine islet xenotransplantation. Curr Opin Organ Transplant. 2020;25(5):449. doi: 10.1097/MOT.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Zheng Y., Huang L., He J. Research progress on mesenchymal stem cells for the treatment of diabetes and its complications. International Journal of Endocrinology. 2023;2023 doi: 10.1155/2023/9324270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen S.J., Zheng X.M., Liu L.F., Li N.N., Mao H.A., Huang L., et al. Effects of primary microglia and astrocytes on neural stem cells in in vitro and in vivo models of ischemic stroke. Neural Regeneration Research. 2021;16(9):1677. doi: 10.4103/1673-5374.306093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan N., Amin L.E., Zaher A.R., Grawish M.E., Scheven B.A. Dental pulp stem cells stimulate neuronal differentiation of PC12 cells. Neural Regeneration Research. 2021;16(9):1821. doi: 10.4103/1673-5374.306089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X.M., Li Z.X., Zhang D.Y., Yang Y.C., Fu S.A., Zhang Z.Q., et al. A systematic summary of survival and death signalling during the life of hair follicle stem cells. Stem Cell Res Ther. 2021;12(1):1–29. doi: 10.1186/s13287-021-02527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahbaz M., Yoshihara E. Immune protection of stem cell-derived islet cell therapy for treating diabetes. Front Endocrinol. 2021;939 doi: 10.3389/fendo.2021.716625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buron F., Reffet S., Badet L., Morelon E., Thaunat O. Immunological monitoring in beta cell replacement: towards a pathophysiology-guided implementation of biomarkers. Curr Diabetes Rep. 2021;21(6):1–11. doi: 10.1007/s11892-021-01386-4. [DOI] [PubMed] [Google Scholar]
- 18.Mohan N., Sunder A. 2022. Molecular challenges and advances in clinical islet transplantation. Type 1 diabetes mellitus. [Google Scholar]
- 19.Cai J., Wu Z., Xu X., Liao L., Chen J., Huang L., et al. Umbilical cord mesenchymal stromal cell with autologous bone marrow cell transplantation in established type 1 diabetes: a pilot randomized controlled open-label clinical study to assess safety and impact on insulin secretion. Diabetes Care. 2016;39(1):149–157. doi: 10.2337/dc15-0171. [DOI] [PubMed] [Google Scholar]
- 20.Gerace D., Martiniello-Wilks R., Nassif N.T., Lal S., Steptoe R., Simpson A.M. CRISPR-targeted genome editing of mesenchymal stem cell-derived therapies for type 1 diabetes: a path to clinical success? Stem Cell Res Ther. 2017;8(1):1–10. doi: 10.1186/s13287-017-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X.W., Feng L.X., Zhu X.J., Liu Q., Wang H.S., Wu X., et al. Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109310. [DOI] [PubMed] [Google Scholar]
- 22.Khosravi N., Pishavar E., Baradaran B., Oroojalian F., Mokhtarzadeh A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: a promising biomimetic nanoplatforms for cancer theranostics. J Contr Release. 2022;348:706–722. doi: 10.1016/j.jconrel.2022.06.026. [DOI] [PubMed] [Google Scholar]
- 23.Műzes G., Sipos F. Mesenchymal stem cell-derived secretome: a potential therapeutic option for autoimmune and immune-mediated inflammatory diseases. Cells. 2022;11(15):2300. doi: 10.3390/cells11152300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elshemy M.M., Asem M., Allemailem K.S., Uto K., Ebara M., Nabil A. Antioxidative capacity of liver-and adipose-derived mesenchymal stem cell-conditioned media and their applicability in treatment of type 2 diabetic rats. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/8833467. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Kumar P., Kandoi S., Misra R., Vijayalakshmi S., Rajagopal K., Verma R.S. The mesenchymal stem cell secretome: a new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. doi: 10.1016/j.cytogfr.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Bermudez M.A., Sendon-Lago J., Eiro N., Trevino M., Gonzalez F., Yebra-Pimentel E., et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest Ophthalmol Vis Sci. 2015;56(2):983–992. doi: 10.1167/iovs.14-15859. [DOI] [PubMed] [Google Scholar]
- 27.Gundala N.K., Naidu V.G., Das U.N. Arachidonic acid and lipoxinA4 attenuate streptozotocin-induced cytotoxicity to RIN5 F cells in vitro and type 1 and type 2 diabetes mellitus in vivo. Nutrition. 2017;35:61–80. doi: 10.1016/j.nut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S.N., Rajivgandhi G., Ramachandran G., Manoharan N. A marine sponge Fascaplysinopsis sp. derived alkaloid fascaplysin inhibits the HepG2 hepatocellular carcinoma cell. Frontiers in Laboratory Medicine. 2018;2(2):41–48. [Google Scholar]
- 29.Rajivgandhi G.N., Chackaravarthi G., Ramachandran G., Chelliah C.K., Maruthupandy M., Alharbi M.S., et al. Morphological damage and increased ROS production of biosynthesized silver nanoparticle against MCF-7 breast cancer cells through in vitro approaches. J King Saud Univ Sci. 2022;34(2) [Google Scholar]
- 30.McFarlin K., Gao X., Liu Y.B., Dulchavsky D.S., Kwon D., Arbab A.S., et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14(4):471–478. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- 31.Ho C.H., Lan C.W., Liao C.Y., Hung S.C., Li H.Y., Sung Y.J. Mesenchymal stem cells and their conditioned medium can enhance the repair of uterine defects in a rat model. J Chin Med Assoc. 2018;81(3):268–276. doi: 10.1016/j.jcma.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Bisse E., Abraham E.C. New less temperature-sensitive microchromatographic method for the separation and quantitation of glycosylated hemoglobins using a non-cyanide buffer system. J Chromatogr B Biomed Sci Appl. 1985;344:81–91. doi: 10.1016/s0378-4347(00)82009-5. [DOI] [PubMed] [Google Scholar]
- 33.Temple R., Clark P.M.S., Hales C.N. Measurement of insulin secretion in type 2 diabetes: problems and pitfalls. Diabet Med. 1992;9(6):503–512. doi: 10.1111/j.1464-5491.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 34.Young D.S., Friedman R.B. Vol. 1. Amer Assn for Clinical Chemistry; 2001. (Effects of disease on clinical laboratory tests). [Google Scholar]
- 35.Warnick G.R., Nauck M., Rifai N. Evolution of methods for measurement of HDL-cholesterol: from ultracentrifugation to homogeneous assays. Clin Chem. 2001;47(9):1579–1596. [PubMed] [Google Scholar]
- 36.Tietz N.W. Clinical guide to laboratory tests. 1995. Clinical guide to laboratory tests; p. 1096. 1096. [Google Scholar]
- 37.Frideewald W. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 38.Satoh K. Method of lipid peroxidation determination in serum. Clin Chim Acta. 1978;90:37. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 39.Ohkawa H., Ohishi W., Yagi K. Determination of lipid peroxidation by MDA. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 40.Beutler E., Duron O., Kelly M. Colorimetric method for determination of glutathione reductase concentration. J Lab Clin Med. 1963;61(882) [PubMed] [Google Scholar]
- 41.Nishikimi M., Roa N.A., Yogi K. Measurement of superoxide dismutase. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 42.Aebi H. Methods in enzymology. Vol. 105. Academic Press; 1984. [13] Catalase in vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 43.DeVita V.T., Lawrence T.S., Rosenberg S.A., editors. Vol. 2. Lippincott Williams & Wilkins; 2008. (DeVita, Hellman, and Rosenberg's cancer: principles & practice of oncology). [Google Scholar]
- 44.Hodge S., Hodge G., Flower R., Han P. Methyl-prednisolone up-regulates monocyte interleukin-10 production in stimulated whole blood. Scand J Immunol. 1999;49(5):548–553. doi: 10.1046/j.1365-3083.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 45.Brouckaert P., Libert C., Everaerdt B., Takahashi N., Cauwels A., Fiers W. Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiology. 1993;187(3–5):317–329. doi: 10.1016/S0171-2985(11)80347-5. [DOI] [PubMed] [Google Scholar]
- 46.Rajivgandhi G., Kumar S.N., Ramachandran G., Manoharan N. Marine sponge alkaloid aaptamine enhances the anti-bacterial and anti-cancer activity against ESBL producing Gram negative bacteria and HepG 2 human liver carcinoma cells. Biocatal Agric Biotechnol. 2019;17:628–637. [Google Scholar]
- 47.Bancroft J.D., Gamble M., editors. Theory and practice of histological techniques. Elsevier health sciences; 2008. [Google Scholar]
- 48.Bain J.M., Mercer F.V. Subcellular organization of the developing cotyledons of Pisum sativum L. Aust J Biol Sci. 1966;19(1):49–68. [Google Scholar]
- 49.Qi L., Wang R., Shi Q., Yuan M., Jin M., Li D. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metabol. 2019;37(3):455–466. doi: 10.1007/s00774-018-0953-9. [DOI] [PubMed] [Google Scholar]
- 50.Krivova Y.S., Proshchina A.E., Barabanov V.M., Barinova I.V., Saveliev S.V. Immunohistochemical detection of vimentin in pancreatic islet β-and α-cells of macrosomic infants of diabetic and nondiabetic mothers. Early Hum Dev. 2018;117:44–49. doi: 10.1016/j.earlhumdev.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Matthaei S., Bierwirth R., Fritsche A., Gallwitz B., Haering H.U., Joost H.G., et al. Medical antihyperglycaemic treatment of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2009;117(9):522–557. doi: 10.1055/s-0029-1239559. [DOI] [PubMed] [Google Scholar]
- 52.Li C.J., Zhang J.Y., Yu D.M., Zhang Q. Adding glimepiride to current insulin therapy increases high-molecular weight adiponectin levels to improve glycemic control in poorly controlled type 2 diabetes. Diabetol Metab Syndrome. 2014;6(41):1–7. doi: 10.1186/1758-5996-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.EL-Kassaby M.I., Salama A.A., Mourad H.H., Abdel-Wahhab K.G. Effect of lemon balm (Melissa officinalis) aqueous extract on streptozotocin-induced diabetic rats. Egypt Pharm J. 2019;18(4):296. [Google Scholar]
- 54.Sabry D., Samar M., Reem Z., Ibrahim H.A., Mai S. Correction to: the effect of exosomes derived from mesenchymal stem cells in the treatment of induced type 1 diabetes mellitus in rats. Biotechnol Lett. 2020;42(12):2761–2762. doi: 10.1007/s10529-020-02975-1. [DOI] [PubMed] [Google Scholar]
- 55.Keshtkar S., Kaviani M., Sarvestani F.S., Ghahremani M.H., Aghdaei M.H., Al-Abdullah I.H., et al. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. EXCLI journal. 2020;19:1064. doi: 10.17179/excli2020-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed O.M., Ali T.M., Abdel Gaid M.A., Elberry A.A. Effects of enalapril and paricalcitol treatment on diabetic nephropathy and renal expressions of TNF-α, p53, caspase-3 and Bcl-2 in STZ-induced diabetic rats. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0214349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed O.M., Saleh A.S., Ahmed E.A., Ghoneim M.M., Ebrahim H.A., Abdelgawad M.A., et al. Efficiency of bone marrow-derived mesenchymal stem cells and hesperetin in the treatment of streptozotocin-induced type 1 diabetes in Wistar rats. Pharmaceuticals. 2023;16:859. doi: 10.3390/ph16060859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roefs M.M., Carlotti F., Jones K., Wills H., Hamilton A., Verschoor M., et al. Increased vimentin in human α- and β-cells in type 2 diabetes. J Endocrinol. 2017;233(3):217–227. doi: 10.1530/JOE-16-0588. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Rocha N.A., McCullough P.A. Contribution of ApoCIII to diabetic dyslipidemia and treatment with volanesorsen. Rev Cardiovasc Med. 2018;19(1):32–37. doi: 10.31083/j.rcm.2018.01.890. [DOI] [PubMed] [Google Scholar]
- 60.Patti A.M., Giglio R.V., Papanas N., Rizzo M., Rizvi A.A. Future perspectives of the pharmacological management of diabetic dyslipidemia. Expet Rev Clin Pharmacol. 2019;12(2):129–143. doi: 10.1080/17512433.2019.1567328. [DOI] [PubMed] [Google Scholar]
- 61.Elbably M., ElNahas E. Ameliorative effect of Sukkari date extract on STZ induced experimentally diabetes in male rats. Benha Veterinary Medical Journal. 2019;37(1):223–228. [Google Scholar]
- 62.Azarkish F., Hashemi K., Talebi A., Kamalinejad M., Soltani N., Pouladian N. Effect of the administration of Solanum nigrum fruit on prevention of diabetic nephropathy in streptozotocin-induced diabetic rats. Pharmacogn Res. 2017;9(4):325. doi: 10.4103/pr.pr_47_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nowak W.N., Taha H., Kachamakova-Trojanowska N., Stępniewski J., Markiewicz J.A., Kusienicka A., et al. Murine bone marrow mesenchymal stromal cells respond efficiently to oxidative stress despite the low level of heme oxygenases 1 and 2. Antioxidants Redox Signal. 2018;29(2):111–127. doi: 10.1089/ars.2017.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeh P.T., Huang H.W., Yang C.M., Yang W.S., Yang C.H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nurdiana S., Goh Y.M., Ahmad H., Dom S.M., Syimal’ain Azmi N., Noor Mohamad Zin N.S., et al. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Compl Alternative Med. 2017;17(1):1–17. doi: 10.1186/s12906-017-1762-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molehin O.R., Oloyede O.I., Adefegha S.A. Streptozotocin-induced diabetes in rats: effects of white butterfly (Clerodendrum volubile) leaves on blood glucose levels, lipid profile and antioxidant status. Toxicol Mech Methods. 2018;28(8):573–586. doi: 10.1080/15376516.2018.1479476. [DOI] [PubMed] [Google Scholar]
- 67.Almalki D.A., Alghamdi S.A., Al-Attar A.M. Comparative study on the influence of some medicinal plants on diabetes induced by streptozotocin in male rats. BioMed Res Int. 2019;2019 doi: 10.1155/2019/3596287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurgul-Convey E., Mehmeti I., Plötz T., Jörns A., Lenzen S. Sensitivity profile of the human EndoC-βH1 beta cell line to proinflammatory cytokines. Diabetologia. 2016;59(10):2125–2133. doi: 10.1007/s00125-016-4060-y. [DOI] [PubMed] [Google Scholar]
- 69.Back S.H., Kang S.W., Han J., Chung H.T. Endoplasmic reticulum stress in the β-cell pathogenesis of type 2 diabetes. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/618396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panigrahy S.K., Bhatt R., Kumar A. Reactive oxygen species: sources, consequences and targeted therapy in type 2 diabetes. J Drug Target. 2017;25(2):93–101. doi: 10.1080/1061186X.2016.1207650. [DOI] [PubMed] [Google Scholar]
- 71.Zou R., Xue J., Huang Q., Dai Z., Xu Y. Involvement of receptor-interacting protein 140 in palmitate-stimulated macrophage infiltration of pancreatic beta cells. Exp Ther Med. 2017;14(1):483–494. doi: 10.3892/etm.2017.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nemecz M., Constantin A., Dumitrescu M., Alexandru N., Filippi A., Tanko G., et al. The distinct effects of palmitic and oleic acid on pancreatic beta cell function: the elucidation of associated mechanisms and effector molecules. Front Pharmacol. 2019;9:1554. doi: 10.3389/fphar.2018.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu P., Xiao J., Chi S. Piperlongumine attenuates oxidative stress, inflammatory, and apoptosis through modulating the GLUT-2/4 and AKT signaling pathway in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2021;35(6):1–12. doi: 10.1002/jbt.22763. [DOI] [PubMed] [Google Scholar]
- 74.Hadi N.R., Rezeg F.A., Al-Amran F., Hussein M.A. Evaluation of the effects of glimepiride (Amaryl) and repaglinide (novoNorm) on atherosclerosis progression in high cholesterol-fed male rabbits. J Cardiovasc Dis Res. 2012;3(1):5–11. doi: 10.4103/0975-3583.91592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elghazaly N.A., Zaatout H.H., Radwan E.H., Elghazaly M.M., Elsheikha E.A. Trigonella foenum graecum extract benefits on hematological, biochemical and male reproductive system as a complementary therapy with glimepiride in treating streptozotocin induced diabetic rats. J Bioinformatics Diabetes. 2019;1(3):45–59. [Google Scholar]
- 76.Zafar S.A., Naveed S., Khan R.U., Sadia H. Comparative study of 3 different brands of glimperide. Liaquat National Journal of Primary Care. 2020;2(2):94–96. [Google Scholar]
- 77.Yuan Y., Shi M., Li L., Liu J., Chen B., Chen Y., et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1α pathway. Clin Sci. 2016;130(23):2181–2198. doi: 10.1042/CS20160235. [DOI] [PubMed] [Google Scholar]
- 78.Mahrouf-Yorgov M., Augeul L., Da Silva C.C., Jourdan M., Rigolet M., Manin S., et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224–1238. doi: 10.1038/cdd.2017.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu B., Ding F.X., Liu Y., Xiong G., Lin T., He D.W., et al. Human umbilical cord-derived mesenchymal stem cells conditioned medium attenuate interstitial fibrosis and stimulate the repair of tubular epithelial cells in an irreversible model of unilateral ureteral obstruction. Nephrology. 2018;23(8):728–736. doi: 10.1111/nep.13099. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Liu J., Yu B., Jin Y., Li J., Ma X., et al. Umbilical cord-derived mesenchymal stem cell conditioned medium reverses neuronal oxidative injury by inhibition of TRPM2 activation and the JNK signaling pathway. Mol Biol Rep. 2022:1–9. doi: 10.1007/s11033-022-07524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abd-Elhalem S.S., Haggag N.Z., El-Shinnawy N.A. Bone marrow mesenchymal stem cells suppress IL-9 in adjuvant-induced arthritis. Autoimmunity. 2018;51(1):25–34. doi: 10.1080/08916934.2018.1428956. [DOI] [PubMed] [Google Scholar]
- 82.Matta C.N., Abdel-Rahim M.H., Youssri M.M., Wazeery A.E.M. Comparative effects of oral hypoglycemic drugs on serum YKL-40 level in type 2 diabetic patients. SVU-International Journal of Medical Sciences. 2020;3(2):41–47. [Google Scholar]
- 83.Kewcharoenwong C., Rinchai D., Utispan K., Suwannasaen D., Bancroft G.J., Ato M., et al. Glibenclamide reduces pro-inflammatory cytokine production by neutrophils of diabetes patients in response to bacterial infection. Sci Rep. 2013;3(1):1–8. doi: 10.1038/srep03363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hashemi S.M., Hassan Z.M., Hossein-Khannazer N., Pourfathollah A.A., Soudi S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology. 2020;28(2):585–601. doi: 10.1007/s10787-019-00661-x. [DOI] [PubMed] [Google Scholar]
- 85.El-Sawah S.G., Althobaiti F., Rashwan H.M., Aldhahrani A., Abdel-Dayem M.A., Fayad E., et al. Anti-inflammatory and antioxidant potential capacities of AD-MSCs and BM-MSCs in suppressing pancreatic β-cells auto-immunity and apoptosis in rats with T1DM induced model. Biocell. 2022;46(3):745. [Google Scholar]
- 86.Salari V., Mengoni F., Del Gallo F., Bertini G., Fabene P.F. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: possible treatments and future perspectives. Int J Mol Sci. 2020;21(24):9683. doi: 10.3390/ijms21249683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Al-Azzawi B., McGuigan D.H., Koivula F.N.M., Elttayef A., Dale T.P., Yang Y., et al. The secretome of mesenchymal stem cells prevents islet beta cell apoptosis via an IL-10-dependent mechanism. Open Stem Cell J. 2020;6(1) [Google Scholar]
- 88.Jin Q.H., Kim H.K., Na J.Y., Jin C., Seon J.K. Anti-inflammatory effects of mesenchymal stem cell-conditioned media inhibited macrophages activation in vitro. Sci Rep. 2022;12(1):1–11. doi: 10.1038/s41598-022-08398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dalirfardouei R., Jamialahmadi K., Jafarian A.H., Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–568. doi: 10.1002/term.2799. [DOI] [PubMed] [Google Scholar]
- 90.Liu W., Yu M., Xie D., Wang L., Ye C., Zhu Q., et al. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res Ther. 2020;11(1):1–15. doi: 10.1186/s13287-020-01756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ooi Y.Y., Dheen S.T., Tay S.S.W. Paracrine effects of mesenchymal stem cells-conditioned medium on microglial cytokines expression and nitric oxide production. Neuroimmunomodulation. 2015;22(4):233–242. doi: 10.1159/000365483. [DOI] [PubMed] [Google Scholar]
- 92.Shen C., Lie P., Miao T., Yu M., Lu Q., Feng T., et al. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol Med Rep. 2015;12(1):20–30. doi: 10.3892/mmr.2015.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Y., Xiang L.X., Shao J.Z., Pan R.L., Wang Y.X., Dong X.J., et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14(6b):1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information is available upon reasonable request from the associated authors.