Abstract
Introduction and importance
Catamenial pneumothorax (CP) is a condition characterized by recurrent spontaneous pneumothoraxes in women, in temporal pattern with menstrual bleeding. This report presents two cases of CP from a family of three sisters affected by this condition.
Case presentation
The first case involved a 38-year-old woman with a history of recurrent pneumothoraxes. Following the diagnosis of CP, she underwent wedge resection with video-assisted thoracoscopic surgery (VATS). However, due to recurrence and the discovery of a diaphragmatic endometrial nodule, she required nodule excision and diaphragm reconstruction through open thoracotomy.
The second case concerned a 47-year-old woman who had a single episode of pneumothorax. After being diagnosed with CP, she also underwent VATS. During the procedure, multiple endometrial diaphragmatic lesions were found, necessitating excision via open thoracotomy. Despite the surgery, the pneumothorax reoccurred, which was managed with a hysterectomy and bilateral salpingo-oophorectomy. At the one-year follow-up, both patients were well.
Clinical discussion
CP is a rare disorder with unclear pathophysiology. Additionally, the impact of genetic factors has not been fully evaluated, and familial cases are rarely reported.
Conclusion
We believe that this report of CP in three sisters suggests a potential genetic role in its occurrence, necessitating further investigation. A better understanding of genetic factors may improve our knowledge of the pathophysiology of CP, ultimately aiding in the management of the condition. We also want to emphasize the importance of collaboration between thoracic surgeons and gynecologists in managing CP, as well as improving prevention of its recurrence.
Keywords: Catamenial pneumothorax, Spontaneous pneumothorax, Recurrent pneumothorax, Thoracic endometriosis syndrome, Video-assisted thoracoscopic surgery, Thoracotomy
Highlights
-
•
The role of genetics in catamenial pneumothorax pathophysiology remains unclear.
-
•
Genetic factors may contribute to catamenial pneumothorax.
-
•
Thorough diaphragm inspection is essential for treating catamenial pneumothorax.
-
•
Small diaphragmatic lesions can lead to disease recurrence.
-
•
Collaboration between thoracic surgeons and gynecologists helps prevent recurrence.
1. Introduction
Catamenial pneumothorax (CP), a condition that was first reported in 1958 [1], is referred to recurrent spontaneous pneumothoraxes in women of reproductive age, in temporal relation with menstrual bleeding [2]. “Catamenial” a Greek word meaning “Monthly”, was used for describing this condition by Lillington et al. [3,4]. The criteria established by Lillington for CP includes: (I) a close temporal relation with menstrual bleeding, (II) involvement of right hemithorax, (III) no occurrence of pneumothorax in other parts of menstrual cycle, (IV) absence of pneumothorax recurrence during ovulation-suppressing treatment or pregnancy, and (V) relatively late onset of disease [3]. However, the definition of CP has changed. In literature CP frequently described as recurrent pneumothorax occurring within 24 h before to 72 h after onset of menstrual bleeding [2,4]. While broader period have been introduced, with the broadest defining the CP period within a week before the onset of menstrual bleeding to a week later its end [5]. Regarding the location of pneumothorax, around 93 % of reported cases [6], pneumothorax involved the right side, while there were CP patients with left-sided [7] or even bilateral [8,9] involvement.
Here we present two sisters diagnosed with CP, suspected to be caused by genetic factors, and treated at this center. They also have an older sister who was previously diagnosed and treated for CP at another center. We would also like to mention that these two cases are reported in line with the SCARE guideline [10].
2. Case presentation
2.1. Case 1
The patient was a thirty-eight year-old woman referred to our clinics, after the fourth occurrence of right-sided pneumothorax. She had no comorbidities, no history of infertility, with past surgical history of cesarean section and appendectomy. Patient was a non-smoker. Notably, there was history of CP in her older sister. She explained that previous pneumothoraxes diagnosed after chest pain, starting at two to three days before onset of menstrual bleedings. Patient became a candidate for video-assisted thoracoscopic surgery (VATS), however, a few days before the date of VATS, she came to emergency room complaining from chest pain predominantly at right hemithorax and dyspnea. Patient was stable. Physical examination revealed decreased breath sounds on the right side, suggesting a recurrence of pneumothorax, which later confirmed by both chest radiography and a chest computed tomography (CT) scan (Fig. 1), which led to the placement of a chest tube.
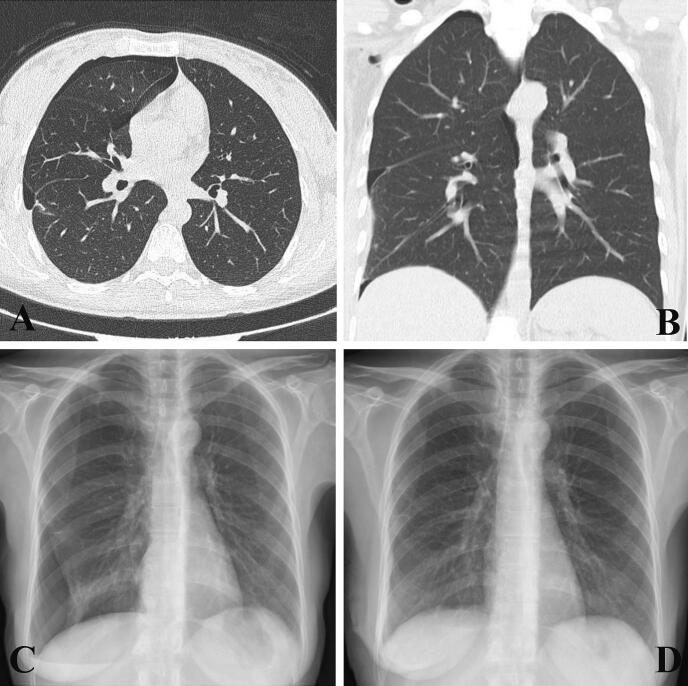
Fig. 1.
Evidence of a right pneumothorax in patient 1 (first pneumothorax at this center) shown on imaging: (A) Chest CT scan, axial view; and (B) chest CT scan, coronal view; (C) chest radiograph, PA view. (D) Normal chest radiography with no evidence of pneumothorax after VATS, taken at discharge.
Afterward, patient underwent VATS from the right side with ports in the lateral 7th intercostal space, anterior and posterior 5th intercostal space. A thorough inspection of the chest cavity revealed brownish nodules on the apex of the right upper lobe. After pneumolysis, a wedge resection of the apex of the right upper lobe was performed using two staplers, followed by parietal pleurectomy. Following scarification and the placement of two size 28 chest tubes, the patient was transferred to the recovery room. Pathology evaluation determined that the resected nodules were endometrial lesions and also demonstrated emphysematous lung tissue, slight patchy interstitial thickening in the resected part of the lung, and inflammation in the parietal pleura and lung bulla.
During post-operative hospitalization patient showed no complications, serial examination was normal and follow-up CT scan showed no evidence of pneumothorax. Therefore, the chest tube was removed and patient was discharged (Fig. 1.D). There were no problems in the follow-up visits during two months post-operation, encompassing two menstrual cycles. In the fourth month after surgery, two days before menstrual bleeding, patient came to emergency room with chest pain, which later diagnosed with the recurrence of pneumothorax. A size 24 chest tube was administrated at sixth intercostal space at midaxillary line, which further removed after four days. Again, one month later patient admitted to emergency room with similar symptoms. After got treated with chest tube for the seventh occurrence of pneumothorax (Fig. 2.A and B), it was decided to presume treatment with open thoracotomy.
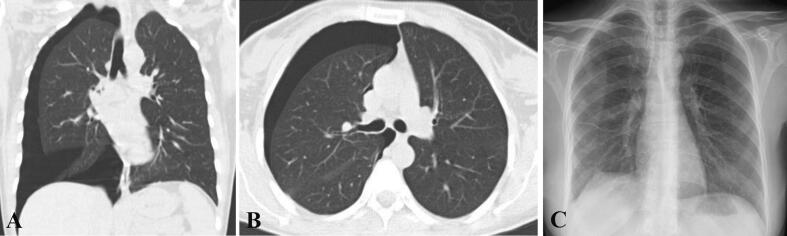
Fig. 2.
Evidence of a second recurrence of pneumothorax after VATS (seventh pneumothorax overall) in patient 1, shown on Chest CT scan: (A) Coronal view and (B) axial view. (C) Normal chest radiography with no evidence of pneumothorax after VATS, taken at discharge.
The thoracotomy was performed with posterolateral approach from right fifth intercostal space. The initial investigation of chest cavity revealed nodule in the right lower lobe and also two cribriform area on the right diaphragm with fenestration to the abdominal cavity. Following the pneumolysis, the nodule was excised with wedge resection. Also, the two mentioned areas on the diaphragm were excised. After complete parietal pleurectomy, the diaphragm reconstruction performed in two layers. At last, two chest tubes were employed. During 10 days of hospitalization, there was no complication. The patient was discharged after chest tube removal (Fig. 2.C).
In the routine monthly follow-up visits, there was no complication and patient only mentioned occasional and positional chest pain, unrelated to menstrual bleedings, at the site of thoracotomy incision.
In the last follow-up, near one and half year after thoracotomy, there was no recurrence of pneumothorax.
2.2. Case 2
The second patient was a forty-seven-year-old non-smoker woman with a medical history of diabetes mellitus, hypothyroidism, and a single episode of right pneumothorax. She was referred to this center complaining of chest pain in the right hemithorax that had started 3–4 days prior, radiating to the right arm, and also experiencing exertional dyspnea. She had a family history of catamenial pneumothorax in both her sisters. Physical examination revealed a slight decrease in breath sounds in the right lung, but her vital signs were stable. A chest CT scan confirmed the diagnosis of non-massive right pneumothorax (Fig. 3.A and B).
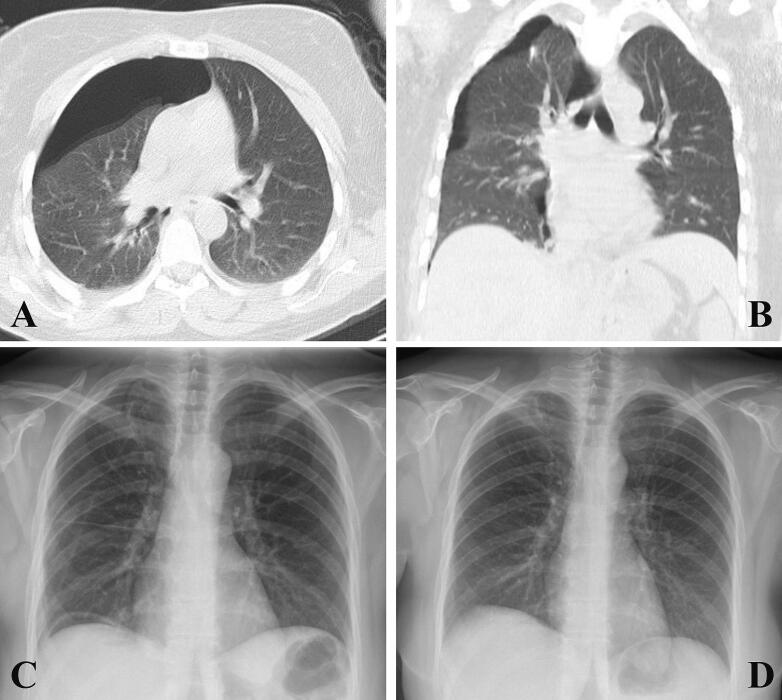
Fig. 3.
Right pneumothorax in patient 2 (first pneumothorax at this center) shown on imaging: (A) Chest CT scan, axial view; (B) chest CT scan, coronal view. (C) Normal chest radiography with no evidence of pneumothorax after VATS, taken at discharge. (D) Normal chest radiography with no evidence of recurrence at the one-year follow-up.
The patient underwent VATS from the right posterior lateral 6th intercostal space. During VATS inspection, multiple endometrial lesions were observed on the tendinous part of the right diaphragm, necessitating conversion to an open thoracotomy to provide better access. The operation continued after thoracotomy from the right posterolateral 6th intercostal space. Pleurectomy was performed, and the endometrial lesions were resected, followed by diaphragmatic reconstruction in two layers. Additionally, a wedge resection of the right upper lobe was performed, and surgery was ended after scarification.
The pathological evaluation of the resected lesions showed evidence of endometriosis and fibromuscular tissue with areas of focal hypercellularity.
During post-operative hospitalization, there were no complications. The patient was discharged 10 days after surgery (Fig. 3.C).
Unfortunately, 10 days after discharge, during the next menstrual cycle, the patient came to the emergency room complaining of dyspnea and right chest pain. A chest CT scan demonstrated the recurrence of right pneumothorax. Therefore, a size 28 chest tube was administered in the right fifth intercostal space. Due to the lack of a gynecologist in our center, a gynecology consultation was done over the phone, which advised referring the patient for further evaluation. After 10 days of hospitalization, the chest tube was removed, and patient referred to gynecologist. The gynecological evaluation revealed endometrial involvement of the uterus and both ovaries, leading to a hysterectomy and bilateral salpingo-oophorectomy. At the one-year follow-up, the patient remained well without any recurrence of pneumothorax (Fig. 3.D).
3. Discussion
In this paper we presented two sisters with CP with positive family history in their older sister. CP was considered a rare disease; however, recent studies have revealed that one third of pneumothoraxes in women needed surgery are due to CP [2]. CP with the estimated prevalence of 7.3–36.7 % in women in the reproductive age [6], is the most common type of Thoracic Endometriosis Syndrome (TES). TES is a broad term describing endometriosis in thorax cavity, the most common site of endometriosis after abdominopelvic cavity, involving lung parenchyma, diaphragm or pleural surface. Although, our understanding about CP has been increased in recent years, there are still aspects that is unclear to us, such as CP's pathophysiology. Many theories have attempted to explain CP and TES, including retrograde menstruation, coelomic metaplasia, migration of endometrial tissue, lymphatic and hematogenous dissemination, stem cell theory, and prostaglandin theory [6,[11], [12], [13]]. While the underlying pathology of CP is still elusive, retrograde menstruation theory, explained by Sampson in 1940 [14], is the most popular. This theory stated that endometrial cells due to retrograde flow in the fallopian tubes, move in the peritoneal cavity and accumulate at the right paracolic gutter and right subdiaphragmatic space. Then these cells infiltrate the diaphragm, leading to formation of endometrial lesion, which can spread to chest cavity through existed holes in diaphragm [11,13]. Also, these lesions can cause necrosis and forming passages to the thorax [4]. This theory seems to explain the underlying reason for the nine times more prevalence of TES at right diaphragm compared to left side [11]. However, it is unable to describe the etiology of TES in cases that have no diaphragmatic defects [12]. In this regard, while the main pathology is unknown to us, we want to mention the possible role of genetic factors. In addition to the three sisters diagnosed with CP presented in this paper, two previous papers also mentioned cases with positive family history for CP [15,16]. A study by Kiss et al. reported genotype findings related to CP [17]. While numerous studies investigated the genetic role in the endometriosis but very few evaluated this matter regarding TES and CP. Therefore, further investigation is essential to better understand the pathology of TES and CP, and the genetic impact.
VATS is the gold standard method for both diagnosis and treatment of CP [11]. Before utilizing VATS, axillary thoracotomy was the main treatment, which offered lower visualization capacity compared to VATS. Also, VATS offered shorter hospital stay, lesser pain and faster recovery [13]. However, while better than thoracotomy, the visualization of diaphragm is difficult through VATS [12], and needed through evaluation by experienced surgeon. Additionally, the timing of surgery is important [2]. It is suggested that perform VATS during menstruation to better observe the small and hidden lesions [2,5,18]. Inspection of TES thorough VATS demonstrated that endometrial implants and diaphragmatic perforations are the most observed findings [2,11,13].
Many different surgical procedures can be helpful in treatment of CP. A systematic review performed on 182 reported patients, found that 90 % of patients underwent surgery, which nearly two thirds of them was pleurodesis alone or in combination with other procedures [2]. Wedge resection, nodule excision, lobectomy, pleurectomy, bullectomy, and diaphragm resection and repair are other procedure utilized for treating CP [2,[4], [5], [6],11]. Pharmacological treatment for ovarian suppression is another treatment plan that utilized in TES and CP treatment similar to pelvic endometriosis. The pharmacological treatment can be used as a single treatment approach or in combination with surgery [5,6,11].
The main challenge of treating CP and the most common post-operative complication is the high rate of recurrence [2]. The reported incidence of recurrence following surgical treatment varies widely among studies. Two systematic evaluations on the reported cases in the literature reported post-operative recurrence rate of 8–40 % [5] and 14.3–55 % [6]. Therefore, it is important to attempt for reducing the recurrence rates. Investigations revealed that the recurrence rate is higher when patient treated with either hormone therapy or surgery alone. Therefore, post-operative administration of ovarian suppressive drugs is advisable to reduce the recurrence rate. In some cases, total hysterectomy and bilateral salpingo-oophorectomy have been reported to be effective in preventing further recurrences [19,20], as needed in the second case presented in this paper. Also, combination of different surgical procedure is helpful to lower the risk of recurrence [21]. Furthermore, it was shown that pleurodesis can reduce the recurrence rate by 20–25 % [11]. Another important point is that the presence of diaphragmatic defect plays a significant role in the recurrence [13], the issue that we believe lead to recurrence in our first patient, hence, it is essential to look for them during surgery to reduce the possible future recurrence.
While our study is one of the first to emphasize the possible role of genetic factors in CP, this study has the limitation that Genetic factors were not evaluated.
4. Conclusion
Catamenial pneumothorax is a relatively rare disease with an unclear pathophysiology. Moreover, the role of genetic factors has not been extensively evaluated in the literature. This paper reports CP in three sisters, highlighting the strong possibility of a genetic impact on the pathophysiology of CP. Additionally, the high risk of recurrence after VATS and even after thoracotomy is another issue, as evidenced by the two cases presented here. Reducing recurrence is essential, including accurate inspection of the diaphragm for possible defects and administering post-operative hormonal therapy.
Ethical approval
At our institution, we have a specific policy regarding ethical approval for deidentified case reports. For these reports, where patient identification and sensitive information have been removed, ethical approval is waived. This policy is overseen by the [name of our ethical committee]. We also affirm our commitment to adhering to the ethical principles outlined in the Declaration of Helsinki (1964). Consent for conducting the case report was obtained from the patient prior to transplantation and is available upon request.
Funding
There was no funding for this case report.
Guarantor
Dr. Mohsen Herik Dizaji accepts all responsibility of this article.
CRediT authorship contribution statement
Conceptualization and methodology: Mojtaba Mokhber Dezfouli, Mohsen Herik Dizaji, Kambiz Sheikhy.
Investigation and Data curation: Arman Hasanzade, Fariba Ghorbani, Mohsen Herik Dizaji.
Writing first draft: Mojtaba Mokhber Dezfouli, Arman Hasanzade, Fariba Ghorbani.
Writing–review & editing: all authors.
All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgement
We would like to express our gratitude to all those in individuals who precipitated in the treatment of this case and help us in the conducting of this case report.
Contributor Information
Kambiz Sheikhy, Email: ksheikhy@sbmu.ac.ir.
Mohsen Herik Dizaji, Email: mohsen.herik2024@gmail.com.
Data availability
Any additional data regarding this case report is available upon request.
References
- 1.Maurer E.R., James A. Surgical by Diaphragm. 2015. Pneumothorax cycle; pp. 17–18. [Google Scholar]
- 2.Bricelj K., Srpčič M., Ražem A., Snoj Ž. Catamenial pneumothorax since introduction of video-assisted thoracoscopic surgery: a systematic review. Wien. Klin. Wochenschr. 2017;129(19–20):717–726. doi: 10.1007/s00508-017-1237-4. [DOI] [PubMed] [Google Scholar]
- 3.Lillington G.A. Catamenial pneumothorax. JAMA. Mar 6 1972;219(10):1328. doi: 10.1001/jama.1972.03190360038009. (Internet) [DOI] [PubMed] [Google Scholar]
- 4.Visouli A.N., Zarogoulidis K., Kougioumtzi I., Huang H., Li Q., Dryllis G., et al. Catamenial pneumothorax. 2014;6(24):448–460. doi: 10.3978/j.issn.2072-1439.2014.08.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marjański T., Sowa K., Czapla A., Rzyman W. Catamenial pneumothorax - a review of the literature. Kardiochirurgia i Torakochirurgia Polska. 2016;13(2):117–121. doi: 10.5114/kitp.2016.61044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gil Y., Tulandi T. Diagnosis and treatment of catamenial pneumothorax: a systematic review. J Minim Invasive Gynecol [Internet]. 2020;27(1):48–53. doi: 10.1016/j.jmig.2019.08.005. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S., Yasuda K., Matsumura Y., Kondo T. 2006. Left-side Catamenial Pneumothorax With Endo; pp. 225–227. [DOI] [PubMed] [Google Scholar]
- 8.Laws H.L., Fox L.S., Younger J.B. Bilateral catamenial pneumothorax. Arch. Surg. 1977;112(5):627–628. doi: 10.1001/archsurg.1977.01370050087015. [DOI] [PubMed] [Google Scholar]
- 9.Nezhat C., King L.P., Paka C., Odegaard J., Beygui R. Bilateral thoracic endometriosis affecting the lung and diaphragm. Journal of the Society of Laparoendoscopic Surgeons. 2012;16(1):140–142. doi: 10.4293/108680812X13291597716384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023;109(5):1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nezhat C., Lindheim S.R., Backhus L., Vu M., Vang N., Nezhat A., et al. Thoracic endometriosis syndrome: a review of diagnosis and management. Journal of the Society of Laparoendoscopic Surgeons. 2019;23(3) doi: 10.4293/JSLS.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topbas Selcuki N.F., Yilmaz S., Kaya C., Usta T., Kale A., Oral E. Thoracic endometriosis: a review comparing 480 patients based on catamenial and noncatamenial symptoms. J Minim Invasive Gynecol [Internet]. 2022;29(1):41–55. doi: 10.1016/j.jmig.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Soares T., Oliveira M.A., Panisset K., Habib N., Rahman S., Klebanoff J.S., et al. Diaphragmatic endometriosis and thoracic endometriosis syndrome: a review on diagnosis and treatment. Horm. Mol. Biol. Clin. Invest. 2022;43(2):137–143. doi: 10.1515/hmbci-2020-0066. [DOI] [PubMed] [Google Scholar]
- 14.Sampson J.A. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol [Internet]. 1940;40(4):549–557. https://www.sciencedirect.com/science/article/pii/S0002937840912388 Available from: [Google Scholar]
- 15.Marcos Sánchez F, Chillón Loarte F, Plaza Díaz R, Rico González LM, Durán Pérez-Navarro A. [2 cases of catamenial pneumothorax with a familial relationship]. Vol. 13, Anales de medicina interna (Madrid, Spain: 1984). Spain; 1996. p. 254. [PubMed]
- 16.Hinson J.M., Brigham K.L., Daniell J. Catamenial pneumothorax in sisters. Chest. 1981;80(5):634–635. doi: 10.1378/chest.80.5.634. [DOI] [PubMed] [Google Scholar]
- 17.Kiss I., Pospisilova E., Kolostova K., Maly V., Stanek I., Lischke R., et al. Circulating endometrial cells in women with spontaneous pneumothorax. Chest [Internet]. 2020;157(2):342–355. doi: 10.1016/j.chest.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Bagan P., Berna P., Assouad J., Hupertan V., Le Pimpec Barthes F., Riquet M. Value of cancer antigen 125 for diagnosis of pleural endometriosis in females with recurrent pneumothorax. Eur. Respir. J. 2008;31(1):140–142. doi: 10.1183/09031936.00094206. [DOI] [PubMed] [Google Scholar]
- 19.Staring G., Monteiro F., Barracha I., Amorim R. Multi-loculated catamenial pneumothorax: a rare complication of thoracic endometriosis. Cureus. 2021;13(8):11–14. doi: 10.7759/cureus.17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arunthari V., Sevin B.U., Krishna M., Johnson M.M. Catamenial pneumothorax with umbilical and diaphragmatic endometriosis: a case report and review of the literature. South. Med. J. 2008;101(10):1043–1045. doi: 10.1097/SMJ.0b013e31817bf9e1. [DOI] [PubMed] [Google Scholar]
- 21.Pathak S., Caruana E., Chowdhry F. Should surgical treatment of catamenial pneumothorax include diaphragmatic repair? Interact. Cardiovasc. Thorac. Surg. 2019;29(6):906–910. doi: 10.1093/icvts/ivz205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any additional data regarding this case report is available upon request.