Highlights
-
•
Mixed linkage (1,3;1,4)-β-D-glucan (MLG) is a pivotal bioactive carbohydrate found in cereals, synthesized by Cellulose Synthase-Like genes (Csl). In this study:
-
•
MLG, a bioactive carbohydrate, shows potential in food industry applications.
-
•
CslF6 gene plays a pivotal role in MLG synthesis in wheat.
-
•
Hybrid breeding increases MLG content by up to 43.3% in bread wheat.
-
•
High MLG lines identified using advanced glycomics tools.
Keywords: Wheat, Aegilops caudata, Dasypyrum villosum, Mixed-linkage glucan, CslF6 gene, Glycomics
Abstract
Mixed linkage (1,3;1,4)-β-d-glucan (MLG) is a well-recognized bioactive carbohydrate and dietary fibre with expanding applications in food industry. The MLG are small components of the cell wall of vegetative tissues of cereals synthetized by members of the Cellulose Synthase-Like genes (Csl). Within the family, the CslF6 has been the major contributor in wheat. It is of significant health and economic benefits to enhance MLG content in wheat, a staple grain with naturally low MLG levels. This study investigated the role of CslF6 gene in MLG synthesis and analysed total MLG contents, cell wall monosaccharide, glycosidic linkage composition, and profile of major comprising oligosaccharides of MLG in various wheat genotypes, their wild relatives (Aegilops caudata and Dasypyrum villosum), and hybrids between them. We observed a relationship between CslF6 gene expression and MLG accumulation across the different wheat lines. While Aegilops caudata and Dasypyrum villosum exhibited higher MLG content than other genotypes, hybrid breeding led to an increase in MLG content by 24.4% in durum wheat and 43.3% in T. aestivum. Variations in the ratios of major oligosaccharides released from MLG by lichenase treatment and in the compositions of cell wall monosaccharides and glycosidic linkages were also found. This study demonstrates that HPAEC-PAD and GC–MS-based glycomics are invaluable tools to assist breeders in selecting high MLG lines.
1. Introduction
Durum wheat (Triticum turgidum L. subsp. durum) is a widely cultivated cereal crop that plays a pivotal role in meeting daily caloric requirements in many countries and regions (Beres et al., 2020). Ranking as the 10th most important cereal globally, it accounts for 5 % of total wheat production and spans a planting area of 16 million hectares worldwide. Durum wheat is a rich source of proteins, starch and non-starch polysaccharides, vitamins, minerals, and other phytochemicals in the human diet (Grant et al., 2012, Marcotuli et al., 2020, Marcotuli et al., 2022). Durum semolina, the product resulting from the milling of the hard-textured durum wheat kernel, is predominantly used in pasta production and also serves as a key ingredient in dishes like couscous and bulgur (Beres et al., 2020). Notably, in countries like Italy, regulatory standards mandate that pasta be made of 100 % durum semolina (Sopiwnyk, 2018).
Mixed linkage (1,3;1,4)-β-d-glucan (MLG), a non-starch polysaccharide characterized by a linear chain of 1,3- and 1,4-linked β-d-glucopyranosyl residues, is predominantly found in the kernels of cereal grains. MLG has demonstrated efficacy in reducing the risks of colorectal cancer, cardiovascular disease, and diabetes, the leading causes of morbidity and mortality in industrialized nations, offering both societal and individual benefits (Bhoite, Satyavrat, & Premasudha Sadananda). Globally recognized regulatory bodies, such as US Food and Drug Administration (FDA), Health Canada, and the European Commission have approved health claims highlighting its role in cholesterol management and associated cardiovascular complications (Mathews, Kamil, & Chu, 2020). These endorsements have enhanced the reputation of MLG, prompting its widespread incorporation in various functional food and nutraceutical formulas. Besides, MLG has been increasingly used as functional additives (e.g., emulsion stabilizer, thickening agent, fat substitute) in food industry due to its water solubility, viscosity-enhancing properties, and gel-forming abilities (Maheshwari et al., 2017, Sun et al., 2021, Sushytskyi et al., 2023).
The biosynthesis of MLG in cereal crops has garnered significant research interest, particularly concerning the functions of associated genes and enzymes, and the effects of mutations on MLG content across various grains. MLG synthesis in grasses is catalyzed by members of Glycosyltransferase Family 2 that use uridine diphosphate glucose (UDP-Glc) for the synthesis process (Bain, van de Meene, Costa, & Doblin). The genes responsible for MLG synthesis share sequence homology with cellulose synthases (CesAs) and belong to the Cellulose synthase-like (Csl) sub-families F, H, and J (Burton et al., 2006, Doblin et al., 2009, Farrokhi et al., 2006, Little et al., 2019). Notably, the CslF and CslH groups were found exclusive to monocotyledons and are believed to either directly or indirectly regulate the accumulation and fine structure of MLG in the grain and other parts of the plant (Burton et al., 2011, Farrokhi et al., 2006). Additionally, it has been demonstrated that the barley CslF6 is responsible for synthesis of β-1,4-linkage, but also as a conserved “switch motif” at the entrance of the enzyme’s transmembrane channel, which is critical to generate (1,3)-linkages (Purushotham et al. 2022).
In Brachypodium distachyon, CslF6 mutants showed altered carbon metabolism in MLG deficient grain (Bain, van de Meene, Costa, & Doblin, 2020). Mutations of CslF6 in barley, rice, and wheat were found associated with reductions in MLG content, but these reductions generally had only moderate effects on vegetative growth and were well-tolerated (Cory et al., 2012, Hu et al., 2014, Nemeth et al., 2010, Taketa et al., 2012, Tonooka et al., 2009, Wong et al., 2015). In wheat, it was observed that targeted RNA knockdowns of CslF6, driven by an endosperm-specific promoter, resulted in a 30–53 % decrease in MLG content in whole grain flour (Nemeth et al., 2010). In another study, knockout mutants of CslF6 in rice exhibited a 97 % reduction in MLG levels in coleoptiles, with undetectable levels in other tissues (Vega-Sanchez et al., 2012). Compared to other cereal species, limited data on biosynthetic pathway of MLG is available for durum wheat, its wild relatives, and their hybrids.
Wheat naturally provides a low amount of MLG. For instance, barley and oat bran have MLG contents of 7 % and 5 %, respectively, whereas the MLG content of wheat bran is less than 1 % (Tiefenbacher, 2017). To introduce wheat-based products with naturally high MLG content to the market, innovative breeding strategies are needed to develop new wheat lines. Due to the limited genetic diversity within the wheat gene pool, incorporating wheat wild relatives into breeding programs is a promising strategy, achievable by introgressing small segments of their genome that carry desirable traits into wheat (Feuillet, Langridge, & Waugh, 2008). Aegilops caudata L. [syn. Ae. markgrafii (Greuter) Hammer], a diploid wild relative (2n = 2x = 14, CC) of hexaploid wheat, has been shown to harbor numerous resistance genes for both biotic and abiotic stresses and established as a valuable source of genetic variation for improving quality traits of wheat (Grewal et al., 2020, Ivanizs et al., 2022). There have been reports of the development of several wheats-Aegilops interspecific hybrids, addition lines, and translocation lines with many agronomically beneficial traits successfully integrated into the wheat gene pool from Aegilops (Schneider, Molnár, & Molnár-Láng, 2008). MLG was found to be an important cell wall component of Aegilops genus (Kishii, 2019). By incorporating chromosome 5U or group 7 chromosomes from the U and M genomes of Aegilops geniculata and Aegilops biuncialis species, the MLG content of T. aestivum significantly increased under various growth conditions (Rakszegi et al., 2017). For Dasypyrum villosum, another wild relative of wheat, there was only one MLG-related report that the incorporation of genes from the V chromosomes into durum wheat genomes improved both grain yield and grain quality; however, the durum wheat with the gene introgression exhibited MLG content similar to that of the wheat parental control (De Pace et al., 2014).
The current study was aimed at investigating and comparing the transcription levels of CslF6 in the endosperm at various developmental stages across a range of genotypes, including Aegilops caudata, Dasypyrum villosum, durum wheat, T. aestivum, and amphyploid lines. We conducted enzymatic-colorimetric MLG assay, GC–MS-based monosaccharide and glycosidic linkage analyses, and HPAEC-PAD-based oligosaccharide profiling to examine the variations in content and structural features of MLG across samples. This study sought to establish a workflow for MLG-specific glycomics, assisting breeders, growers, and processors in selecting MLG-rich cereal products.
2. Materials and methods
2.1. Wheat materials
A set of six genotypes, described in Table 1, were grown in an experimental field in Valenzano, Bari, Italy. The seeds included the durum variety Creso, the D. villosum genotype, the durum-villosum amphyploid genotype (from now named as Amphyploid 1), the T. aestivum variety Alcedo, the Ae. Caudata genotype and the T. aestivum-caudata Amphyploid (from now named as Amphyploid 2). Seeds from these genotypes were germinated and, during the growing season, 10 g of nitrogen per m2 was applied at the beginning of planting following standard cultivation practices. Single plants were hand-harvested at maturity, and the grain was stored at 4 °C. The grain was ground using a 1093 Cyclotec Sample Mill (Tecator Foss, Hillerød, Denmark) and passed through a 1-µm sieve. Endosperm samples from each genotype were collected at five developmental stages (6, 12, 18, 24, and 32 days post anthesis) and stored at −80 °C for subsequent RNA extraction and analysis.
Table 1.
Genome, whole grain MLG content, and MLG major oligosaccharide ratios (DP3/DP4) for wheats, wild relatives, and their hybrid lines.
| Line | Genome | MLG (w%) | St.dev. | DP3/DP4 | St.dev. | |
|---|---|---|---|---|---|---|
| T. durum | AABB | 0.45 | 0.02 | 2.5 | 0.09 | |
| D. villosum | VV | 4.36 | 0.04 | 1.8 | 0.09 | |
| Amphyploid 1 | AABBVV | 0.56 | 0.05 | 1.8 | 0.02 | |
| T. aestivum | AABBDD | 0.90 | 0.01 | 1.8 | 0.04 | |
| Ae. caudata | CC | 4.15 | 0.19 | 1.9 | 0.09 | |
| Amphyploid 2 | AABBDDCC | 1.29 | 0.03 | 1.8 | 0.03 |
2.2. Determination of MLG content using enzymatic colorimetric assay
The total content of MLG was determined in wholegrain flour obtained from mature kernels and in immature endosperm sampled at each developmental stage previously described using the commercially available Mixed-Linkage β-d-glucan Assay Kit (Megazyme International Ireland Ltd., Wicklow, Ireland), following the methodology described by the literature (McCleary & Codd, 1991). Barley material with known MLG content of 4.1 % (w/w) was included in the kit as a reference for the analysis. Three biological replicates, each with two technical replicates, were performed for each genotype.
2.3. Oligosaccharide profile of lichenase digest by high performance anion exchange chromatography coupled to amperometric detection (HPAEC-PAD)
HPAEC-PAD analysis was used to profile the oligosaccharides enzymatically released from MLG from kernels with different genotypes, as described in our previous report (Chang et al., 2023). This involved subjecting the oligosaccharides released by lichenase digestion in the Megazyme assay to solid phase extraction using Varian Bond Elut Carbon columns (Agilent Technologies Inc., USA) with a concentration of 50 mg/mL. Elution was performed with 55 % (v/v) acetonitrile on a Dionex ICS-5000 HPAEC-PAD system (Thermo Fisher Scientific Inc., USA). The ratio of the trisaccharide G4G3G to the tetrasaccharide G4G4G3G was calculated and denoted as DP3/DP4.
2.4. Cell wall preparation
The whole cell walls of the durum wheats were prepared as de-starched alcohol insoluble residues (AIRs) following the published procedure (Wood et al., 2018), except that the hexane and ethyl acetate soaking steps were omitted, only thermostable amylase digestion was performed (replacing the combined use of amylase and amyloglucosidase), and the de-starched samples were dialyzed with a molecular weight cut off of 6,000–8,000 Da instead of 3,500 Da (Klassen et al., 2023). The dry pellets of AIRs were finely powdered by ball-milling in preparation for subsequent monosaccharide and linkage analyses.
2.5. Monosaccharide analysis
Monosaccharides were released from around 10 mg of dry AIR powder by heating in 2 mL of 1.25 M anhydrous methanolic hydrochloric acid at 80 °C for 16 h with gentle magnetic stirring, followed by evaporation under nitrogen until dryness and additional two rounds of evaporation to dryness in 2 mL of anhydrous methanol (Casillo et al., 2022, Gottstein et al., 2021, Smith et al., 2020). The samples were then magnetically stirred in 2 mL of deionized water containing 10 mg/mL of sodium borodeuteride at 4 °C for 24 h to carboxyl reduce uronic acid methyl esters to their C-6 dideuterated neutral sugar counterparts (Bacic et al., 1986, Badhan et al., 2022, Low et al., 2020). Excess reductant was quenched by glacial acetic acid, the reaction mixture was evaporated to dryness under nitrogen, and then the product was acetylated by heating in a mixture of 0.5 mL of trifluoroacetic acid and 2.5 mL of acetic anhydride at 60 °C for 1 h min (Chang et al., 2023, Jones et al., 2020, Robb et al., 2022, Voiges et al., 2012). After evaporation to dryness under nitrogen, the product were redissolved in 3 mL of dichloromethane followed by partition with 3 mL of saturated sodium bicarbonate deionized water one time then 3 mL of deionized water three times, passing the final lower phase through a glass wool plugged Pasteur pipette loaded with anhydrous sodium sulfate, and evaporation to dryness by nitrogen (Robb et al., 2022, Voiges et al., 2012, Yu et al., 2017). The resulting acetylated methyl glycosides were converted to alditol acetates by 2 M trifluoroacetic acid hydrolysis (100 °C, 2 h), reduction with sodium borodeuteride, and acetylation in heated mixture of trifluoroacetic acid and acetic anhydride (1:5, v/v) (Chang et al., 2023, Jones et al., 2020, Robb et al., 2022, Voiges et al., 2012). The derivatives were then cleaned up using partition and sodium sulfate column as described above, redissolved in ethyl acetate, and tested on a 6890 N GC-FID system (Agilent, United States) installed with an Supelco SP-2380 column (30 m × 0.25 mm × 0.20 μm, Sigma-Aldrich, United States) with oven temperature to start at 180 °C (hold 1 min) followed by increasing at 3 °C/min to 250 °C (hold 20 min). The same sample was also tested on a 7890B-5977B GC–MS system (Agilent, United States) installed with an Supelco SP-2380 column (100 m × 0.25 mm × 0.20 μm, Sigma-Aldrich, United States) with oven temperature to start at 220 °C followed by increasing at 1 °C/min to 250 °C (hold 30 min). Both systems had the same settings of inlet temperature (250 °C) and constant column helium flow of 0.8 mL/min. Splitless injection and split injection (10:1 ratio) were used for the GC-FID and GC–MS, respectively. The relative composition of alditol acetates was quantified using FID response factors obtained from standards, and the C-6 deuterated alditol acetates of uronic acids and alditol acetates generated from corresponding neutral monosaccharides were distinguished and determined based on GC–MS data, as described in the literature (Pettolino, Walsh, Fincher, & Bacic, 2012). Two separate experiments were conducted for each sample.
2.6. Glycosidic linkage analysis
Uronic acids in around 10 mg of dry AIR powder were carboxyl reduced to 6,6-dideuterated neutral sugars by sodium borodeuteride reduction of their methyl esters generated by weak methanolysis (0.5 M methanolic HCl, 80 °C, 20 min) (Chong et al., 2019, Hosain et al., 2019, Muhidinov et al., 2020). After quenching excess reductant with acetic acid and removal of borate by repeated evaporation to dryness in mixture of acetic acid and methanol (1:10, v/v) then in anhydrous methanol, the sample was methylated in a mixture of 1.2 mL of methyl iodide and 2 mL of DMSO with 100 mg of dry sodium hydroxide powder (Badhan et al., 2022, Jones et al., 2020, Low et al., 2020). The methylation product was cleaned up by partition between 3 mL of dichloromethane and 6 mL of 10 % acetic acids over ice two times then with 6 mL of deionized water two times. The lower phase was evaporated to dryness, and the methylation process was repeated one more time. For the first round of methylation, the sample was magnetically stirred in DMSO at 60 °C overnight, with the tube headspace filled with N2, prior to the methylation reaction. Before the second round of methylation, the same process of stirring in DMSO was conducted overnight but at room temperature, instead of 60 °C. The methylated samples were then converted to partially methylated alditol acetates (PMAAs) by first underwent 2 M trifluoracetic acid hydrolysis (120 °C, 2 h). The hydrolysis product was then reduced by sodium borodeuteride before all free hydroxyl groups were acetylated under a mixture of trifluoroacetic acid and acetic anhydride (1:5, v/v) (Chang et al., 2023, Robb et al., 2022, Voiges et al., 2012). The PMAAs were redissolved in ethyl acetate and tested on the Agilent 7890B-5977B GC–MS system with the same 100 m Supelco SP-2380 column as described above for monosaccharide analysis, except that the oven temperature was programmed to start at 100 °C followed by increasing at 1.5 °C/min to 220 °C then at 1.25 °C/min to 250 °C (hold 20 min). The EI-MS spectra of the PMAAs were interpreted by comparing them with those of reference derivatives and by referring to the literature (Carpita & Shea, 1989). The glycosidic linkage composition (mol%) was calculated following the published protocol (Pettolino et al., 2012).
2.7. RNA extraction and cDNA synthesis
The expression analysis of the gene in the endosperm of the two wheat varieties, Ae. caudata, D. villosum, and amphyploid genotypes was conducted using the primer pairs described in our previous report (I. Marcotuli, P. Colasuonno, A. Blanco, & A. Gadaleta, 2018). To analyze the expression level of CslF6, total RNA was extracted from the endosperm of each genotype using the RNeasy Plant Mini Kit (Qiagen, Valencia, USA) and assessed on a 1.5 % denaturing agarose gel. All RNA samples were adjusted to the same concentration (1 μg/mL) and reverse-transcribed into double-stranded cDNA with the QuantiTect Reverse Transcriptase Kit (Qiagen, Valencia, USA). Data were normalized using three reference genes: Cell Division Control AAA-Superfamily of ATPases (CDC), ADP-Ribosylation Factor (ADP-RF), and RNase L Inhibitor-like protein (RLI). These genes had a stability value of approximately 0.035 when evaluated with NormFinder software (Andersen, Jensen, & Ørntoft, 2004).
2.8. qPCR for cellulose synthase gene
Quantitative real-time PCR (qRT-PCR) was performed using Cyber® GREEN on a CFX96TM Real-Time PCR Systems (Bio-Rad Laboratories, Hercules, USA), adhering to the amplification procedure described in our previous report (Marcotuli et al., 2018). The specificity of the amplicons was validated through several means: by observing a single band of the expected size for each primer pair on a 2 % (w/v) agarose gel, by noting a singular peak in the melting curves of the PCR products, and by sequencing the amplified fragments using a 3500 Genetic Analyzer (Applied Biosystems, Waltham, USA). qRT-PCR data were derived from the mean values of three independent amplification reactions conducted on five distinct plants harvested at the same phenotypic stage (biological replicates). All calculations and analyses were conducted using the ΔCt method with the CFX Manager 2.1 software (Bio-Rad Laboratories, Hercules, USA). Standard deviations were utilized to normalize values for the highest or lowest individual expression levels as per the CFX Manager 2.1 software user manual. ANOVA and the LSD test were employed to evaluate significant differences in the expression of the CslF6 gene between genotypes.
3. Results and discussion
The health and economic benefits associated with MLG have led to a search for wild alleles that can increase the level of MLG in wheat, primarily focusing on species within the genus Triticum (Marcotuli et al., 2015, Marcotuli et al., 2016, Marcotuli et al., 2016). Wild relatives of wheat from the genus Aegilops and Dasypyrum, which showed MLG content around 4 % and are critical sources of new genes and alleles for wheat breeding, have been largely overlooked in breeding efforts aimed at developing high MLG wheat. The current study investigated the role of the CslF6 gene in MLG accumulation in wheat, its wild relatives Aegilops caudata and Dasypyrum villosum, and their hybrids, and also aimed to enhance the efficiency and accuracy of identifying high MLG lines using MLG-specific glycomics.
3.1. CslF6 expression in mature kernels and endosperm at different developmental stages
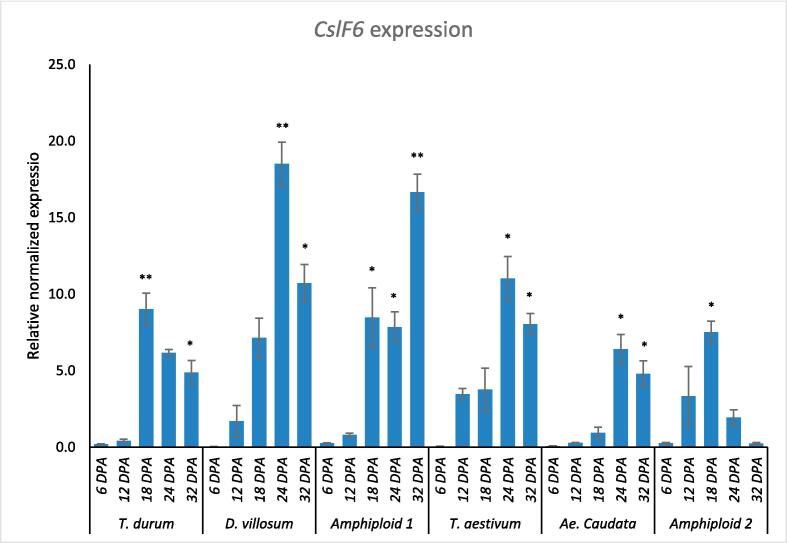
The transcript levels of CslF6 mRNA were determined in the endosperm of bread and durum wheats, Ae. caudata, D. villosum, and the two corresponding amphyploid during various developmental stages, spanning from 6 to 32 days post anthesis (DPA) (Fig. 1). For T. durum, the normalized expression of CslF6 mRNA remained relatively low during the initial days, peaking significantly at 18 DPA with a value of 9.0, then declining in subsequent measurements. D. villosum displayed a drastic surge at 24 DPA, reaching the highest expression level of 18.5 before descending to 10.7 at 32 DPA. The hybrid of T. durum and D. villosum (Amphyploid 1) exhibited a steady increase in expression up to 32 DPA, reaching a peak value of 16.7. T. aestivum showed a notable spike at 24 DPA with a value of 11.0. It seems that Ae. Caudata reached peak expressions similar to that of D. villosum at 24 DPA.. The hybrid of T. aestivum and Ae. caudata demonstrated high expression levels at 18 DPA (7.5), followed by a dramatic drop to 0.2 by 32 DPA. In comparison, both T. durum and Ae. caudata reached peak expressions around the middle of the developmental stages, while D. villosum and T. aestivum presented higher expressions slightly later, around 24 DPA. Amphyploid 1 showed a sharp increase at 18DPA rather than consistent increase.Amphyploid, while the Amphyploid 2 demonstrated a severe decline post its peak. Such variances in expression patterns could be indicative of the genetic and regulatory influences that each species or hybrid inherits and how these influences affect the expression of the CslF6 gene during endosperm development. These results were in good agreement with previous studies on the expression patterns of CslF6 in developing endosperm of durum wheat. For instance, similar trends of low CslF6 expression were previously reported in the early stages of endosperm development, followed by a significant increase in expression during the mid to late stages (Ilaria Marcotuli, Pasqualina Colasuonno, Antonio Blanco, & Agata Gadaleta, 2018). These findings suggested a conserved regulatory mechanism governing CslF6 expression during endosperm development across different genotypes. The observed increase in CslF6 expression at 18 DPA was in good agreement with the previous report that a set of genes in Brachypodium, including CslF6, exhibited peak expression levels during this stage of endosperm development (Francin-Allami et al., 2023). The upregulation of CslF6 at 18 DPA might indicate its involvement in specific developmental processes, such as cell division or cell wall synthesis, crucial for endosperm development during this period. A distinct expression pattern of CslF6 in Triticum aestivum, showing a gradual increase in CslF6 expression throughout development, was previously reported (Nemeth et al., 2010). It is worth noting that while our study focused on the expression levels of CslF6 mRNA during endosperm development, the functional implications of CslF6 in this context should not be overlooked. A previous study demonstrated that down-regulation of CslF6 expression resulted in altered cell wall composition and reduced grain filling in transgenic lines (Nemeth et al., 2010). This suggested that CslF6 plays a critical role in endosperm development, particularly in the biosynthesis and organization of cell wall components. The current study sheds light on the role of CslF6 in endosperm development, but further research is needed to pinpoint its precise functions, its contribution in various genotypes, and its interplay with genes involved in cell wall biosynthesis and grain filling.
Fig. 1.
Normalized expression levels for CslF6 gene in the developing endosperm at various times (days) following the initiation of maturation at 6, 12, 18, 24, and 32 days post anthesis (DPA) analysed in durum wheat (T. durum), T. aestivum, Ae. Caudata, D. villosum, and amphyploid lines. Amphyploid 1 refers to the hybrid between T. durum and D. villosum. Amphyploid 2 denotes the hybrid between T. aestivum and Ae. caudata. The height of each bar represents the mean value, with the standard deviation denoted by error bars labelled at the top. Asterisks indicate genotypes significantly different within the developmental stages (** P ≤ 0.01; * P ≤ 0.05).
3.2. Total MLG content determined by enzymatic colorimetric assay
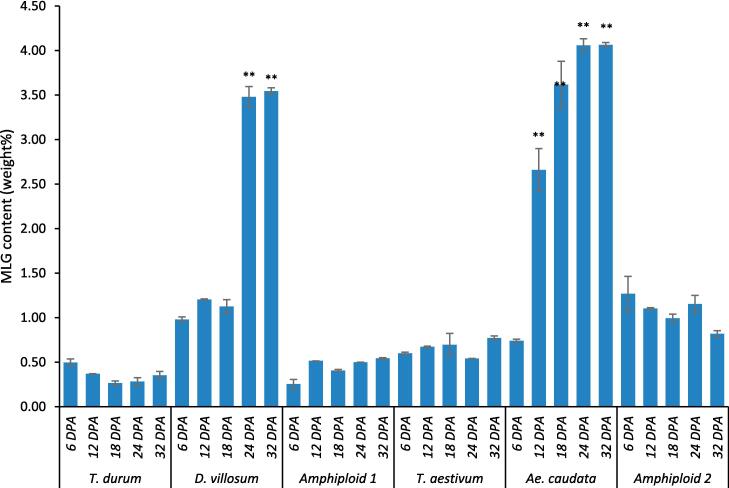
The total MLG amount in mature grains was tested using a well-established enzymatic and colorimetric method with commercial kit from Megazyme (McCleary et al., 1991). The amount of MLG was expressed as weight percentage (w%) based on the dry weight of the kernel. Results showed that the whole grain MLG values ranged from 0.45 % (durum wheat) to 4.15 % (Ae. caudata), with significant differences (p ≤ 0.01) between the genotypes (Table 1). The T. aestivum-caudata amphyploidamphyploid genotype displayed a much lower whole grain MLG content (1.29 %) than the caudata line (4.15 %). Similarly, the durum wheat-villosum amphyploidamphyploid genotype had a low whole grain MLG content of 0.56 %. Highest value was shown from D. villosum (4.36 %). Both amphyploidamphyploid lines exhibited low MLG amounts similar to those of the parental lines. The results were in good agreement with previous reports that bread and durum wheats were not significant sources of MLG because of their low content in the grain, typically less than 1 % on a dry weight basis (Collins et al., 2010, Marcotuli et al., 2016, Marcotuli et al., 2016). However, it is worth noting that higher MLG values, up to 2.3 %, have been reported in T. aestivum (Fincher & Stone, 2004). The relatively high concentration of MLG in wheat grain was mainly found in the sub-aleurone layer, with lower amounts present in the rest of the endosperm (Beresford & Stone, 1983).
In the developing endosperm (6, 12, 18, 24, and 32 DPA), the MLG contents across the different species showed distinct patterns (Fig. 2). T. durum started with an MLG content of 0.50 % at 6 DPA, saw a slight decline by 18 DPA to 0.27 %, and then gradually rebounded to 0.35 % by 32 DPA, suggesting relative stability in its low MLG levels over time. D. villosum, with an initial value of 0.98 % at 6 DPA, consistently increased, reaching its highest value of 3.54 % by 32 DPA. The hybrid of T. durum and D. villosum maintained a relatively stable trend, initiating at 0.25 % and increasing slowly to 0.54 % over the 32 days. T. aestivum exhibited a narrow range of fluctuation, starting at 0.60 % and showing a minor peak of 0.77 % at 32 DPA. Ae. caudata, with its onset at 0.74 %, saw a significant increase in its MLG content, reaching 4.06 % by 24 DPA, and preserved this level until 32 DPA. The hybrid lineage of T. aestivum and Ae. caudata had a relatively high value of 1.27 % at 6 DPA, but then stabilized around 1.0 %, concluding at a slightly reduced value of 0.82 % by 32 DPA. These results demonstrated the dynamic changes in MLG content during kernel development across different varieties. The variations observed in MLG content among the developmental stages and varieties suggested the potential influence of genetic factors and developmental processes on MLG accumulation in kernels. A noticeable rise in the content of MLG from 4 to 28 DPA during T. aestivum grain development was observed in previous study (Palmer, Cornuault, Marcus, Knox, Shewry, & Tosi, 2015). That study also revealed that the most pronounced increase occurred during the early stages of development (4–12 DPA), after which the growth rate decelerated as it approached maturity.
Fig. 2.
MLG content detected in the developing endosperm at various times (days) following the initiation of maturation at 6, 12, 18, 24, and 32 days post anthesis (DPA) analysed in durum wheat (T. durum), T. aestivum, Ae. Caudata, D. villosum, and amphyploid lines. Amphyploid 1 refers to the hybrid between T. durum and D. villosum. Amphyploid 2 denotes the hybrid between T. aestivum and Ae. caudata. The height of each bar represents the mean value, with the standard deviation denoted by error bars labelled at the top. Asterisks indicate genotypes significantly different within the developmental stages (** P ≤ 0.01; * P ≤ 0.05).
3.3. HPAEC-PAD analysis of oligosaccharides released from kernels by lichanase treatment
The molecular traits of MLG are typically derived from HPAEC-PAD analysis of oligosaccharides generated by lichenase with a 3-linked reducing end, while all other residues are 4-linked (Chang et al., 2023). Variations exist within the same cereal species due to factors such as genotype and environmental conditions (Jiang and Vasanthan, 2000, Miller et al., 1993, Storsley et al., 2003). Chromosome 5U was found to contribute to a reduction in the DP3/DP4 ratio of MLG (Rakszegi et al., 2017). The DP3/DP4 ratio has been used to profile MLG from different sources of cereals because, among all oligosaccharides detectable by HPAEC-PAD, trisaccharide and tetrasaccharide units make up 90–95 % of total oligosaccharides, with longer chains accounting for only 5–10 % (Lante, Canazza, & Tessari, 2023). Barley, wheat, oat, and rice were reported to have DP3/DP4 ratio ranges of 2.3–3.4, 1.5–2.3, 3.0–4.5, and 2.4–2.7, respectively (Lante et al., 2023). In the current study, the DP3/DP4 ratios were determined to be 1.8 for villosum, wheat-villosum amphyploid, T. aestivum, and T. aestivum-caudata amphyploid genotypes, 1.9 for the caudata line, and 2.5 for the durum wheat line (Table 1).
3.4. Monosaccharide and linkage analysis by GC–MS/FID
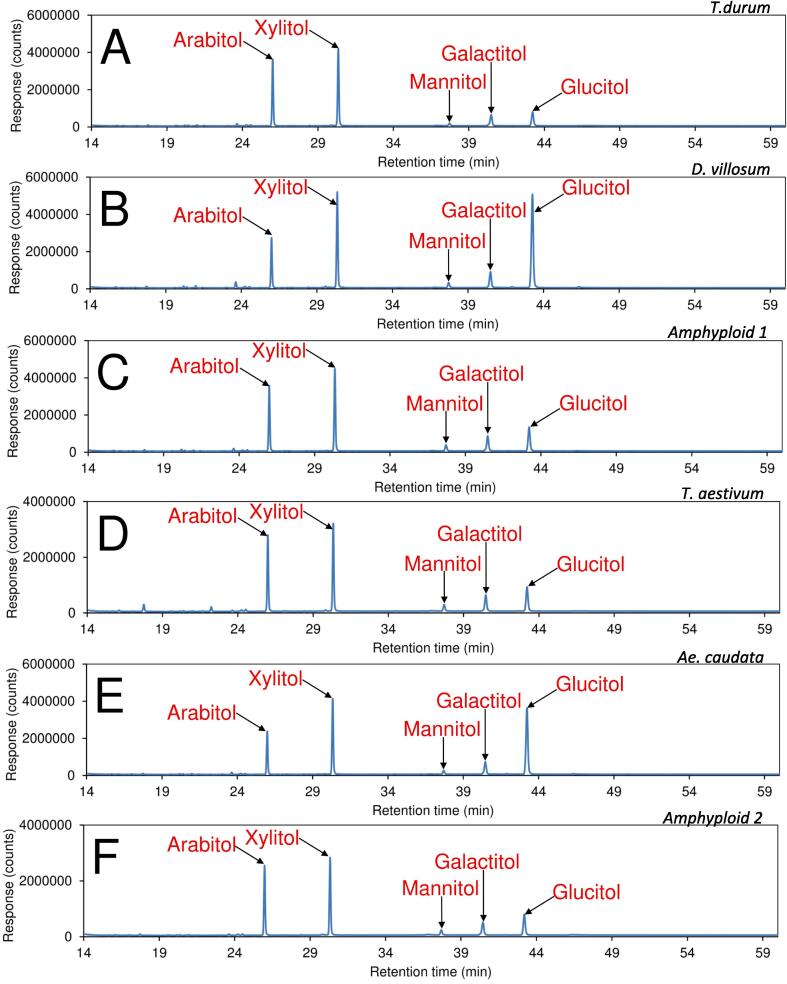
Whole cell wall polysaccharides of the durum wheat kernel were prepared as de-starched AIR using a well-established procedure that has been proven effective on many higher plant samples (e.g., chickpea kernel, canola meals) (Badhan et al., 2022, Klassen et al., 2023, Li et al., 2022, Li et al., 2019, Low et al., 2020, Wood et al., 2018). DMSO soaking was employed to solubilize all starches, including resistant starches (Sowinski, Gilbert, Lam, & Carpita, 2019). These can be hydrolysed to monosaccharides and oligosaccharides and then further removed by dialysis. It is commonly known that higher plant cell wall polysaccharides consist mainly of hemicellulose, cellulose, and pectins (Pettolino et al., 2012). Cellulose and MLG are both β-d-glucans with linear chains of glucopyranosyl residues, with the distinction that the glucopyranosyl residues are 4-linked only in the former but a mixture of 3- and 4-linked in the latter. The homo-linkage nature of cellulose results in it forming a highly crystalline structure in the cell wall by strong hydrogen bonding formed between neighbouring polysaccharide chains, even though a minor amount of amorphous cellulose also exits naturally in higher plant cell walls. In contrast, the mixed-linkage structural nature of MLG does not form strong crystalline regions because 3-linked residues introduce a kink that prevents the formation of strong interchain hydrogen bonding and thus crystalline region. It is also well-known that weak acid-catalysed hydrolysis methods, such as TFA and methanolysis, cannot break the crystalline structures of cellulose but can hydrolyse non-crystalline cell polysaccharides such as pectins (e.g., homogalacturonan and rhamnogalacturonans) and hemicelluloses (e.g., arabinoxylan, heteroxylan, heteromannans, and MLG) (Bertaud et al., 2002, Biswal et al., 2022, Foster et al., 2010, Schäfer et al., 2020, Tingley et al., 2021, Willför et al., 2009, Wilson et al., 2021). In the current study, methanolysis (1.25 M HCl in methanol, 80 °C, 16 h) was used to release monosaccharides from non-crystalline cell wall polysaccharides of durum wheat kernels. Results showed that the relative compositions of glucose in the AIRs of D. villosum (45.1 %) and Ae. caudata (40.1 %) were much higher than those in the T. durum (9.7 %), Amphyploid 1 (14.5 %), T. aestivum (14.3 %), and Amphyploid 2 (13.5 %) (Table 2, Fig. 3). This finding could be due to the presence of other genes responsible for MLG synthesis in wheats, the quantitative nature of the trait and the polygenic control by genes with additive effects (Marcotuli et al., 2016, Marcotuli et al., 2016, Houston et al., 2014). Given that crystalline cellulose and MLG are the two major glucose-containing non-starch polysaccharides in the kernel and that the glucose in crystalline cellulose cannot be released by acid methanolysis, the detected glucose was mainly from MLG. Therefore, the result indicated that the relative MLG composition in the cell walls of DV and AC kernels were much higher than in the other samples.
Table 2.
Monosaccharide composition (mol%) of non-crystalline cell wall polysaccharides of kernels of wheats, wild relatives, and their hybrid lines.
| Monosaccharide | TD | DY | A1 | TA | AC | A2 |
|---|---|---|---|---|---|---|
| Ara | 43.2 | 19.2 | 37.0 | 38.3 | 21.6 | 41.4 |
| Gal | 6.1 | 5.4 | 7.4 | 7.7 | 5.5 | 6.3 |
| GalA | 1.6 | 1.2 | 1.2 | 1.2 | 1.2 | 1.3 |
| Glc | 9.7 | 45.1 | 14.5 | 14.3 | 40.1 | 13.5 |
| GlcA | 1.7 | 0.9 | 1.8 | 2.1 | 0.9 | 1.9 |
| Man | 1.8 | 2.1 | 3.7 | 3.8 | 1.9 | 3.1 |
| Xyl | 35.9 | 26.1 | 34.4 | 32.5 | 28.7 | 32.4 |
Note: Each experiment was conducted in duplicate to generate a mean. TD: T. durum; DV: D. villosum; A1: Amphyploid 1; TA: T. aestivum; AC: Ae. caudata; A2: Amphyploid 2.
Fig. 3.
Total ion current (TIC) chromatograms of alditol acetate derivatives of the six kernel cell wall samples. TD: T. durum; DV: D. villosum; A1: Amphyploid 1; TA: T. aestivum; AC: Ae. caudata; A2: Amphyploid 2. Amphyploid 1 refers to the hybrid between T. durum and D. villosum. Amphyploid 2 denotes the hybrid between T. aestivum and Ae. caudata.
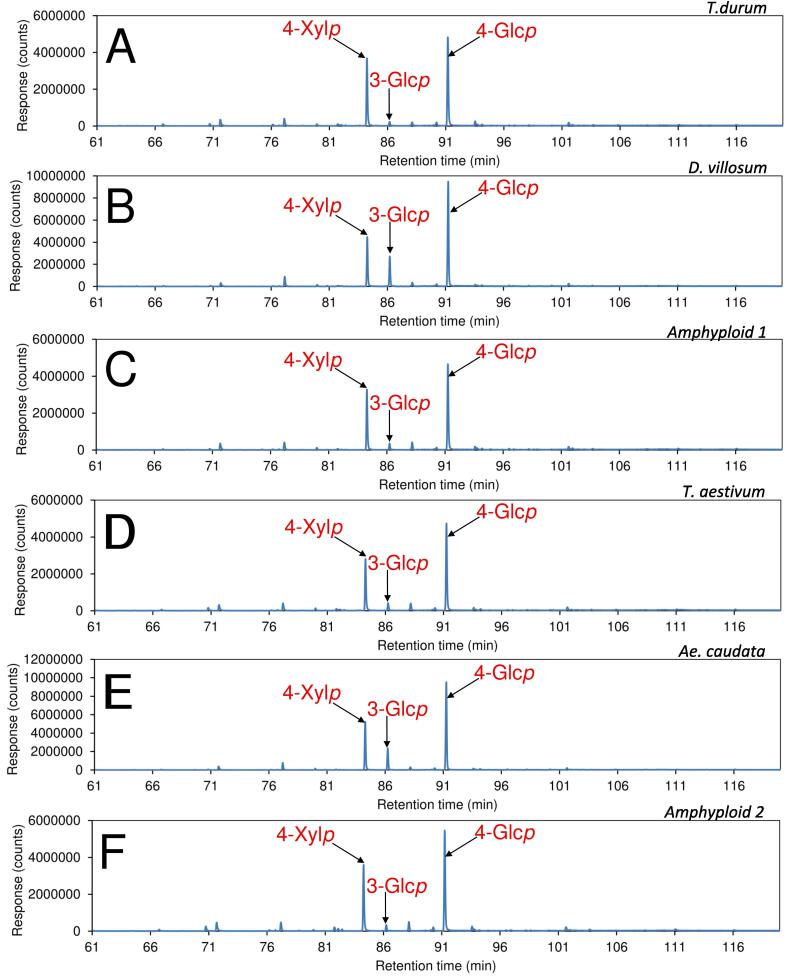
Crystalline cellulose in insoluble cell wall samples can be depolymerized by hydrolysis using water solution of strong acid (e.g., sulphuric acid); however, the hydrolysis condition is so harsh that degradation of released monosaccharides can occur, resulting in a reduced level of detected monosaccharides (Black, Heiss, & Azadi, 2019). Per-O-methylation of polysaccharide by methyl iodide in DMSO in the presence of dry sodium hydroxide powder (the Ciucanu Method) has been proved to be effective in completely decrystallising and methylating crystalline cellulose structure (Black et al., 2019, Ciucanu, 2006). The per-O-methylated decrystalised cellulose can be depolymerized by relatively weak TFA hydrolysis, and the released methylated monosaccharides can be converted to their partially methylated alditol acetate (PMAA) derivatives for analysis of glycosidic linkage composition using GC–MS/FID (Pettolino et al., 2012). This methylation analysis procedure has been successfully conducted to many whole cell wall samples (Badhan et al., 2022, Klassen et al., 2021, Low et al., 2020, Pham et al., 2019, Pham et al., 2019, Wood et al., 2018). For the determination of pectin, carboxyl reduction of uronic acids to their corresponding C-6 dideuterium labelled neutral sugar is required before the methylation step by sodium borodeuteride reduction of uronic acid esters generated by carbodiimide activation (Kim & Carpita, 1992) or weak methanolysis treatment (0.5 M methanolic HCl, 80 ˚C, 20 min) (Chong et al., 2019, Hosain et al., 2019, Muhidinov et al., 2020). In the current study, the latter carboxy reduction method was used for the whole kernel cell walls, followed by methylation-GC–MS/FID analysis of the carboxyl reduced samples. Results showed that the D. villosum and T. aestivum cell walls contained much higher levels of 3-linked glucopyranoses than the others (Table 2, Fig. 4). The levels of 3-linked glucopyranoses were very similar among T. durum, Amphyploid 1, T. aestivum, and Amphyploid 2. The results of 3-linked glucopyranoses were in good agreement with the results of total MLG contents. As expected, abundant 4-linked glucopyranoses were detected, originating from the 4-linked residues of MLG and cellulose that were fully decrystallised by methylation. EI-MS spectra of PMAAs from 3-linked glucopyranose and 4-linked glucopyranose from D. villosum kernel are shown in Fig. 5.
Fig. 4.
Total ion current (TIC) chromatograms of partially methylated alditol acetate (PMAA) derivatives of the kernel cell walls of (A) T. durum, (B) D. villosum, (C) Amphyploid 1, (D) T. aestivum; (E) Ae. Caudata, and (F) Amphyploid 2. Amphyploid 1 refers to the hybrid between T. durum and D. villosum. Amphyploid 2 denotes the hybrid between T. aestivum and Ae. caudata.
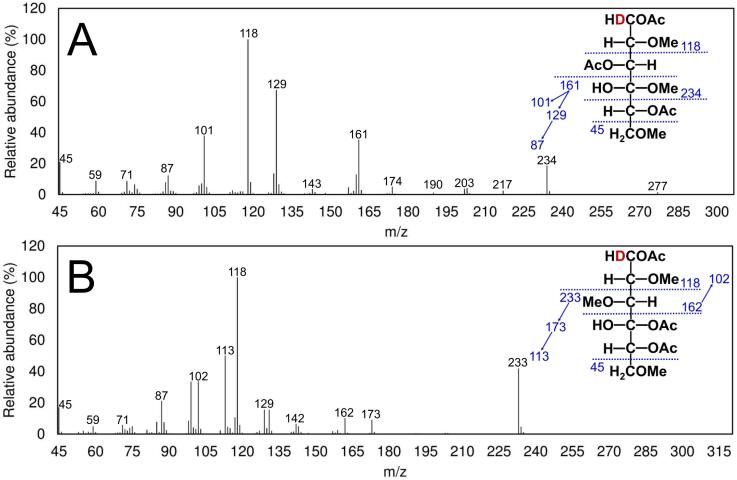
Fig. 5.
EI-MS fragmentation patterns of the partially methylated alditol acetates from (A) 1,3-linked glucopyranose and (B) 1,4-linked glucopyranose.
In addition to the linkages from MLG and cellulose, we also found in the samples many other linkages (Table 2) from various cell wall polysaccharide structures such as arabinoxylan, arabinan, heteroxylan, heteromannan, arabinogalactan, and homogalacturonan according to previous studies on linkage assignment of higher plant cell walls (Badhan et al., 2022, Li et al., 2022, Li et al., 2019, Low et al., 2020, Pettolino et al., 2012, Wood et al., 2018). The weak metanalysis (0.5 M methanolic HCl, 80 ˚C, 20 min)-sodium borodeuteride method is a quicker, less expensive carboxyl reduction method that allows larger sample amount (e.g., 10 mg of AIR) and larger throughput compared to the conventional carbodiimide activation-sodium borodeuteride method. This metanalysis-based method has been recently used for linkage analysis of some cell wall polysaccharide mixtures and purified fractions containing uronic acids (Chang et al., 2023, Robb et al., 2022, Voiges et al., 2012). This was the first instance of the method being used for carboxyl reduction of kernel cell walls of wheat and its wild relatives. This technique allowed the detection of galacturonic acid linkages in our samples. However, we noted that the method resulted in significant debranching of arabinoxylans. This was evident from the diminished intensity of various arabinofuranose linkages, a reduced presence of branching xylopyranosyl residues, and a pronounced peak of 4-linked xylopyranose residues (the building unit of the debranched xylan backbone). It was apparent that the methyl glycosides of arabinose, resulting from the debranching of arabinoxylan by weak methanolysis, were transformed into highly volatile permethylated methyl glycosides that were lost by repeated evaporation-to-dryness during methylation analysis. Given the minimal amounts of pectins in wheat and its wild relatives, we recommend bypassing the carboxyl reduction for kernel cell walls in future studies unless the primary objective is to extract pectin linkage information. If linkage details for both pectins and arabinoxylans are crucial for future studies, the traditional carbodiimide activation-sodium borodeuteride reduction method should be employed.
3.5. Correlation between CslF6 expression and MLG accumulation and the significance of kernel MLG content enhancement achieved through hybrid breeding
The synthesis of MLG in barley grains is influenced by the expression of CslF6, as demonstrated by the significant enhancement in MLG content resulting from endosperm-specific gene overexpression (Burton et al. in 2011). Similar trends have been observed in wheat and rice, where CslF6 orthologs play analogous roles (Nemeth et al., 2010). We analyzed the correlation between gene expression and MLG accumulation in our genetic material. We found that the correlation between CslF6 gene expression and MLG accumulation in amphyploid lines was slightly positive but not remarkable (p = 0.08, Pearson test). The expression level of CslF6 in the endosperm (32 DPA) of the durum wheat amphyploid was 3 times that of the durum wheat parent line (Fig. 1), while the kernel MLG content was moderately increased from 0.45 % in the parental line to 0.56 % in the hybrid, an enhancement of 24.4 % (Table 1). The kernel MLG content in the hybrid line of T. aestivum was 43.3 % higher than in the parent line (Table 1). In contrast, the CslF6 expression levels in the endosperm harvested at 24 and 32 DPA of the T. aestivum parental lines were 5.7 and 31.9 times those of the hybrid lines, respectively, indicating a strong negative correlation between the endosperm CslF6 expression and kernel MLG content. These observations could be attributed to the presence of a degradation complex, formed as a result of past breeding programs primarily aimed at enhancing wheat productivity. The final products of this complex serve as precursors for the biosynthesis and accumulation of starch in the kernel. This was supported by a previous report of a strong and inverse relationship between MLG and starch biosynthesis (Burton et al., 2008). It was also reported that MLG and amylose levels are controlled by numerous genes interconnected through expression networks, all contributing to the regulation of MLG and amylose metabolism and biosynthesis (Islamovic et al., 2013). The presence of a conserved 'switch motif' at the entrance of the enzyme's transmembrane channel plays a crucial role in the production of 1,3-linked β-d-glucopyranoses, and within this region, a single-point mutation can significantly impact formation of the 1,3-linkage, leading to increased synthesis of cellulosic polysaccharides (Purushotham, Ho, Yu, Fincher, Bulone, & Zimmer, 2022). Therefore, further investigation is needed to clarify the genetic mechanism and to study protein accumulation and structure. See (Table 3).
Table 3.
Glycosidic linkage composition (mol%) of different kernel cell walls with pretreatment of weak methanolysis (0.5 M methanolic HCl, 80 °C, 20 min)-sodium borodeuteride reduction.
| Linkage | TD | DY | A1 | TA | AC | A2 |
|---|---|---|---|---|---|---|
| t-Arap | 1.5 | 0.6 | 1.0 | 2.0 | 0.6 | 3.0 |
| t-Araf | 1.1 | 0.3 | 0.6 | 0.8 | 0.4 | 1.0 |
| 2-Araf | 0.8 | 0.2 | 0.4 | 0.4 | 0.3 | 0.6 |
| 3-Araf | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 |
| 5-Araf | 1.0 | 0.4 | 0.8 | 0.9 | 0.5 | 2.0 |
| t-GalAp | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 4-GalAp | 1.1 | 0.7 | 0.7 | 0.8 | 0.6 | 1.0 |
| t-Galp | 0.9 | 0.8 | 1.1 | 1.2 | 0.8 | 0.5 |
| 3-Galp | 1.3 | 0.2 | 1.7 | 1.7 | 0.2 | 0.1 |
| 4-Galp | 0.5 | 0.3 | 0.4 | 0.5 | 0.3 | 0.6 |
| 6-Galp | 0.8 | 0.5 | 0.6 | 0.7 | 0.6 | 0.4 |
| 3,6-Galp | 0.2 | 0.2 | 0.5 | 0.6 | 0.2 | 0.5 |
| t-GlcAp | 2.3 | 1.0 | 1.8 | 2.0 | 1.0 | 1.9 |
| t-Glcp | 1.5 | 3.7 | 2.5 | 2.0 | 3.4 | 1.7 |
| 3-Glcp | 2.2 | 12.8 | 3.1 | 3.6 | 10.4 | 2.4 |
| 4-Glcp | 40.5 | 45.6 | 42.0 | 42.0 | 45.9 | 40.1 |
| 6-Glcp | 0.4 | 0.2 | 0.3 | 0.4 | 0.3 | 0.3 |
| 2,4-Glcp | 0.3 | 0.3 | 0.4 | 0.3 | 0.3 | 0.3 |
| 3,4-Glcp | 0.3 | 0.3 | 0.6 | 0.3 | 0.4 | 0.2 |
| 3,6-Glcp | 0.1 | 0.3 | 0.4 | 0.2 | 0.3 | 0.2 |
| 4,6-Glcp | 0.5 | 0.2 | 0.6 | 0.4 | 0.2 | 0.5 |
| t-Manp | 0.3 | 0.2 | 0.5 | 0.4 | 0.3 | 0.4 |
| 4-Manp | 1.0 | 1.7 | 1.8 | 1.9 | 1.4 | 3.7 |
| t-Xylp | 4.1 | 2.3 | 4.1 | 4.4 | 2.9 | 4.9 |
| 2-Xylp | 2.5 | 1.3 | 2.2 | 2.5 | 1.5 | 2.8 |
| 4-Xylp | 30.8 | 23.5 | 28.4 | 26.6 | 24.9 | 26.4 |
| 2,4-Xylp | 1.7 | 0.6 | 1.3 | 1.2 | 0.6 | 1.6 |
| 3,4-Xylp | 0.7 | 0.3 | 0.8 | 0.5 | 0.4 | 0.8 |
| 2,3,4-Xylp | 1.5 | 1.1 | 1.3 | 1.6 | 1.1 | 1.6 |
Note: Each experiment was conducted in duplicate to generate a mean. TD: T. durum; DV: D. villosum; A1: Amphyploid 1; TA: T. aestivum; AC: Ae. caudata; A2: Amphyploid 2.
In the current study, disproving the existence of a remarkable positive correlation between CslF6 gene expression and MLG content in kernel does not diminish the significance of the MLG content enhancement by 24.4 % for durum wheat and 43.3 % for T. aestivum achieved through hybrid breeding. Wheat naturally contain less MLG than oat and barley (Tiefenbacher, 2017). However, people consume wheat products more than those of oat or barley. The inherent properties of MLG as a bioactive polysaccharide and dietary fiber and the omnipresence nature of wheat mean that even a slight increase in MLG content in wheat can significantly enhance the nutritional quality of the human diet. Enhancing MLG synthesis in wheat through innovative breeding techniques could lead to the production of wheat flour with the higher nutritional and functional quality that food processor seeks. This advancement would also elevate the potential of wheat bran, a byproduct of the milling process of wheat grain, as a material for the extraction and production of high-quality purified MLG, a value-added food additive in the industry (Maheshwari et al., 2017).
4. Conclusions
The results presented in this study shed light on the complex relationship between gene expression and MLG accumulation in wheat and its related species at various developmental stages and among different genotypes. While the expression of CslF6 has been previously associated with MLG content in grains, our findings suggested a relationship between CslF6 gene expression and MLG accumulation across different lines. Although the correlation was not strongly pronounced in amphyploid lines, the noteworthy increase in MLG content by 24.4 % in durum wheat and 43.3 % in T. aestivum through hybrid breeding presents promising opportunities for enhancing the nutritional profile of these staple grains. Given the global prominence of wheat in human diets, even slight increases in MLG content can lead to significant advancements in dietary quality. Our research paints an optimistic picture for the future of high MLG wheat breeding by introducing genes from wheat wild relatives. Moreover, the study offers insights into the composition and structural features of MLG and other non-starch cell wall polysaccharides in the kernels of wheats, their wild relatives, and hybrid lines between them, providing valuable data for upcoming research in this domain. The study also demonstrated that HPAEC-PAD and GC–MS-based glycomics can be potent tools for plant breeders to enhance the efficiency and accuracy of selecting high MLG wheat relatives and hybrid lines.
CRediT authorship contribution statement
Ilaria Marcotuli: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Xiaohui Xing: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Davide Caranfa: Formal analysis. Stefania L. Giove: Formal analysis. Yves S.Y. Hsieh: Writing – review & editing, Data curation. Shu-Chieh Chang: Formal analysis. D. Wade Abbott: Writing – review & editing, Writing – original draft, Data curation. Agata Gadaleta: Writing – review & editing, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Agata Gadaleta reports article publishing charges and equipment, drugs, or supplies were provided by university of Bari. Agata Gadaleta reports a relationship with University of Bari that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Research. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.Can-04-0496. [DOI] [PubMed] [Google Scholar]
- Bacic A., Moody S.F., Clarke A.E. Structural analysis of secreted root slime from maize (Zea mays L.) Plant Physiology. 1986;80(3):771–777. doi: 10.1104/pp.80.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhan A., Low K.E., Jones D.R., Xing X., Milani M.R.M., Polo R.O., McAllister T.A. Mechanistic insights into the digestion of complex dietary fibre by the rumen microbiota using combinatorial high-resolution glycomics and transcriptomic analyses. Computational and Structural Biotechnology Journal. 2022;20:148–164. doi: 10.1016/j.csbj.2021.12.009. https://www.sciencedirect.com/science/article/pii/S2001037021005171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain, M., van de Meene, A., Costa, R., & Doblin, M. S. Characterisation of Cellulose Synthase Like F6 (CslF6) Mutants Shows Altered Carbon Metabolism in β-D-(1,3;1,4)-Glucan Deficient Grain in Brachypodium distachyon. (1664-462X (Print)). [DOI] [PMC free article] [PubMed]
- Bain M., van de Meene A., Costa R., Doblin M.S. Characterisation of cellulose synthase like F6 (CslF6) mutants shows altered carbon metabolism in beta-D-(1,3;1,4)-glucan deficient grain in brachypodium distachyon. Front Plant Sci. 2020;11 doi: 10.3389/fpls.2020.602850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres B.L., Rahmani E., Clarke J.M., Grassini P., Pozniak C.J., Geddes C.M., Ransom J.K. A systematic review of durum wheat: Enhancing production systems by exploring genotype, environment, and management (G × E × M) synergies. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.568657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford G., Stone B.A. (1→3), (1→4)-β-D-glucan content of Triticum grains. Journal of Cereal Science. 1983;1(2):111–114. doi: 10.1016/S0733-5210(83)80028-9. [DOI] [Google Scholar]
- Bertaud F., Sundberg A., Holmbom B. Evaluation of acid methanolysis for analysis of wood hemicelluloses and pectins. Carbohydrate Polymers. 2002;48(3):319–324. https://www.sciencedirect.com/science/article/pii/S0144861701002491 [Google Scholar]
- Bhoite, R., Satyavrat, V., & Premasudha Sadananda, M. Clinical benefits of β-glucan supplementation in children: a review. LID - 37. (2731-4286 (Electronic)).
- Biswal A.K., Hengge N.N., Black I.M., Atmodjo M.A., Mohanty S.S., Ryno D., Mohnen D. Composition and yield of non-cellulosic and cellulosic sugars in soluble and particulate fractions during consolidated bioprocessing of poplar biomass by Clostridium thermocellum. Biotechnology for Biofuels and Bioproducts. 2022;15(1):23. doi: 10.1186/s13068-022-02119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I., Heiss C., Azadi P. Comprehensive monosaccharide composition analysis of insoluble polysaccharides by permethylation to produce methyl alditol derivatives for gas chromatography/mass spectrometry. Analytical Chemistry. 2019;91(21):13787–13793. doi: 10.1021/acs.analchem.9b03239. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Collins H.M., Kibble N.A.J., Smith J.A., Shirley N.J., Jobling S.A., Fincher G.B. Over-expression of specific HvCslF cellulose synthase-like genes in transgenic barley increases the levels of cell wall (1,3;1,4)-β-d-glucans and alters their fine structure. Plant Biotechnology Journal. 2011;9(2):117–135. doi: 10.1111/j.1467-7652.2010.00532.x. [DOI] [PubMed] [Google Scholar]
- Burton R.A., Jobling S.A., Harvey A.J., Shirley N.J., Mather D.E., Bacic A., Fincher G.B. The genetics and transcriptional profiles of the cellulose synthase-like HvCslF gene family in barley. Plant Physiology. 2008;146(4):1821–1833. doi: 10.1104/pp.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R.A., Wilson S.M., Hrmova M., Harvey A.J., Shirley N.J., Medhurst A., Fincher G.B. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science. 2006;311(5769):1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
- Carpita, N. C., & Shea, E. M. (1989). Linkage structure of carbohydrates by gas chromatography-mass spectrometry (GC-MS) of partially methylated alditol acetates. In B. C.J. & G. D. McGinnis (Eds.), Analysis of Carbohydrates by GLC and MS (pp. 157-216). Boca Raton, Florida: CRC Press, Inc.
- Casillo A., D’Angelo C., Parrilli E., Tutino M.L., Corsaro M.M. Membrane and extracellular matrix glycopolymers of Colwellia psychrerythraea 34H: Structural changes at different growth temperatures. Frontiers in Microbiology. 2022;13 doi: 10.3389/fmicb.2022.820714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.-C., Kao M.-R., Saldivar R.K., Díaz-Moreno S.M., Xing X., Furlanetto V., Hsieh Y.S.Y. The Gram-positive bacterium Romboutsia ilealis harbors a polysaccharide synthase that can produce (1,3;1,4)-β-D-glucans. Nature Communications. 2023;14(1):4526. doi: 10.1038/s41467-023-40214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong H.H., Cleary M.T., Dokoozlian N., Ford C.M., Fincher G.B. Soluble cell wall carbohydrates and their relationship with sensory attributes in Cabernet Sauvignon wine. Food Chemistry. 2019;298 doi: 10.1016/j.foodchem.2019.05.020. https://www.sciencedirect.com/science/article/pii/S0308814619308155 [DOI] [PubMed] [Google Scholar]
- Ciucanu I. Per-O-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Analytica Chimica Acta. 2006;576(2):147–155. doi: 10.1016/j.aca.2006.06.009. http://www.sciencedirect.com/science/article/pii/S0003267006012372 [DOI] [PubMed] [Google Scholar]
- Collins H.M., Burton R.A., Topping D.L., Liao M.-L., Bacic A., Fincher G.B. Variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: Potential importance in human health and nutrition. Cereal Chemistry. 2010;87(4):272–282. doi: 10.1094/cchem-87-4-0272. [DOI] [Google Scholar]
- Cory A.T., Båga M., Anyia A., Rossnagel B.G., Chibbar R.N. Genetic markers for CslF6 gene associated with (1,3;1,4)-β-glucan concentration in barley grain. Journal of Cereal Science. 2012;56(2):332–339. https://www.sciencedirect.com/science/article/pii/S0733521012000446 [Google Scholar]
- De Pace, C., Bizzarri, M., Vittori, D., Vaccino, P., Caceres, M. E., Ceccarelli, M., . . . Vida, G. (2014). The progeny from the [(T. turgidum X Dasypyrum villosum) amphiploid X Triticum aestivum] hybridization is an effective source of new durum wheat inbred lines. In E. Porceddu, C. O. Qualset & A. B. Damania (Eds.), Proceedings of the International Symposium on Genetics and breeding of durum wheat (pp. 189-200): Bari : CIHEAM.
- Doblin M.S., Pettolino F.A., Wilson S.M., Campbell R., Burton R.A., Fincher G.B., Bacic A. A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis. Proceedings of the National Academy of Sciences. 2009;106(14):5996–6001. doi: 10.1073/pnas.0902019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi N., Burton R.A., Brownfield L., Hrmova M., Wilson S.M., Bacic A., Fincher G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnology Journal. 2006;4(2):145–167. doi: 10.1111/j.1467-7652.2005.00169.x. [DOI] [PubMed] [Google Scholar]
- Feuillet C., Langridge P., Waugh R. Cereal breeding takes a walk on the wild side. Trends in Genetics. 2008;24(1):24–32. doi: 10.1016/j.tig.2007.11.001. https://www.sciencedirect.com/science/article/pii/S0168952507003484 [DOI] [PubMed] [Google Scholar]
- Fincher G.B., Stone B.A. In: Encyclopedia of Grain Science. Wrigley C., editor. Elsevier; Oxford: 2004. Chemistry of nonstarch polysaccharides; pp. 206–223. [Google Scholar]
- Foster C.E., Martin T.M., Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. Journal of Visual Experiments. 2010;37 doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francin-Allami M., Bouder A., Geairon A., Alvarado C., Le-Bot L., Daniel S., Sibout R. Mixed-linkage glucan is the main carbohydrate source and starch is an alternative source during Brachypodium grain germination. International Journal of Molecular Sciences. 2023;24(7) doi: 10.3390/ijms24076821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottstein V., Bernhardt M., Dilger E., Keller J., Breitling-Utzmann C.M., Schwarz S., Bunzel M. Coffee silver skin: Chemical characterization with special consideration of dietary fiber and heat-induced contaminants. Foods. 2021;10(8):1705. doi: 10.3390/foods10081705. https://www.mdpi.com/2304-8158/10/8/1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant C.A., Cubadda F., Carcea M., Pogna N.E., Gazza L. In: Durum Wheat: Chemistry and Technology. 2nd edition: Sissons M., Abécassis J., Marchylo B., Carcea M., editors. AACC; 2012. Vitamins, minerals, and nutritional value of durum wheat. [Google Scholar]
- Grewal S., Othmeni M., Walker J., Hubbart-Edwards S., Yang C.-Y., Scholefield D., King J. Development of wheat-Aegilops caudata introgression lines and their characterization using genome-specific KASP markers. Frontiers in Plant Science. 2020;11 doi: 10.3389/fpls.2020.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosain N.A., Ghosh R., Bryant D.L., Arivett B.A., Farone A.L., Kline P.C. Isolation, structure elucidation, and immunostimulatory activity of polysaccharide fractions from Boswellia carterii frankincense resin. International Journal of Biological Macromolecules. 2019;133:76–85. doi: 10.1016/j.ijbiomac.2019.04.059. https://www.sciencedirect.com/science/article/pii/S0141813019301837 [DOI] [PubMed] [Google Scholar]
- Houston K., Russell J., Schreiber M., Halpin C., Oakey H., Washington J.M., Booth A., Shirley N., Burton R.A., Fincher G.B., Waugh R. A genome wide association scan for (1,3;1,4)-β- glucan content in the grain of contemporary 2-row Spring and Winter barleys. BMC Genomics. 2014;15(1):907. doi: 10.1186/1471-2164-15-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G., Burton C., Hong Z., Jackson E. A mutation of the cellulose-synthase-like (CslF6) gene in barley (Hordeum vulgare L.) partially affects the β-glucan content in grains. Journal of Cereal Science. 2014;59(2):189–195. doi: 10.1016/j.jcs.2013.12.009. [DOI] [Google Scholar]
- Islamovic E., Obert D.E., Oliver R.E., Marshall J.M., Miclaus K.J., Hang A., Jackson E.W. A new genetic linkage map of barley (Hordeum vulgare L.) facilitates genetic dissection of height and spike length and angle. Field Crops Research. 2013;154:91–99. https://www.sciencedirect.com/science/article/pii/S0378429013002104 [Google Scholar]
- Ivanizs L., Marcotuli I., Rakszegi M., Kalapos B., Szőke-Pázsi K., Farkas A., Molnár I. Identification of New QTLs for Dietary Fiber Content in Aegilops biuncialis. International Journal of Molecular Sciences. 2022;23(7) doi: 10.3390/ijms23073821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G., Vasanthan T. MALDI-MS and HPLC quantification of oligosaccharides of lichenase-hydrolyzed water-soluble β-glucan from ten barley varieties. Journal of Agricultural and Food Chemistry. 2000;48(8):3305–3310. doi: 10.1021/jf0001278. [DOI] [PubMed] [Google Scholar]
- Jones D.R., Xing X., Tingley J.P., Klassen L., King M.L., Alexander T.W., Abbott D.W. Analysis of active site architecture and reaction product linkage chemistry reveals a conserved cleavage substrate for an endo-alpha-mannanase within diverse yeast mannans. Journal of Molecular Biology. 2020;432(4):1083–1097. doi: 10.1016/j.jmb.2019.12.048. http://www.sciencedirect.com/science/article/pii/S0022283620300346 [DOI] [PubMed] [Google Scholar]
- Kim J.B., Carpita N.C. Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiology. 1992;98(2):646–653. doi: 10.1104/pp.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishii M. An update of recent use of Aegilops species in wheat breeding. Frontiers in Plant Science. 2019;10 doi: 10.3389/fpls.2019.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen L., Reintjes G., Li M., Jin L., Amundsen C., Xing X., Abbott D.W. Fluorescence activated cell sorting and fermentation analysis to study rumen microbiome responses to administered live microbials and yeast cell wall derived prebiotics. Frontiers in Microbiology. 2023;13 doi: 10.3389/fmicb.2022.1020250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen L., Xing X., Tingley J.P., Low K.E., King M.L., Reintjes G., Abbott D.W. Approaches to investigate selective dietary polysaccharide utilization by human gut microbiota at a functional level. Frontiers in Microbiology. 2021;12(308) doi: 10.3389/fmicb.2021.632684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lante A., Canazza E., Tessari P. Beta-glucans of cereals: Functional and technological properties. Nutrients. 2023;15(9):2124. doi: 10.3390/nu15092124. https://www.mdpi.com/2072-6643/15/9/2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Hsiung S.-Y., Kao M.-R., Xing X., Chang S.-C., Wang D., Hsieh Y.S.Y. Structural compositions and biological activities of cell wall polysaccharides in the rhizome, stem, and leaf of Polygonatum odoratum (Mill.) Druce. Carbohydrate Research. 2022;521 doi: 10.1016/j.carres.2022.108662. https://www.sciencedirect.com/science/article/pii/S000862152200163X [DOI] [PubMed] [Google Scholar]
- Li J., Wang D., Xing X., Cheng T.-J.-R., Liang P.-H., Bulone V., Hsieh Y.S.Y. Structural analysis and biological activity of cell wall polysaccharides extracted from Panax ginseng marc. International Journal of Biological Macromolecules. 2019;135:29–37. doi: 10.1016/j.ijbiomac.2019.05.077. http://www.sciencedirect.com/science/article/pii/S014181301931150X [DOI] [PubMed] [Google Scholar]
- Little A., Lahnstein J., Jeffery D.W., Khor S.F., Schwerdt J.G., Shirley N.J., Bulone V. A novel (1,4)-β-linked glucoxylan is synthesized by members of the cellulose synthase-like F gene family in land plants. ACS Central Science. 2019;5(1):73–84. doi: 10.1021/acscentsci.8b00568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K.E., Xing X., Moote P.E., Inglis G.D., Venketachalam S., Hahn M.G., Abbott D.W. Combinatorial glycomic analyses to direct CAZyme discovery for the tailored degradation of canola meal non-starch dietary polysaccharides. Microorganisms. 2020;8(12):1888. doi: 10.3390/microorganisms8121888. https://www.mdpi.com/2076-2607/8/12/1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari G., Sowrirajan S., Joseph B. Extraction and isolation of β-glucan from grain sources—A review. Journal of Food Science. 2017;82(7):1535–1545. doi: 10.1111/1750-3841.13765. [DOI] [PubMed] [Google Scholar]
- Marcotuli I., Houston K., Schwerdt J.G., Waugh R., Fincher G.B., Burton R.A., Blanco A., Gadaleta A. Genetic diversity and genome wide association study of β-glucan content in tetraploid wheat grains. PLoS ONE. 2016;11(4):e0152590. doi: 10.1371/journal.pone.0152590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotuli I., Colasuonno P., Blanco A., Gadaleta A. Expression analysis of cellulose synthase-like genes in durum wheat. Scientific Reports. 2018;8(1):15675. doi: 10.1038/s41598-018-34013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotuli I., Colasuonno P., Hsieh Y.S.Y., Fincher G.B., Gadaleta A. Non-starch polysaccharides in durum wheat: A review. International Journal of Molecular Sciences. 2020;21(8):2933. doi: 10.3390/ijms21082933. https://www.mdpi.com/1422-0067/21/8/2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotuli I., Houston K., Waugh R., Fincher G.B., Burton R.A., Blanco A., Gadaleta A. Genome wide association mapping for arabinoxylan content in a collection of tetraploid wheats. PLoS ONE. 2015;10(7):e0132787. doi: 10.1371/journal.pone.0132787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotuli I., Hsieh Y.S.Y., Lahnstein J., Yap K., Burton R.A., Blanco A., Gadaleta A. Structural variation and vontent of arabinoxylans in endosperm and bran of durum wheat (Triticum turgidum L.) Journal of Agricultural and Food Chemistry. 2016;64(14):2883–2892. doi: 10.1021/acs.jafc.6b00103. [DOI] [PubMed] [Google Scholar]
- Marcotuli I., Soriano J.M., Gadaleta A. A consensus map for quality traits in durum wheat based on genome-wide association studies and detection of ortho-meta QTL across cereal species. Frontiers in Genetics. 2022;13 doi: 10.3389/fgene.2022.982418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews R., Kamil A., Chu Y. Global review of heart health claims for oat beta-glucan products. Nutrition Reviews. 2020;78(Supplement_1):78–97. doi: 10.1093/nutrit/nuz069. [DOI] [PubMed] [Google Scholar]
- McCleary B.V., Codd R. Measurement of (1 → 3), (1 → 4)-β-D-glucan in barley and oats: A streamlined enzymic procedure. Journal of the Science of Food and Agriculture. 1991;55(2):303–312. doi: 10.1002/jsfa.2740550215. [DOI] [Google Scholar]
- Miller S.S., Vincent D.J., Weisz J., Fulcher R.G. Oat β-glucans: An evaluation of eastern Canadian cultivars and unregistered lines. Canadian Journal of Plant Science. 1993;73:429–436. [Google Scholar]
- Muhidinov Z.K., Bobokalonov J.T., Ismoilov I.B., Strahan G.D., Chau H.K., Hotchkiss A.T., Liu L. Characterization of two types of polysaccharides from Eremurus hissaricus roots growing in Tajikistan. Food Hydrocolloids. 2020;105 https://www.sciencedirect.com/science/article/pii/S0268005X19316777 [Google Scholar]
- Nemeth C., Freeman J., Jones H.D., Sparks C., Pellny T.K., Wilkinson M.D., Shewry P.R. Down-regulation of the CSLF6 gene results in decreased (1,3;1,4)-β-D-glucan in endosperm of wheat. Plant Physiology. 2010;152(3):1209–1218. doi: 10.1104/pp.109.151712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Cornuault V., Marcus S.E., Knox J.P., Shewry P.R., Tosi P. Comparative in situ analyses of cell wall matrix polysaccharide dynamics in developing rice and wheat grain. Planta. 2015;241(3):669–685. doi: 10.1007/s00425-014-2201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettolino F.A., Walsh C., Fincher G.B., Bacic A. Determining the polysaccharide composition of plant cell walls. Nature Protocols. 2012;7(9):1590–1607. doi: 10.1038/nprot.2012.081. [DOI] [PubMed] [Google Scholar]
- Pham T.A.T., Kyriacou B.A., Schwerdt J.G., Shirley N.J., Xing X., Bulone V., Little A. Composition and biosynthetic machinery of the Blumeria graminis f. sp. hordei conidia cell wall. The Cell Surface. 2019;5 doi: 10.1016/j.tcsw.2019.100029. http://www.sciencedirect.com/science/article/pii/S2468233019300076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham T.A.T., Schwerdt J.G., Shirley N.J., Xing X., Hsieh Y.S.Y., Srivastava V., Little A. Analysis of cell wall synthesis and metabolism during early germination of Blumeria graminis f. sp. hordei conidial cells induced in vitro. The Cell Surface. 2019;5 doi: 10.1016/j.tcsw.2019.100030. http://www.sciencedirect.com/science/article/pii/S2468233019300088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushotham P., Ho R., Yu L., Fincher G.B., Bulone V., Zimmer J. Mechanism of mixed-linkage glucan biosynthesis by barley cellulose synthase–like CslF6 (1,3;1,4)-β-glucan synthase. Science Advances. 2022;8(45) doi: 10.1126/sciadv.add1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakszegi M., Molnár I., Lovegrove A., Darkó É., Farkas A., Láng L., Shewry P. Addition of Aegilops U and M chromosomes affects protein and dietary fiber content of wholemeal wheat flour. Frontiers in Plant Science. 2017;8 doi: 10.3389/fpls.2017.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb C.S., Hobbs J.K., Pluvinage B., Reintjes G., Klassen L., Monteith S., Boraston A.B. Metabolism of a hybrid algal galactan by members of the human gut microbiome. Nature Chemical Biology. 2022;18(5):501–510. doi: 10.1038/s41589-022-00983-y. [DOI] [PubMed] [Google Scholar]
- Schäfer J., Hale J., Hoffmann C.M., Bunzel M. Mechanical properties and compositional characteristics of beet (Beta vulgaris L.) varieties and their response to nitrogen application. European Food Research and Technology. 2020;246(10):2135–2146. doi: 10.1007/s00217-020-03562-4. [DOI] [Google Scholar]
- Schneider A., Molnár I., Molnár-Láng M. Utilisation of Aegilops (goatgrass) species to widen the genetic diversity of cultivated wheat. Euphytica. 2008;163(1):1–19. doi: 10.1007/s10681-007-9624-y. [DOI] [Google Scholar]
- Smith P.J., O’Neill M.A., Backe J., York W.S., Peña M.J., Urbanowicz B.R. Analytical techniques for determining the role of domain of unknown function 579 proteins in the synthesis of O-methylated plant polysaccharides. SLAS Technology: Translating Life Sciences Innovation. 2020;25(4):345–355. doi: 10.1177/2472630320912692. [DOI] [PubMed] [Google Scholar]
- Sopiwnyk E. Sustainable Production of Durum Wheat in Canada. Barilla America Inc; Bannockburn, IL: 2018. Durum production and consumption, a global perspective; pp. 5–9. [Google Scholar]
- Sowinski E.E., Gilbert S., Lam E., Carpita N.C. Linkage structure of cell-wall polysaccharides from three duckweed species. Carbohydrate Polymers. 2019;223 doi: 10.1016/j.carbpol.2019.115119. http://www.sciencedirect.com/science/article/pii/S0144861719307866 [DOI] [PubMed] [Google Scholar]
- Storsley J.M., Izydorczyk M.S., You S., Biliaderis C.G., Rossnagel B. Structure and physicochemical properties of β-glucans and arabinoxylans isolated from hull-less barley. Food Hydrocolloids. 2003;17(6):831–844. https://www.sciencedirect.com/science/article/pii/S0268005X03001048 [Google Scholar]
- Sun L., Hu M., Zhao J., Lv L., Zhang Y., Liu Q., Zhang Y. Molecular characteristics, synthase, and food application of cereal β-glucan. Journal of Food Quality. 2021;2021:6682014. doi: 10.1155/2021/6682014. [DOI] [Google Scholar]
- Sushytskyi L., Synytsya A., Čopíková J., Lukáč P., Rajsiglová L., Tenti P., Vannucci L.E. Perspectives in the application of high, medium, and low molecular weight oat β-D-glucans in dietary nutrition and food technology-A short overview. Foods. 2023;12(6):1121. doi: 10.3390/foods12061121. https://www.mdpi.com/2304-8158/12/6/1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketa S., Yuo T., Tonooka T., Tsumuraya Y., Inagaki Y., Haruyama N., Jobling S.A. Functional characterization of barley betaglucanless mutants demonstrates a unique role for CslF6 in (1,3;1,4)-β-D-glucan biosynthesis. Journal of Experimental Botany. 2012;63(1):381–392. doi: 10.1093/jxb/err285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiefenbacher K.F. In: Wafer and Waffle. Tiefenbacher K.F., editor. Academic Press; 2017. Technology of main Ingredients-water and flours; pp. 15–121. [Google Scholar]
- Tingley J.P., Low K.E., Xing X., Abbott D.W. Combined whole cell wall analysis and streamlined in silico carbohydrate-active enzyme discovery to improve biocatalytic conversion of agricultural crop residues. Biotechnology for Biofuels. 2021;14(1):16. doi: 10.1186/s13068-020-01869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonooka T., Aoki E., Yoshioka T., Taketa S. A novel mutant gene for (1–3, 1–4)-β-D-glucanless grain on barley (Hordeum vulgare L.) chromosome 7H. Breeding Science. 2009;59(1):47–54. doi: 10.1270/jsbbs.59.47. [DOI] [Google Scholar]
- Vega-Sanchez M.E., Verhertbruggen Y., Christensen U., Chen X., Sharma V., Varanasi P., Ronald P.C. Loss of cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiology. 2012;159(1):56–69. doi: 10.1104/pp.112.195495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voiges K., Adden R., Rinken M., Mischnick P. Critical re-investigation of the alditol acetate method for analysis of substituent distribution in methyl cellulose. Cellulose. 2012;19(3):993–1004. doi: 10.1007/s10570-012-9663-y. [DOI] [Google Scholar]
- Willför S., Pranovich A., Tamminen T., Puls J., Laine C., Suurnäkki A., Holmbom B. Carbohydrate analysis of plant materials with uronic acid-containing polysaccharides–A comparison between different hydrolysis and subsequent chromatographic analytical techniques. Industrial Crops and Products. 2009;29(2):571–580. https://www.sciencedirect.com/science/article/pii/S0926669008002082 [Google Scholar]
- Wilson L.A., Deligey F., Wang T., Cosgrove D.J. Saccharide analysis of onion outer epidermal walls. Biotechnology for Biofuels. 2021;14(1):66. doi: 10.1186/s13068-021-01923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.C., Shirley N.J., Little A., Khoo K.H.P., Schwerdt J., Fincher G.B., Mather D.E. Differential expression of the HvCslF6 gene late in grain development may explain quantitative differences in (1,3;1,4)-β-glucan concentration in barley. Molecular Breeding. 2015;35(1):20. doi: 10.1007/s11032-015-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J.A., Tan H.T., Collins H.M., Yap K., Khor S.F., Lim W.L., Tucker M.R. Genetic and environmental factors contribute to variation in cell wall composition in mature desi chickpea (Cicer arietinum L.) cotyledons. Plant, Cell & Environment. 2018;41(9):2195–2208. doi: 10.1111/pce.13196. [DOI] [PubMed] [Google Scholar]
- Yu L., Yakubov G.E., Zeng W., Xing X., Stenson J., Bulone V., Stokes J.R. Multi-layer mucilage of Plantago ovata seeds: Rheological differences arise from variations in arabinoxylan side chains. Carbohydrate Polymers. 2017;165:132–141. doi: 10.1016/j.carbpol.2017.02.038. http://www.sciencedirect.com/science/article/pii/S0144861717301583 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.