Abstract
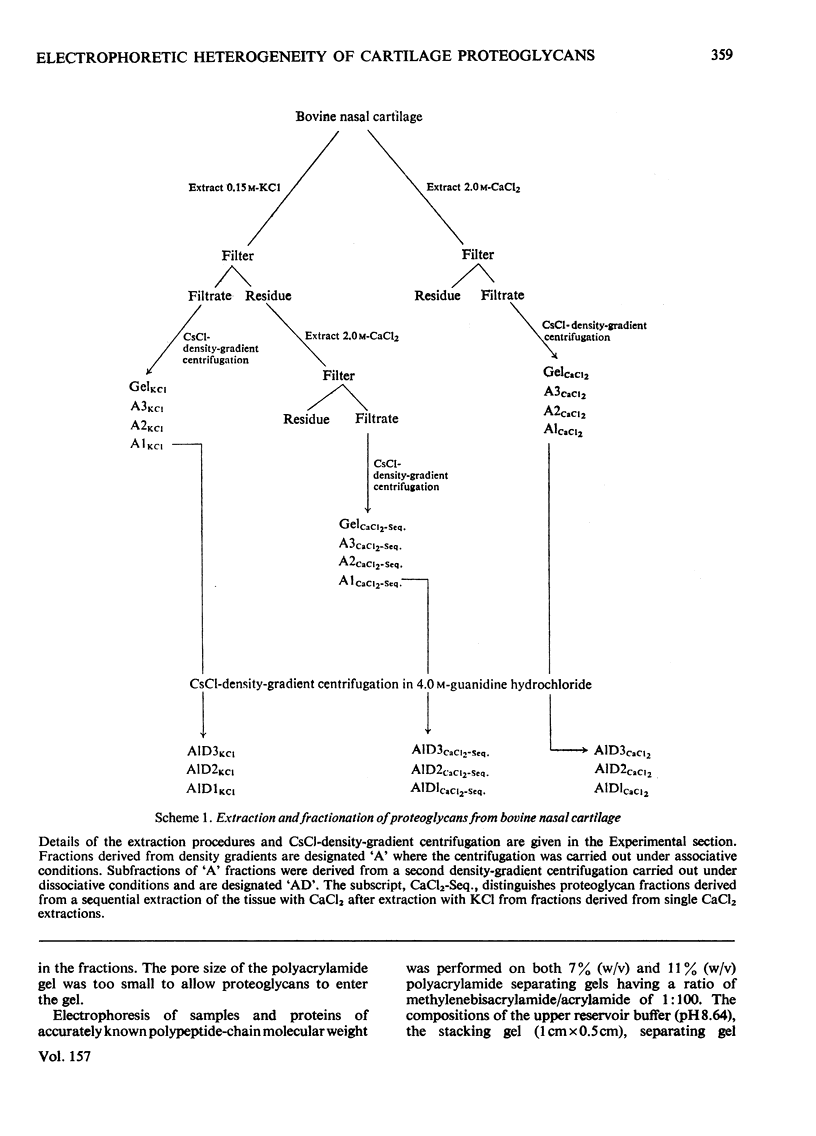
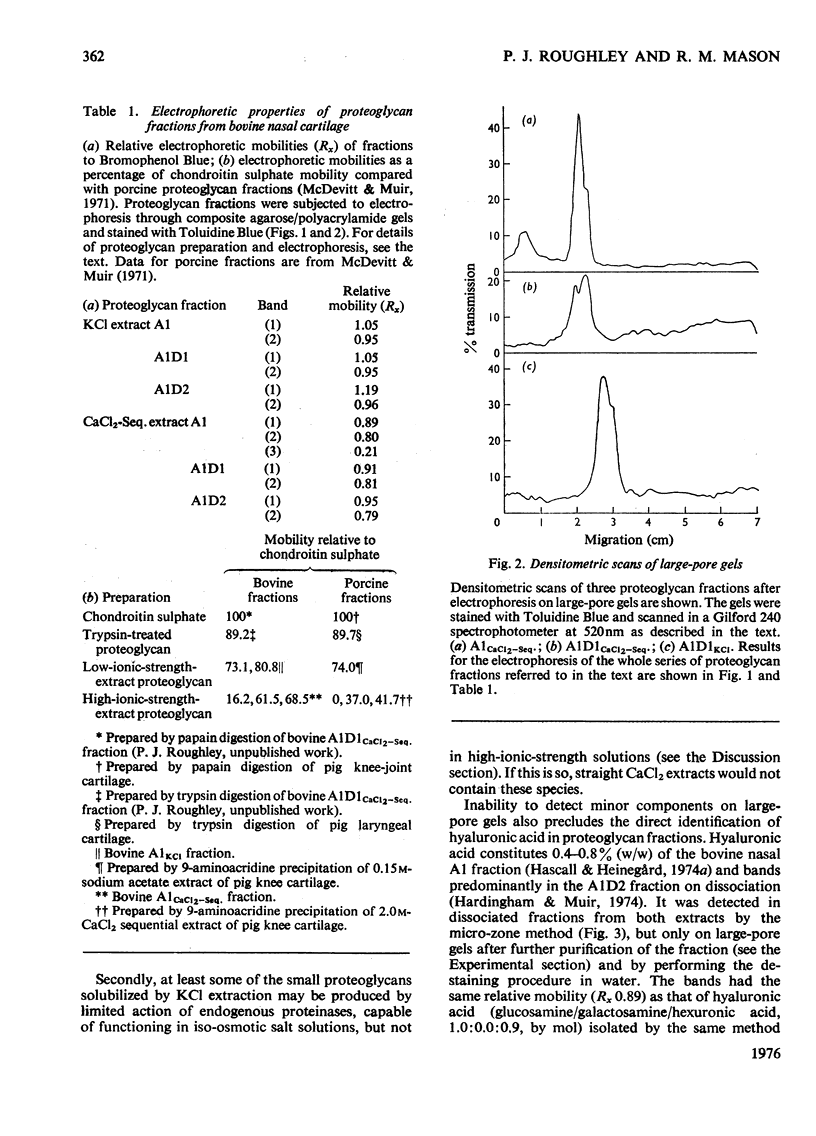
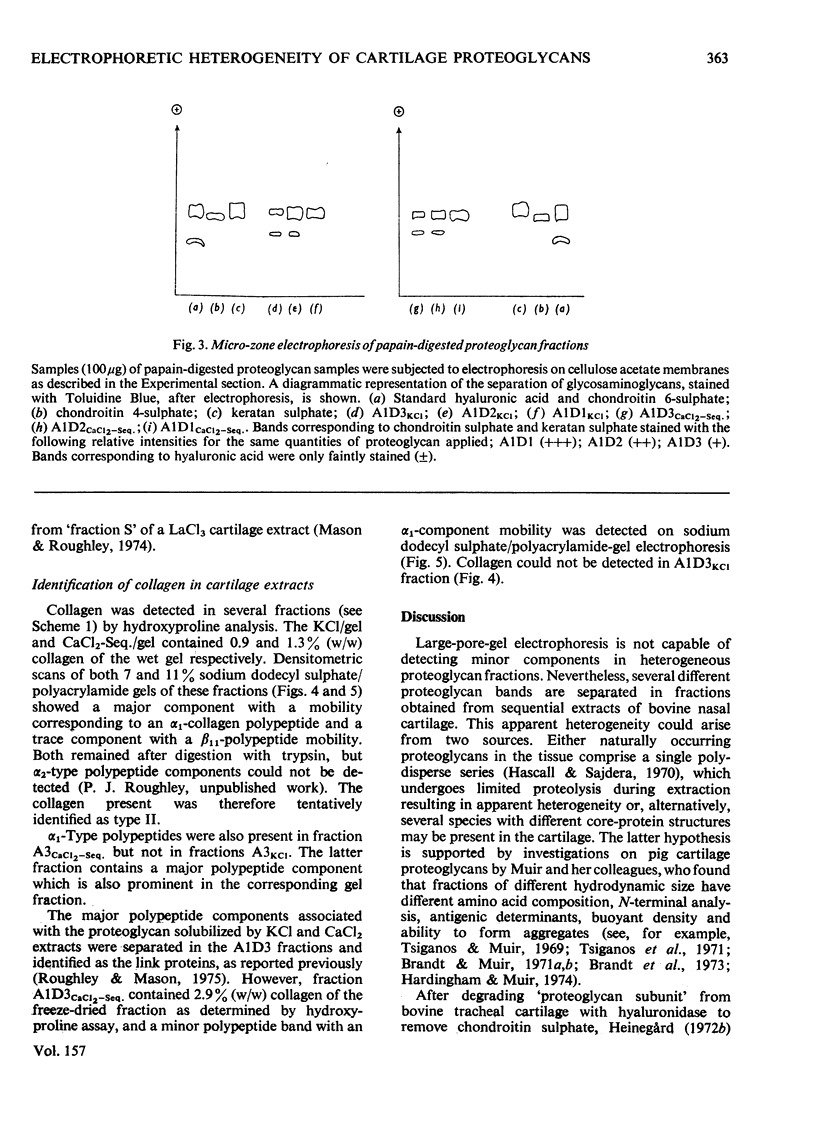
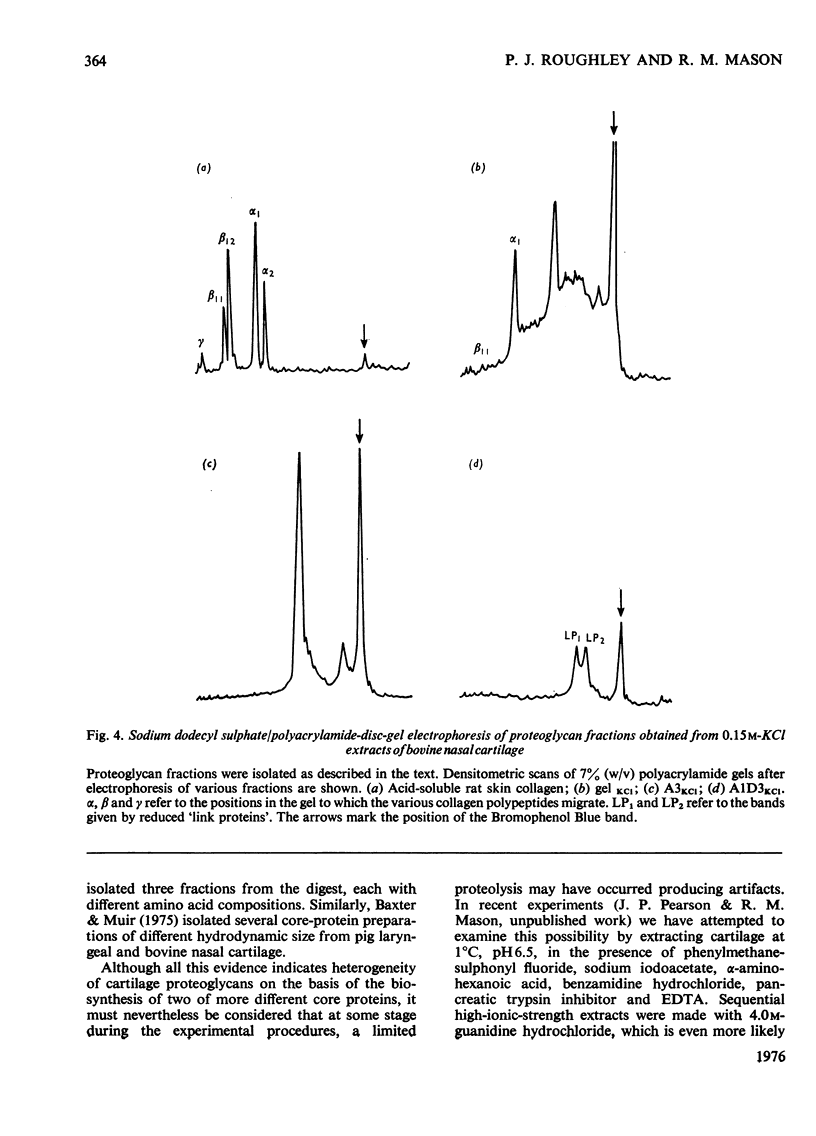
1. Proteoglycans were extracted from bovine nasal cartilage with 2.0M-CaC2 or with 0.15M-KCl followed by 2.0M-CaC2.. Proteoglycan fractions were prepared from the extracts by density-gradient centrifugation in CsCl under 'associative' and 'dissociative' conditions. 2. The heterogeneity of the proteoglycan fractions was investigated by large-pore-gel electrophoresis. It was concluded that extracts made with 2.0M-CaCl2 or sequential 2.0M-CaCl2 contain two major species of proteoglycan 'subunit' of different hydrodynamic size, together with proteoglycan aggregates. Both 'subunits' have mobilities that are greater than those of proteoglycans obtained from pig articular cartilage McDevitt & Muir (1971) Anal. Biochem. 44, 612-622] and are therefore probably smaller in size than the latter. 3. Proteoglycan fractions isolated from cartilage extracted lith 0.15M-KCl separated into two main components on large-pore-gel electrophoresis with mobilities greater than those of proteoglycans extracted with 2.0M-CaCl2. Proteoglycans extracted at low ionic strength from bovine nasal cartilage are of similar hydrodynamic size to those extracted from pig articular cartilage under the same conditions [McDevitt & Muir (1971) Anal. Biochem. 44, 612-622]. 4. The role of endogenous proteolytic enzymes in producing proteoglycan heterogeneity, particularly in low-ionic-strength cartilage extracts is discussed. 5. Hyaluronic acid and 'link proteins' were present in the proteoglycan fraction separated from KCl extracts as well as in the fraction separated from CaCl2 extracts. Hyaluronic acid can only be identified in proteoglycan fractions by large-pore-gel electrophoresis after proteolysis and further purification of the fraction. 6. Collagen was extracted by both salt solutions and was tentatively identified as type II. Small amounts of collagen appear to be associated with the proteoglycan-aggregate fraction from the high-ionic-strength extract but not with the corresponding fraction from the KCl extract.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONOPOULOS C. A., BORELIUS E., GARDELL S., HAMNSTROM B., SCOTT J. E. The precipitation of polyanions by long-chain aliphatic ammonium compounds. IV. Elution in salt solutions of mucopolysaccharide-quaternary ammonium complexes adsorbed on a support. Biochim Biophys Acta. 1961 Dec 9;54:213–226. doi: 10.1016/0006-3002(61)90360-2. [DOI] [PubMed] [Google Scholar]
- Baxter E., Muir H. The nature of the protein moieties of cartilage proteoglycans of pig and ox. Biochem J. 1975 Sep;149(3):657–668. doi: 10.1042/bj1490657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The chondroitin sulphate proteins associaterd with collagen. Biochem J. 1971 Aug;123(5):747–755. doi: 10.1042/bj1230747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The sequential extraction of chondroitin sulphate-proteins with iso-osmotic neutral sodium acetate. Biochem J. 1971 Jan;121(2):261–270. doi: 10.1042/bj1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D., Tsiganos C. P., Muir H. Immunological relationships between proteoglycans of different hydrodynamic size from articular cartilage of foetal and mature pigs. Biochim Biophys Acta. 1973 Sep 14;320(2):453–468. doi: 10.1016/0304-4165(73)90326-7. [DOI] [PubMed] [Google Scholar]
- Di Ferrante N., Donnelly P. V., Sajdera S. W. A segregated antigen in cartilage matrix. J Lab Clin Med. 1972 Sep;80(3):364–372. [PubMed] [Google Scholar]
- Eyre D. R., Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975 Dec;151(3):595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochem J. 1973 Dec;135(4):905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L. Characteristics of the protein-keratan sulfate core and of keratan sulfate prepared from bovine nasal cartilage proteoglycan. J Biol Chem. 1972 Jul 25;247(14):4529–4538. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Isolation and characterisation of different keratan sulfate proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):193–207. doi: 10.1016/0005-2795(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Keiser H., Shulman H. J., Sandson J. I. Immunochemistry of cartilage proteoglycan. Immunodiffusion and gel-electrophoretic studies. Biochem J. 1972 Jan;126(1):163–169. doi: 10.1042/bj1260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S. E., Ray R. D., Kuettner K. E. Microchemical studies on acid glycosaminoglycans of the epiphyseal zones during endochondral calcification. Calcif Tissue Res. 1973 Dec 31;13(4):271–285. doi: 10.1007/BF02015417. [DOI] [PubMed] [Google Scholar]
- Mashburn T. A., Jr, Hoffman P. Comparative fractionation studies of cartilage proteinpolysaccharides. J Biol Chem. 1971 Nov;246(21):6497–6506. [PubMed] [Google Scholar]
- Mason R. M., Mayes R. W. Extraction of cartilage protein-polysaccharides with inorganic salt solutions. Biochem J. 1973 Mar;131(3):535–540. doi: 10.1042/bj1310535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes R. W., Mason R. M., Griffin D. C. The composition of cartilage proteoglycans. An investigation using high- and low-inonic-strength extraction procedures. Biochem J. 1973 Mar;131(3):541–553. doi: 10.1042/bj1310541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Pasternack S. G., Veis A., Breen M. Solvent-dependent changes in proteoglycan subunit conformation in aqueous guanidine hydrochloride solutions. J Biol Chem. 1974 Apr 10;249(7):2206–2211. [PubMed] [Google Scholar]
- Rosenberg L. C., Pal S., Beale R. J. Proteoglycans from bovine proximal humeral articular cartilage. J Biol Chem. 1973 May 25;248(10):3681–3690. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- Roughley P. J., Mason R. M. Proteins and hyaluronic acid associated with proteoglycans extracted from bovine nasal cartilage with low-ionic-strength salt solution. Biochem Soc Trans. 1975;3(1):140–142. doi: 10.1042/bst0030140. [DOI] [PubMed] [Google Scholar]
- SCOTT J. E. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Methods Biochem Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Simůnek Z., Muir H. Changes in the protein-polysaccharides of pig articular cartilage during prenatal life, development and old age. Biochem J. 1972 Feb;126(3):515–523. doi: 10.1042/bj1260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C. A specific collagenase from rabbit fibroblasts in monolayer culture. Biochem J. 1974 Feb;137(2):373–385. doi: 10.1042/bj1370373. [DOI] [PMC free article] [PubMed] [Google Scholar]


